Abstract
Background and Aims:
Prolonged mechanical ventilation after cardiac surgery is associated with serious complications that increase morbidity and mortality. The present study was designed to compare ketamine-propofol (KP) and ketamine-dexmedetomidine (KD) combinations for sedation and analgesia in patients after coronary artery bypass graft (CABG) surgery as regards hemodynamics, total fentanyl dose, time of weaning from mechanical ventilation, time of extubation, and any adverse outcome.
Materials and Methods:
Seventy post-CABG patients were sedated using ketamine 1 mg/kg IV then 0.25 mg/kg/h infusion combined with either dexmedetomidine or propofol to maintain Ramsay sedation score ≥4 during assisted ventilation. Group KP received ketamine + propofol 1 mg/kg bolus followed by 25–50 μg/kg/min. Group KD received ketamine + dexmedetomidine 1.0 μg/kg over 20 min and then 0.2–0.7 μg/kg/h. Total dose of fentanyl in the first 24 h, time of weaning, time of extubation, mean arterial blood pressure, heart rate, and Intensive Care Unit (ICU) stay time were recorded.
Statistics:
Sample size of 35 patients was calculated for 90% power, α = 0.05, β = 0.1, and anticipated effect size = 0.40 using sample size software (G*Power version 3.00.10, Germany). Analytic statistics was performed on IBM compatible computer using SPSS version 11.5 (IBM, New York, United States) software package under Windows XP operating system. All results presented in the form of mean ± standard deviation. Data compared using unpaired Student's t-test, P < 0.05 was considered as statistically significant.
Results:
Group KD showed a significant decrease in mean time of weaning and extubation in group KD in comparison with group KP (374.05 ± 20.25 min vs. 445.23 ± 21.7 min, respectively, P < 0.001) (432.4 ± 19.4 min and 504 ± 28.7 min, respectively, P < 0.0001). Fentanyl consumption showed a significant decrease in group KD in comparison with group KP (41.94 ± 20.43 μg and 152.8 ± 51.2 μg, respectively, with P < 0.0001). There were insignificant difference between both groups as regards hemodynamic stability and length of ICU stay.
Conclusion:
Using KD combination for sedation, post-CABG surgery provided short duration of mechanical ventilation with less fentanyl dose requirement in comparison with KP with insignificant difference in both groups as regards hemodynamic stability and length of the ICU stay.
Keywords: Ketamine, dexmedetomidine, propofol, CABG, sedation
Introduction
In postcardiac surgery, many cardiovascular and other complications may occur that lead to increase mortality and hospital stays. Meticulous perioperative management is important to avoid these adverse events. Tachycardia was the main cause of post-coronary artery bypass graft (CABG) myocardial ischemia which can be decreased by sedation and analgesia.[1]
Dexmedetomidine is a highly specific alpha-2-adrenoreceptor agonist. Its sedative effect results from stimulation of alpha-2-adrenoreceptors in the central nervous system (in the locus coeruleus) independent of GABA system contrary to other sedative drugs dexmedetomidine has better sedative effect and similar respiratory and hemodynamic effects to midazolam. It does not depress respiratory drive or decrease arterial oxygen saturation so intravenous (IV) continuous sedation with dexmedetomidine does not change the normal course of ventilator weaning and extubation.[2] It produces a unique EEG pattern of sleep that closely resembles that of normal physiological sleep that allows easy arousal.[3] Dexmedetomidine has also analgesic effect. All these properties make dexmedetomidine a first-line drug for the cooperative sedation management in the Intensive Care Unit (ICU).[4]
Propofol is a sedative-hypnotic agent commonly used in the ICU for short-term (24 h) sedation of postoperative mechanically ventilated patients, propofol is not known to be analgesic, so opioids are given for pain. It facilitates inhibitory neurotransmission mediated by GABA. Its main advantages are its rapid induction and recovery, antiemetic effects, and anticonvulsant effects. Its main disadvantages lie in its dose-dependent hypotension, bradycardia, and respiratory depression;[5] hypotension is related to its moderate vasodilator effects, clinically significant hypotension occurs in patients who are hemodynamically unstable or those with limited myocardial reserve. Respiratory depression is more prominent in the presence of opioids.[6] Hence, discontinuing propofol before weaning is important.
Ketamine is a phencyclidine nonbarbiturate derivative that binds with N-methyl-d-aspartate and sigma opioid receptors to produce dissociative anesthesia, analgesia, and amnesia with little or no respiratory or cardiovascular depression. Ketamine inhibits endothelial nitric oxide production leading to positive inotropic action and vasoconstriction which preserves hemodynamic stability.[7]
Clinical studies concluded that combination of propofol and ketamine was safe and effective.[8]
The antiemetic and anxiolytic properties of propofol counteract the vomiting and emergence reactions induced by ketamine, whereas the sympathomimetic effect of ketamine counteracts the hypotension induced by propofol.[9] Using ketamine and propofol in combination allows sedation to be achieved with lower total doses of each drug, resulting in less toxicity than either drug alone and favorable recovery time profiles.[10]
Dexmedetomidine can effectively and safely attenuate the ketamine-induced hemodynamic pressor response and psychomimetic effects.[11]
Dexmedetomidine expected to prevent the tachycardia, hypertension, salivation, and emergence phenomena associated with ketamine. Ketamine may prevent the bradycardia and hypotension that have been reported with dexmedetomidine.[12]
Materials and Methods
After obtaining the Institutional Ethics Committee approval and informed written consent from patients, this prospective, randomized study was performed between June and November 2016 over 70 patients of 40–60 years old hemodynamically stable with normal or moderately impaired left ventricular function ejection fraction >40% that underwent elective CABG surgery for single vessel under high-dose opioid anesthesia on mechanical ventilation. Pregnancy, neurologic disease, liver or renal insufficiency, hemodynamic instability both intraoperative, postbypass or postoperative, and patients on vasopressor or inotropes are the exclusion criteria. All patients were sedated using ketamine 1 mg/kg IV bolus, followed by 0.25 mg/kg/h infusion combined with either dexmedetomidine or propofol to maintain Ramsay sedation score ≥4 during assisted ventilation. Group KP received ketamine + propofol 1 mg/kg bolus followed by 25–50 μg/kg/min. Group KD received ketamine + dexmedetomidine 1.0 μg/kg over 20 min and then 0.2–0.7 μg/kg/h. Sedation level was assessed using Ramsay Sedation Scale.[13] If awake, Ramsay 1 – anxious, agitated, or restless; Ramsay 2 – cooperative, oriented, tranquil; and Ramsay 3 – responsive to commands only. Moreover, if asleep, Ramsay 4 – brisk response to light glabellar tap or loud auditory stimulus; Ramsay 5 – sluggish response to light glabellar tap or loud auditory stimulus; and Ramsay 6 – no response to light glabellar tap or loud auditory stimulus. All patients received fentanyl for postoperative analgesia start at 1 μg/kg/h infusion then adjusted according to adult nonverbal pain score. Pain assessed using adult nonverbal pain score[14] [Table 1].
Table 1.
Pain assessed using adult non verbal pain score
| Categories | 0 | 1 | 2 |
|---|---|---|---|
| Face | No particular expression or smile | Occasional grimace, tearing, frowning, wrinkled forehead | Frequent grimace, tearing, frowning, wrinkled forehead |
| Activity (movement) | Laying quietly normal position | Seeking attention through movement or slow cautious movement | Restlessness excessive activity and/or withdrawal reflexes |
| Guarding | Laying quietly, no positioning of hand over areas of the body | Splinting area of the body tense | Rigid, stiff |
| Physiology (vital signs) | Stable vital signs | Change any of the following SBP>20 mmHg HR>20/min |
Change in any of the following SBP >30 mmHg HR >25/min |
| Respiration | Baseline RR/SpO2 compliant with ventilator | RR >10 above baseline, SpO2 decrease 5%, mild asynchrony with the ventilator | RR >20°C/min above baseline 10% decrease in SpO2 severe asynchrony with the ventilator |
SBP: Systolic blood pressure, HR: Heart rate, RR: Respiratpry rate
Results
There was a significant decrease in total dose of fentanyl, time to weaning, and extubation in group ketamine-dexmedetomidine (KD) in comparison with group ketamine-propofol (KP) with insignificant change between both groups as regards duration of stay in ICU, mean arterial blood pressure (MAP), and heart rate (HR). After extubation, one case developed nausea in KD group and one case developed skin allergy in KP group [Table 2 and Figures 1 and 2].
Table 2.
Demographic data, Total fentanyl dose, Time of weaning, Time of extubation and Duration of ICU stay
| Group KP | Group KD | P | |
|---|---|---|---|
| Age (years) | 54.88±5.3 | 53.5±4.9 | 0.14 |
| Weight (kg) | 81.44±6.9 | 79.7±6.4 | 0.43 |
| Height (cm) | 167.73±5.6 | 170.76±4.5 | 0.2 |
| Gender (male:female) | 20:15 | 18:17 | |
| Total fentanyl dose (µg) | 152.8±51.2 | 41.94±20.43* | <0.0001 |
| Time of weaning (min) | 445.23±21.7 | 374.05±20.25* | <0.001 |
| Time of extubation (min) | 504±28.7 | 432.4±19.4* | <0.0001 |
| Duration of ICU stay (h) | 44.97±3.3 | 43.7±3.04 | 0.13 |
*Statistical significance in comparison with group KP. ICU: Intensive Care Unit, KP: Ketamine-propofol, KD: Ketamine-dexmedetomidine
Figure 1.
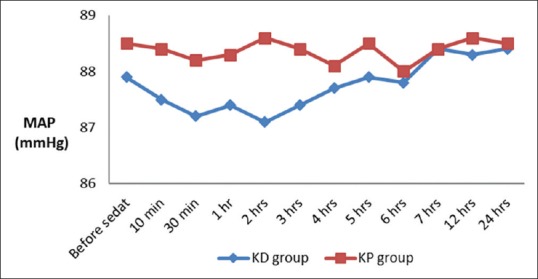
Mean arterial blood pressure changes in both groups
Figure 2.
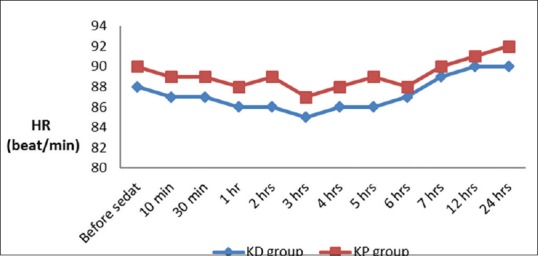
Heart rate changes in both groups
Discussion
CABG represents a great percentage of all cardiac surgeries in many cardiothoracic centers. One of the most important causes of postoperative mortality and morbidity is prolonged mechanical ventilation.[15] Type of sedation affects the duration of postoperative mechanical ventilation.[1] Selection of a sedative should depend on the agent's onset of action, side effects, and time to regain cognitive function after drug discontinuation.[16] Use of shorter-acting sedatives and opioids favors early tracheal extubation, decreases pain anxiety and cardiac instability that result from sympathetic output, helps earlier mobilization and hence rapid ICU and hospital discharge thus saving health-care costs.[17] Prolonged mechanical ventilation increases incidence of morbidity and mortality, due to increased liability to infection such as ventilator associated pneumonias, stress ulcer, gastrointestinal bleeding, pulmonary barotrauma, and decreased cardiac output.[18]
We made our study over 35 patients admitted to cardiothoracic ICU after CABG surgery on mechanical ventilation under sedation using (KP combination) and another 35 patients who received sedation using (KD combination). Although, to our knowledge, no other study had evaluated the effect of adding ketamine to either dexmedetomedine or propofol after CABG surgery, many studies have evaluated the effects of those combinations in sedation of pediatric and adult patients in procedures other than post-CABG surgery. There were no difference between the two groups as regards to patient characteristics including age weight and height.
The current study suggested that dexmedetomidine-ketamine combination is associated with shorter length of mechanical ventilation (374.05 ± 20.25 min vs. 445.23 ± 21.7 min, respectively, with P < 0.001), and earlier extubation compared with propofol-ketamine combination (432.4 ± 19.4 vs. 504 ± 28.7 min, respectively, with P < 0.0001). However, there was insignificant difference in both hemodynamics and duration of ICU stay between both groups. Early weaning and shorter duration of mechanical ventilation with dexmedetomidine may be contributed to the absent respiratory depressant effect, in addition to its better analgesic effect that decreased the total amount of fentanyl consumption which was 41.94 ± 20.43 μg in group KD and 152.8 ± 51.2 μg in group KP with P < 0.0001. Barletta et al. believed that dexmedetomidine is a good sedative in postoperative cardiac patients as it has no effect on respiratory function, minimizes opioid use, and decreases sympathetic discharge thus decreases times to extubation and intensive care length of stay and hospital length of stay.[19] Furthermore, Herr et al. compared dexmedetomidine-based versus propofol-based sedation regimens and found that propofol group patients needed a 4 times total dose of morphine than that of dexmedetomidine group patients and showed that dexmedetomidine was more effective compared with propofol in achieving a goal level of sedation. The median time to extubation was lesser by about 1 h in the dexmedetomidine group.[20]
Dexmedetomidine has opioid-sparing effects[21] and has found its way into every anesthesia techniques.[22] On the other hand, Anger et al. suggested that dexmedetomidine was not “narcotic-sparing” as patients treated with dexmedetomidine needed more opioids than patients treated with propofol. These studies reported that dexmedetomidine increased morphine use from 3.6% to 39.3% and ketorolac use from 3.6% to 25% over propofol in postcardiac surgery patients.[23]
The beneficial effects of dexmedetomedine-ketamine combination over propofol-ketamine combination as regards to earlier extubation and lesser time of the ICU stay were found in agreement with many previous studies. James et al. in a single-center retrospective analysis of effects of sedation on achievement of early extubation in postoperative cardiac surgery patients compared propofol-based versus dexmedetomidine-based sedation in cardiac surgery and found that postoperative extubation time and ICU stay were shorter with dexmedetomidine-based sedation. While length of hospital stay was nearly similar in both groups.[16] Barletta et al. said that dexmedetomidine use in postoperative cardiac surgery has rapidly expanded and found that dexmedetomidine achieved early extubation than propofol; this may be due to it did not affect respiration and had sympatholytic activity thus decreasing opiate doses.[19] Stephan et al. in a study of dexmedetomidine versus midazolam or propofol for sedation during prolonged mechanical ventilation thought that dexmedetomidine was a good choice for long-term sedation in intensive care patients with lesser duration of mechanical ventilation. Patients with dexmedetomidine had lesser duration of mechanical ventilation as compared with those with midazolam, lesser time to extubation as compared with both midazolam and propofol but had no effect on length of ICU or hospital stay. Dexmedetomidine caused bradycardia and hypotension more than midazolam but nearly equal to propofol.[24]
On the other hand, a previous study showed that there was no difference in extubation time between patients receiving dexmedetomidine or propofol in postoperative cardiac surgery patients.[25] As propofol has short duration of action and rapid return of cognition when stopping sedation.[26] In 2011, Reichert et al. study, effect of a dexmedetomidine substitution during a nationwide propofol shortage in 70 patients undergoing CABG surgery. They found no differences between the propofol and dexmedetomidine-sedated patients for either opioid requirement in the first 12 h after the ICU admission or time to extubation.[27] Janette et al. used a combination of dexmedetomidine and ketamine during spinal anesthesia in children and thought that the drawbacks of these two drugs may be prevented by their combination. Unfavorable adverse effects of dexmedetomidine include bradycardia, hypotension, and xerostomia. Ketamine, with its side effect profile of hypertension, tachycardia, and increased secretions, seems to be a good choice with dexmedetomidine during sedation as its side effects may abolish the unfavorable effects of dexmedetomidine and vice versa. On the other point of view, ketamine has powerful analgesic properties and has no respiratory depressant effect.[28]
Our hemodynamic data including MAP and HR showed an insignificant difference between the two groups. We believed that the use of ketamine in addition to dexmedetomedine or propofol for sedation was associated with fewer incidences of marked hemodynamic changes. We attribute that to the sympathomimetic effect of ketamine which counterbalance the unfavorable hemodynamic effects of both propofol and dexmedetomidine. As both drugs produce hypotension and dexmedetomidine produce bradycardia.
No patient required inotropic or chronotropic medication during our study. However, using either dexmedetomidine or propofol alone in some previous studies produce unfavorable hemodynamic outcomes; for example, Triltsch et al. compared a group of ventilated postsurgical patients sedated with dexmedetomidine and a placebo group and found that dexmedetomidine is associated with more incidence of bradycardia.[29] Results of Herr et al. in a study of 300 ICU sedated patients after CABG surgery showed that dexmedetomidine is associated with more decrease in BP than propofol, 36% versus 24%.[20] Furthermore, marked bradycardia was noticed in a meta-analysis when a loading dose and a high maintenance dose exceeds 0.7 μg/kg/h.[30] This may be due to dexmedetomidine is a selective and potent alpha-2 receptor agonist with dual vasomotor effects: vasoconstriction due to activation of alpha-2-adrenoceptors on vascular smooth muscle cells and vasodilatation due to activation of alpha-2-adrenoceptors on endothelial cells and inhibition of sympathetic nervous activity.[31,32] Furthermore, the vasomotor effects of propofol is generalized vasodilatation all over the arteriolar tree.[33]
Previous studies evaluating hemodynamic effects of dexmedetomidine in cardiac surgery have variable results; some results showed that the incidence of hypotension was no worse while others reported significant decreases in blood pressure which necessitates vasopressors.[34] One of them was a study over 44 children with acyanotic congenital heart disease undergoing cardiac catheterization. Tosun et al. compared a sedation of dexmedetomidine and ketamine combination with propofol and ketamine combination. They concluded that the propofol-ketamine regimen was superior.[35] Mester et al. evaluated the combination of dexmedetomidine and ketamine for sedation during cardiac catheterization in 16 children with congenital heart disease, they found that the combination of ketamine and dexmedetomidine provided effective sedation for cardiac catheterization in infants and children without marked hemodynamic or ventilator effects.[36] Janette et al. thought that using ketamine and dexmedetomidine combination increases success rate than dexmedetomidine alone or an equivalent success rate to the high doses of dexmedetomidine, without the risks of adverse cardiovascular effects as bradycardia.[28]
Conclusion
Using ketamine + dexmedetomidine combination for sedation post-CABG surgery provided short duration of mechanical ventilation with less fentanyl dose requirement in comparison with ketamine + propofol with insignificant difference in both groups as regards hemodynamic stability and length of hospital stay.
Financial support and sponsorship
This study was supported by Faculty of Medicine Tanta University Hospital.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zhang X, Zhao X, Wang Y. Dexmedetomidine: A review of applications for cardiac surgery during perioperative period. J Anesth. 2015;29:102–11. doi: 10.1007/s00540-014-1857-z. [DOI] [PubMed] [Google Scholar]
- 2.Mantz J, Josserand J, Hamada S. Dexmedetomidine: New insights. Eur J Anaesthesiol. 2011;28:3–6. doi: 10.1097/EJA.0b013e32833e266d. [DOI] [PubMed] [Google Scholar]
- 3.Huupponen E, Maksimow A, Lapinlampi P, Särkelä M, Saastamoinen A, Snapir A, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52:289–94. doi: 10.1111/j.1399-6576.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 4.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 5.Bassett KE, Anderson JL, Pribble CG, Guenther E. Propofol for procedural sedation in children in the emergency department. Ann Emerg Med. 2003;42:773–82. doi: 10.1016/s0196-0644(03)00619-x. [DOI] [PubMed] [Google Scholar]
- 6.Leino K, Mildh L, Lertola K, Seppälä T, Kirvelä O. Time course of changes in breathing pattern in morphine-and oxycodone-induced respiratory depression. Anaesthesia. 1999;54:835–40. doi: 10.1046/j.1365-2044.1999.00946.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen RM, Chen TL, Lin YL, Chen TG, Tai YT. Ketamine reduces nitric oxide biosynthesis in human umbilical vein endothelial cells by down-regulating endothelial nitric oxide synthase expression and intracellular calcium levels. Crit Care Med. 2005;33:1044–9. doi: 10.1097/01.ccm.0000163246.33366.51. [DOI] [PubMed] [Google Scholar]
- 8.Alletag MJ, Auerbach MA, Baum CR. Ketamine, propofol, and ketofol use for pediatric sedation. Pediatr Emerg Care. 2012;28:1391–5. doi: 10.1097/PEC.0b013e318276fde2. [DOI] [PubMed] [Google Scholar]
- 9.Camu F, Vanlersberghe C. Pharmacology of systemic analgesics. Best Pract Res Clin Anaesthesiol. 2002;16:475–88. doi: 10.1053/bean.2002.0262. [DOI] [PubMed] [Google Scholar]
- 10.Willman EV, Andolfatto G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;49:23–30. doi: 10.1016/j.annemergmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Gupta K, Gupta A, Gupta PK, Rastogi B, Agarwal S, Lakhanpal M. Dexmedetomidine premedication in relevance to ketamine anesthesia: A prospective study. Anesth Essays Res. 2011;5:87–91. doi: 10.4103/0259-1162.84193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levänen J, Mäkelä ML, Scheinin H. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology. 1995;82:1117–25. doi: 10.1097/00000542-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Sessler CN, Grap MJ, Ramsay MA. Evaluating and monitoring analgesia and sedation in the Intensive Care Unit. Crit Care. 2008;12(Suppl 3):S2. doi: 10.1186/cc6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wegman DA. Tool for pain assessment. Crit Care Nurse. 2005;25:14–5. [PubMed] [Google Scholar]
- 15.Gumus F, Polat A, Yektas A, Totoz T, Bagci M, Erentug V, et al. Prolonged mechanical ventilation after CABG: Risk factor analysis. J Cardiothorac Vasc Anesth. 2015;29:52–8. doi: 10.1053/j.jvca.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Curtis JA, Hollinger MK, Jain HB. Propofol-based versus dexmedetomidine-based sedation in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2013;27:1289–94. doi: 10.1053/j.jvca.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Trouillet JL, Combes A, Vaissier E, Luyt CE, Ouattara A, Pavie A, et al. Prolonged mechanical ventilation after cardiac surgery: Outcome and predictors. J Thorac Cardiovasc Surg. 2009;138:948–53. doi: 10.1016/j.jtcvs.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 18.Fougères E, Teboul JL, Richard C, Osman D, Chemla D, Monnet X. Hemodynamic impact of a positive end-expiratory pressure setting in acute respiratory distress syndrome: Importance of the volume status. Crit Care Med. 2010;38:802–7. doi: 10.1097/CCM.0b013e3181c587fd. [DOI] [PubMed] [Google Scholar]
- 19.Barletta JF, Miedema SL, Wiseman D, Heiser JC, McAllen KJ. Impact of dexmedetomidine on analgesic requirements in patients after cardiac surgery in a fast-track recovery room setting. Pharmacotherapy. 2009;29:1427–32. doi: 10.1592/phco.29.12.1427. [DOI] [PubMed] [Google Scholar]
- 20.Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: Dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17:576–84. doi: 10.1016/s1053-0770(03)00200-3. [DOI] [PubMed] [Google Scholar]
- 21.Martin E, Ramsay G, Mantz J, Sum-Ping ST. The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the Intensive Care Unit. J Intensive Care Med. 2003;18:29–41. doi: 10.1177/0885066602239122. [DOI] [PubMed] [Google Scholar]
- 22.Sudheesh K, Harsoor S. Dexmedetomidine in anaesthesia practice: A wonder drug? Indian J Anaesth. 2011;55:323–4. doi: 10.4103/0019-5049.84824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anger KE, Szumita PM, Baroletti SA, Labreche MJ, Fanikos J. Evaluation of dexmedetomidine versus propofol-based sedation therapy in mechanically ventilated cardiac surgery patients at a tertiary academic medical center. Crit Pathw Cardiol. 2010;9:221–6. doi: 10.1097/HPC.0b013e3181f4ec4a. [DOI] [PubMed] [Google Scholar]
- 24.Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, et al. Dexmedetomidine vs. midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA. 2012;307:1151–60. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 25.Corbett SM, Rebuck JA, Greene CM, Callas PW, Neale BW, Healey MA, et al. Dexmedetomidine does not improve patient satisfaction when compared with propofol during mechanical ventilation. Crit Care Med. 2005;33:940–5. doi: 10.1097/01.ccm.0000162565.18193.e5. [DOI] [PubMed] [Google Scholar]
- 26.Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell-Bright S, Levitt J, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006;34:1326–32. doi: 10.1097/01.CCM.0000215513.63207.7F. [DOI] [PubMed] [Google Scholar]
- 27.Reichert MG, Jones WA, Royster RL, Slaughter TF, Kon ND, Kincaid EH. Effect of a dexmedetomidine substitution during a nationwide propofol shortage in patients undergoing coronary artery bypass graft surgery. Pharmacotherapy. 2011;31:673–7. doi: 10.1592/phco.31.7.673. [DOI] [PubMed] [Google Scholar]
- 28.McVey JD, Tobias JD. Dexmedetomidine and ketamine for sedation during spinal anesthesia in children. J Clin Anesth. 2010;22:538–45. doi: 10.1016/j.jclinane.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Triltsch AE, Welte M, von Homeyer P, Grosse J, Genähr A, Moshirzadeh M, et al. Bispectral index-guided sedation with dexmedetomidine in intensive care: A prospective, randomized, double blind, placebo-controlled phase II study. Crit Care Med. 2002;30:1007–14. doi: 10.1097/00003246-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: A meta-analysis. Intensive Care Med. 2010;36:926–39. doi: 10.1007/s00134-010-1877-6. [DOI] [PubMed] [Google Scholar]
- 31.Miranda ML, Balarini MM, Bouskela E. Dexmedetomidine attenuates the microcirculatory derangements evoked by experimental sepsis. Anesthesiology. 2015;122:619–30. doi: 10.1097/ALN.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 32.Yeh YC, Sun WZ, Ko WJ, Chan WS, Fan SZ, Tsai JC, et al. Dexmedetomidine prevents alterations of intestinal microcirculation that are induced by surgical stress and pain in a novel rat model. Anesth Analg. 2012;115:46–53. doi: 10.1213/ANE.0b013e318253631c. [DOI] [PubMed] [Google Scholar]
- 33.Koch M, De Backer D, Vincent JL, Barvais L, Hennart D, Schmartz D. Effects of propofol on human microcirculation. Br J Anaesth. 2008;101:473–8. doi: 10.1093/bja/aen210. [DOI] [PubMed] [Google Scholar]
- 34.Mukhtar AM, Obayah EM, Hassona AM. Preliminary experience with dexmedetomidine in pediatric anesthesia. Anesth Analg. 2006;103:250. doi: 10.1213/01.ANE.0000228303.92422.73. [DOI] [PubMed] [Google Scholar]
- 35.Tosun Z, Akin A, Guler G, Esmaoglu A, Boyaci A. Dexmedetomidine-ketamine and propofol-ketamine combinations for anesthesia in spontaneously breathing pediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anesth. 2006;20:515–9. doi: 10.1053/j.jvca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Mester R, Easley RB, Brady KM, Chilson K, Tobias JD. Monitored anesthesia care with a combination of ketamine and dexmedetomidine during cardiac catheterization. Am J Ther. 2008;15:24–30. doi: 10.1097/MJT.0b013e3180a72255. [DOI] [PubMed] [Google Scholar]


