Abstract
Background
Sympathovagal imbalance has been associated with poor prognosis in chronic diseases, but there is conflicting evidence in multiple sclerosis.
Objectives
The objective of this study was to investigate the autonomic nervous system dysfunction correlation with inflammation and progression in multiple sclerosis.
Methods
Heart rate variability was analysed in 120 multiple sclerosis patients and 60 healthy controls during supine rest and head-up tilt test; the normalised units of low frequency and high frequency power were considered to assess sympathetic and vagal components, respectively. Correlation analyses with clinical and radiological markers of disease activity and progression were performed.
Results
Sympathetic dysfunction was closely related to the progression of disability in multiple sclerosis: progressive patients showed altered heart rate variability with respect to healthy controls and relapsing–remitting patients, with higher rest low frequency power and lacking the expected low frequency power increase during the head-up tilt test. In relapsing–remitting patients, disease activity, even subclinical, was associated with lower rest low frequency power, whereas stable relapsing–remitting patients did not differ from healthy controls. Less sympathetic reactivity and higher low frequency power at rest were associated with incomplete recovery from relapse.
Conclusions
Autonomic balance appears to be intimately linked with both the inflammatory activity of multiple sclerosis, which is featured by an overall hypoactivity of the sympathetic nervous system, and its compensatory plastic processes, which appear inefficient in case of worsening and progressive multiple sclerosis.
Keywords: Sympathetic nervous system, autonomic nervous system, autonomic nervous system disorder, primary progressive multiple sclerosis, relapsing remitting multiple sclerosis, neuronal plasticity
Introduction
Autonomic nervous system (ANS) impairment frequently occurs in multiple sclerosis (MS).1–4 The ANS may be intimately linked with the altered immune regulation in MS. It is well established that central nervous system (CNS) activation affects peripheral mononuclear cell function through the release of catecholamines.5 Although evidence suggests widespread involvement of ANS in MS and preclinical data support a pathogenic role of ANS alterations, the correlation with disease activity among MS patients is still unclear.1,2,6,7 Even less explored is the relationship between ANS function and prognosis in MS, although sympathovagal imbalance has been associated with poor prognosis in other acute and chronic diseases.8,9 Acute disability impacts short-term health and quality of life, whereas two processes, the worsening and progression of disability, concur in limiting long-term health in MS, the former related to incomplete recovery from inflammatory relapses, the latter independent from relapses.10
Spectral analysis of heart rate variability (HRV) has emerged as the most valuable non-invasive measure of ANS function. Alterations in HRV have been reported in a wide spectrum of other chronic conditions.11
The aim of this study was to investigate HRV in a large number of MS patients and healthy controls (HCs). Additional analyses investigated the correlation between ANS dysfunction and markers of MS severity, including measures of acute, worsening and progressive disability.
Methods
This study complied with the principles of the Declaration of Helsinki, and was approved by the ethical committee of the Policlinico Università Tor Vergata in Rome. All the subjects gave their written informed consent to the study.
Subjects and study design
One hundred and twenty MS patients, diagnosed according to the revised McDonald criteria,12 regardless of symptoms of orthostatic intolerance or other autonomic dysfunction, were included. Of them, 84 had relapsing–remitting multiple sclerosis (RRMS) and 36 had progressive multiple sclerosis (PMS; primary PMS, n=20; secondary PMS, n = 16). The control group consisted of 60 age and sex-matched healthy volunteers, with no evidence of MS or other neurological illnesses.
None of the patients and controls had confounding cardiovascular illnesses or other diseases including diabetes mellitus, hypertension or ischaemic heart disease. None of the tested subjects were on medications known to affect the cardiovascular or autonomic systems. Patients could not be enrolled if they had received steroid treatment during the previous month. All the patients and controls refrained from alcohol and caffeine consumption for the 24 hours preceding the tests.
Demographic and clinical information was derived from medical records. MS onset was defined as the first episode of focal neurological deficit indicative of MS. Disease duration was calculated as years from onset to the last assessment of disability.
Ongoing first or second-line disease-modifying therapies were noted. Patients treated with Fingolimod were excluded because of the drug’s potential cardiac effects.
A subgroup of RRMS patients (n = 36) was in the active phase of disease at the moment of HRV assessment, either experiencing a relapse (n = 20) or showing radiological activity on magnetic resonance imaging (MRI) (n = 16). Relapses were defined as the development of new or recurrent neurological symptoms, without fever or infection, with onset at least 24 hour before the HRV assessment.
All patients underwent autonomic function tests, MRI and disability assessment within 24 hours, whereas only autonomic function was tested in the HC group.
Disability assessment
Disability was determined by a specially trained and certified examining neurologist using the Expanded Disability Status Scale (EDSS), a 10-point disease severity score derived from nine ratings of individual neurological domains.13 EDSS evaluation was performed, among relapsing MS patients, at the relapse and during a one-year period of follow-up, and then compared to the last EDSS available before the relapse, in order to assess relapse severity and disability recovery.
Recovery was considered as the lowest EDSS and functional systems scores from the second to the twelfth months after the attack. If the pre-event EDSS was 0, recovery was considered complete if the follow-up EDSS score was 0, incomplete if the follow-up EDSS was 1.0 or greater. If the pre-event EDSS was greater than 0, recovery was defined as complete if no residual signs or symptoms remained other than those present prior to the attack, incomplete if exceeding prior scores.
Autonomic function tests
HRV analysis was performed under standardised environmental conditions14,15 by a trained technician unaware of the subjects’ clinical details. All subjects were studied in a temperature-controlled room (23 ± 1℃). They were asked to abstain from alcohol and caffeine for at least the preceding 24 hours. All tests were performed between 8 a.m. and 10 a.m.
ECG (Click EGC USB 3–12 leads; ET Medical Devices SpA) was monitored by standard methods.
HRV analysis was performed in each subject in the frequency domain using a dedicated software (Light-SNV software) by a trained neurologist unaware of the subjects’ clinical details. Stable heart rate (HR) periods of 5 minutes duration were chosen in the last 6 minutes of a 30-minute supine rest. Power spectral analysis (PSA) was calculated using both a parametric (AR) and a non-parametric algorithm (FFT). We considered a high frequency (HF) component (centred 0.16–0.4 Hz), reflecting mostly vagal activity, and a low frequency (LF) component (0.04–0.15 Hz), reflecting mostly sympathetic activity. The oscillatory components between (0–0.03 Hz) were considered direct current (DC) noise. We calculated the spectral components in normalised units (LFnu, HFnu) by dividing it by the total power minus the DC component. The LF/HF ratio was used as an index of sympathovagal balance. After 30 minutes of supine rest, the subject was tilted up at 65° on a tilt table for 10 minutes. At each minute of head-up tilt test (HUTT), the changes in and HR were calculated with respect to basal values. Stable HR epochs of 5 minutes duration were chosen in the last between the fourth and ninth minute of HUTT.
The ratio between LFnu after HUTT and LFnu at rest, in a percentage, was used as an index of sympathetic reactivity.
Magnetic resonance imaging
Brain and cervical spinal cord 3 Tesla MRI scans were taken into account when performed within 7 days from HRV assessment. The number and distribution of T2 lesions and the presence of gadolinium (Gd)-enhancing (0.2 ml/Kg iv) lesions were assessed by a neuroradiologist unaware of patients’ clinical details and autonomic assessments. An active scan was defined as showing any Gd-enhancing lesion. Patients were stratified according to the presence or absence of infratentorial demyelinating lesions involving the brainstem and cervical spinal cord.
Statistical analysis
Fisher’s exact test was used for contingency analyses. Comparisons between two groups were performed by Student’s t-test or by the Mann–Whitney test. Multiple comparisons were performed by analysis of variance, followed by Tukey’s HSD test or by the Kruskal–Wallis test followed by the Mann–Witney test. Correlations were performed by calculating Spearman coefficients.
HRV parameters were used to construct multivariate models, also including confounding factors as age, gender and baseline HRV values.
All statistical analyses were conducted using Stata 9.2 (Stata Corp, College Station, TX, USA). Data were presented as mean ± standard deviation (SD). A P value lower than 0.05 was considered statistically significant.
Results
Subject characteristics
Demographic features and clinical characteristics of MS patients and HCs are shown in Table 1. The two groups were age and gender matched. All subjects correctly performed all the autonomic tests and none of them had symptoms of orthostatic intolerance while testing. The basal values of HR were comparable between the two groups.
Table 1.
Demographic and clinic characteristics of subjects.
| Controls | Patients | P level | PMS | RRMS | P level | |
|---|---|---|---|---|---|---|
| Number | 60 | 120 | n.a. | 36 | 84 | n.a. |
| Gender (M/F) | 20/40 | 47/73 | 0.51 | 18/18 | 29/55 | 0.15 |
| Age (years) | 37.45 ± 15.2 | 39.28 ± 10.7 | 0.35 | 47.16 ± 11.5 | 35.9 ± 8.4 | <0.001 |
| Baseline HR (bpm) | 67.53 ± 6.2 | 68.35 ± 5.9 | 0.38 | 67.90 ± 5.6 | 68.81 ± 6.8 | 0.48 |
| Disease duration (years) | n.a. | 6.99 ± 4.7 | n.a. | 7.97 ± 4.7 | 6.57 ± 4.7 | 0.14 |
| EDSS | n.a. | 2.75 ± 1.5 | n.a. | 4.40 ± 0.8 | 2.04 ± 1.1 | <0.01 |
| Autonomic symptoms (Y/N) | n.a. | 22/98 | n.a. | 7/29 | 15/69 | 0.80 |
| Treatment (none/IFN/GA/NTZ) | n.a. | 42/42/29/7 | n.a. | 29/7/0/0 | 13/35/29/7 |
PMS: progressive multiple sclerosis; RRMS: relapsing–remitting multiple sclerosis; HR: heart rate; EDSS: Expanded Disability Status Scale; IFN: interferon; GA: Glatiramer Acetate; NTZ: Natalizumab
HRV highlighted a gender difference with an LF/HF ratio higher in men (2.25 ± 1.3 vs. 1.50 ± 1.15; P = 0.02). The minimum and maximum EDSS values were, respectively, 0 and 6.5, being higher in the PMS group. Ten patients showed abnormal bladder function, three had sexual symptoms, five had sweat dysfunction and four had defecation symptoms. The presence of autonomic symptoms was assumed as covariate in multivariate analyses. Most PMS patients were not on immunomodulatory or immunosuppressive treatments. Differences in ongoing MS treatment were taken into account for subsequent analyses.
Disease duration affects HRV in MS
HRV analysis in the frequency domain showed no significant differences in LFnu, HFnu and the LF/HF ratio at rest between MS patients and controls (Figure 1(a)). The within-group analysis revealed that the expected changes in PSA components during orthostatic stress (significant increase in the LF component and significant reduction of the HF component) were observed both in controls and MS patients (P < 0.01 for all parameters), with a comparable percentage of increase of the LFnu component (Figure 1(b)). The same results were replicated using FFT analysis (not shown).
Figure 1.
Basal heart rate variability (HRV) parameters of the study subjects. The graphs show that there was no difference in basal HRV parameters between multiple sclerosis (MS) patients and healthy controls (HC), in terms of LF component (a), HF component (b) and LF/HF ratio (c). The same HRV parameters showed similar variations after tilting in both patients with MS and HC (d-f).
Correlation analyses between LF/HF and clinical characteristics revealed a significant association between age (r = 0.30, P < 0.001), disease duration (r = 0.35, P < 0.001) and EDSS (r = 0.28, P = 0.01). Of note, a positive correlation was found between sympathovagal balance and disease duration, indicating a disease-related progressive disruption of normal autonomic balance. We divided our sample into two groups: group 1 within 5 years from establishment of diagnosis and group 2 with more than 5 years, respectively. We confirmed that patients with more than 5 years from the diagnosis had lower HFnu and higher LFnu, with a consequent higher LF/HF ratio (P < 0.05 for each comparison).
A longer duration of disease might lead to widespread areas of demyelination throughout the CNS and consequently to greater damage of cerebral pathways located in the brainstem and spinal cord and involved in autonomic control of HR.16
In our sample, sympathovagal balance was not influenced by the distribution of demyelinating lesions: no significant differences were observed comparing patients with and without infratentorial involvement. Neither the presence of demyelinating lesions in the cervical spinal cord nor in the brainstem was correlated with significant differences in the LF/HF ratio (infratentorial 1.59 ± 1.44 vs. 2.00 ± 1.72, P = 0.16; cervical spinal cord 1.84 ± 1.52 vs. 1.75 ± 1.77, P = 0.79, total 1.87 ± 1.59 vs. 1.60 ± 1.67, P = 0.42).
Multivariate regression analysis confirmed that, among MS patients, disease duration predicted the LF/HF ratio independently from sex, EDSS, distribution of demyelinating lesions, presence of autonomic symptoms and ongoing disease-modifying therapies, as shown in Table 2.
Table 2.
Multivariate linear regression analysis (LF/HF ratio as outcome).
| Coefficient | SE | 95% CI | P level | |
|---|---|---|---|---|
| Age | 0.02 | 0.01 | –0.01–0.05 | 0.17 |
| Disease duration | 0.08 | 0.03 | 0.02–0.15 | 0.007 |
| Gender | 0.35 | 0.30 | –0.21–0.94 | 0.24 |
| EDSS | 0.18 | 0.11 | –0.04–0.40 | 0.10 |
| Infratentorial lesions | 0.09 | 0.32 | –0.55–0.75 | 0.76 |
| Autonomic symptoms (Y/N) | 0.21 | 0.36 | –0.49–0.93 | 0.54 |
| IFN | 0.33 | 0.36 | –0.39–1.06 | 0.36 |
| GA | –0.09 | 0.39 | –0.86–0.68 | 0.81 |
| NTZ | –0.16 | 0.63 | –1.41–1.08 | 0.79 |
LF: low frequency; HF: high frequency; CI: confidence interval; EDSS: Expanded Disability Status Scale; IFN: interferon; GA: Glatiramer Acetate; NTZ: Natalizumab
Sympathetic system is overactive and exhausted in PMS
In order to clarify autonomic involvement in MS better, we analysed HRV in patients stratified according to disease form. We therefore considered PMS and RRMS patients in two different groups with respect to HCs.
Disease form significantly affected the LF/HF ratio (F = 13.23, P < 0.001): PMS patients revealed a disruption of sympathovagal balance, showing a predominance of the sympathetic activity at rest. Post hoc analysis revealed a higher LF/HF ratio among PMS patients with respect to both HCs and RRMS (P = 0.001 with respect to RRMS patients, P = 0.01 with respect to HCs; Figure 2(a)). Higher LFnu and lower HFnu accounted for the higher LF/HF ratio of the PMS group. Significantly, no differences between RRMS and HC groups were recorded (P > 0.05; Figure 2(a)).
Figure 2.
Progressive disease affects heart rate variability (HRV). Patients with progressive disease showed higher sympathetic tone at rest and lower sympathetic reactivity (a, b). Higher disease severity was associated with greater HRV alteration in progressive multiple sclerosis (PMS) subjects (c). *P < 0.05 with respect to healthy controls (HCs) and relapsing–remitting multiple sclerosis (RRMS) patients.
The magnitude of HRV of each frequency component during orthostatic stress was then calculated. Disease form significantly affected also sympathetic reactivity (F = 3.7, P = 0.02). We observed a significant decrease in HF power and increase in LF power only in HC and RRMS groups (P < 0.001 for each group). Progressive patients failed to show any significant HRV change after HUTT (P = 0.8) (Figure 2(b)).
Progressive disease was related to loss of sympathetic reactivity in patients with equal values of EDSS, disease duration, demographics, symptoms of autonomic dysfunction, presence of infratentorial lesions and basal LFnu (Table 3).
Table 3.
Multivariate logistic regression analysis (preservation of sympathetic reactivity as outcome).
| OR | SE | 95% CI | P level | |
|---|---|---|---|---|
| Disease duration | 1.03 | 0.06 | 0.92–1.15 | 0.53 |
| Gender | 2.18 | 1.24 | 0.71–6.69 | 0.17 |
| Age | 0.92 | 0.03 | 0.87–0.98 | 0.01 |
| EDSS | 1.66 | 0.43 | 0.99–2.76 | 0.05 |
| Infratentorial lesions | 0.37 | 0.23 | 0.10–1.29 | 0.12 |
| Autonomic symptoms (Y/N) | 2.22 | 1.60 | 0.55–9.12 | 0.25 |
| LFnu | 0.91 | 0.02 | 0.87–0.96 | <0.01 |
| PMS disease form | 0.17 | 0.14 | 0.03–0.90 | 0.03 |
CI: confidence interval; EDSS: Expanded Disability Status Scale; LFnu: low frequency power; PMS: progressive multiple sclerosis.
Disease severity significantly affected the LF/HF ratio (F = 6.73, P < 0.01) in PMS patients. In fact, a greater LF/HF ratio was found with increasing disability, passing from mildly disabled (EDSS < 4.5) patients to patients with moderate disability (EDSS 4.5–5.5) and then patients with more severe impairment (EDSS > 5.5) (Figure 2(c)). However, physiological sympathetic reactivity was partially preserved in PMS patients with mild disability, because the percentage of LFnu increase after HUTT among those with EDSS less than 4.5 was higher but not statistically significant compared to the other PMS (114.1% vs. 94.0%, P = 0.07).
Sympathetic system is defective in active MS
ANS has been reported to be involved in the regulation of immune activation.17
RRMS subjects were stratified according to presence (Gd + , n = 36) or absence (Gd–, n = 48) of contrast-enhancing lesions at MRI. Our analysis showed altered LF/HF ratio with significantly lower LFnu and higher HFnu in the Gd + group compared to the Gd– group (LFnu, HFnu P < 0.01; LF/HF ratio 0.01), suggesting an involvement of autonomic alterations in the acute stage of inflammation. Furthermore, we observed a significant increase in LF power after HUTT in both groups, with higher sympathetic reactivity among Gd + patients (P = 0.01, Figure 3).
Figure 3.
Sympathetic system is defective in active multiple sclerosis (MS). An altered low frequency (LF)/high frequency (HF) ratio with significantly lower LF power and higher HF power was found in the gadolinium (Gd)+ group compared to the Gd– group and healthy controls (HCs). Active MS was also associated with greater sympathetic reactivity. *P < 0.05 with respect to HCs.
The supratentorial or infratentorial location of acute lesions did not influence the sympathovagal balance (LF/HF 0.74 ± 0.69 vs. 0.87 ± 0.72, P = 0.36).
Multivariate regression analysis confirmed that radiological activity independently predicted lower LFnu at equal value of disease duration, EDSS and gender (coefficient –8.14, standard error (SE) 2.41, 95% confidence interval (CI) –12.94 to –3.35, P = 0.001). Conversely, radiological activity did not predict higher sympathetic reactivity at equal values of basal LFnu (coefficient –0.19, SE 0.11, 95% CI –0.42 to 0.03, P = 0.08).
PSA components and sympathetic reactivity were similar between Gd– RRMS patients and HCs (Figure 3). The same results were replicated using FFT analysis (not shown).
Sympathetic system is ineffective in worsening MS
Two processes, progression and worsening, have been described at the basis of irreversible disability in MS.10 We have shown specific HRV alterations in progressive disease, but we also aimed to study HRV involvement in worsening processes. We therefore analysed HRV parameters in active patients, according to relapse severity and recovery.
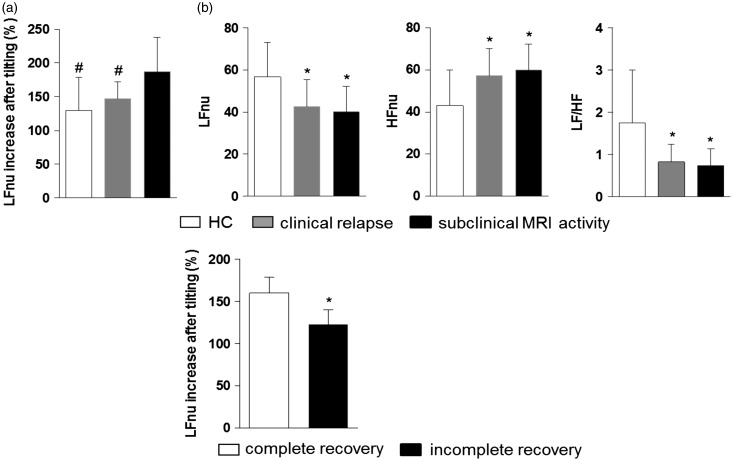
First, we found significant differences in HRV response to orthostatic stress between relapsing patients and subjects with only radiological signs of inflammation. Active non-relapsing patients showed greater LFnu increase after HUTT with respect to relapsing subjects, suggesting the involvement of sympathetic reactivity in the clinical expression of brain inflammation (percentage of LFnu increase after HUTT 187 ± 51% vs. 147 ± 25%, P = 0.03, Figure 4(a)). Notably, no significant differences in HRV parameters were found at rest between the two groups (LF/HF 0.83 ± 0.42 0.74 ± 0.40, P = 0.99), LF/HF being lower than HCs for both relapsing and non-relapsing active patients, underlying the effect of subclinical inflammation on HRV alterations (Figure 4(b)).
Figure 4.
Sympathetic system is ineffective in worsening multiple sclerosis (MS). Active not relapsing patients showed a higher increase of low frequency power (LFnu) after head-up tilt test (HUTT) with respect to relapsing subjects (a), despite comparable heart rate variability parameters at rest (b). Incomplete recovery from relapse was associated with lower LFnu increase after HUTT (c). LF/HF: low frequency/high frequency ratio. *P < 0.05 with respect to healthy controls; #P < 0.05 with respect to active not relapsing MS.
Second, we found that subjects with poor recovery from relapse showed less increase after HUTT during the relapse, compared to subjects with complete recovery (LFnu percentage increase 159.9 ± 19.2% vs. 123.15 ± 17.12%, P < 0.001, Figure 4(c)). In a logistic regression analysis the LFnu percentage increase after HUTT was an independent predictor of complete recovery at equal values of sex, age, disease duration and EDSS during the relapse (odds ratio 0.91, SE 0.04, 95% CI 0.83–0.99, P = 0.03).
Notably, the severity of relapse, assessed by EDSS increase during the relapse, had no correlation with the HRV parameters (not shown).
Discussion
Although recent evidences point to a widespread and early involvement of ANS in MS,2 with sympathetic cardiovascular dysregulation and impaired sudomotor function found even in clinically isolated syndrome patients,18 available data are far from defining clearly the pattern and evolution of autonomic dysfunction in MS. Here we have shown specific altered patterns of HRV in MS related to different phases of disease.
A disease-specific influence on HRV is here shown by the correlation between autonomic balance and disease duration, regardless of the extension of focal demyelination in brain areas controlling these systems, as supposed for years.16 Furthermore, the correlation between autonomic balance and disease duration was independent from the presence of autonomic symptoms, suggesting that autonomic dysfunction could easily be overlooked by sole clinical evaluation. Therefore autonomic testing might be helpful in detecting subclinical autonomic changes in MS and in the future could be implemented as an outcome measure in clinical trials.
PMS patients revealed a clear disruption of sympathovagal balance, indicative of a predominance of basal sympathetic activity. Our findings showed that overactivity of the sympathetic system was more evident among severely disabled progressive patients, thus correlating with irreversible disability. Our hypothesis is that the chronic overactivation of the sympathetic system could induce the neurodegenerative processes evident in progressive MS patients. Excitotoxicity has been implicated as a mechanism of neurodegeneration and disability progression in MS.19,20 Adrenoreceptor antagonists provided neuroprotective effects in experimental settings, partially due to the attenuation of glutamate release.21 The stimulation of β-adrenoreceptors on HeLa cells has been found to interfere with cellular homeostatic functions such as the activity of the sodium–calcium exchanger, the regulation of calcium homeostasis and apoptotic processes leading to cellular degeneration.22 Of interest, transgenic mice that systemically overexpress the alpha1-adrenergic receptors show changes in the expression of genes involved in neurotransmission. Microarray analysis of transgenic brains showed increased N-methyl-d-aspartate receptors and decreased GABAA suggesting a glutamate imbalance as a molecular rationale for excitotoxicity.23 A correlation between the overactive sympathetic system and bad prognosis has also been revealed in other chronic diseases, such as cardiac diseases.24 Low resting vagal tone and high sympathetic tone have also been interpreted as stress vulnerability markers in psychiatric diseases, in which increased levels of sympathetic tone were correlated with a worse clinical presentation of the psychiatric conditions.25,26
Moreover, we have demonstrated here that the increased basal sympathetic tone was associated with the failure of physiological sympathetic reactivity during orthostatic stress. While the HRV analysis shows a statistically significant variation in HRV components during HUTT compared to the supine position in RRMS groups and HCs, such variation does not occur in PMS individuals. The lack of variability at HUTT could reflect an impaired sympathetic reactivity to orthostatic stress in PMS, and this is a further index of autonomic impairment in these patients. On the other hand, the diminished reflex activation of the sympathetic nervous system could be related to inactivity of severely disabled progressive patients and consequent cardiovascular deconditioning. Humans subjected to prolonged periods of bed rest undergo deconditioning of the cardiovascular system, characterised by resting tachycardia, reduced exercise capability and a predisposition for orthostatic intolerance. These changes in cardiovascular function are likely to be due to a combination of factors, including changes in the control of body fluid balance or cardiac alterations resulting in inadequate maintenance of stroke volume, altered arterial or venous vascular function, reduced activation of cardiovascular hormones and diminished autonomic reflex function.
Furthermore, we found that sympathetic reactivity was related to plastic reserve in RRMS, because patients with higher sympathetic reactivity did not show any clinical signs of ongoing brain inflammation. The ongoing brain inflammation in MS is sometimes clinically evident, as patients can present with new inflammatory lesions in parallel to clinical relapses; other times it is subclinical, as patients do not show any signs of neurological dysfunction at equal brain damage. We found with the present study that the sympathetic reactivity is associated with better recovery from MS relapse at equal value of relapse severity, as previously described for measures of cortical plasticity, already related to the concept of plastic reserve in MS.27 Accordingly, norepinephrine has been proved crucial in neuronal plasticity in healthy and injured human brains.28–30 We have thus here postulated that the loss of sympathetic reactivity is a specific marker of progressive MS and that higher reactivity is involved in compensative mechanisms such as adaptive plasticity, recently shown to be crucial in counteracting neuronal damage and to limit the expression of acute and worsening disability.31 Of note, the inactivity of patients with clinical disability in comparison with asymptomatic active MS patients could again induce cardiovascular deconditioning and consequent diminished reflex activation of the sympathetic nervous system. On the other hand, our multivariate analysis demonstrated that sympathetic reactivity is an independent predictor of recovery, ruling out the association with relapse severity, disability and inactivity.
Concerning the relationship between sympathetic activity and inflammation, previous studies showed that alterations of the sympathetic system can alter immunological cascades: increased sympathetic tone can induce an anti-inflammatory and T helper (Th) 2 type milieu, whereas decreased sympathetic tone results in a pro-inflammatory milieu and, in such an environment, Th1 type autoimmune diseases can occur.32,33 In line with this, chemical sympathectomy has been shown to increase the severity of experimental allergic encephalomyelitis,34 whereas adrenoreceptor agonists can reduce it.34–36 Furthermore, previous studies demonstrated reduced norepinephrine levels in blood mononuclear cells of MS patients during relapses.37 Consistently with these studies, our results showed a significant difference in measures of sympathetic activity between active RRMS patients and both stable MS and HCs, with the former showing a lower sympathetic activity than the latter. We thus hypothesised that patient-specific autonomic balance could predict the risk of reactivation in MS, reflecting endogenous inhibitory signals inefficiency against overshooting inflammation. Accordingly, if the hypoactivity of the sympathetic system was involved in inflammatory reactivation, its hyperactivity should be protective against neuroinflammation; indeed fewer or no relapses occurred in progressive MS, in which we had found a sympathetic predominance. Neurodegeneration and progressive disease could be the price for the continuous autonomic control of acute inflammatory reactivation in MS.
In MS, adaptive plasticity could play a protective role in disease expression, because a certain degree of inflammatory white matter damage is generally well tolerated; the reversible or irreversible clinical disability might only appear when the adaptive abilities of the brain fail. Long-term potentiation of the efficacy of glutamate synapses in unaffected areas is believed to mediate clinical recovery from focal brain lesions, but excessive glutamate transmission is detrimental for neuronal survival through excitotoxic processes. In this respect, glutamate neurotransmission double game in learning and memory and in excitotoxicity is best known. A plasticity–pathology continuum, from plasticity through excitotoxicity, has in fact here been proposed for the sympathetic system activation in MS. A prone sympathetic reactivity could be necessary in all stages of disease to limit the clinical expression of brain inflammation and favour compensatory plasticity, which is involved in recovery from both worsening and progressive disability, the first related to incomplete recovery from acute inflammatory relapses and focal demyelination in RRMS patients, the second regardless of relapses.
In conclusion, altered HRV may be linked to reduced global neurological performance, above and beyond traditional cardiovascular risk factors.
A few limitations of the present study should be addressed. First, the number of outcomes was too small to assess the relations of HRV measures with individual neurological endpoints. Further prospective investigations are needed to verify the predictive value of HRV in imminent MS reactivation and disability worsening. Second, the values obtained from short-term recordings could differ from those based on longer periods of observation. Finally, the involvement of the sympathetic system in disease processes is here only postulated, but the demonstration of a cause–effect relationship is still lacking.
A better understanding of the molecular mechanisms underlying progression and recovery in MS will substantially aid the development of new treatment strategies able to exert neuroprotective and restorative effects.
Acknowledgement
The authors would like to thank all the patients who consented to be enrolled in the research.
Conflicts of interest.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Rossi acted as an advisory board member of Biogen Idec, Bayer Schering, Merck Serono, Teva, Novartis and Genzyme, and received funding for travelling and honoraria for speaking or writing from Biogen Idec, Merck Serono, Teva, Novartis, Bayer Schering, Genzyme, Almirall. She received support for research projects by Teva, Merck Serono and Bayer Schering and is involved as principal investigator in clinical trials for Teva, Novartis and Roche.
Dr Centonze acted as an advisory board member of Merck-Serono, Teva, Bayer Schering, Biogen Idec, Novartis, Almirall, Genzyme, GW Pharmaceuticals, and received funding for travelling and honoraria for speaking or consultation fees from Merck Serono, Teva, Novartis, Bayer Schering, Sanofi-aventis, Biogen Idec, Almirall, Genzyme, GW Pharmaceuticals. He is the principal investigator in clinical trials for Novartis, Merck Serono, Teva, Bayer Schering, Mitsubishi, Sanofi-aventis, Biogen Idec, Roche, Almirall. His preclinical and clinical research was supported by grants from Bayer, Biogen, Merck Serono, Novartis and Teva.
The other authors declare that there are no conflicts of interests.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Frontoni M, Fiorini M, Strano S, et al. Power spectrum analysis contribution to the detection of cardiovascular dysautonomia in multiple sclerosis. Acta Neurol Scand 1996; 93: 241–245. [DOI] [PubMed] [Google Scholar]
- 2.Nasseri K, Uitdehaag BM, van Walderveen MA, et al. Cardiovascular autonomic function in patients with relapsing remitting multiple sclerosis: a new surrogate marker of disease evolution? Eur J Neurol 1999; 6: 29–33. [DOI] [PubMed] [Google Scholar]
- 3.Acevedo AR, Nava C, Arriada N, et al. Cardiovascular dysfunction in multiple sclerosis. Acta Neurol Scand 2000; 101: 85–88. [DOI] [PubMed] [Google Scholar]
- 4.Karaszewski JW, Reder AT, Maselli R, et al. Sympathetic skin responses are decreased and lymphocyte beta-adrenergic receptors are increased in progressive multiple sclerosis. Ann Neurol 1990; 27: 366–372. [DOI] [PubMed] [Google Scholar]
- 5.Zalli A, Bosch JA, Goodyear O, et al. Targeting β2 adrenergic receptors regulate human T cell function directly and indirectly. Brain Behav Immun 2015; 45: 211–218. [DOI] [PubMed] [Google Scholar]
- 6.Gunal DI, Afsar N, Tanridag T, et al. Autonomic dysfunction in multiple sclerosis: correlation with disease-related parameters. Eur Neurol 2002; 48: 1–5. [DOI] [PubMed] [Google Scholar]
- 7.Flachenecker P, Reiners K, Krauser M, et al. Autonomic dysfunction in multiple sclerosis is related to disease activity and progression of disability. Mult Scler 2001; 7: 327–334. [DOI] [PubMed] [Google Scholar]
- 8.Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci 2009; 148: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suorsa E, Korpelainen JT, Ansakorpi H, et al. Heart rate dynamics in temporal lobe epilepsy – a long-term follow-up study. Epilepsy Res 2011; 93: 80–83. [DOI] [PubMed] [Google Scholar]
- 10.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xhyheri B, Manfrini O, Mazzolini M, et al. Heart rate variability today. Prog Cardiovasc Dis 2012; 55: 321–331. [DOI] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 14.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 1986; 59: 178–193. [DOI] [PubMed] [Google Scholar]
- 15.Mathias C and Bannister R. Autonomic failure. A textbook of clinical disorders of autonomic nervous system. 4th ed. Oxford: Oxford University Press, 1999.
- 16.Mahovic D, Lakusic N. Progressive impairment of autonomic control of heart rate in patients with multiple sclerosis. Arch Med Res 2007; 38: 322–325. [DOI] [PubMed] [Google Scholar]
- 17.Elenkov IJ, Wilder RL, Chrousos GP, et al. The Sympathetic nerve – an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 2000; 52: 595–638. [PubMed] [Google Scholar]
- 18.Mario Habek M, Crnošija L, Lovrić M, et al. Sympathetic cardiovascular and sudomotor functions are frequently affected in early multiple sclerosis. Clin Auton Res 2016; 26: 385–393. [DOI] [PubMed] [Google Scholar]
- 19.Rossi S, Studer V, Moscatelli A, et al. Opposite roles of NMDA receptors in relapsing and primary progressive multiple sclerosis. PLoS One 2013; 8: e67357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi S, Motta C, Studer V, et al. Tumor necrosis factor is elevated in progressive multiple sclerosis and causes excitotoxic neurodegeneration. Mult Scler 2014; 20: 304–312. [DOI] [PubMed] [Google Scholar]
- 21.Goyagi T, Nishikawa T, Tobe Y. Neuroprotective effects and suppression of ischemia-induced glutamate elevation by β1-adrenoreceptor antagonists administered before transient focal ischemia in rats. J Neurosurg Anesthesiol 2011; 23: 131–137. [DOI] [PubMed] [Google Scholar]
- 22.Markova J, Hudecova S, Soltysova A, et al. Sodium/calcium exchanger is upregulated by sulfide signaling, forms complex with the β1 and β3 but not β2 adrenergic receptors, and induces apoptosis. Pfluügers Arch Eur J Physiol 2014; 466: 1329–1342. [DOI] [PubMed] [Google Scholar]
- 23.Yun J, Gaivin RJ, McCune DF, et al. Gene expression profile of neurodegeneration induced by alpha1B-adrenergic receptor overactivity: NMDA/GABAA dysregulation and apoptosis. Brain 2003; 126(Pt 12): 2667–2681. [DOI] [PubMed] [Google Scholar]
- 24.Nolan RP, Jong P, Barry-Bianchi SM, et al. Effects of drug, biobehavioral and exercise therapies on heart rate variability in coronary artery disease: a systematic review. Eur J Cardiovasc Prev Rehabil 2008; 15: 386–396. [DOI] [PubMed] [Google Scholar]
- 25.Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry 1996; 39: 255–266. [DOI] [PubMed] [Google Scholar]
- 26.Kemp AH, Quintana DS, Gray MA, et al. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry 2010; 67: 1067–1074. [DOI] [PubMed] [Google Scholar]
- 27.Mori F, Nicoletti CG, Rossi S, et al. Growth factors and synaptic plasticity in relapsing–remitting multiple sclerosis. NeuroMolecular Med 2014; 16: 490–498. [DOI] [PubMed] [Google Scholar]
- 28.Tegenthoff M, Cornelius B, Pleger B, et al. Amphetamine enhances training-induced motor cortex plasticity. Acta Neurol Scand 2004; 109: 330–336. [DOI] [PubMed] [Google Scholar]
- 29.Plewnia C, Hoppe J, Cohen LG, et al. Improved motor skill acquisition after selective stimulation of central norepinephrine. Neurology 2004; 62: 2124–2126. [DOI] [PubMed] [Google Scholar]
- 30.Sczesny-Kaiser M, Bauknecht A, Höffken O, et al. Synergistic effects of noradrenergic modulation with atomoxetine and 10 Hz repetitive transcranial magnetic stimulation on motor learning in healthy humans. BMC Neurosci 2014; 15: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori F, Rossi S, Piccinin S, et al. Synaptic plasticity and PDGF signaling defects underlie clinical progression in multiple sclerosis. J Neurosci 2013; 33: 19112–19119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woiciechowsky C, Asadullah K, Nestler D, et al. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med 1998; 4: 808–813. [DOI] [PubMed] [Google Scholar]
- 33.Shahabi S, Hassan ZM, Jazani NH, et al. Sympathetic nervous system plays an important role in the relationship between immune mediated diseases. Med Hypotheses 2006; 67: 900–903. [DOI] [PubMed] [Google Scholar]
- 34.Chelmicka-Schorr E, Checinski M, Arnason BG. Chemical sympathectomy augments the severity of experimental allergic encephalomyelitis. J Neuroimmunol 1988; 17: 347–350. [DOI] [PubMed] [Google Scholar]
- 35.Simonini MV, Polak PE, Sharp A, et al. Increasing CNS noradrenaline reduces EAE severity. J Neuroimmune Pharmacol 2010; 5: 252–259. [DOI] [PubMed] [Google Scholar]
- 36.Wiegmann K, Muthyala S, Kim DH, et al. Beta-adrenergic agonists suppress chronic/relapsing experimental allergic encephalomyelitis (CREAE) in Lewis rats. J Neuroimmunol 1995; 56: 201–206. [DOI] [PubMed] [Google Scholar]
- 37.Rajda C, Bencsik K, Vécsei LL, et al. Catecholamine levels in peripheral blood lymphocytes from multiple sclerosis patients. J Neuroimmunol 2002; 124: 93–100. [DOI] [PubMed] [Google Scholar]