Abstract
Background:
Echocardiographic analysis of mitral valve (MV) has become essential for diagnosis and management of patients with MV disease. Currently, the various software used for MV analysis require manual input and are prone to interobserver variability in the measurements.
Aim:
The aim of this study is to determine the interobserver variability in an automated software that uses artificial intelligence for MV analysis.
Settings and Design:
Retrospective analysis of intraoperative three-dimensional transesophageal echocardiography data acquired from four patients with normal MV undergoing coronary artery bypass graft surgery in a tertiary hospital.
Materials and Methods:
Echocardiographic data were analyzed using the eSie Valve Software (Siemens Healthcare, Mountain View, CA, USA). Three examiners analyzed three end-systolic (ES) frames from each of the four patients. A total of 36 ES frames were analyzed and included in the study.
Statistical Analysis:
A multiple mixed-effects ANOVA model was constructed to determine if the examiner, the patient, and the loop had a significant effect on the average value of each parameter. A Bonferroni correction was used to correct for multiple comparisons, and P = 0.0083 was considered to be significant.
Results:
Examiners did not have an effect on any of the six parameters tested. Patient and loop had an effect on the average parameter value for each of the six parameters as expected (P < 0.0083 for both).
Conclusion:
We were able to conclude that using automated analysis, it is possible to obtain results with good reproducibility, which only requires minimal user intervention.
Keywords: Artificial intelligence, eSie Valve Software, interobserver variability, mitral valve, mitral valve analysis
Introduction
The concept of artificial intelligence (AI) was proposed in the 1920s, and it has been growing exponentially throughout the last century.[1] AI combines its computing powers with the input provided by experts and results in machines that are capable of self-learning and adapting to changes. AI has been used in medicine for the last three decades for diagnostic assistance and planning patient management.[2,3] For example, DXplain developed by the laboratory of computer science at Massachusetts General Hospital during the 1980s continues to assist in clinical decision-making and establishing diagnoses.[4]
Machine learning is a subfield of AI that makes it possible for computers to learn from experience through artificial neural networks. Using this technology, it is possible to “train” a machine by process of presentation of repetitive data and develop the ability to recognize patterns. During the process, the machine system continues to learn, adjust, and adapt from corrections made by a human observer.[5,6] This is a form of supervised machine learning technique. The ability of AI to learn from image recognition and interpretation has resulted in the development of automated electroencephalogram, electrocardiography analyses, and facial recognition technology. There are multiple possibilities in medicine where AI and machine learning can be of practical use. Tracing cardiac structures and analyses of the same is an entity where AI has been used recently.
Objective and quantitative echocardiographic analyses are critical in diagnosis, therapeutic planning, and perioperative management of patients with mitral valve (MV) disease. Currently, there are multiple algorithmic software available for such analyses. While some are semi-automated, most software requires considerable user input with potential for error magnification and variability.[7] Since the geometric valvular assessment is also based on pattern recognition and appreciation of subtle variations from normal, AI has been integrated into such programs. Recent advances in AI and machine learning have led to the development of fully automated valvular analyses packages with minimal to no user input. Whereas these are automated and self-learning, there is considerable skepticism regarding their clinical feasibility and reproducibility of their analyses. With the popularity of minimally invasive or percutaneous interventions, direct visual valve assessment has become limited or impossible. Echocardiographic MV quantification has assumed importance and clinical relevance. Therefore, we decided to assess the feasibility and reproducibility of one such automated MV analytical software.
Materials and Methods
This study was conducted as part of an ongoing Institutional Review Board approved protocol of retrospective analysis of intraoperative echocardiographic data with a waiver of informed consent from August 2015 to September 2015. Intraoperative three-dimensional (3D) transesophageal echocardiography (TEE) data were collected from four patients with normal MV undergoing routine coronary artery bypass graft (CABG) surgery. A Siemens SC-2000 TEE system with a Z6M TEE machine (Siemens Medical, Mountain View, CA, USA) capable of real-time 3D imaging probe was used for this study.
Data acquisition
Data were acquired immediately after induction of general anesthesia. Patients who had normal biventricular function and scheduled for elective CABG surgery were included in this study. After esophageal intubation with the TEE probe, it was positioned in the mid-esophageal location, and scan plane rotation was set at zero degrees. The two-dimensional image was optimized with gain and compression settings, and the region of interest was identified. Using the 3D control button, a full volume of MV was acquired over 2–3 beats at an average volume rate of 10–14 volumes/s without R-wave gating. The quality of the data was ensured with the absence of any dropout, inclusion of the entire mitral annulus, coaptation zone, and the leaflets. Three different data were acquired for each patient by an experienced echocardiographer (FM).
Data analysis
The data were then exported through USB drive in DICOM format to offline windows workstation equipped with the eSie Valve Software (Siemens Healthcare, Mountain View, CA, USA). Within the environment of the DICOM viewer software, eSie valve function was accessed and the volumetric data were analyzed. As a first step using the cine control function, the end-systolic (ES) frame was identified visually as the last frame before the MV begins to open. Further analyses were based on this ES frame as the starting point. In total, three examiners performed the static MV analysis in three ES frames for each of the four patients. A total of 12 ES frames analyzed by three different examiners have been included in the study. After selection of the frame of interest, the subsequent tracking of the valve is automated and minimal user input is required. After the software performs an automated tracing of the valve, there is an option to review and make manual correction of the software identified anatomical landmarks and surfaces in multiple planes [Figure 1]. Six geometric parameters of interest were selected for this study: (1) mitral annulus anterolateral posteromedial (ALPM) diameter, (2) mitral annulus anteroposterior (AP) diameter, (3) mitral annular area, (4) mitral annulus nonplanarity angle, (5) mitral annulus total perimeter, and (6) anterior and posterior leaflet areas. The measurements for each frame were then individually exported into.csv files.
Figure 1.

Steps performed for every frame that was selected and analyzed in the study. Manual correction of the automatically traced contours, if needed, can be performed in an overview of mitral valve, a rotational view, or in a parallel view of the mitral valve. Once the tracing is complete, the software analyzes various parameters and displays the result
Statistical methods
The.csv output from each MV analysis was imported to R Software (R Core Team, Vienna, Austria) for further analysis. For each of the selected six parameters computed by the eSie Valve Software, a multiple mixed-effects ANOVA model was constructed to identify whether the examiner, the patient, and the loop had a significant effect on the parameter average value. Therefore, the ANOVA was constructed such that the response was parameter average value, and the effects were: examiner, patient, and loop. More specifically, parameter average value was modeled against examiner, patient, and a patient-loop interaction term (patient-loop was defined as an interaction term because the loops were specific to each patient.).
The P value computed for each of the effects, therefore, tested the null hypothesis that the examiner, the patient, or the loop had no effect on the parameter. A Bonferroni correction was used to correct for multiple comparisons; since six parameters were studied, P = 0.0083 was considered to be significant.
Results
Three examiners successfully obtained outputs for the MVs of four different patients, visualized at three different loops during their examinations. No data were missing. On qualitative examination of the.csv outputs, the values of mitral annulus ALPM diameter, mitral annulus AP diameter, mitral annulus area, mitral annulus nonplanarity angle, mitral annulus total perimeter, and total leaflet area were all plausible. A mixed-effects ANOVA was successfully fit to the data for each of the six parameters.
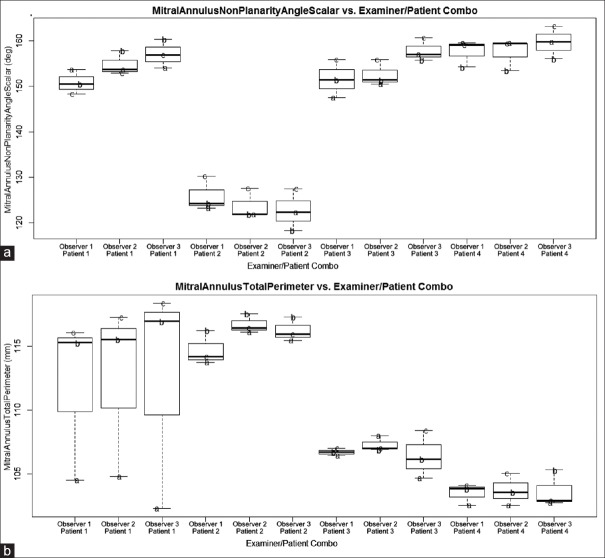
Examiner did not have an effect on any of the six parameters tested; APLM diameter (P = 0.72), AP diameter (P = 0.25), annulus area (P = 0.07), nonplanarity angle (P = 0.03), annulus perimeter (P = 0.13), and leaflet area (P = 0.15). These parameters are therefore robust to different examiners obtaining such values [Figures 2–4 and Table 1].
Figure 2.
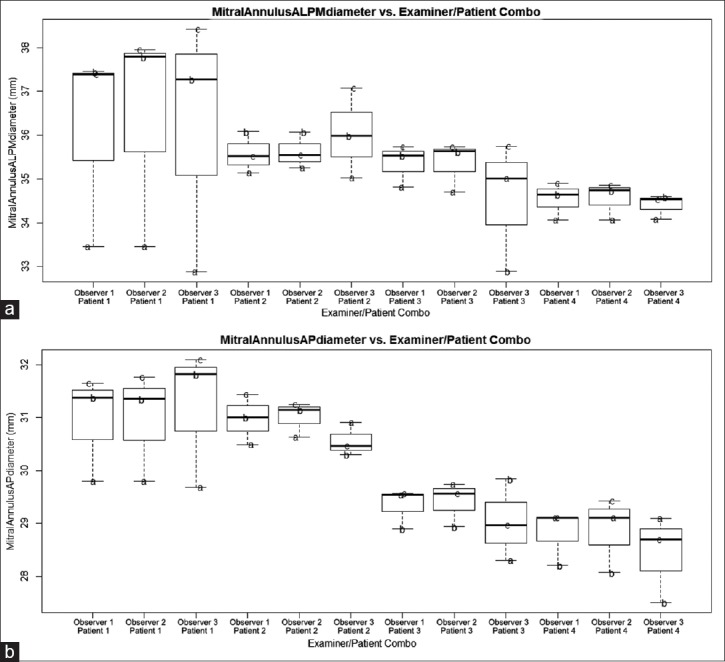
Plot of anterolateral posteromedial (panel A) and anteroposterior (panel B) diameter against examiner/patient combination. Overlaid text letters (a and b) denote different loop selections for each particular patient
Figure 4.
Plot of nonplanarity angle (panel A) and annulus perimeter (panel B) against examiner/patient combination. Overlaid text letters (a and b) denote different loop selections for each particular patient
Table 1.
Effect of examiner, patient, and loop on average parameter value in mixed-effects ANOVA modeling of each parameter as a response to the three effects terms
| Effect of examiner, patient, and loop on average parameter value in mixed-effects ANOVA | |||
|---|---|---|---|
| Parameter | P | ||
| Examiner | Patient | Loop | |
| ALPM diameter | 0.13 | 7.2E-08* | 2.7E-11* |
| AP diameter | 0.32 | 1.1E-15* | 1.9E-05* |
| Annulus area | 0.07 | 6.6E-24* | 3.1E-16* |
| Nonplanarity angle | 0.03 | 6.0E-26* | 5.0E-05* |
| Annulus perimeter | 0.13 | 1.6E-21* | 4.1E-13* |
| Leaflet area | 0.15 | 6.0E-20* | 2.3E-12* |
*A Bonferroni-corrected P=0.0083 was considered significant. ALPM: Anterolateral posteromedial, AP: Anteroposterior
Figure 3.

Plot of annulus area (panel A) and leaflet area (panel B) against examiner/patient combination. Overlaid text letters (a and b) denote different loop selections for each particular patient
Patient selection had an effect on average parameter value for each of the six parameters investigated as was expected [P < 0.0083, Table 1]. Loop had an effect on the average parameter for each of the six parameters investigated [P < 0.0083, Table 1].
Discussion
The results from our study demonstrate that it is feasible to perform automated analyses of MV with good reproducibility. Commonly used geometric parameters of MV analysis can be readily acquired without significant user input. Ability to readily perform such clinically relevant measurements is likely to introduce more uniformity and accuracy into quantitative analyses. Furthermore, there is less likelihood of individual bias impacting these measurements. Furthermore, with MV interventions becoming minimally invasive or percutaneous in nature, the interventionists have limited access to MV, and the 3D measurements are the starting and end points of interventions. Such interventions are entirely based on geometric measurements that are provided by echocardiographers; it is crucial to ensure accuracy and reproducibility of such analyses.[8,9] Therefore, MV quantification is evolving into an objective and precise process. Further, the software is capable of analyzing the valve throughout systole and diastole and tracks the entire surface of leaflets automatically.
Intraoperative geometric analyses of mitral, aortic, and tricuspid valves have assumed significant clinical importance. The currently available systems are either entirely manual or require significant user input and interaction during the process. Conventionally, the static analyses are based on the ES frame or the systolic phase of the cardiac cycle only. Dynamic analyses throughout the cardiac cycle have been reported with customized software or with user identification of multiple individual frames.[10,11] These are not only cumbersome and time consuming but also not clinically feasible. Commercial availability of such a system addresses a clinical need and will fulfill a knowledge gap. Whereas our study did not include the reproducibility of the dynamic analysis, we analyzed the most commonly utilized parameters in the clinical arena. They are useful for diagnosing mitral annular dilation, flattening, and establishing thresholds for intervention.
The maximum input that is required from the user is obtaining optimal image quality, adequate data sets, and verifying the automatic valve tracing after it is completed. Moreover, the automated analysis is independent of R-wave gating and requirement of sinus rhythm that explains the high reproducibility of the results. Apart from the regularly monitored parameters, the software generates many other MV analytical data majority of which pertain only to research interest and are not routinely used in the clinical scenario currently. For this study, we chose the most important clinical parameters that are used frequently on a day-to-day basis.
Conclusion
We were able to conclude that though automated analysis is prone to slight inconsistencies, it is possible to obtain results with good reproducibility that requires minimal user intervention. It thereby decreases the time taken to analyze cardiac structures and possibly expedites clinical decision-making. With introduction of machine learning algorithms into such software, any minor inconsistency can be further reduced as the software learns to adapt and adjust according to the user inputs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Mario Montealegre-Gallegos, MD, and Jayant Jainandunsing, MD, for their contribution toward analysis of the data.
References
- 1.Shortliffe EH. The adolescence of AI in medicine: Will the field come of age in the ‘90s? Artif Intell Med. 1993;5:93–106. doi: 10.1016/0933-3657(93)90011-q. [DOI] [PubMed] [Google Scholar]
- 2.Szolovits P, Patil RS, Schwartz WB. Artificial intelligence in medical diagnosis. Ann Intern Med. 1988;108:80–7. doi: 10.7326/0003-4819-108-1-80. [DOI] [PubMed] [Google Scholar]
- 3.Berner ES, Webster GD, Shugerman AA, Jackson JR, Algina J, Baker AL, et al. Performance of four computer-based diagnostic systems. N Engl J Med. 1994;330:1792–6. doi: 10.1056/NEJM199406233302506. [DOI] [PubMed] [Google Scholar]
- 4.Barnett GO, Hoffer EP, Packer MS, Famiglietti KT, Kim RJ, Cimino C, et al. DXPLAIN – Demonstration and discussion of a diagnostic clinical decision support system. Proc Annu Symp Comput Appl Med Care. 1991:878. [PMC free article] [PubMed] [Google Scholar]
- 5.Mayr A, Binder H, Gefeller O, Schmid M. The evolution of boosting algorithms. From machine learning to statistical modelling. Methods Inf Med. 2014;53:419–27. doi: 10.3414/ME13-01-0122. [DOI] [PubMed] [Google Scholar]
- 6.Narula S, Shameer K, Salem Omar AM, Dudley JT, Sengupta PP. Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J Am Coll Cardiol. 2016;68:2287–95. doi: 10.1016/j.jacc.2016.08.062. [DOI] [PubMed] [Google Scholar]
- 7.Warraich HJ, Shahul S, Matyal R, Mahmood F. Bench to bedside: Dynamic mitral valve assessment. J Cardiothorac Vasc Anesth. 2011;25:863–6. doi: 10.1053/j.jvca.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Mahmood F, Matyal R. A quantitative approach to the intraoperative echocardiographic assessment of the mitral valve for repair. Anesth Analg. 2015;121:34–58. doi: 10.1213/ANE.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 9.Wijdh-den Hamer IJ, Bouma W, Lai EK, Levack MM, Shang EK, Pouch AM, et al. The value of preoperative 3-dimensional over 2-dimensional valve analysis in predicting recurrent ischemic mitral regurgitation after mitral annuloplasty. J Thorac Cardiovasc Surg. 2016;152:847–59. doi: 10.1016/j.jtcvs.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rim Y, Choi A, McPherson DD, Kim H. Personalized computational modeling of mitral valve prolapse: Virtual leaflet resection. PLoS One. 2015;10:e0130906. doi: 10.1371/journal.pone.0130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai W, Li H, Tang H, Zhang Q, Zhu Y, Rao L. Assessment of aortic and mitral annuli dynamics during the cardiac cycle using speckle tracking echocardiography. Echo Res Pract. 2014;1:11–6. doi: 10.1530/ERP-14-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]