Abstract
Objective
Evaluate safety/tolerability/efficacy of MK-8242 in subjects with refractory/recurrent AML.
Methods
MK-8242 was dosed p.o. QD (30–250 mg) or BID (120–250 mg) for 7on/7off in 28-day cycle. Dosing was modified to 7on/14off, in 21-day cycle (210 or 300 mg BID).
Results
26 subjects enrolled (24 evaluable for response); 5/26 discontinued due to AEs. There were 7 deaths; 1 (fungal pneumonia due to marrow aplasia) possibly drug-related. With the 7on/7off regimen, 2 subjects had DLTs in the 250 mg BID group (both bone marrow failure and prolonged cytopenia). With the 7on/14off, no DLTs were observed in 210 mg BID or 300 mg BID (doses >300 mg not tested). Best responses were: 1/24 PR (11 weeks;120 mg QD, 7on/7off); 1/24 CRi (2 weeks;210 mg BID, 7on/14off); 1/24 morphologic leukemia-free state (4 weeks; 250 mg BID, 7on/7off). PK on Day7 at 210 mg BID revealed AUC0-12hr 8.7 μM*hr, Cmax 1.5 μM (n=5, Tmax, 2–6 hr), T1/2 7.9 hr, CLss/F 28.8 L/hr, and Vss/F 317 L.
Conclusions
The 7on/14off regimen showed a more favorable safety profile; no MTD was established. Efficacy was seen using both regimens providing impetus for further study of HDM2 inhibitors in subjects with AML.
Keywords: Human Double Minute 2 Inhibitor, MK-8242, Acute Myelogenous Leukemia, p53, Phase I
1. Introduction
Acute myelogenous leukemia (AML) is the most common form of myeloid leukemia, with an overall incidence rate of approximately 4.0 cases per 100,000 persons and a median age of onset of 67 [1, 2]. It is a clonal hematopoietic stem cell disorder with the accumulation of poorly differentiated progenitor cells that fail to respond to the normal regulators of proliferation and differentiation [3]. When left untreated, this uncontrolled hematopoietic proliferation generally leads to rapid death due to cellular infiltration of the bone marrow and organs, leading to bleeding and infection [4, 5].
In patients younger than 60 years, treatment of AML typically consists of cytotoxic chemotherapy with a general cure rate of 35–40% depending on the cytogenetic classification [5]. In contrast, standard chemotherapy produces a similar outcome in only 5–15 % of older patients, primarily because of their inability to tolerate intensive treatment and their generally higher risk disease features [4, 5]. Treatment of older or frail patients usually involves supportive care, low-dose cytarabine and hypomethylating agents; however, no consensus treatment algorithm exists for this difficult to treat population [5, 6]. Unfortunately, treatment outcomes in older patients who are unable to receive intensive chemotherapy are extremely poor, with a median survival of 5 to 10 months [6]. For the majority of treated AML patients who achieve remission, relapse will occur within 3 years of the initial diagnosis [4, 7]. In general, the prognosis for AML patients following disease relapse is poor and the treatment options are unsatisfactory [6, 8–11]. Therefore an urgent unmet medical need exists for the development of new AML therapies, particularly in older and relapsed patients.
The transcription factor p53 is a powerful tumor suppressor that plays a critical role in maintaining genomic stability and protecting against malignant transformation [12]. Nearly 50% of all human cancers harbor loss of function mutations in the p53 gene [13]. However, the frequency of p53 mutations in hematologic cancers is low with only 3–8% of AML cases demonstrating a detectable p53 mutation [14]. Even in the presence of wild type p53, other aberrations can deleteriously influence the function and activity of p53, including over-expression of negative regulators [15]. The interaction of p53 with one such negative regulator, murine double minute 2 (MDM2; sometimes called HDM2 for its human analog), normally plays a central role in controlling the cell cycle by arresting p53 function [16]. HDM2 directly binds to p53 thereby inhibiting its transcriptional activity and mediating its ubiquitination and subsequent degradation [17]. Additionally, inactivation of the p53 pathway by HDM2 overexpression allows cancer cells to avoid p53-driven apoptosis in response to treatment with cytotoxic agents and radiation therapy. In cancer cells, the tight control of the HDM2:p53 protein-protein interaction is disrupted, thereby minimizing the tumor-suppressive activities of HDM2 on p53 and promoting a cell growth advantage [12]. These data collectively support the notion that HDM2 inhibition may hold promise as a potential anti-cancer therapeutic strategy in a wide variety of human malignancies, either alone or in combination with traditional chemotherapy, by restoring normal p53 function [18].
Prior research has shown that HDM2 over-expression, frequently resulting from increased mRNA expression rather than gene amplification, is a signature associated with certain malignancies including AML [19–23]. Furthermore, leukemia cells over-expressing HDM2 are often resistant to conventional therapy resulting in a poor overall prognosis [24]. Taken together, these findings suggest that HDM2 inhibitors may be effective in patients with AML as well as other types of cancer. Indeed, small molecule HDM2 inhibitors have shown significant activity in animal models of leukemia and in preclinical experiments using patient AML cells and leukemic cell lines [25, 26]. Given these promising preclinical results, several HDM2 inhibitors have progressed to clinical evaluation in patients with AML and other hematologic malignancies (NCT02319369, NCT02098967, NCT02545283, NCT02319369; NCT02143635) [27].
MK-8242 (otherwise known as SCH 900242) is a potent, orally bioavailable, small-molecule inhibitor of the HDM2:p53 protein-protein interaction. MK-8242 has an IC50 value as low as 20 nM resulting in growth arrest and cell death [28]. This report describes the results of a Phase I clinical dose-ranging study designed to establish the recommended phase 2 dose (RP2D) of MK-8242 based on safety, tolerability and pharmacokinetics (PK) in adult patients with refractory or recurrent AML. Other objectives of this study were to determine the complete response (CR) rate, the complete response with incomplete platelet recovery (CRi) [29], and the duration of response following treatment with MK-8242.
2. Methods
2.1. Study design
This was a multi-center, non-randomized, open-label, 2-part (dose escalation and confirmation) study (Study Sponsor: Merck & Co., Inc., Kenilworth, New Jersey; Clinical Protocol MK-8242-005; www.clinicaltrials.gov NCT01451437) conducted between December 2011 and September 2014. The study was terminated in June 2014 by the Sponsor for reasons unrelated to safety (i.e., reprioritization of oncology portfolio) and only the monotherapy cohort (i.e., Arm A, Part 1) was enrolled.
2.2. Treatments
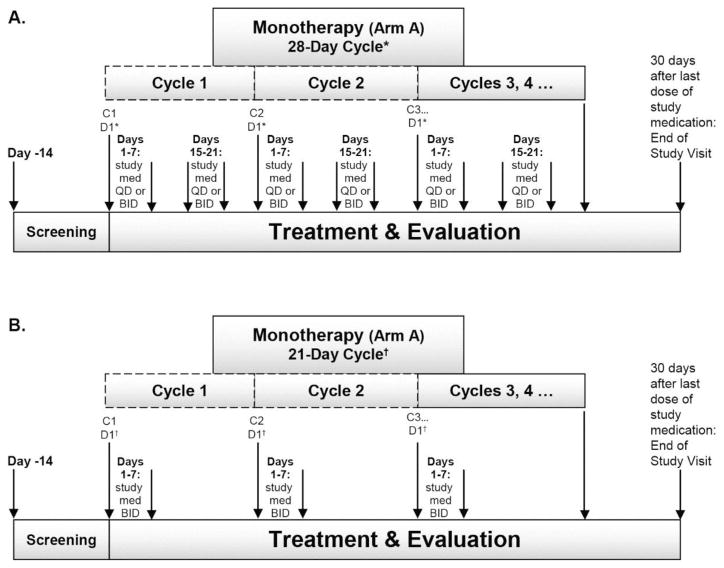
MK-8242 was initially dosed p.o. either QD (30 mg-250 mg) or BID (120 mg-250 mg) for 7 days on/7 days off (7 on/7 off) in a 28-day cycle (Fig. 1A). To improve the safety/tolerability profile of myelosuppression with prolonged cytopenia and infection, the dosing schedule was later modified to 7 on/14 off in a 21-day cycle (210 mg or 300 mg BID) (Fig. 1B).
Fig. 1.
Study diagram for Arm A, MK-8242 monotherapy using a (A) 28-day treatment cycle and (B) 21-day treatment cycle.
Subjects were initially enrolled in cohorts of single subjects and treated at accelerated, escalating dose levels of MK-8242 as per Simon et al. [30]. When ≥1 dose-limiting toxicity (DLT) occurred at a particular treatment level, escalation was converted to a modified 3+3 design [31]. When ≥1 subject at a given dose level experienced a Grade 2 or greater adverse event (AE) that did not meet DLT criteria and was not clearly attributable to another cause, the level was expanded to 3 subjects. If no additional Grade 2 or greater AEs occurred, escalation continued with the accelerated design. If at any time ≥2 subjects out of 3 experienced a Grade 2 or greater AE that was not clearly attributable to another cause, dose escalation converted to a 3+3 design for the remainder of the study. In Part 1, monotherapy escalation continued until a preliminary maximum tolerated dose (MTD) was identified. Patients continued to receive treatment until withdrawal criterion was met or up to 4 cycles of study treatment was received with additional cycles allowed at the Investigator’s/Sponsor’s discretion.
Each subject provided written informed consent prior to the conduct of any study procedures. The study protocols were approved by the Ethics Review Committees for the individual study centers. The study protocols were conducted in accordance with the guidelines on good clinical practice and with ethical standards for human experimentation established by the Declaration of Helsinki.
2.3. Eligibility criteria
Eligible patients were aged ≥18 years with a diagnosis of refractory or recurrent AML. They had to have an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 with adequate organ function (creatinine <1.5 upper limit of normal [ULN] or calculated creatinine clearance ≥60 mL/min, total bilirubin <1.5 ULN aspartate aminotransferase/serum glutamic-oxalecetic transaminases and alanine aminotransferase/serum glutamic-pyruvic transaminases (SGPT) <3 x ULN) and were ineligible for standard therapy. Subjects enrolled in the 3+3 escalation portion of Part 1 were required to have a confirmed WT p53 status (as per Roche Molecular Systems AmpliChip p53 Assay). Significant exclusion criteria included: active malignancy other than AML; history of leptomeningeal leukemia requiring intrathecal therapy; myelodysplasic syndrome (Part 1, only); isolated extramedullary leukemia without meeting bone marrow criteria for AML; diagnosis of AML blast crisis of chronic myelogenous leukemia; bone marrow transplant with active graft-versus-host disease or who received immunosuppressive therapy.
Patients could be withdrawn from the study for failure to comply with the study requirements, pregnancy, disease recurrence or progression, dose delay >2 weeks, intercurrent illness preventing further administration of treatment, unacceptable AEs not manageable by symptomatic therapy, or a serious or life-threatening AE. A subject who discontinued from the study was allowed to be replaced if they did not complete the evaluation period for DLTs in Cycle 1 for reasons other than study treatment-related toxicity.
Dose delays of up to 14 days were allowed for any ≥Grade 3 hematologic AE not clearly attributable to underlying disease. In addition, dose delays were allowed for ≥Grade 3 non-hematologic AE not clearly attributable to underlying disease or another cause, and persist beyond 72 hours following maximal supportive care. Subjects were assessed weekly until the AE resolved.
2.4. Study endpoints
The primary safety endpoint was to identify DLTs and establish the RP2D. Other safety endpoints included AEs, laboratory safety assessments, ECOG performance status, electrocardiograms (ECGs), vital signs and physical examinations. All laboratory assessments were performed on-site. Pre-specified AEs of clinical interest associated with AML included myelosuppression, neutropenia, thrombocytopenia, fever, infection, bleeding, and fatigue.
The primary efficacy endpoint was to find the percentage of evaluable subjects with clinically relevant responses including CR, or CRi, as defined by Cheson et al. at the RP2D [29]. Other endpoints included AEs and laboratory safety assessments; CR and CRi rates at dose levels other than RP2D; and duration of CR and CRi response.
2.5. Study assessments
AEs were graded according to the National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0. A DLT was any ≥Grade 3 non-hematological toxicity considered drug-related during Cycle 1, except: (1) infection, fatigue, anorexia or alopecia; (2) Grade 3 nausea, vomiting, diarrhea or dehydration responsive to treatment (i.e., resolving <72 hours with maximal supportive care); (3) any ≥Grade 3 toxicities not related to an underlying disease or another event.
A hematological DLT was defined as neutropenia and/or thrombocytopenia (i.e., neutrophil count <500 mm3 and/or platelet count <25,000 mm3) observed in Cycle 1 by Day 42 or Day 35 for the 7 on/7 off and 7 on/14 off schedules, respectively, and lasting >6 weeks in the context of no morphological evidence of recurrent or residual acute leukemia at Day 28 (i.e., bone marrow blasts <5% and no clusters of blasts).
The preliminary MTD was the dose at which the percentage of subjects experiencing a DLT was less than 33%. Dose escalation in Part 1 was scheduled to continue until an MTD was established at which point dose confirmation in Part 2 was scheduled to begin followed by response assessment.
Physical examinations, ECGs, vital signs, urinalysis, blood chemistry and laboratory safety test were performed. AEs were followed for ≥30 days following the administration of the last dose of study therapy. Subjects who were discontinued from the study due to AEs were followed until resolution or stabilization of the AE. Bone marrow biopsies and aspirates for p53 status were shipped to assigned laboratories for analysis.
The PK profiles of MK-8242 and M16 (a metabolite of MK-8242 with similar in-vitro potency) were characterized following oral administration of multiple doses of MK-8242 during Cycle 1. Blood samples for the determination of plasma MK-8242 concentrations were collected from each subject pre-dose and at Days 1, 6, 7 and 8 post-dose following administration of MK-8242 in Cycle 1. These samples were collected in chilled 6 mL K2 EDTA vacutainers and were centrifuged between 1000–1300 RCF (x g) at 4°C for 15 minutes, and stored at −20°C or colder until analysis. Plasma samples were analyzed for MK-8242 using a validated assay liquid chromatography–mass spectrometric assay with a lower limit of quantitation of 20 ng/mL and an analytical range of 20 to 10000 ng/mL.
2.6. Response methodology
Bone marrow aspirates and biopsies were obtained monthly for Cycles 2–4, then every other cycle, until the discontinuation visit. If the morphologic result was ambiguous, a second bone marrow examination was performed one week later. Responses were evaluated following Cycle 1 according to the International Working Group criteria adapted from Cheson et al. for CR, CRi, and partial remission (PR) [29].
Briefly, the designation of morphologic leukemia-free state required less than 5% blasts in an aspirate sample with marrow spicules and with a count of at least 200 nucleated cells. There could not be any blasts with Auer rods or persistence of extramedullary disease. The presence of a unique phenotype (by flow cytometry) identical to that found in the pretreatment specimen (e.g., CD34, CD7 coexpression) was considered persistence of leukemia. CR was defined as a morphologic leukemia-free state with a neutrophil count ≥1,000/μL, a platelet count ≥100,000/μL, no extramedullary disease, and red blood cell transfusion independence.
For CRi, subjects had to fulfill all of the criteria for CR except for residual neutropenia (<1,000/μL), thrombocytopenia (<100,000/μL), or red blood cell transfusion dependence. PR was defined as a ≥50% decrease in bone marrow blasts to 5% to 25% in the bone marrow. A value of <5% blasts was also considered a PR if Auer rods were present. A determination of PR required a neutrophil count ≥1,000/μL, a platelet count ≥100,000/μL, and no extramedullary disease. Disease progression was defined as an increase of 50% or more in bone marrow or circulating blasts, new development of circulating blasts on at least 2 consecutive determinations, or development of extramedullary disease. Relapse was defined as a reappearance of leukemic blasts in the peripheral blood or >5% blasts in the bone marrow not attributable to any other cause (e.g., bone marrow regeneration) following the determination of CR. The appearance of new dysplastic changes also was considered a relapse. Stable disease was defined as any disease state not meeting the criteria for CR, CRi, PR, disease progression, or relapse.
2.7. Statistical analyses
Safety and tolerability were assessed by clinical review of all relevant parameters including AEs, laboratory tests, vital signs, and ECG measurements. Toxicities were recorded according to NCI-CTCAE 4.0 and summarized by dose level. The number and percentage of DLTs in each dose level were provided. AEs were summarized as counts and frequencies for each dose level. Laboratory assessments, vital signs, and other safety endpoints were summarized as appropriate.
MK-8242 PK parameters were estimated and summarized by dose level using descriptive statistics in Parts 1 and 2. The following MK-8242 PK parameters were estimated: maximum observed plasma concentration (Cmax), area under the plasma concentration time curve (AUC), time of maximum observed plasma concentration (Tmax), accumulation ratio (R), and if feasible, apparent terminal half-life (t1/2), apparent total body clearance (CL/F), and volume of distribution (Vd/F). For all PK parameters except Tmax, the geometric mean and coefficient of variation (median and range for Tmax) were calculated at each dose level. To assess dose proportionality, the power model at a 90% confidence interval was applied to log-transformed PK parameters. The geometric mean accumulation ratio from Day 1 to Day 7 was measured using AUC0-24hr for QD dosing, or AUC0-12hr for BID dosing.
3. Results
3.1. Subject characteristics
At the time of trial termination, 26 subjects (all with AML) had been enrolled in Arm A, Part 1 and received study treatment across 9 dose levels. Subject characteristics are summarized in Table 1. The median age was 66 years (range: 29–81) and the majority of subjects were males (77%). Per the study inclusion criteria, all enrolled patients were diagnosed with recurrent or refractory acute myelogenous leukemia (AML) at study entry (N=26; Supplementary Table 1). Three (3/26=12%) patients in this study had been previously diagnosed and only once treated for AML, excluding the current recurrence that led to study entry. All 3 of these patients had ‘progressive disease’ as their best response prior to entering the study. Of the patients with multiple prior recurrences of relapsed/refractory AML, 13 (57%), 7 (30%), 2 (9%), and 1 (4%) patients had 1, 2, 3 and 6 previous recurrence(s) of disease, respectively, excluding the current recurrence that led to study entry. A total of 23 (23/26= 89%) enrolled patients had genetic testing performed at baseline to determine WTp53 status. Of the enrolled patients with genetic testing, 7 (30%) were confirmed positive for WT p53 and 16 (70%) had abnormal genetic findings (i.e., p53 mutations).
Table 1.
Baseline demographics and disease characteristics
| Dosing schedule | 7 on/7 off in a 28-day cycle | 7 on/14 off in a 21-day cycle | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MK-8242 dose | 30 mg QD | 60 mg QD | 120 mg QD | 250 mg QD | 120 mg BID | 170 mg BID | 250 mg BID | 210 mg BID | 300 mg BID | |
| Number enrolled | 1 | 1 | 3 | 1 | 4 | 3 | 3 | 6 | 4 | 26 |
| Sex (male), no. (%) | 1 (100) | 1 (100) | 2 (66.7) | 1 (100) | 4 (100) | 2 (66.7) | 3 (100) | 3 (50) | 3 (75) | 20 (76.9) |
| Age (years), mean ± SD | 63.0 | 74.0 | 72.3 ± 6.7 | 77.0 | 58.8 ± 18.5 | 63.3 ± 14.6 | 72.3 ± 12.5 | 63.5 ± 11.5 | 41.0 ± 14.3 | 62.2 ± 15.5 |
| Race, no. (%) | ||||||||||
| White | 1 (100) | 1 (100) | 3 (100) | 1 (100) | 4 (100) | 3 (100) | 3 (100) | 5 (83.3) | 3 (75) | 24 (92.3) |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 1 (25) | 2 (7.6) |
| Primary Diagnosis | ||||||||||
| Recurrent AML | 0 | 0 | 2 (66.7) | 1 (100) | 0 | 0 | 2 (66.7) | 3 (50) | 2 (50) | 10 (38.5) |
| Refractory AML | 1 (100) | 1 (100) | 1 (33.3) | 0 | 4 (100) | 3 (100) | 1 (33) | 3 (50) | 2 (50) | 16 (61.5) |
| WT p53 positive | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (33.3) | 0 | 3 (50.0) | 2 (50.0) | 7 (26.9) |
| WT p53 negative | 1 (100) | 1 (100) | 2 (66.7) | 1 (100) | 4 (100) | 2 (66.7) | 1 (33.3) | 3 (50.0) | 1 (25.0) | 16 (61.5) |
| Genetic testing not performed | 0 | 0 | 0 | 0 | 0 | 0 | 2 (66.7) | 0 | 1 (25.0) | 3 (11.5) |
3.2. Subject disposition and treatment exposure
All 26 subjects discontinued from the study early, with 14 (54%) discontinuing due to progressive disease, 5 (19%) discontinuing due to AEs and 7 (27%) due to other reasons, 4 subjects (15.4%) discontinuing due to the physician’s decision, 2 subjects (7.7%) discontinuing due to withdrawal of consent, and 1 subject (3.8%) discontinuing due to a protocol violation (concomitant treatment with an antineoplastic agent; Table 2). Overall, subjects received a median of 1 treatment cycle (range: 1 to 7) with a median of 21.0 days on therapy.
Table 2.
Summary of subject disposition and treatment exposure
| Dosing schedule | 7 on/7 off in a 28-day cycle | 7 on/14 off in a 21- day cycle | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MK-8242 dose | 30 mg QD | 60 mg QD | 120 mg QD | 250 mg QD | 120 mg BID | 170 mg BID | 250 mg BID | 210 mg BID | 300 mg BID | |
| Number enrolled | 1 | 1 | 3 | 1 | 4 | 3 | 3 | 6 | 4 | 26 |
| Discontinued, no (%) | 1 (100) | 1 (100) | 3 (100) | 1 (100) | 4 (100%) | 3 (100) | 3 (100) | 6 (100) | 4 (100) | 26 (100) |
| AE | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 2 (66.7) | 1 (16.7) | 1 (25) | 5 (19.2) |
| Physician decision | 1 (100) | 1 (100) | 2 (66.7) | 0 | 0 | 0 | 0 | 0 | 0 | 4 (15.4) |
| Progressive disease | 0 | 0 | 1 (33.3) | 1 (100) | 3 (75) | 2 (66.7) | 1 (33.3) | 3 (50) | 3 (75) | 14 (53.8) |
| Protocol violation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (3.8) |
| Withdrawal by subject | 0 | 0 | 0 | 0 | 1 (25) | 0 | 0 | 1 (16.7) | 0 | 2 (7.7) |
| No. days on therapy, median (range) | 91 (91-91) | 21 (21-21) | 21 (21–190) | 20 (20-20) | 43 (6–104) | 77 (47–79) | 21 (21-21) | 7 (7–63) | 7 (7–57) | 21 (6–190) |
| No. treatment cycles, median (range) | 4 (4-4) | 1 (1-1) | 1 (1–7) | 1 (1-1) | 2 (1–4) | 3 (2–3) | 1 (1-1) | 1 (1–3) | 1 (1–2) | 1 (1–7) |
AE = adverse event
3.3. Overall AE summary
All subjects had ≥1 AE, with 18 (69.2%) of all subjects experiencing ≥1 drug-related AE of any grade (Supplementary Table 2). Commonly-reported, drug-related AEs of any grade included diarrhea (23.1%), nausea (42.3%), vomiting (34.6%), fatigue (19.2%), and decreased appetite (23.1%). All were Grades 1– 2, except one Grade 3 fatigue. Nausea occurred more frequently in subjects dosed BID 10/20 (50.0%) versus those dosed QD 1/6 (16.7%). All of these AEs were considered expected for MK-8242.
Sixteen subjects (61.5%) experienced an SAE; only 2 (7.7%) were considered to be drug-related. One subject, dosed at 210 mg BID, experienced Grade 3 atrial fibrillation and Grade 2 acute renal failure. The other subject, dosed at 250 mg BID, experienced a DLT of bone marrow failure (Grade 4) associated with Grade 5 fungal pneumonia, Grade 4 febrile neutropenia, Grade 3 Escherichia infection, and Grade 2 cellulitis.
In total, 7 deaths occurred within 52 days (range, 20–52 days) after the first dose of MK-8242, including 5 deaths in the 7 on/7 off dosing schedule and 2 deaths in the 7 on/14 off schedule. The most frequently reported causes of death were respiratory-related (4; i.e., 3 pneumonia and 1 respiratory failure) followed by AML progression (2) and sepsis (1). One death (i.e., fungal pneumonia due to marrow aplasia) was reported to be drug-related.
Five (19.2%) subjects experienced ≥1 Grade 3–5 drug-related AE, including 2 (1 each in the 120 mg QD and 250 mg BID dose groups) in the 7 on/7 off dosing schedule and 3 (2 in the 210 BID and 1 in the 300 mg BID dose groups) in the 7 on/14 off schedule (Table 3).
Table 3.
Overall summary of drug-related Grade 3–5 AEs (n/N; %) presented by MK-8242 dose
| Dosing schedule | 7 on/7 off in a 28-day cycle | 7 on/14 off in a 21-day cycle | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MK-8242 dose | 30 mg QD n=1 |
60 mg QD n=1 |
120 mg QD n=3 |
250 mg QD n=1 |
120 mg BID n=4 |
170 mg BID n=3 |
250 mg BID n=3 |
210 mg BID n=6 |
300 mg BID n=4 |
N=26 |
| Subjects with ≥1 drug-related AE, Grade 3–5 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (33.3) | 2 (33.3) | 1 (25.0) | 5 (19.2) |
| Blood and lymphatic system disorders | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 2 (7.7) |
| Bone marrow failure | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) |
| Leukopenia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) |
| Neutropenia | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 2 (7.7) |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) |
| Cardiac disorders | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (3.8) |
| Atrial fibrillation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (3.8) |
| General disorders and administration site conditions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (3.8) |
| Fatigue | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (3.8) |
| Infections and infestations | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) |
| Escherichia infection | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) |
| Pneumonia fungal | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) |
| Investigations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (3.8) |
| Blood bilirubin increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (3.8) |
| Metabolism and nutrition disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (25.0) | 2 (7.7) |
| Hypocalcaemia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) |
| Hypokalaemia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (25.0) | 2 (7.7) |
| Hypomagnesaemia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) |
| Hypophosphataemia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) |
| Vascular disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) |
| Hypotension | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.8) |
Every subject is counted a single time for each applicable row and column
3.4. DLT summary
Subjects were initially treated at 30, 60, 120, and 250 mg QD and at 120, 170, and 250 mg BID, using a 7 day on/7 day off dosing schedule in a 28-day treatment cycle. Hematological DLTs occurred in 2 out of 3 patients who were enrolled in the 250 mg BID group (Grade 4 bone marrow failure and Grade 1 bone marrow failure with prolonged cytopenia which met the hematologic DLT criteria; Supplementary Table 3) which was dosed above the MTD as defined by the study protocol. The trajectory of decreases in peripheral blood counts in these patients suggested that Cycle 1, Day 14 was likely at or near the nadir. Re-dosing MK-8242 on Cycle 1 Day 15 could have reintroduced toxicity prior to potential recovery. Holding the Day 15 dose and re-dosing on Day 21 (i.e. 7 days on/14 days off) was expected to result in less hematologic toxicity and a greater likelihood of recovery. Unpublished data from subjects with advanced solid tumors enrolled in a separate study, which utilized BID dosing and a 7 days on/14 days off dosing schedule in a 21-day treatment cycle, provided additional support for this hypothesis. Therefore the dosing schedule was changed to a 7 day on/14 day off schedule in a 21-day treatment cycle. After switching to the 7 on/14 off schedule, no DLTs were observed for the 210 mg BID or 300 mg BID groups (doses above 300 mg not tested). No MTD was established for 7 on/14 off dosing schedule.
Since doses above 300 mg BID were not tested in this study (unrelated to safety), a RP2D for MK-8242 monotherapy could not be established utilizing the 7-day on/14-day off dosing schedule.
3.5. Hematologic response rates
Bone marrow biopsies were available for 24/26 (92%) subjects in this study; however, 2 subjects did not have Day 28 and Day 42 bone marrow biopsies due to medical reasons. Across both dosing schedules, the best responses were as follows: 1 PR observed after 11 weeks of treatment (4%; 120 mg QD, 7 on/7 off) with a response duration of 113 days (i.e., this patient had a diagnosis of refractory AML and was confirmed positive for WT p53 at baseline); 1 CRi observed after 2 weeks of treatment (4%; 210 mg BID, 7 on/14 off) with a response duration of 51 days (i.e., this patient had a diagnosis of recurrent AML and was confirmed positive for WT p53 at baseline); 1 morphologic leukemia-free state after 4 weeks of treatment (4%; 250 mg BID, 7 on/7 off) with a response duration of 22 days (i.e., this patient had a diagnosis of recurrent AML and had an unknown genetic status at baseline).
3.6. Pharmacokinetics
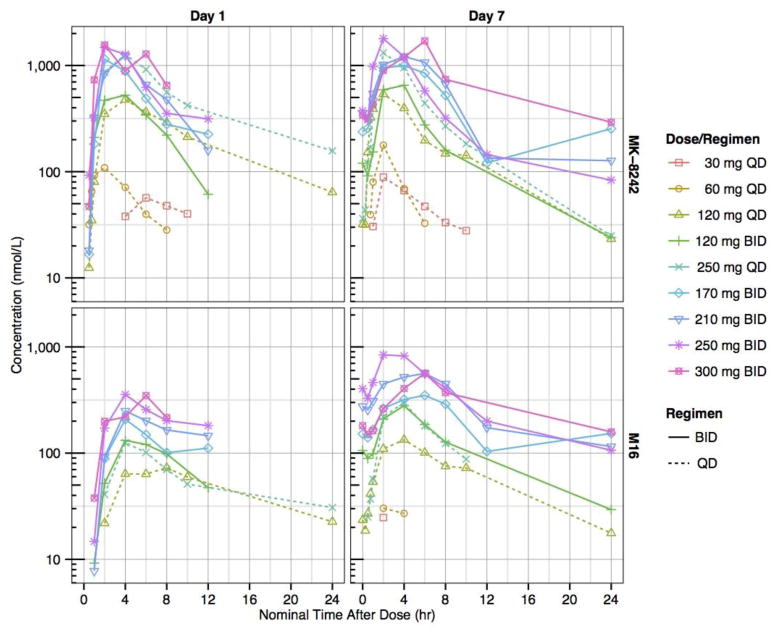
The PK profiles of MK-8242 and M16 (a metabolite of MK-8242 with similar in vitro potency) were characterized on Days 1 and 7 (Fig. 2, Table 4 and Supplementary Table 4). On Day 7 (following 7 days of dosing) across all treatment arms, the median Tmax range was 1.6 to 4.6 hr for MK-8242, and 2.0 to 4.8 hr for M16; and geometric mean half-life range was 4.6 to 10.3 hr for MK-8242, and 3.8 to 9.7 hr for M16. The 210 mg BID dose revealed a geometric mean AUC0-12hr of 8.7 μM*hr, Cmax of 1.5 μM (n=5, Tmax, 2–6 hr), t1/2 of 7.9 hr, CLss/F of 28.8 L/hr, and Vss/F of 317 L. Moderate to high inter-subject variability in AUC and Cmax was observed, as measured by the coefficient of variation (CV).
Fig. 2.
Mean plasma-concentration time profiles of MK-8242 and metabolite M16 on Days 1 and 7 following treatment with MK-8242 30–300 mg doses administered once-daily (QD) or twice-daily (BID). Only one dose was administered on Day 7 in BID panels to support PK characterization.
Table 4.
Summary of pharmacokinetic parameters of MK-8242 following 30 – 250 mg doses of MK-8242 administered once-daily (QD) or twice-daily (BID) on Days 1 and 7 (only 1 dose was administered on Day 7 in BID panels to support PK characterization). PK parameters are maximum concentration (Cmax), time of maximum concentration (Tmax), area under the concentration-time curve from 0 to 12 hr (AUC0-12hr), area under the concentration-time curve from 0 to 24 hr (AUC0-24hr), terminal half-life (t1/2), apparent total body steady-state clearance (CLss/F), volume of distribution at steady-state (Vss/F). All PK parameters are geometric means (% coefficient of variation), except for Tmax, which is median (range).
| Dose | N | Day | Cmax (nM)a | Tmax (hr)b | AUC0-12hr (hr*nM)a | AUC0-24hr (hr*nM)a | t1/2 (hr)a | CLss/F (L/hr)a | Vss/F (L)a |
|---|---|---|---|---|---|---|---|---|---|
| 30 mg QD | 1 | 1 | 56.7 | 6.2 | 398 | 604 | -- | -- | -- |
| 1 | 7 | 88.8 | 2.0 | 522 | 665 | 4.7 | 53.7 | 364 | |
| 60 mg QD | 1 | 1 | 109 | 2.0 | 510 | 510 | -- | -- | -- |
| 1 | 7 | 178 | 2.0 | 515 | 515 | -- | 139 | -- | |
| 120 mg QD | 3 | 1 | 416 (81) | 4.0 (2.1 – 4.0) | 2780 (107) | 3710 (126) | -- | -- | -- |
| 3 | 7 | 530 (56) | 2.0 (1.0 – 2.0) | 2800 (52) | 3460 (55) | 5.33 (24) | 41.3 (55) | 318 (34) | |
| 120 mg BID | 4 | 1 | 561 (52) | 4.0 (2.1 – 4.0) | 2810 (90)c | 3230 (101)c | -- | -- | -- |
| 3 | 7 | 744 (56) | 4.0 (2.0 – 4.0) | 3360 (42) | 4180 (41) | -- | 42.5 (42) | -- | |
| 250 mg QD | 1 | 1 | 1270 | 4.0 | 7930 | 10900 | -- | -- | -- |
| 1 | 7 | 1320 | 2.0 | 6070 | 6840 | 4.57 | 43.5 | 287 | |
| 170 mg BID | 3 | 1 | 984 (93) | 2.0 (2.0 – 4.1) | 5940 (135)d | 6940 (127)d | -- | -- | -- |
| 3 | 7 | 1200 (65) | 4.2 (2.2 – 6.0) | 6220 (83) | 8440 (105) | 10.30e | 32.5 (83) | 775e | |
| 210 mg BID | 5 | 1 | 1310 (52) | 2.0 (1.9 – 4.1) | 5500 (64)f | 6400 (76)f | -- | -- | -- |
| 5 | 7 | 1550 (51) | 4.0 (2.0 – 6.0) | 8670 (48) | 11200 (53) | 7.92 (32)c | 28.8 (48) | 317 (50)c | |
| 250 mg BID | 3 | 1 | 1510 (66) | 2.0 (2.0 – 4.0) | 6710 (76) | 7910 (82) | -- | -- | -- |
| 3 | 7 | 1500 (108) | 2.0 (1.9 – 4.1) | 7240 (74) | 8800 (57) | 5.21 (18)d | 41.1 (74) | 222 (70)d | |
| 300 mg BID | 3 | 1 | 1940 (26) | 2.1 (2.0 – 6.0) | 8300e | 9480e | -- | -- | -- |
| 3 | 7 | 1920 (81) | 4.0 (4.0 – 6.0) | 10100 (50) | 14200 (62) | 7.06 (37)d | 35.4 (50) | 384 (31)d |
Geometric mean (%CV);
Median (min - max);
N=3;
N=2;
N=1;
N=4;
The daily exposure target of MK-8242 was established in the preclinical xenograft model as 25.0 μM*hr. On Day 7, there were no doses for which the geometric mean projected daily exposure of MK-8242 or M16 was greater than the daily exposure target. On Day 7 following 7 days of dosing and across all treatment arms, the geometric mean projected daily exposure (based on geometric mean AUC0-24hr for QD dosing, or 2x geometric mean AUC0-12hr for BID dosing) range was 515 to 20200 nM*hr for MK-8242, and 37.1 to 11000 for M16. The geometric mean projected daily exposure of MK-8242 + M16 was greater than the target exposure for doses of 210 mg BID (27.5 μM*hr), 250 mg BID (25.5 μM*hr), and 300 mg BID (28.3 μM*hr). On Day 7, the projected daily exposure increased in a supra-proportional manner with respect to the total daily dose for MK-8242, M16, and their sum. The geometric mean accumulation ratio range across all treatment arms was 0.63 to 1.33 for MK-8242, and 1.77 to 3.11 for M16.
4. Discussion
In this study, MK-8242 was initially administered orally either QD or BID across 7 dose levels ranging from 30 mg to 250 mg using a 7-day on/7-day off dosing schedule in a 28-day cycle. Following the observation of hematologic toxicities, the dosing schedule was subsequently modified whereby MK-8242 was administered orally at 250 mg and 300 mg BID using a 7-day on/14-day off schedule in a 21-day cycle. A limited number of cycles were administered (median of 1) to patients in this study primarily due to either disease progression or complications related to treatment. A total of 2 DLTs out of 3 patients were observed at the 250 mg BID dose using the 7-day on/7-day off schedule. The observed DLTs were bone marrow failure with associated prolonged cytopenia (i.e., Grade 4 bone marrow failure and Grade 1 bone marrow failure with prolonged cytopenia which met the hematologic DLT criteria). No DLTs were observed at the 210 mg BID and 300 mg BID dosing levels using the 7-day on/14-day off dosing schedule in a 21-day treatment cycle (doses above 300 mg not tested). The results of this study show that treatment with MK-8242 as monotherapy had an acceptable safety and toxicity profile. The modified 7-day on/14-day off schedule demonstrated a more favorable safety profile compared to the 7-day on/7-day off schedule; however, an MTD was not established for the 7-day on/14-day off schedule. Thus, a RP2D was not identified in this study.
Overall, the pattern of toxicity seen in this study was consistent with previous findings of another HDM2 inhibitor[32] and included gastrointestinal toxicities, particularly diarrhea, nausea, vomiting, as well as hematologic toxicities, which included neutropenia and thrombocytopenia. Most of the commonly reported, drug-related AEs observed in this study were less than Grade 3 and manageable. However, there were instances of Grade 3–5 hematologic SAEs consisting of bone marrow failure, leukopenia, febrile neutropenia, neutropenia and thrombocytopenia (all occurring in 2 patients at the 250 mg BID dose using the 7-day on/7-day off schedule). One drug-related death of fungal pneumonia occurred in a subject who also experienced a Grade 4 DLT of bone marrow failure at the 250 mg BID dose using the 7-day on/7-day off schedule. The hematologic AEs and infections seen in this study are common and expected in patients with advanced AML, thus rendering it difficult to distinguish between those that may have been due to the drug and those related to underlying leukemia. In addition, 1 subject had a Grade 3 drug-related AE of atrial fibrillation. Atrial fibrillation also was reported in a modest proportion of patients (~10%) treated with another clinically tested HDM2 inhibitor [32]. The mechanism(s) underlying the potential anti-rhythmic effects of MDM2 inhibitors is unclear and has not been reported widely in the literature. A knockout study in mice showed that full ablation of MDM2 gene induces cardiomyocyte loss resulting in embryonic lethality [33]. In the case of ibrutinib (another small molecule inhibitor), the proposed mechanism of cardiotoxicity involves possible inhibition of cardiac PI3K-AKT signaling [34, 35]. It is possible that MK-8242 and other similar compounds also may inhibit cardiac muscle enzymes and signaling molecules.
Pharmacokinetic analysis revealed that MK-8242 and its bioactive metabolite, M16, exhibited non-linear kinetics in that the increase in exposure with respect to dose was supra-proportional (i.e., greater than linear). Neither MK-8242 nor M16 individually met the preclinically established daily exposure target at any dose tested in this study; however, the sum of MK-8242 and M16 exceeded the target for doses of 210, 250, and 300 mg BID.
The preliminary assessment of efficacy in this study demonstrated modest single-agent activity of MK-8242 in patients with very advanced AML. All patients had recurrent or refractory disease at study entry, with refractory disease being the most common, and the median age was 62 years. Among 26 evaluable patients, 3 experienced an objective response following treatment with MK-8242 monotherapy according to IWG criteria. The best responses included 1 PR after 11 weeks (120 mg QD, 7on/7off) in a patient diagnosed with refractory AML and confirmed positive for WT p53; 1 CRi after 2 weeks (210 mg BID, 7on/14off) in a patient diagnosed with recurrent AML and confirmed positive for WT p53; and 1 morphologic leukemia-free state after 4 weeks (250 mg BID, 7on/7off) in a patient diagnosed with recurrent AML and an unknown genetic status at baseline. The limited clinical activity seen in this study may be due to the generally more advanced disease state of the study population and limited number of treatment cycles. It is unclear if MK-8242 might show greater efficacy in less heavily pre-treated or younger patients, longer duration of treatment, or when administered in combination with other traditional chemotherapeutic agents.
MDM2 Inhibitors have been shown to have an acceptable tolerability profile in preclinical studies with normal cells showing less susceptibility toward drug-induced death compared with cancer cells [36–38]. However, given the adverse events seen in this and other clinical studies the preclinical findings may not be fully predictive of the human toxicity profile. In addition, previous in vitro reports showed that non-genotoxic HD2M inhibitors can sensitize cells to the effects of conventional chemotherapeutic agents, such as cytarabine and selumetinib, thus providing a strong rationale for moving combination regimens forward into clinical testing [26, 39–42]. It should be possible to use MK-8242 in combination with cytotoxic chemotherapy, if continuous dosing of the drug is not employed during the treatment cycle. For example, if cytarabine is used in combination with MK-8242 on a 21-day treatment cycle, MK-8242 for the combination therapy would be administered on Days 1–7 and cytarabine on Days 1–4 of each treatment cycle to allow sufficient time for bone marrow recovery prior to the start of next treatment cycle. To this end, the safety and efficacy profiles of several HDM2/chemotherapeutic combination regimens are being extensively explored in early clinical trials of patients with hematological and solid tumors [28, 43]. Our preclinical data suggests that MK-8242 and cytarabine enhance survival when coadministered using an EOL-1 Luc animal model at a range of clinically relevant doses including those used in this study. Unfortunately, the present clinical study was terminated before the combination cytarabine treatment arm was enrolled. Nevertheless, several studies using other HDM2 inhibitors either have explored or are currently exploring the clinical efficacy and safety/tolerability of combination therapy with cytarabine in patients with AML (NCT01635296, NCT01773408, NCT02545283). The gastrointestinal, hematologic and potential cardiac AEs seen in this study with the administration of MK-8242 should be taken into consideration in the design of future studies to minimize toxicities and maximize clinical efficacy. This is especially important for combination therapy studies in light of the fact that standard chemotherapeutic agents also elicit similar toxicities that may be exacerbated when administered together with HDM2 inhibitors.
Modest clinical efficacy was observed in this study with single-agent MK-8242. Using the 7-day on/14 day-off dosing schedule MK-8242 showed adequate safety at doses up to 300 mg BID with no significant AEs and no DLTs. An MTD and RP2D were not established because MK-8242 development was not further pursued by the sponsor for reasons unrelated to safety. Thus adequate exposure for single agent activity, at least based on preclinical models, was not reached in this study. In addition, combination tolerance and activity was not explored in this study. Thus, additional studies are required to more thoroughly evaluate the potential clinical activity of H2DM inhibitors both as monotherapy and in combination in patients with AML. The results of the current study should guide improved trail design, patient selection and treatment outcomes for future studies.
5. Conclusion
In summary, MK-8242 showed adequate safety in patients with recurrent/relapsed AML at doses up to 300 mg BID, using a modified 7-day on/14-day off dosing schedule. No DLT’s or significant adverse effects were observed. However, a MTD and RP2D were not established for the better tolerated 7-day on/14-day off dosing schedule because the study ended early. Further single agent tolerance was not explored by the sponsor since doses above 300 mg BID were not tested in this study. The observed toxicity profile of MK-8242 was consistent with that reported for other HDM2 inhibitors, and included gastrointestinal and hematologic AEs and one event of atrial fibrillation. The incidence of these AEs appeared to be dose related. Single agent clinical activity was observed in this study, providing impetus for further evaluation of HDM2 inhibitors administered either as a single agent or in combination with standard cytotoxic chemotherapy in patients with AML.
Supplementary Material
Highlights.
This study evaluated MK-8242 in subjects with wild type p53 refractory/recurrent AML.
Adequate safety was seen at doses ≤300mg BID using 7-day on/14-day off dosing.
Observed dose-related toxicities included gastrointestinal and hematologic AEs.
An MTD and RP2D were not established for 7-day on/14-day off dosing schedule.
Single agent clinical activity was observed in this study.
Acknowledgments
The authors would like to acknowledge Kristen Lewis (Merck & Co., Inc., Kenilworth, NJ) and Sheila Erespe (Merck & Co., Inc., Kenilworth, NJ) for their assistance preparing this paper for publication.
Funding for this study was provided by Merck & Co., Inc., Kenilworth, New Jersey, USA. The funding organization was involved in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Footnotes
Contributions
FR, IG, MMP, MDM, HK, AJL-L, CF, RL, MBJ, CDK, SR, PSP and RT are responsible for the work described in this paper. All authors were involved in at least one of the following: [conception, design, acquisition, analysis, statistical analysis, interpretation of data] and [drafting the manuscript and/or revising/reviewing the manuscript for important intellectual content]. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The study was sponsored by Merck & Co., Inc, Kenilworth, New Jersey, USA. AOJ-L, CF, RL, MBJ, CDK, SR, PSP are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA, and may own stock or stock options in the company. IG reports advisory board fees from Merck & Co., Inc., outside of the submitted work. FR, MDM, HK, RT and MMP have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Cancer Institute. SEER Stat Fact Sheets: Acute Myeloid Leukemia (AML) 2015 http://seer.cancer.gov/statfacts/html/amyl.html.
- 2.Roboz GJ. Novel approaches to the treatment of acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:43–50. doi: 10.1182/asheducation-2011.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Yang YG, Zhou M, et al. Meta-analysis of randomised clinical trials comparing idarubicin + cytarabine with daunorubicin + cytarabine as the induction chemotherapy in patients with newly diagnosed acute myeloid leukaemia. PLoS One. 2013;8:e60699. doi: 10.1371/journal.pone.0060699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 5.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 6.Longo DL, Armitage JO. Controversies in the treatment of early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1667–9. doi: 10.1056/NEJMe1502888. [DOI] [PubMed] [Google Scholar]
- 7.Ravandi F. Are adjuncts to induction chemotherapy worthwhile in the treatment of acute myeloid leukemia? Best Pract Res Clin Haematol. 2014;27:241–6. doi: 10.1016/j.beha.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Craddock C, Tauro S, Moss P, Grimwade D. Biology and management of relapsed acute myeloid leukaemia. Br J Haematol. 2005;129:18–34. doi: 10.1111/j.1365-2141.2004.05318.x. [DOI] [PubMed] [Google Scholar]
- 9.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23:1969–78. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Giles F, O’Brien S, Cortes J, et al. Outcome of patients with acute myelogenous leukemia after second salvage therapy. Cancer. 2005;104:547–54. doi: 10.1002/cncr.21187. [DOI] [PubMed] [Google Scholar]
- 11.Estey EH. Treatment of relapsed and refractory acute myelogenous leukemia. Leukemia. 2000;14:476–9. doi: 10.1038/sj.leu.2401568. [DOI] [PubMed] [Google Scholar]
- 12.Duffy MJ, Synnott NC, McGowan PM, Crown J, O’Connor D, Gallagher WM. p53 as a target for the treatment of cancer. Cancer Treat Rev. 2014;40:1153–60. doi: 10.1016/j.ctrv.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 14.Nahi H, Selivanova G, Lehmann S, et al. Mutated and non-mutated TP53 as targets in the treatment of leukaemia. Br J Haematol. 2008;141:445–53. doi: 10.1111/j.1365-2141.2008.07046.x. [DOI] [PubMed] [Google Scholar]
- 15.Saha MN, Qiu L, Chang H. Targeting p53 by small molecules in hematological malignancies. J Hematol Oncol. 2013;6:23. doi: 10.1186/1756-8722-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013;13:83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 18.Saiki AY, Caenepeel S, Yu D, et al. MDM2 antagonists synergize broadly and robustly with compounds targeting fundamental oncogenic signaling pathways. Oncotarget. 2014;5:2030–43. doi: 10.18632/oncotarget.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onel K, Cordon-Cardo C. MDM2 and prognosis. Mol Cancer Res. 2004;2:1–8. [PubMed] [Google Scholar]
- 20.Bueso-Ramos CE, Yang Y, deLeon E, McCown P, Stass SA, Albitar M. The human MDM-2 oncogene is overexpressed in leukemias. Blood. 1993;82:2617–23. [PubMed] [Google Scholar]
- 21.Quesnel B, Preudhomme C, Oscier D, et al. Over-expression of the MDM2 gene is found in some cases of haematological malignancies. Br J Haematol. 1994;88:415–8. doi: 10.1111/j.1365-2141.1994.tb05044.x. [DOI] [PubMed] [Google Scholar]
- 22.Moller MB, Nielsen O, Pedersen NT. Oncoprotein MDM2 overexpression is associated with poor prognosis in distinct non-Hodgkin’s lymphoma entities. Mod Pathol. 1999;12:1010–6. [PubMed] [Google Scholar]
- 23.Wojcik I, Szybka M, Golanska E, et al. Abnormalities of the P53, MDM2, BCL2 and BAX genes in acute leukemias. Neoplasma. 2005;52:318–24. [PubMed] [Google Scholar]
- 24.Gu L, Zhu N, Findley HW, Zhou M. MDM2 antagonist nutlin-3 is a potent inducer of apoptosis in pediatric acute lymphoblastic leukemia cells with wild-type p53 and overexpression of MDM2. Leukemia. 2008;22:730–9. doi: 10.1038/leu.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Sun W, Zhao Y, et al. SAR405838: an optimized inhibitor of MDM2-p53 interaction that induces complete and durable tumor regression. Cancer Res. 2014;74:5855–65. doi: 10.1158/0008-5472.CAN-14-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima K, Konopleva M, Samudio IJ, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–9. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Aguilar A, Bernard D, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction (MDM2 Inhibitors) in clinical trials for cancer treatment. J Med Chem. 2015;58:1038–52. doi: 10.1021/jm501092z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Zeng SX, Lu H. Targeting p53-MDM2-MDMX loop for cancer therapy. Subcell Biochem. 2014;85:281–319. doi: 10.1007/978-94-017-9211-0_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 30.Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–47. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 31.Ji Y, Li Y, Nebiyou BB. Dose-finding in phase I clinical trials based on toxicity probability intervals. Clin Trials. 2007;4:235–44. doi: 10.1177/1740774507079442. [DOI] [PubMed] [Google Scholar]
- 32.Ray-Coquard I, Blay JY, Italiano A, et al. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. Lancet Oncol. 2012;13:1133–40. doi: 10.1016/S1470-2045(12)70474-6. [DOI] [PubMed] [Google Scholar]
- 33.Grier JD, Xiong S, Elizondo-Fraire AC, Parant JM, Lozano G. Tissue-specific differences of p53 inhibition by Mdm2 and Mdm4. Mol Cell Biol. 2006;26(1):192–8. doi: 10.1128/MCB.26.1.192-198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanafelt TD, Chaffee KG, Call TG, et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL) Blood. 2015;126(23):2950. doi: 10.1080/10428194.2016.1257795. [DOI] [PubMed] [Google Scholar]
- 35.Yang T, Moslehi JJ, Roden DM. Proarrhythmic Effects of Ibrutinib, a Clinically Approved Inhibitor of Bruton’s Tyrosine Kinase (BTK) Used in Cancer Therapy. Circulation. 2015;132:A14587. [Google Scholar]
- 36.Shangary S, Qin D, McEachern D, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci U S A. 2008;105(10):3933–8. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Secchiero P, Barbarotto E, Tiribelli M, et al. Functional integrity of the p53-mediated apoptotic pathway induced by the nongenotoxic agent nutlin-3 in B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2006;107(10):4122–9. doi: 10.1182/blood-2005-11-4465. [DOI] [PubMed] [Google Scholar]
- 38.Tovar C, Graves B, Packman K, et al. MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models. Cancer Res. 2013;73(8):2587–97. doi: 10.1158/0008-5472.CAN-12-2807. [DOI] [PubMed] [Google Scholar]
- 39.Kojima K, Konopleva M, Tsao T, Nakakuma H, Andreeff M. Concomitant inhibition of Mdm2-p53 interaction and Aurora kinases activates the p53-dependent postmitotic checkpoints and synergistically induces p53-mediated mitochondrial apoptosis along with reduced endoreduplication in acute myelogenous leukemia. Blood. 2008;112:2886–95. doi: 10.1182/blood-2008-01-128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kojima K, Kornblau SM, Ruvolo V, et al. Prognostic impact and targeting of CRM1 in acute myeloid leukemia. Blood. 2013;121:4166–74. doi: 10.1182/blood-2012-08-447581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saha MN, Micallef J, Qiu L, Chang H. Pharmacological activation of the p53 pathway in haematological malignancies. J Clin Pathol. 2010;63:204–9. doi: 10.1136/jcp.2009.070961. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Konopleva M, Burks JK, et al. Blockade of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase and murine double minute synergistically induces Apoptosis in acute myeloid leukemia via BH3-only proteins Puma and Bim. Cancer Res. 2010;70:2424–34. doi: 10.1158/0008-5472.CAN-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khoo KH, Verma CS, Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. Nat Rev Drug Discov. 2014;13:217–36. doi: 10.1038/nrd4236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.