Abstract
The effect of delayed-release dimethyl fumarate (DMF; also known as gastro-resistant DMF) on the Multiple Sclerosis Functional Composite (MSFC) was assessed using integrated Phase 3 DEFINE and CONFIRM data. Patients treated with DMF (n = 769) demonstrated significant superiority on the MSFC, and each component, compared with placebo (n = 771) over two years: mean change for DMF vs placebo was 0.054 vs −0.053 on MSFC; −0.088 vs −0.286 on Timed 25-Foot Walk, 0.047 vs 0.003 on 9-Hole Peg Test and 0.178 vs 0.123 on Paced Auditory Serial Addition Test. DMF was an efficacious treatment for patients with MS.
Keywords: Delayed-release dimethyl fumarate, multiple sclerosis, disability evaluation, Multiple Sclerosis Functional Composite, disease progression, neuropsychological tests
Introduction
Multiple sclerosis (MS) is an inflammatory, demyelinating disease of the central nervous system (CNS) often associated with the loss of motor function. While the Expanded Disability Status Scale (EDSS) score is currently the standard objective measure to assess clinical efficacy of MS treatments on disability, it is sometimes criticized because of its lack of standardization, inability to differentiate motor disabilities, and disregard of cognitive function.1 Cognitive impairments can be hidden by more visible deficits (e.g. motor and sensory), emotional complaints, fatigue or pain.2 While less apparent, cognitive impairment can be detrimental, especially considering its impact on quality of life of patients with MS.3 Therefore, it is necessary to specifically evaluate such impairments.
The Multiple Sclerosis Functional Composite (MSFC) is a multidimensional tool used to assess disability using quantitative composites of arm and hand function (9-Hole Peg Test (9HPT)), leg function and ambulation (Timed 25-Foot Walk (T25W)), and cognitive function (Paced Auditory Serial Addition Test (PASAT-3)).4 The MSFC was developed to improve the standard measure of disability and provide a multidimensional metric of overall clinical status.5 While MSFC has been shown to correlate with the EDSS and predict subsequent changes, the use of the MSFC may provide additional information on dimensions not covered in the EDSS.4
This post-hoc analysis describes the effect of dimethyl fumarate (DMF; also known as gastro-resistant DMF) on the MSFC using integrated data from the two-year Phase 3 Efficacy and Safety of Oral BG00012 in Relapsing–Remitting Multiple Sclerosis (DEFINE) and Results of the Comparator and an Oral Fumarate in Relapsing–Remitting Multiple Sclerosis (CONFIRM) trials.
Materials and methods
Patients and study design
The designs of the DEFINE (NCT00420212) and CONFIRM (NCT00451451) trials have been described elsewhere.6,7 Patients were randomized to receive DMF 240 mg twice (BID) or thrice daily (TID), placebo, or glatiramer acetate (GA; reference comparator in CONFIRM only) for up to 96 weeks. The integrated analysis was pre-specified prior to the unblinding of CONFIRM and was considered valid due to the many similarities between DEFINE and CONFIRM, (e.g. inclusion/exclusion criteria, regions from which patients were recruited, overall design, measurement criteria, observed efficacy). This analysis included data from patients randomized to receive placebo or DMF BID (the approved dosage).
Disability was measured by the change in the MSFC from baseline to Year 2 according to established guidelines.8 The MSFC comprised three measures: T25W (time to walk 25 feet), 9HPT (time to place and remove nine pegs into a pegboard), and PASAT-3 (auditory information processing speed and/or efficiency). The scores for the three components were converted into z-scores and then averaged together to create the overall MSFC score. The MSFC was performed every 12 weeks, with all three tests performed on each day of testing (with ≥5 days separating each test) to minimize any learning effect. Decreases in the z-scores represented deterioration in neurologic function.
Disease progression was defined as ≥20% increase on 9HPT or T25W compared with baseline confirmed ≥6 months later and also at last study visit using either of these measures.9 Patients must have had ≥2 post-baseline visits to be considered as having progressed.
Statistical analysis
Z-scores were calculated based on a reference population (all patients in each study) baseline mean. Observed data after patients switched to alternative MS medications were excluded from the analyses. Missing data prior to alternative MS medications and visits after patients switched to alternative MS medications were imputed using the last observation carried forward method, if available. Otherwise, the mean of the data for each treatment group/visit was used. P values for statistical comparison between DMF and placebo groups were based on analysis of covariance (ANOVA) on rank data, adjusted for study, region and MSFC z-score at baseline. Odds ratios (ORs) for progression were based on logistic regression, adjusted for baseline value (time) of the corresponding MSFC component, region and study.
Results
Patients
The intent-to-treat population for the integrated analysis comprised 2301 patients, of whom 769 were treated with DMF and 771 were treated with placebo. Baseline demographic and disease characteristics were similar across treatment groups (Table 1). The mean EDSS score was 2.5, and the mean number of relapses was 1.3 within the past 12 months, in both treatment groups.
Table 1.
Baseline demographics of the ITT population.
| DMF (n = 769) | PLACEBO (n = 771) | |
|---|---|---|
| Characteristica,b | ||
| Age, years | 37.9 ± 9.22 | 37.7 ± 9.22 |
| Female, n (%) | 541 (70) | 557 (72) |
| Time since diagnosis, years (mean/median) | 5.3/4.0 | 5.3/4.0 |
| EDSS | 2.5 ± 1.25 | 2.5 ± 1.21 |
| Number of relapses | ||
| Within prior three years | 2.5 ± 1.36 | 2.5 ± 1.52 |
| Within prior one year | 1.3 ± 0.65 | 1.3 ± 0.73 |
| Relapse-free patientsc, n (%) | 26 (3) | 27 (4) |
| Time since prior relapse, months | 6.4 ± 5.88 | 6.6 ± 6.59 |
| T25W, seconds | 7.4 ± 9.86 | 7.4 ± 9.91 |
| T25W, seconds, median (minimum, maximum) | 5.4 (2.6, 121.7) | 5.5 (2.6, 157.6) |
| 9HPTd, seconds | 24.8 ± 27.50 | 24.6 ± 24.67 |
| 9HPTd, seconds, median (minimum, maximum) | 20.6 (10.2, 402.6) | 20.8 (8.9, 406.1) |
| PASAT-3, number of items | 48.6 ± 11.20 | 47.9 ± 11.59 |
| PASAT-3, number of items, median (minimum, maximum) | 52.5 (5, 60) | 52.0 (0, 60) |
DMF: delayed-release DMF (also known as gastro-resistant DMF); EDSS: Expanded Disability Status Scale; ITT: intention-to-treat; MSFC: Multiple Sclerosis Functional Composite; T25W: Timed 25-Foot Walk; 9HPT: 9-Hole Peg Test; PASAT-3: Paced Auditory Serial Addition Test with a 3-second interstimulus interval.
Data are expressed as mean ± SD, unless otherwise indicated.
p > 0.05 for all comparisons.
Number of relapses within the past 12 months.
Data are expressed as the average of the mean time (seconds) taken to complete the test from both hands.
MSFC outcomes
In the integrated analysis of DEFINE and CONFIRM, there were continuous increases in the median MSFC z-scores throughout the 96 weeks for DMF patients compared with placebo. At the end of the studies, the mean (median) changes in MSFC from baseline to Year 2 were 0.054 (0.075) and −0.053 (0.023) for DMF and placebo, respectively (p = 0.0001). Benefits were evident within each individual component of the MSFC, compared with placebo. The mean (median) changes in T25W z-score were −0.088 (−0.013) and −0.286 (−0.022) for DMF and placebo (p = 0.0351; Figure 1(a)). Mean (median) changes in 9HPT z-scores were 0.047 (0.054) for DMF and 0.003 (0.012) for placebo (p = 0.0112; Figure 1(b)). Lastly, the mean change in PASAT-3 z-scores was 0.178 (0.175) for DMF and 0.123 (0.088) for placebo (p = 0.0016; Figure 1(c)). At Year 2, 91% of DMF patients did not experience a progression (defined as an increase of ≥20% increase from baseline confirmed at six months and at end of study) in T25W compared with 89% of placebo-treated patients (OR vs placebo: 0.82; p = 0.232). Both in DMF- and placebo-treated patients, 93% did not experience progression on 9HPT (OR vs placebo: 0.89; p = 0.578).
Figure 1.
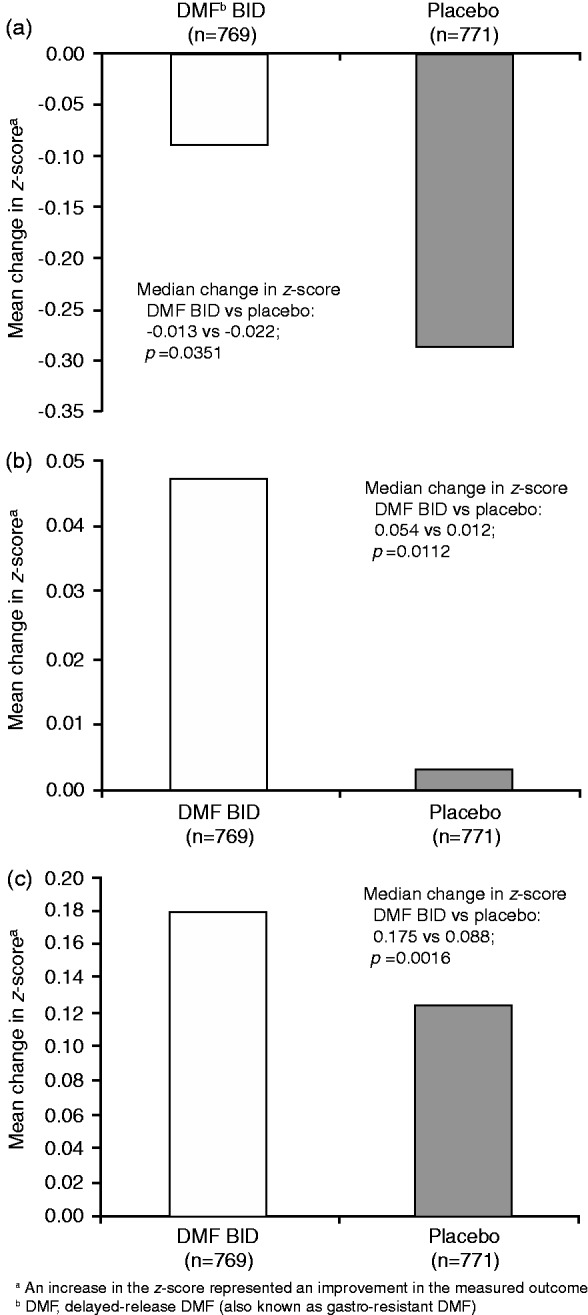
Change in T25W (a), 9HPT (b), and PASAT-3 (c) z-scores from baseline to Year 2a. BID: Twice daily; DMF: Delayed-release dimethyl fumarate (also known as gastro-resistant DMF); PASAT-3: Paced Auditory Serial Addition Test; T25W: Timed 25-Foot Walk; 9HPT: 9-Hole Peg Test.
Discussion
In this integrated analysis of the DEFINE and CONFIRM studies, DMF demonstrated statistically significant superiority on the MSFC, and each of its components, compared with placebo throughout the two years. Although the utilization of the EDSS in clinical trials supersedes that of the MSFC, the MSFC is being implemented into many recent and ongoing clinical studies. The MSFC has not only been shown to correlate with the EDSS, it was also shown to be associated with disease course, patient self-reported symptoms and health-related quality of life, and magnetic resonance imaging (MRI)-measured lesion load and cerebral atrophy.10 The MSFC had excellent intra-rater and inter-rater reliability.9 Thus, the MSFC may provide a more comprehensive assessment of an individual patient’s disability.
Conclusion
The functional improvements, as measured by the MSFC, further support the value of DMF as an efficacious treatment for patients with relapsing–remitting MS.
Acknowledgments
Biogen provided funding for medical writing support in the development of this paper; Gina Rocco, PhD, from Complete Medical Communications wrote the first draft of the manuscript based on input from the authors, and Elise Chahine from Complete Medical Communications copyedited and styled the manuscript per journal requirements. Biogen reviewed and provided feedback on the paper. The authors had full editorial control of the paper, and provided their final approval of all content.
Funding
This work was supported by Biogen.
Conflicts of interest.
GG: Honoraria from Abbvie, Bayer HealthCare, Biogen, Canbex Therapeutics, Five Prime Therapeutics, Genzyme, GlaxoSmithKline, GW Pharma, Merck, Merck Serono, Novartis, Protein Discovery Laboratories, Roche, Synthon, Teva Neuroscience, UCB, and Vertex; research grant support from Biogen, Ironwood, Merck Serono, Merz, and Novartis; compensation from Elsevier as co-chief editor of Multiple Sclerosis and Related Disorders.
RG: Consulting fees from Bayer HealthCare, Biogen, Merck Serono, Novartis, Teva Neuroscience, and Genzyme; grant/research support from Bayer HealthCare, Biogen, Genzyme, Merck Serono, Novartis, and Teva Neuroscience; compensation from Sage as editor of Therapeutic Advances in Neurological Disorders.
LK: Institution (University Hospital Basel) received and used exclusively for research support: steering committee, advisory board and consultancy fees from Actelion, Addex, Bayer Health Care, Biogen, Biotica, Genzyme, Lilly, Merck, Mitsubishi, Novartis, Ono Pharma, Pfizer, Receptos, Sanofi-Aventis, Santhera, Siemens, Teva, UCB, Xenoport; speaker fees from Bayer Health Care, Biogen, Merck, Novartis, Sanofi-Aventis, Teva; support of educational activities from Bayer Health Care, Biogen, CSL Behring, Genzyme, Merck, Novartis, Sanofi, Teva; royalties from Neurostatus Systems GmbH; grants from Bayer Health Care, Biogen, Merck, Novartis, Roche, Swiss MS Society, the Swiss National Research Foundation, the European Union, and Roche Research Foundations.
DLA: Honoraria/revenue/consultancy: Acorda Therapeutics, Biogen, EMD Serono, Genentech, Genzyme, GlaxoSmithKline, MedImmune, Mitsubishi, Novartis, Opexa Therapeutics, Receptos, Roche, Sanofi-Aventis, Teva; employee and stockholder of NeuroRx; research support from Novartis and Biogen.
ABO: Honoraria or research support from Biogen, DioGenix, Genentech, GlaxoSmithKline, Guthy-Jackson Charitable Foundation, MedImmune, Merck Serono, Novartis, Ono Pharmacia, Receptos, Roche, Sanofi-Aventis, and Teva Neuroscience; research support from Biogen, DioGenix, Genentech, and GlaxoSmithKline.
JLM, MY, AL: Employees of and hold stock/stock options in Biogen.
References
- 1.D’Souza M, Kappos L, Czaplinski A. Reconsidering clinical outcomes in multiple sclerosis: Relapses, impairment, disability and beyond. J Neurol Sci 2008; 274: 76–79. [DOI] [PubMed] [Google Scholar]
- 2.Guimarães J, Sá MJ. Cognitive dysfunction in multiple sclerosis. Front Neurol 2012; 3: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao SM, Leo GJ, Ellington L, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology 1991; 41: 692–696. [DOI] [PubMed] [Google Scholar]
- 4.Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 1999; 122(Pt 5): 871–882. [DOI] [PubMed] [Google Scholar]
- 5.Fischer JS, Rudick RA, Cutter GR, et al. The Multiple Sclerosis Functional Composite Measure (MSFC): An integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 1999; 5: 244–250. [DOI] [PubMed] [Google Scholar]
- 6.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 7.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 8.Multiple Sclerosis Functional Composite-Administration and Scoring Manual, National Multiple Sclerosis Society. Available at: http://main.nationalmssociety.org/docs/HOM/MSFC_Manual_and_Forms.pdf (10 January 2001, accessed 6 May 2015).
- 9.Polman CH, Rudick RA. The multiple sclerosis functional composite: A clinically meaningful measure of disability. Neurology 2010; 74(Suppl 3): S8–S15. [DOI] [PubMed] [Google Scholar]
- 10.Goldman MD, Motl RW, Rudick RA. Possible clinical outcome measures for clinical trials in patients with multiple sclerosis. Ther Adv Neurol Disord 2010; 3: 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]


