Abstract
Background
People with multiple sclerosis (MS) have functional disability and may have reduced muscle mitochondrial capacity.
Objective
The objective of this paper is to measure muscle mitochondrial capacity of leg muscles using near-infrared spectroscopy (NIRS) and compare to functional status.
Materials and methods
People with MS (n = 16) and a control (CON) group (n = 9) were evaluated for 25-ft walk time. Mitochondrial capacity of both gastrocnemius muscles were measured with NIRS as the rate of recovery of oxygen consumption in after exercise.
Results
Mitochondrial capacity was lower in the MS group compared to the CON group (rate constants: 1.13 ± 0.29 vs. 1.68 ± 0.37 min−1, p < 0.05). There was a tendency for people with MS who used assistive devices to have lower mitochondrial capacity in the weaker leg (p = 0.07).
Conclusion
NIRS measurements of mitochondrial capacity suggest a 40% deficit in people with MS compared to CONs and this may contribute to walking disability.
Keywords: Near-infrared spectroscopy, 25-foot walk
Introduction
Multiple sclerosis (MS) is a progressive disorder that is associated with a number of impairments that affect all aspects of daily life, such as cognition, sensation, and physical function.1 Furthermore, impairments in walking can lead to reduced activity levels and may exacerbate clinical symptoms.2 Previous studies have reported changes in skeletal muscle characteristics in people with MS.3,4 This includes smaller type 1 skeletal muscle fiber diameters with impaired skeletal mitochondrial succinate dehydrogenase activity and complex I function.3,4 People with MS have also been reported to have lower muscle mitochondrial capacities using the recovery rate of phosphocreatine measured with 31P magnetic resonance spectroscopy (31P MRS).5 People with MS report having one side of the body present with more or a greater intensity of symptoms.6 This primarily refers to muscle strength; however, bilateral differences in peak muscle oxygen uptake during one-legged cycling have been observed.7 Whether impaired muscle mitochondrial capacity is a result of changes related to MS or a consequence of inactivity related to MS symptoms is still under investigation. However, research has shown walking ability is related to mitochondrial capacity in various types of MS.8 Therefore, characterizing mitochondrial capacity in persons with MS and identifying bilateral differences may be of clinical importance. There is a need for practical and noninvasive assessments of mitochondrial capacity in people with MS. Assessments of muscle mitochondrial function that use muscle biopsies or expensive 31P MRS methodologies may not be ideal for evaluating bilateral changes in mitochondrial capacity in people with MS. Mitochondrial capacity can be measured noninvasively in vivo using near-infrared spectroscopy (NIRS).9 NIRS measures the decrease in oxygen saturation under ischemic conditions as muscle metabolic rate (mVO2), and the rate constant of recovery of mVO2 after either exercise or muscle electrical stimulation has been used as an index of muscle mitochondrial capacity (mVO2max).10 NIRS has been shown to be reproducible,11 independent of exercise intensity,12 and able to identify changes due to training status13 or disability.14
The aim of this study was to use NIRS to evaluate skeletal muscle mitochondrial capacity of the gastrocnemius muscle in people with MS compared to a group of healthy controls without MS (CON). mVO2max was evaluated in the medial gastrocnemius muscles in both legs. The gastrocnemius muscle was chosen to represent a primary muscle using in walking. We hypothesized that: (1) muscle mitochondrial capacity would be reduced in the MS group compared to the CON group, (2) in the MS group there would be a relationship between muscle oxidative capacity and walking speed, and (3) in the MS group muscle oxidative capacity would be lower in the weaker leg compared to the stronger leg.
Methods
Study participants
People with a diagnosis of MS were recruited from the Shepherd Center (Atlanta, GA) and via local advertisements both in Atlanta, GA, and Athens, GA. Prospective participants for the MS group must have been diagnosed with MS for at least 12 months, been able to ambulate at least 25 feet (ft), had stable use of disease-modifying drugs, and were not suffering from other neuromuscular injury or chronic diseases. Prospective participants for the able-bodied CON group had to be considered physically inactive as defined by <2 days/week of structured exercise, measured by the International Physical Activity Questionnaire Short Form (IPAQ-SF),14 and have no other neurological or orthopedic condition. Participants were excluded if they met any of the following criteria: body mass index (BMI)>30 kg/m2 and/or adipose tissue thickness of >20 mm over their gastrocnemius muscle, current or chronic orthopedic injuries of the lower limbs, inability to tolerate arterial occlusions as determined by involuntary spasms or extreme discomfort, inability to follow series of directions, or any females currently pregnant.
The study was approved by the Research Review Committee at the Shepherd Center (Atlanta, GA) and by the institutional review board at the University of Georgia (Athens, GA). All participants provided written informed consent prior to data collection.
Study design
Participants with MS underwent one to two testing sessions, depending on availability, scheduled within one week of each other. After providing informed consent, each MS group participant completed questionnaires about medication usage, MS symptoms, physical activity, and spasm frequency, with assistance provided by an investigator if necessary. Legs were assigned as weaker (most-affected) or stronger (least-affected) on a self-reported basis. CON group participants filled out physical activity and fatigue questionnaires, underwent NIRS assessment in both legs, and completed a timed 25-ft walk test within one testing session.
Descriptive outcome measures
Daily physical activity, related to sports and recreational activities, household activities, transportation, labor activities, and sitting time was evaluated by the IPAQ-SF.15 From this questionnaire, the metabolic equivalent (MET)*hours/week of activity was calculated for each participant. Spasm severity was assessed by the Assessment of Spasm Frequency scale used in a previous study.16 Participants rated on a scale of mild (1) to severe (5) including affected muscle(s), intensity, duration, frequency, sensitivity, and medication usage resulting in an overall score ranging from 0 to 30.
Clinical outcome measures
A trained rater assessed spasticity of the ankle dorsiflexor, ankle plantar flexor, and knee extensor of each leg using the Modified Ashworth Scale (MAS) as described by Ansari et al.17 The timed 25-ft walk test is an assessment of walking speed and is one of the quantitative assessments included in functional assessments of people with MS. To remove rater bias, a digital timing system (Brower IRD-T175, Salt Lake City, UT) was used as previously described.18 An assistive device was used if necessary. The average of the two trials was used as participants’ final score.
NIRS mitochondrial capacity assessment
Muscle mitochondrial capacity was quantified as the rate of recovery of muscle metabolism after a short bout of exercise or electrical stimulation to increase metabolic rate.19,20 Muscle metabolism was measured as changes in NIRS-measured oxygen saturation (Oxymon MK III, Artinis Medical Systems, The Netherlands) during short (5–10 seconds) bouts of ischemia. Measurements were made in both gastrocnemius muscles. If the participants could exercise their calf muscles, they completed ∼5–7 seconds of rapid, voluntary plantar flexion through their full range of motion against a commercially available rubberized resistance band pulled back to the knee by an assistant (Thera-Band Red model, Hygenic Corporation, Akron, OH). If the participants were not able to exercise both legs, electrical stimulation (15 s at 4 Hz) was used to activate the muscle. Electrical stimulation current was established as the amount necessary to produce vigorous contractions without eliciting discomfort. Previous studies have shown no differences in recovery rates from tests using voluntary or electrical stimulation.12 Two recovery kinetic tests were completed on each leg and averaged. A representation of the experimental setup is shown in Figure 1.
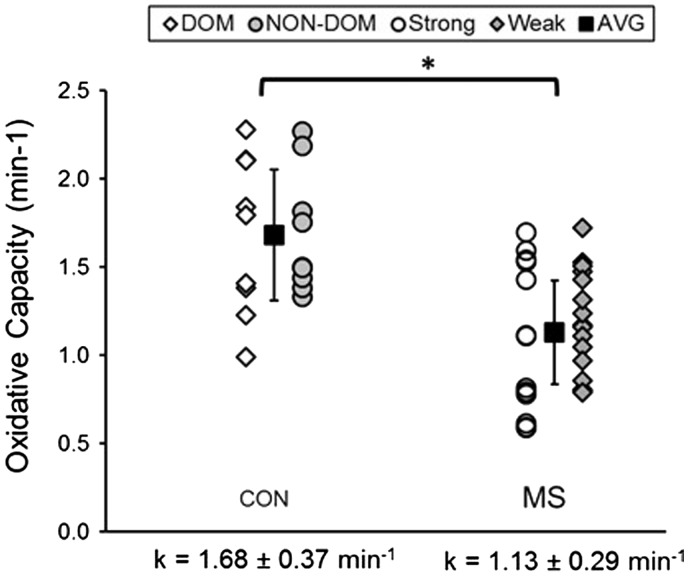
Figure 1.
Individual values for mitochondrial capacity of dominant (DOM) and non-dominant (NON-DOM) legs in the control participants (CON) and the strong and weak legs in people with multiple sclerosis (MS). AVG: average. Mean values are solid squares with SD.
Statistical analysis
Data are presented as mean ± SD. Statistical analyses were performed using SPSS 19.0 (IBM, Armonk, NY). Statistical analysis of rate constants of the MS group vs. CON group was conducted using a student’s unpaired t test. A paired student’s t test was used to analyze rate constants between legs. Linear regression was applied to rate constants, 25-ft walk completion time, and fatigue questionnaire scores to identify potential relationships.
Results
Participant characteristics
Sixteen participants with MS and nine CON participants were included in the study. Average age of the MS group was different from the CON group (49.7 ± 10.4 vs. 40.1 ± 9.8 years, p = 0.04). The MS group consisted of 13 females and three males (81% female): and the CON group consisted of eight females and one male (88% female). Comparison of participant characteristics can be found in Table 1.
Table 1.
Comparison of MS and control groups.
| MS | CON | p | |
|---|---|---|---|
| n | 16 | 9 | |
| Sex (M/F) | 3/13 | 1/8 | |
| Age (years) | 49 ± 10 | 40 ± 9 | 0.04a |
| Height (cm) | 164 ± 8 | 165 ± 8 | 0.87 |
| Weight (kg) | 70 ± 9 | 65 ± 13 | 0.32 |
| BMI (kg/m2) | 26 ± 3 | 24 ± 3 | 0.14 |
| ATT (cm) | Weak 1.0 ± 0.3 | ND 1.1 ± 0.3 | 1.00 |
| Strong 1.0 ± 0.3 | D 1.1 ± 0.3 | 1.00 | |
| Walking (MET-hour/week)b | 6.9 ± 5.2 | 3.5 ± 2.4 | 0.05 |
| Total PA (MET-hour/week)b | 20.4 ± 26.6 | 10.4 ± 12.1 | 0.13 |
MS: multiple sclerosis; CON: control; M: male; F: female; BMI: body mass index; ATT: adipose tissue thickness; MET: metabolic equivalent; PA: physical activity; Weak: most-affected limb; Strong: least-affected limb; ND: non-dominant limb; D: dominant limb. Data presented as mean ± SD; ap < 0.05; bn = 13 because of outliers defined as >2 SD from mean.
Participants with MS varied in diagnosis (nine relapsing–remitting, four secondary progressive, three no determination of MS type). Disease duration ranged from 1 to 36 years (17 ± 11 years). Nine of the 16 participants with MS required an assistive device during the 25-ft walk assessment. The following assistive devices were used: rollator (n = 5), Lofstrand crutch (n = 2), and single-point cane (n = 2). On average, low scores of spasticity were reported by the Spasm Frequency Questionnaire (10.5 ± 6.9 out of 30) and only six participants showed evidence of spasticity via the MAS.
Participants with MS reported taking the following medications: dalfampridine (n = 6), glatiramer acetate (n = 3), natalizumab (n = 1), interferons (n = 1), immunomodulators (n = 3), analgesics (n = 8), and muscle-relaxing drugs (n = 5). Participants were also on a variety of non-prescribed vitamins and supplements. There were no adverse events during testing in either group.
NIRS mitochondrial capacity
On average, the MS group had 40% lower mitochondrial capacity compared to the CON group (MS 1.13 ± 0.29 vs. CON 1.68 ± 0.37 min−1; p = 0.001) as seen in Figure 1. There was no significant difference observed in mitochondrial capacity between self-reported dominant and non-dominant legs in the CON group (p = 0.97) or between strong and weak legs in the MS group (p = 0.27).
25-ft walk test
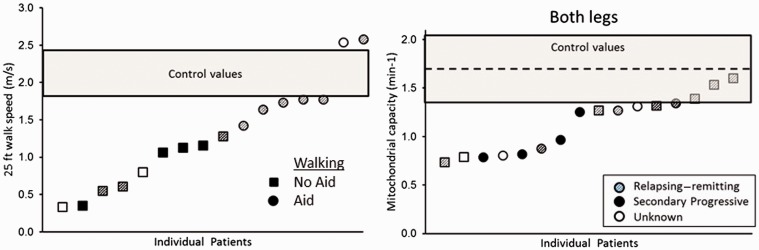
The MS group, on average, completed the 25-ft walk slower than the CON group as shown in Figure 2(a) (MS, 8.50 ± 6.23 vs. CON, 3.72 ± 0.40 s, p = 0.03). Participants in the MS group who used an assistive device during the 25-ft walk test had significantly slower completion times compared to participants who did not (p = 0.004) as shown in Figure 2(b). Participants with MS who used an aid not only walked slower, but trended to have an average lower mitochondrial capacity, 1.05 ± 0.24 min−1, compared to participants who did not use an aid, 1.23 ± 0.34 min−1 (p = 0.07).
Figure 2.
(a) Results of timed 25-foot walk test. The multiple sclerosis (MS) group is ordered from fastest to slowest walking speeds. The gray shaded box represents the 95% confidence interval of completion times observed in the control group. (b) Mitochondrial capacity for the MS group ordered from the lowest to the highest mitochondrial capacity. Circles represent those participants with MS who used an assistive device and squares represent those who did not. Shaded symbols represent relapsing–remitting MS, solid symbols secondary progressive MS, and open symbols type of MS unknown.
Discussion
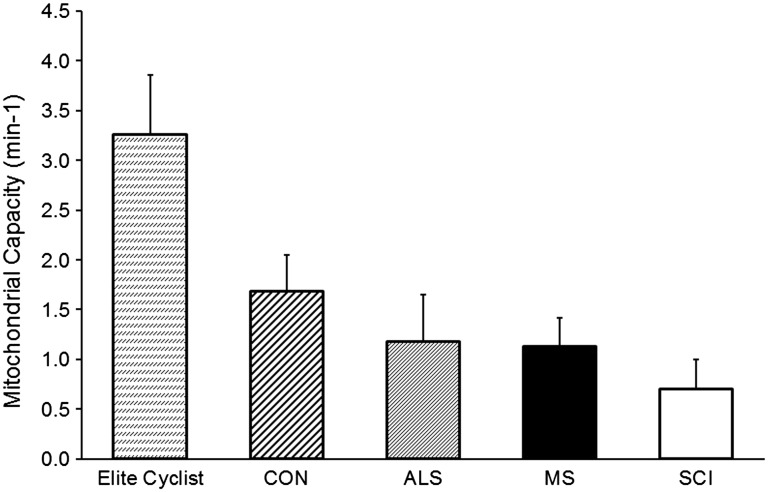
This is the first study to use the NIRS recovery kinetics test to measure muscle oxidative capacity (mVO2max) in people with MS. We found the test protocol to be well tolerated by the study participants. One of the advantages of the NIRS method of measuring mVO2max is that the rate constants can be directly compared between studies (Figure 3). Our participants with MS had similar oxidative capacity compared to people with amyotrophic lateral sclerosis,21 slightly higher than people with spinal cord injury,20 but only one-third the oxidative capacity of highly trained cyclists.22
Figure 3.
Comparisons of muscle mitochondrial capacity between groups of participants. Multiple sclerosis (MS) and control groups (CON) are from this study. Previously published data from highly trained cyclists,22 people with amyloid lateral sclerosis (ALS)21 and motor complete spinal cord injury (SCI).20 All the presented groups were significantly different from their respective control groups (p < 0.05).
Our participants with MS had 40% lower mVO2max in the MS group compared to the CON group. This agrees with previously published data by Kent-Braun and colleagues using the recovery rate of phosphocreatine measured with 31P MRS.5 An interesting aspect of the study by Kent-Braun and colleagues is that a large (four-fold) range in muscle oxidative capacity values was reported in their group with MS. It is possible that the wide range of values reflects a wide range of muscle oxidative capacities, potentially related to functional status. We did not find a wider range of muscle oxidative capacity values (only two-fold) in our group with MS compared to CONs. However, all participants were able to walk at least 25 ft, indicating a narrower range of functional ability compared to the range reported in the study by Kent-Braun and colleagues.5 Spasticity, while considered a negative symptom in terms of function, does result in muscle activation not accounted for in physical activity questionnaires. In the current study, spasticity was shown to be minimal while Kent-Braun and colleagues did not report spasticity, which may explain some of their reported variability not seen in the current study. A recent study has suggested that resting mVO2 may be an important variable of skeletal muscle metabolic impairments in MS, since they found resting mVO2 to be higher in an MS group compared to healthy CONs.23 The current study did not see differences in resting mVO2 between groups. We are not sure what accounts for the differences between studies. We have not found differences in resting metabolic rate in other neuromuscular disease or injury populations compared to CONs.21,20
A secondary aim of this study was to explore relationships between functional status and muscle oxidative capacity in people with MS who are ambulatory. While the Expanded Disability Status Scale (EDSS) score is clinically used as a measure of functional disability, it also takes into account neurologic and cognitive impairments.24 We chose the 25-ft walk test as a measure of functional disability, specifically walking function. We did not find significant correlations between walking speed and oxidative capacity. However, muscle mitochondrial capacity in the weaker leg in people who used an assistive device to walk compared to people who did not use an assistive device had a p value of 0.07. This effect appeared to be greater in the weaker leg than in the stronger leg. Larson and colleagues observed significant asymmetry in strength, oxygen uptake, and workload in participants with MS compared to healthy CONs.7 Future studies should examine the relationship between disease severity and the functional and metabolic differences between legs. Hansen and colleagues found a significant relationship between 6-minute walk test results and muscle oxidative capacity as assessed by exercise-onset oxygen uptake kinetics.8 Together these findings suggest that oxidative capacity may be an important component of walking endurance. Further characterizing the relationship between mitochondrial capacity and physical function could potentially provide insight into the mechanisms contributing to the progression of disability in MS and guide rehabilitation interventions that target skeletal muscle.
There were several limitations to this study. Some of our participants were not able to activate their plantar flexor muscles enough with voluntary exercise to measure recovery rates. In the participants who could not voluntarily activate their muscles, we found electrical stimulation effective. We have previously demonstrated that mVO2max values are not different between voluntary and electrical stimulation modes of activing muscle in healthy CONs,12 but this has not been demonstrated in MS. Calf muscle strength was not quantified in our study. We feel our participants could accurately identify their stronger and weaker legs, but quantifying the differences would have helped with comparisons between the stronger and weaker legs and legs with higher and lower mitochondrial capacity. We used 25-ft walking time and the use of walking assistive devices as our indicators of symptom severity. Assessments including the Multiple Sclerosis Functional Composite battery2 and self-reported fatigue scales such as the Modified Fatigue Impact Scale,25 or the Mental and Physical State and Trait Energy and Fatigue scales26 may have been useful to quantify symptom severity in terms of perceived fatigue. We have unpublished data with these scales on a subset of participants with MS that suggest that they would be useful in future studies. We attempted to capture physical activity through a self-report questionnaire, but participants with MS may have over-reported the intensity of their daily activities because of complications of their disease. We chose a self-report method for the convenience of the assessment given it was not a main outcome. However, an objective measure is suggested for a more accurate assessment of physical activity. An additional limitation in this study is the inability to make NIRS measures in individuals with excessive adipose tissue thickness over the muscle of interest.11,27 This exclusion of people with adipose tissue thicknesses over the muscle of interest greater than ∼2 cm limited our participant population, but this is an inherent limitation of the current NIRS technology.
In conclusion, we were able to successfully measure muscle oxidative capacity using NIRS in participants with mild MS. We observed reduced oxidative capacity compared to healthy CONs, and consistent with what has been reported for other neuromuscular diseases/disorders. The NIRS approach is noninvasive and less expensive to perform than other noninvasive muscle measurements (31P MRS). Determination of skeletal muscle mitochondrial capacity can benefit clinicians by providing insight on the extent to which muscle disuse is occurring in their patients, as well as providing predictions on the ability of a patient to perform rehabilitation or wellness programs.
Acknowledgments
The authors would like thank all volunteers who participated in this study, Blake Burdett for his assistance with recruitment, and T. Bradley Willingham and Melissa Erickson for their assistance with editing the manuscript.
Declaration of conflicting interests.
K.K. McCully is the president of Infrared Rx Inc. This company develops software analysis solutions for NIRS-based measurements of skeletal muscle. The other authors have nothing to declare.
Funding
This work was funded in part by the Eula C. and Andrew C. Carlos MS Rehabilitation and Wellness Program.
References
- 1.White LJ, Dressendorfer RH. Exercise and multiple sclerosis. Sports Med 2004; 34: 1077–1100. [DOI] [PubMed] [Google Scholar]
- 2.Rudick RA, Cutter G, Reingold S. The Multiple Sclerosis Functional Composite: A new clinical outcome measure for multiple sclerosis trials. Mult Scler 2002; 8: 359–365. [DOI] [PubMed] [Google Scholar]
- 3.Kumleh HH, Riazi GH, Houshmand M, et al. Complex I deficiency in Persian multiple sclerosis patients. J Neurol Sci 2006; 243: 65–69. [DOI] [PubMed] [Google Scholar]
- 4.Kent-Braun JA, Ng AV, Castro M, et al. Strength, skeletal muscle composition, and enzyme activity in multiple sclerosis. J Appl Physiol (1985) 1997; 83: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 5.Kent-Braun JA, Sharma KR, Miller RG, et al. Postexercise phosphocreatine resynthesis is slowed in multiple sclerosis. Muscle Nerve 1994; 17: 835–841. [DOI] [PubMed] [Google Scholar]
- 6.Chung LH, Remelius JG, Van Emmerik RE, et al. Leg power asymmetry and postural control in women with multiple sclerosis. Med Sci Sport Exer 2008; 40: 1717–1724. [DOI] [PubMed] [Google Scholar]
- 7.Larson RD, McCully KK, Larson DJ, et al. Bilateral differences in lower-limb performance in individuals with multiple sclerosis. J Rehabil Res Dev 2013; 50: 215–221. [DOI] [PubMed] [Google Scholar]
- 8.Hansen D, Feys P, Wens I, et al. Is walking capacity in subjects with multiple sclerosis primarily related to muscle oxidative capacity or maximal muscle strength? A pilot study. Mult Scler Int 2014; 2014: 759030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamaoka T, McCully KK, Quaresima V, et al. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt 2007; 12: 062105. [DOI] [PubMed] [Google Scholar]
- 10.Ryan TE, Southern WM, Reynolds MA, et al. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol (1985) 2013; 115: 1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Southern WM, Ryan TE, Reynolds MA, et al. Reproducibility of near-infrared spectroscopy measurements of oxidative function and postexercise recovery kinetics in the medial gastrocnemius muscle. Appl Physiol Nutr Metab 2014; 39: 521–529. [DOI] [PubMed] [Google Scholar]
- 12.Ryan TE, Brizendine JT, McCully KK. A comparison of exercise type and intensity on the noninvasive assessment of skeletal muscle mitochondrial function using near-infrared spectroscopy. J Appl Physiol (1985) 2013; 114: 230–237. [DOI] [PubMed] [Google Scholar]
- 13.Ryan TE, Southern WM, Brizendine JT, et al. Activity-induced changes in skeletal muscle metabolism measured with optical spectroscopy. Med Sci Sports Exerc 2013; 45: 2346–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson ML, Ryan TE, Brizendine JT, et al. Measuring skeletal muscle metabolism with near-infrared spectroscopy. Med Sci Sport Exer 2012; 44: 231. [Google Scholar]
- 15.Booth M. Assessment of physical activity: An international perspective. Res Q Exerc Sport 2000; 71(2 Suppl): S114–S120. [PubMed] [Google Scholar]
- 16.Erickson ML. Evaluation of skeletal muscle oxidative capacity in persons with spinal cord injury with near-infrared spectroscopy. Master’s Thesis, University of Georgia, USA, 2012.
- 17.Ansari NN, Naghdi S, Arab TK, et al. The interrater and intrarater reliability of the Modified Ashworth Scale in the assessment of muscle spasticity: Limb and muscle group effect. NeuroRehabilitation 2008; 23: 231–237. [PubMed] [Google Scholar]
- 18.Larson RD, Larson DJ, Baumgartner TB, et al. Repeatability of the timed 25-foot walk test for individuals with multiple sclerosis. Clin Rehabil 2013; 27: 719–723. [DOI] [PubMed] [Google Scholar]
- 19.Ryan TE, Erickson ML, Brizendine JT, et al. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: Correcting for blood volume changes. J Appl Physiol (1985) 2012; 113: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erickson ML, Ryan TE, Young HJ, et al. Near-infrared assessments of skeletal muscle oxidative capacity in persons with spinal cord injury. Eur J Appl Physiol 2013; 113: 2275–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan TE, Erickson ML, Verma A, et al. Skeletal muscle oxidative capacity in amyotrophic lateral sclerosis. Muscle Nerve 2014; 50: 767–774. [DOI] [PubMed] [Google Scholar]
- 22.Brizendine JT, Ryan TE, Larson RD, et al. Skeletal muscle metabolism in endurance athletes with near-infrared spectroscopy. Med Sci Sports Exerc 2013; 45: 869–875. [DOI] [PubMed] [Google Scholar]
- 23.Malagoni AM, Felisatti M, Lamberti N, et al. Muscle oxygen consumption by NIRS and mobility in multiple sclerosis patients. BMC Neurol 2013; 13: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer-Moock S, Feng YS, Maeurer M, et al. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurology 2014; 14: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson RD. Psychometric properties of the Modified Fatigue Impact Scale. Int J MS Care 2013; 15: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connor P. Mental and Physical State and Trait Energy and Fatigue scales, Athens, GA: University of Georgia, 2016. [Google Scholar]
- 27.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol 2004; 29: 463–487. [DOI] [PubMed] [Google Scholar]