Abstract
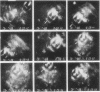
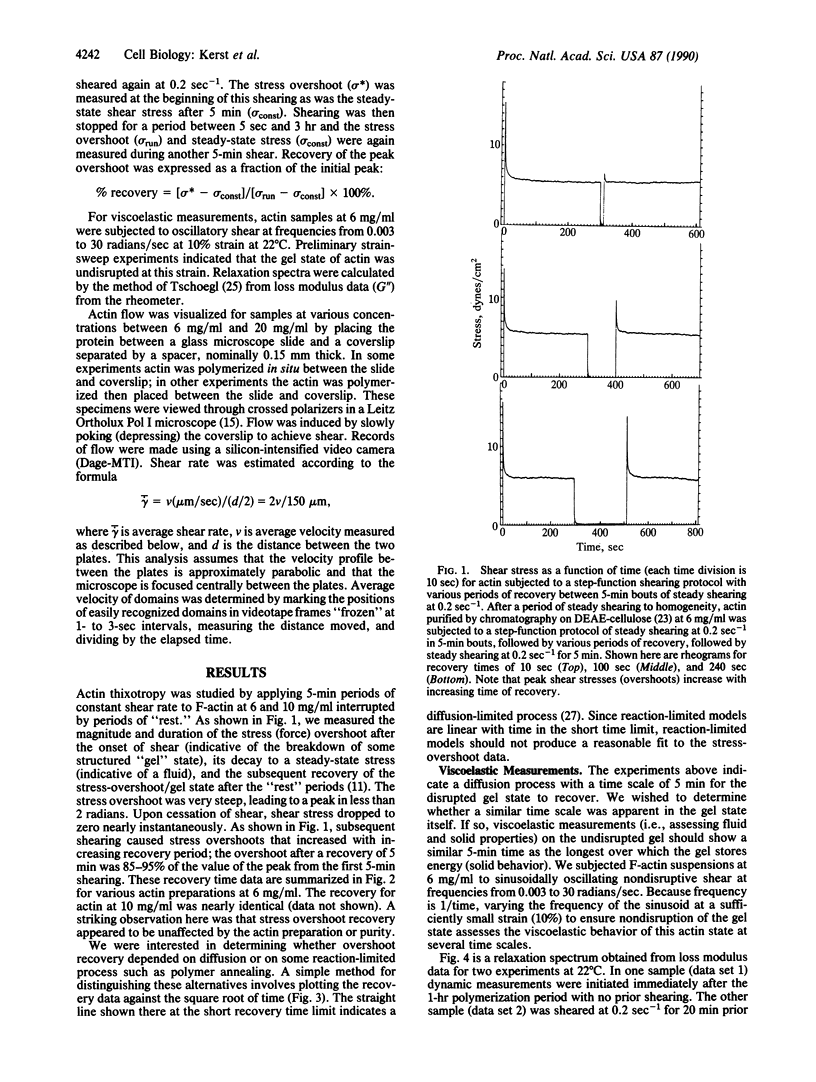
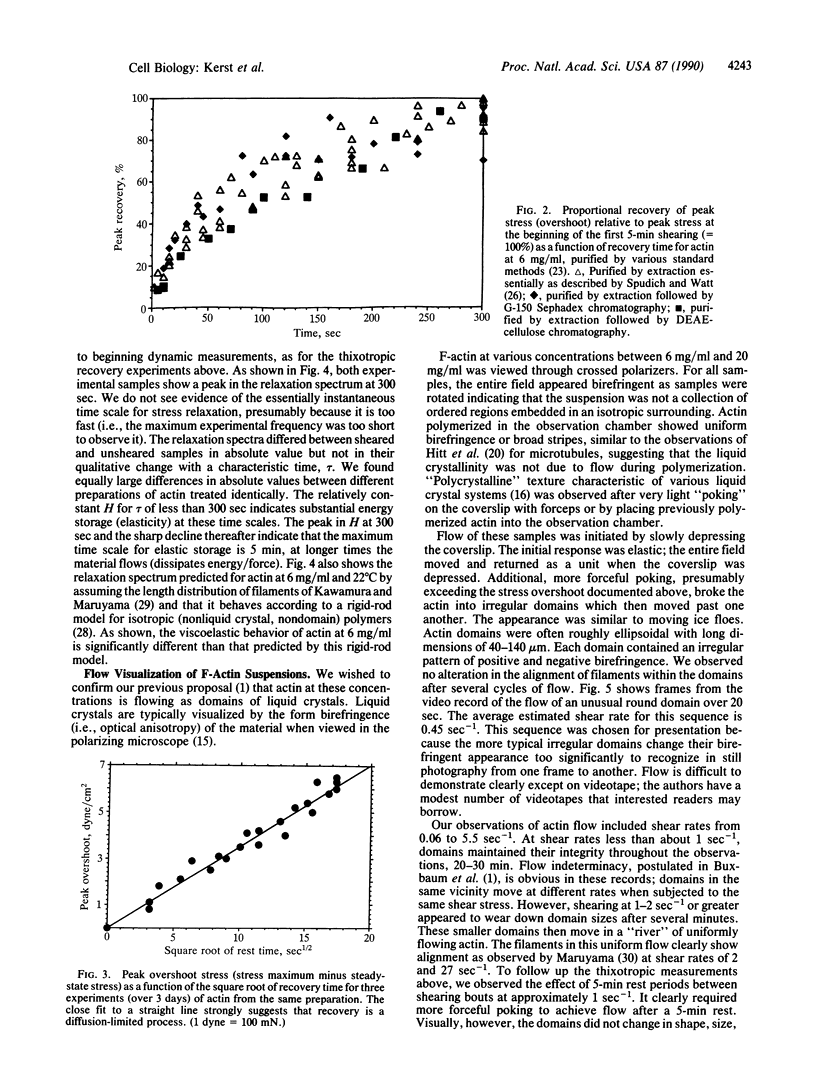
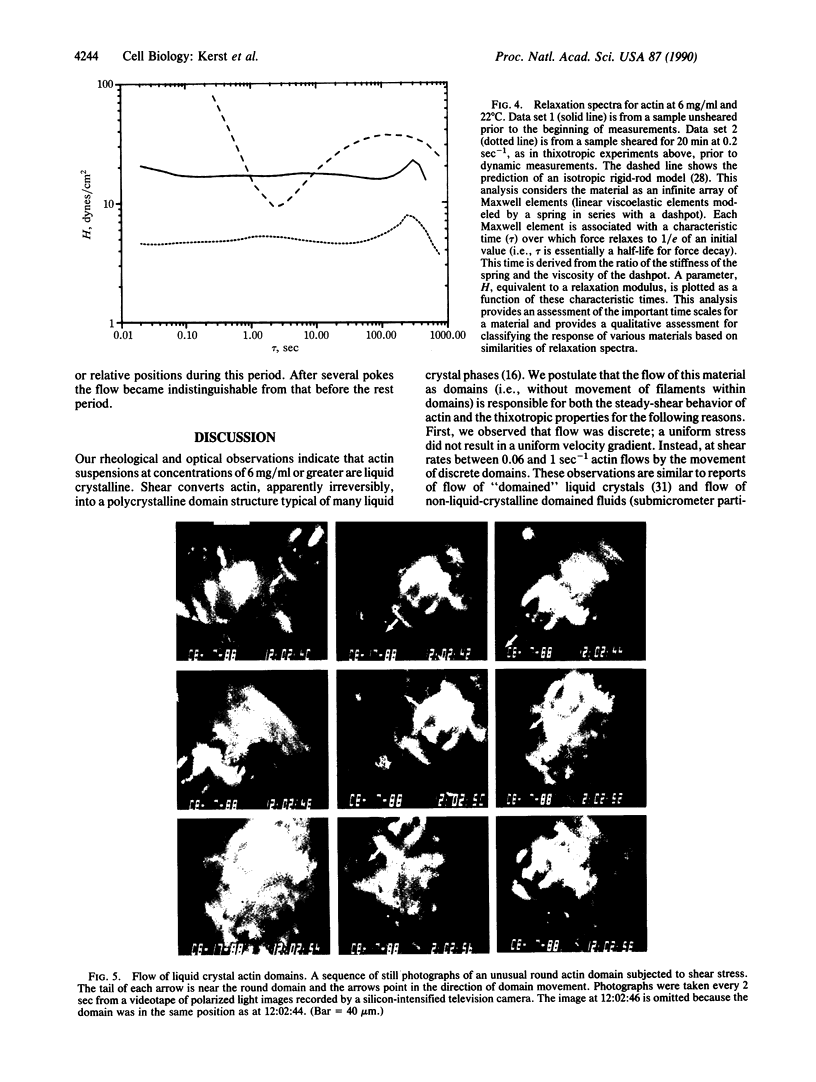
The thixotropic properties of filamentous actin suspensions were examined by a step-function shearing protocol. Samples of purified filamentous actin were sheared at 0.2 sec-1 in a cone and plate rheometer. We noted a sharp stress overshoot upon the initiation of shear, indicative of a gel state, and a nearly instantaneous drop to zero stress upon cessation of shear. Stress-overshoot recovery was almost complete after 5 min of "rest" before samples were again sheared at 0.2 sec-1. Overshoot recovery increased linearly with the square root of rest time, suggesting that gel-state recovery is diffusion limited. Actin suspensions subjected to oscillatory shearing at frequencies from 0.003 to 30 radians/sec confirmed the existence of a 5-min time scale in the gel, similar to that for stress-overshoot recovery. Flow of filamentous actin was visualized by polarized light observations. Actin from 6 mg/ml to 20 mg/ml showed the "polycrystalline" texture of birefringence typical for liquid crystal structure. At shear rates less than 1 sec-1, flow occurred by the relative movement of irregular, roughly ellipsoidal actin domains 40-140 microns long; the appearance was similar to moving ice floes. At shear rates greater than 1 sec-1, domains decreased in size, possibly by frictional interactions among domains. Eventually domains flow in a "river" of actin aligned by the flow. Our observations confirm our previous domain-friction model for actin rheology. The similarities between the unusual flow properties of actin and cytoplasm argue that cytoplasm also may flow as domains.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray D., White J. G. Cortical flow in animal cells. Science. 1988 Feb 19;239(4842):883–888. doi: 10.1126/science.3277283. [DOI] [PubMed] [Google Scholar]
- Brown P. A., Berlin R. D. Packing volume of sedimented microtubules: regulation and potential relationship to an intracellular matrix. J Cell Biol. 1985 Oct;101(4):1492–1500. doi: 10.1083/jcb.101.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum R. E., Dennerll T., Weiss S., Heidemann S. R. F-actin and microtubule suspensions as indeterminate fluids. Science. 1987 Mar 20;235(4795):1511–1514. doi: 10.1126/science.2881354. [DOI] [PubMed] [Google Scholar]
- Cortese J. D., Frieden C. Microheterogeneity of actin gels formed under controlled linear shear. J Cell Biol. 1988 Oct;107(4):1477–1487. doi: 10.1083/jcb.107.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitt A. L., Cross A. R., Williams R. C., Jr Microtubule solutions display nematic liquid crystalline structure. J Biol Chem. 1990 Jan 25;265(3):1639–1647. [PubMed] [Google Scholar]
- Jen C. J., McIntire L. V., Bryan J. The viscoelastic properties of actin solutions. Arch Biochem Biophys. 1982 Jun;216(1):126–132. doi: 10.1016/0003-9861(82)90196-5. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Maruyama K. Electron microscopic particle length of F-actin polymerized in vitro. J Biochem. 1970 Mar;67(3):437–457. doi: 10.1093/oxfordjournals.jbchem.a129267. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Taylor D. L., Lanni F. Probing the structure of cytoplasm. J Cell Biol. 1986 Jun;102(6):2015–2022. doi: 10.1083/jcb.102.6.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUYAMA K. A FLOW BIREFRINGENCE STUDY OF F-ACTIN. J Biochem. 1964 Mar;55:277–286. doi: 10.1093/oxfordjournals.jbchem.a127881. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Kaibara M., Fukada E. Rheology of F-actin. I. Network of F-actin in solution. Biochim Biophys Acta. 1974 Nov 5;371(1):20–29. doi: 10.1016/0005-2795(74)90150-0. [DOI] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Sato M., Leimbach G., Schwarz W. H., Pollard T. D. Mechanical properties of actin. J Biol Chem. 1985 Jul 15;260(14):8585–8592. [PubMed] [Google Scholar]
- Sato M., Wong T. Z., Allen R. D. Rheological properties of living cytoplasm: endoplasm of Physarum plasmodium. J Cell Biol. 1983 Oct;97(4):1089–1097. doi: 10.1083/jcb.97.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Tait J. F., Frieden C. Polymerization and gelation of actin studied by fluorescence photobleaching recovery. Biochemistry. 1982 Jul 20;21(15):3666–3674. doi: 10.1021/bi00258a022. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Condeelis J. S. Cytoplasmic structure and contractility in amoeboid cells. Int Rev Cytol. 1979;56:57–144. doi: 10.1016/s0074-7696(08)61821-5. [DOI] [PubMed] [Google Scholar]
- Valberg P. A., Feldman H. A. Magnetic particle motions within living cells. Measurement of cytoplasmic viscosity and motile activity. Biophys J. 1987 Oct;52(4):551–561. doi: 10.1016/S0006-3495(87)83244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaner K. S., Stossel T. P. Physical basis of the rheologic properties of F-actin. J Biol Chem. 1983 Sep 25;258(18):11004–11009. [PubMed] [Google Scholar]
- Zaner K. S., Valberg P. A. Viscoelasticity of F-actin measured with magnetic microparticles. J Cell Biol. 1989 Nov;109(5):2233–2243. doi: 10.1083/jcb.109.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]