Abstract
Background
The worst Ebola virus disease (EVD) outbreak in history has resulted in more than 28,000 cases and 11,000 deaths. We present the final results of two phase 1 trials of an attenuated, replication-competent, recombinant vesicular stomatitis virus (rVSV)–based vaccine candidate designed to prevent EVD.
Methods
We conducted two phase 1, placebo-controlled, double-blind, dose-escalation trials of an rVSV-based vaccine candidate expressing the glycoprotein of a Zaire strain of Ebola virus (ZEBOV). A total of 39 adults at each site (78 participants in all) were consecutively enrolled into groups of 13. At each site, volunteers received one of three doses of the rVSV-ZEBOV vaccine (3 million plaque-forming units [PFU], 20 million PFU, or 100 million PFU) or placebo. Volunteers at one of the sites received a second dose at day 28. Safety and immunogenicity were assessed.
Results
The most common adverse events were injection-site pain, fatigue, myalgia, and headache. Transient rVSV viremia was noted in all the vaccine recipients after dose 1. The rates of adverse events and viremia were lower after the second dose than after the first dose. By day 28, all the vaccine recipients had seroconversion as assessed by an enzyme-linked immunosorbent assay (ELISA) against the glycoprotein of the ZEBOV-Kikwit strain. At day 28, geometric mean titers of antibodies against ZEBOV glycoprotein were higher in the groups that received 20 million PFU or 100 million PFU than in the group that received 3 million PFU, as assessed by ELISA and by pseudovirion neutralization assay. A second dose at 28 days after dose 1 significantly increased antibody titers at day 56, but the effect was diminished at 6 months.
Conclusions
This Ebola vaccine candidate elicited anti-Ebola antibody responses. After vaccination, rVSV viremia occurred frequently but was transient. These results support further evaluation of the vaccine dose of 20 million PFU for preexposure prophylaxis and suggest that a second dose may boost antibody responses. (Funded by the National Institutes of Health and others; rVSVΔG-ZEBOV-GP ClinicalTrials.gov numbers, NCT02269423 and NCT02280408.)
The worst Ebola virus disease (EVD) outbreak in recorded history has resulted in more than 28,000 cases and 11,000 reported deaths.1 Although the primary strategy to stop the transmission of Ebola remains the identification and isolation of contacts and the use of appropriate personal protective equipment, the development of a safe and efficacious vaccine would provide an important public health tool. Numerous Ebola virus vaccine candidates are in preclinical development, and some have proceeded to human trials.2-5
An Ebola virus vaccine candidate based on an attenuated, replication-competent, recombinant vesicular stomatitis virus (rVSV) has shown promise in preclinical studies. The vaccine candidate (rVSV-ZEBOV) is genetically engineered to replace the VSV glycoprotein with the glycoprotein from a Zaire strain of Ebola virus (ZEBOV). Vaccination induces replication of viral particles similar to VSV but expressing the ZEBOV surface glycoprotein. ZEBOV glycoprotein is responsible for receptor binding and membrane fusion between ZEBOV and host target cells and the induction of functional antibodies, including neutralizing antibodies.6
Preclinical testing of rVSV-ZEBOV supports its potential efficacy. The rVSV-ZEBOV vaccine has been shown to be attenuated in normal and immunocompromised nonhuman primates in safety and immunogenicity studies.7, 8 Multiple studies in cynomolgus macaques have shown that a single administration of the vaccine confers a high level of protection against lethal challenge.9,10 Various methods of vaccine delivery (oral, intranasal, or intramuscular) have shown protective efficacy in animal models.11
On the basis of this preclinical experience, we conducted phase 1, double-blind, placebo-controlled, dose-escalation studies of rVSV-ZEBOV at two locations in the United States: the Walter Reed Army Institute of Research (WRAIR), in Silver Spring, Maryland, and the National Institutes of Health (NIH) Clinical Center, in Bethesda, Maryland. Although the studies were designed as two independent studies, the assessments and data collections were largely harmonized. The WRAIR evaluated a single-dose strategy, whereas the NIH evaluated a homologous, two-dose regimen administered at study days 0 and 28. Safety and humoral-immunogenicity data through day 180 after vaccination, generated by the same laboratories for both trials, are presented here for the three vaccine dose levels (3 million plaque-forming units [PFU], 20 million PFU, and 100 million PFU) that were under consideration for human use. On the basis of the data presented here and additional clinical and preclinical data, the rVSV-ZEBOV vaccine (at the dose of 20 million PFU) was selected for inclusion in the Partnership for Research on Ebola Vaccines in Liberia (PREVAIL) trial (ClinicalTrials.gov number, NCT02344407), a phase 3 efficacy study in Guinea,12 and the phase 3 Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE, NCT02378753).
Methods
Vaccine
The rVSV-ZEBOV vaccine candidate is a live attenuated recombinant virus consisting of the VSV strain Indiana, with the glycoprotein of the ZEBOV Kikwit 1995 strain replacing the gene for the VSV envelope glycoprotein. The resultant rVSV construct contains surface ZEBOV glycoprotein that exhibits a narrower host-cell tropism in vitro than wild-type VSV, as well as considerable attenuation of replication.13 A 2015 analysis estimated a 3.4% nucleotide divergence (approximately 1.6% amino acid divergence) between the ZEBOV Kikwit 1995 strain and a limited number of genomic sequences for the currently circulating strain,14 although no conclusions regarding the effect on immunogenicity can be made.
The vaccine was developed by the Public Health Agency of Canada, licensed to BioProtection Systems (NewLink Genetics), and most recently sublicensed to Merck, which is responsible for ongoing research and development. The sponsor of the investigational new drug (IND) application, BioProtection Systems, was involved in discussions of the study design and in the study monitoring and statistical analysis; it also provided the vaccine candidate. The vaccine, which was manufactured according to current Good Manufacturing Practices, was formulated with recombinant human serum albumin and tris(hydroxymethyl)aminomethane buffer and was dispensed in a vial containing 100 million PFU per milliliter (lot number 003 05 13). Normal saline was used as a diluent by the study pharmacists to formulate the doses of 3 million PFU or 20 million PFU.
Volunteers and Study Design
Both trials were phase 1, double-blind, placebo-controlled, dose-escalation trials. The trials were designed to assess the safety, reactogenicity, and immunogenicity of rVSV-ZEBOV across three dose levels: 3 million PFU, 20 million PFU, and 100 million PFU. A total of 78 healthy adult men and women from the Washington, D.C.–Balti-more metropolitan area were recruited according to protocols that were approved by the institutional review board at each site. Written informed consent was obtained from all the volunteers before enrollment. Exclusion criteria were active involvement in clinical care of patients; substantial contact with immunocompromised populations, children 5 years of age or younger, or animals at risk for VSV infection; and a history of infection with filoviruses or VSV, predisposition for exposure to filoviruses or VSV, or previous receipt of a filovirus vaccine or VSV-vectored vaccine. Pregnant or lactating women and persons found to have the human immunodeficiency virus, hepatitis B or C virus infection, or clinically significant medical conditions at screening were excluded.
Study Procedures
A total of 39 adults at each site were consecutively enrolled into groups of 13 each. In each group, 3 volunteers were randomly assigned in a blinded manner to receive the control (saline placebo), and 10 were assigned to receive the rVSV-ZEBOV vaccine at a dose of 3 million PFU, 20 million PFU, or 100 million PFU. Each participant received a 1-ml injection in the deltoid muscle; at the NIH site, a second identical dose was administered 28 days after the first. Volunteers were assessed on days 1, 3, 7, 14, and 28 after the first and (if applicable) second injection. Data on solicited adverse events related to injection-site and systemic reactogenicity were collected for 14 days after each injection. Data on unsolicited adverse events, changes in medical status, and concomitant medication use were collected for 28 days after each injection. Blood samples were obtained for assessment of safety and immunologic end points. All the volunteers had safety laboratory evaluations (including a complete blood count with differential; measurements of serum creatinine, alanine aminotrans-ferase, and aspartate aminotransferase levels; determination of the prothrombin time and partial-thromboplastin time; and urinalysis [red-cell count and levels of protein and glucose]) at baseline and 7 days and 28 days after each injection. In addition, the WRAIR site evaluated these laboratory variables 1 day and 3 days after injection. Grading of adverse events was based on Food and Drug Administration toxicity grading.15 Positivity for vaccine ZEBOV-glycoprotein nucleic acid sequences was assessed in plasma, saliva, and urine. At the WRAIR, samples were obtained before the injection and on days 1, 3, 7, and 14 after the injection. At the NIH site, specimens were obtained on days 3 and 7 after each injection. Further details are available in the trial protocols, available with the full text of this article at NEJM.org.
rVSV-ZEBOV Surveillance by RT-PCR
A reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay was used to measure potential rVSV virus in the plasma, saliva, and urine, through amplification of the Ebola Zaire glycoprotein gene insert of the vaccine. The assay was performed at the WRAIR. Details are provided in the Supplementary Appendix, available at NEJM.org.
Measurement of Antibody Responses to Ebola Glycoprotein
The primary assays for antibody response were an enzyme-linked immunosorbent assay (ELISA) against the homologous Zaire–Kikwit strain glycoprotein and a pseudovirion neutralization assay (PsVNA) against the homologous Zaire–Kikwit strain glycoprotein. These assays were performed at the U.S. Army Medical Research Institute of Infectious Diseases (see the Methods section of the Supplementary Appendix). A limited number of samples were also tested with the use of an ELISA against the Zaire–Mayinga strain glycoprotein at the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases, with the use of methods described previously,16 to allow cross-vaccine comparisons of immunogenicity.
Statistical Analysis
Statistical analyses were performed with the use of R software, version 3.3.1. For each serologic variable, data were summarized by assessment day and included the geometric mean titer and 95% confidence interval, the median value, and minimum and maximum values. A two-sample t-test was performed for comparison of geometric mean titers between dose levels and study sites. A paired t-test was used for comparisons between time points within a dose level. All calculations and comparisons of geometric mean titers were performed on the log10 scale.
A positive response for the Kikwit strain ELISA was defined as a titer of 50 or more, with titers of less than 50 assigned values of 25 for calculation. A positive response for the PsVNA was defined as a titer of 20 or more, with titers of less than 20 assigned values of 10 for calculation. Seroconversion on these assays was defined as a quadrupling of the titer over the baseline value. Baseline values were subtracted from the postvaccination values for determination of the Mayinga strain ELISA titers, as described previously.3,4
Results
Study Participants
A total of 78 volunteers (55 men [71%] and 23 women [29%]), with a mean age of 36 years (range, 20 to 64), were enrolled in a consecutive manner; injections were administered between October 10, 2014, and January 6, 2015. A total of 60 volunteers were randomly assigned to receive rVSV-ZEBOV, and 18 volunteers were randomly assigned to receive saline placebo. At the NIH site, all the participants received a second identical dose of vaccine 28 days after the initial dose. All the volunteers completed the follow-up visits that were scheduled during the 28-day windows after vaccination; however, 4 volunteers were lost to follow-up by the conclusion of the trial (Fig. S1 in the Supplementary Appendix). Additional details regarding the demographic characteristics of the volunteers are provided in Table 1.
Table 1. Characteristics of the Participants at Enrollment*.
| Characteristic | Vaccine, 3 Million PFU (N = 20) |
Vaccine, 20 Million PFU (N = 20) |
Vaccine, 100 Million PFU (N = 20) |
Placebo (N = 18) |
Overall (N = 78) |
|---|---|---|---|---|---|
| Sex — no. (%) | |||||
| Male | 13 (65) | 16 (80) | 15 (75) | 11 (61) | 55 (71) |
| Female | 7 (35) | 4 (20) | 5 (25) | 7 (39) | 23 (29) |
| Age — yr | 36.9 ±11.8 | 34.6±12.2 | 38.8±14.7 | 32.7±10.7 | 35.8±12.4 |
| Race — no. (%)† | |||||
| Asian | 4 (20) | 3 (15) | 1 (5) | 2 (11) | 10 (13) |
| Black | 4 (20) | 7 (35) | 8 (40) | 4 (22) | 23 (29) |
| White | 10 (50) | 10 (50) | 10 (50) | 12 (67) | 42 (54) |
| Multiracial | 2 (10) | 0 | 1 (5) | 0 | 3 (4) |
| Hispanic ethnic group — no. (%)† | 1 (5) | 4 (20) | 1 (5.0) | 0 | 6 (8) |
| Body-mass index‡ | 26.3±4.7 | 26.7±5.2 | 28.7±7.9 | 27±6.6 | 27±6.2 |
Plus–minus values are means ±SD. There were no significant between-group differences at baseline. PFU denotes plaque-forming units.
Race and ethnic group were self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Safety
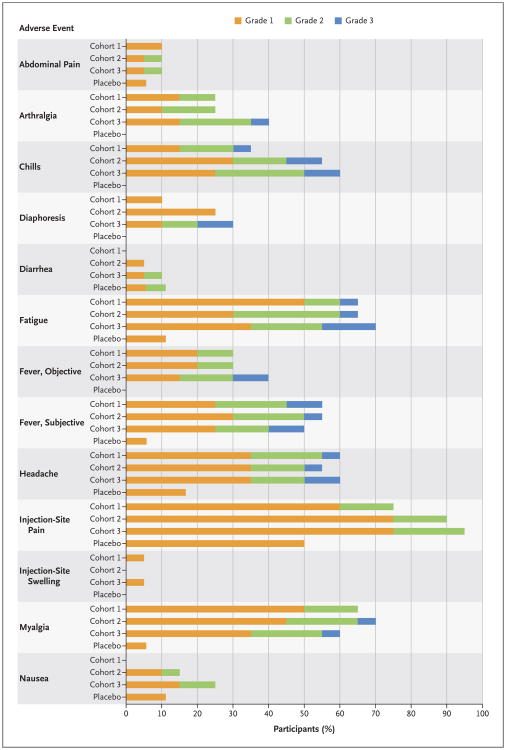
There were no deaths, serious adverse events, or adverse events resulting in withdrawal from the study. There was no association between vaccine dose and the frequency or severity of adverse events (Fig. 1, and Figs. S2 and S3 and Table S10 in the Supplementary Appendix). After a single inoculation of vaccine, mild-to-moderate injection-site pain was observed in the majority of participants. Systemic reactogenicity was transient and, in the majority of volunteers, mild to moderate in severity. Objective fever was noted in 20 of the 60 vaccinees: 11 (18%) had grade 1 fever (temperature range, 38.0 to 38.4°C), 7 (12%) had grade 2 fever (temperature range, 38.5 to 38.9°C), and 2 (3%) had grade 3 fever (temperature range, 39.0 to 40.0°C). Fever onset and frequency did not appear to be dose-dependent (Fig. 1, and Fig. S2 in the Supplementary Appendix); fever typically developed 12 to 24 hours after vaccination and resolved by the end of postvaccination day 1. One volunteer who received a dose of 3 million PFU had grade 1 fever 7 days after vaccination that resolved within 24 hours without development of other symptoms.
Figure 1. Frequency of Solicited Adverse Events According to Cohort and Grade.
Cohort 1 received a dose of 3 million plaque-forming units (PFU) of the vaccine, Cohort 2 a dose of 20 million PFU, and Cohort 3 a dose of 100 million PFU. All adverse events were assessed for relatedness to the vaccine; events that were judged by the investigating physicians not to be related to the vaccine are not shown. Adverse events were graded for severity on the basis of Food and Drug Administration toxicity grading.15 Unsolicited adverse events and laboratory adverse events are shown in the Supplementary Appendix.
Other commonly reported systemic symptoms among vaccinees were headache, myalgia, and fatigue, with typical onset 12 to 24 hours after vaccination. Notable adverse events were unilateral conjunctivitis that developed in one volunteer 1 day after inoculation and oral ulcers that developed in five vaccinated volunteers 4 to 16 days after vaccination. PCR analysis of swabs of the affected areas (a conjunctival swab and swabs of three of the five oral ulcers) was negative for the Ebola glycoprotein gene insert. Three vaccinated participants had cervical lymphadenopathy; one of the three also reported an oral ulcer. Infectious colitis developed in one participant 21 days after vaccination; symptoms included severe abdominal pain on the left side and four episodes of mild diarrhea with blood. Computed tomography of the abdomen at an outside hospital showed mild thickening of the descending colon. All conditions resolved without complications. Among participants who received a second dose of the vaccine, reactogenicity at the injection site and systemic reactogenicity were less severe after the second dose than after the first dose. A complete list of solicited and unsolicited adverse events is provided in Table S10 in the Supplementary Appendix.
Safety laboratory values were generally unremarkable, with the majority of adverse events occurring after the first dose of vaccine. Transient mild-to-moderate lymphopenia occurred in 24 of 60 participants, typically on day 1, with abatement by day 3 after vaccination. Mild-to-moderate neutropenia, which occurred in 14 of 60 participants, was most notable on day 3 after vaccination and typically abated within 2 to 4 days. An asymptomatic grade 2 thrombocytopenia, associated with grade 1 lymphopenia, was noted on day 1 after vaccination in one volunteer who received a dose of 20 million PFU; the condition resolved by day 7.
After a report from a phase 1 study in Geneva of the onset of arthritis in 22% of the participants starting the second week after injection,17,18 volunteers were specifically queried about the development of new arthralgia, arthritis, or rash during the second week or later after vaccination. A total of 19 participants reported arthralgia, typically soon after vaccination. Five participants had an onset of arthralgia 7 to 14 days after vaccination, and 3 participants had arthralgia that began after the second vaccination. No clinical cases of arthritis were diagnosed.
rVSV-ZEBOV on PCR Assay
PCR results are shown in Table 2. All the vaccinated volunteers had detectable vaccine viremia at the first visit after vaccination (day 1 at the WRAIR and day 3 at the NIH). Twelve of the 60 vaccinated volunteers (20%) had viremia on day 7 after vaccination. Viremia was undetectable by day 14 in all vaccinees tested at that time point (30 volunteers at the WRAIR). In the group that received a dose of 3 million PFU, there was one positive urine sample on day 3 and one on day 7. Across the vaccine groups, a small number of saliva samples were PCR-positive on days 1, 3, 7, and 14. Two subsequent saliva samples were PCR-negative in the single volunteer who had a positive PCR saliva sample on day 14. Cycle-threshold values for the positive urine sample on day 7 and saliva sample on day 14 were near the lower limit of detection for the assay.
Table 2. Vaccine Virus Detection by Means of Qualitative Reverse-Transcriptase–Polymerase-Chain-Reaction Assay.
| Type of Specimen | Day 1* | Day 3 | Day 7 | Day 14* | Day 31† | Day 35† |
|---|---|---|---|---|---|---|
| no. of positive samples/no. of samples tested (%) | ||||||
| Blood | ||||||
| Vaccine, 3 million PFU | 10/10 (100) | 20/20 (100) | 1/20 (5) | 0/10 | 0/10 | 0/10 |
| Vaccine, 20 million PFU | 10/10 (100) | 20/20 (100) | 5/20 (25) | 0/10 | 0/9 | 0/10 |
| Vaccine, 100 million PFU | 10/10 (100) | 19/20 (95) | 6/20 (30) | 0/10 | 1/8 (12) | 0/9 |
| Urine | ||||||
| Vaccine, 3 million PFU | 0/10 | 1/10 (10) | 1/10 (10) | 0/10 | 0/10 | 0/10 |
| Vaccine, 20 million PFU | 0/10 | 0/17 | 0/20 | 0/10 | 0/9 | 0/10 |
| Vaccine, 100 million PFU | 0/10 | 0/19 | 0/19 | 0/10 | 0/8 | 0/9 |
| Saliva | ||||||
| Vaccine, 3 million PFU | 0/10 | 2/20 (10) | 0/20 | 0/10 | 0/10 | 0/10 |
| Vaccine, 20 million PFU | 0/10 | 0/19 | 5/20 (25) | 1/10 (10) | 0/10 | 0/10 |
| Vaccine, 100 million PFU | 1/10 (10) | 0/19 | 1/19 (5) | 0/10 | 0/9 | 0/9 |
Data for days 1 and 14 are from the Walter Reed Army Institute of Research only.
Data for days 31 and 35 (after the second dose) are from the National Institutes of Health (NIH) Clinical Center only.
After administration of a second vaccine dose at the NIH site, a single volunteer in the group that received a dose of 100 million PFU had viremia 3 days later. PCR results were otherwise negative in blood, urine, and saliva.
ELISA for Ebola Glycoprotein
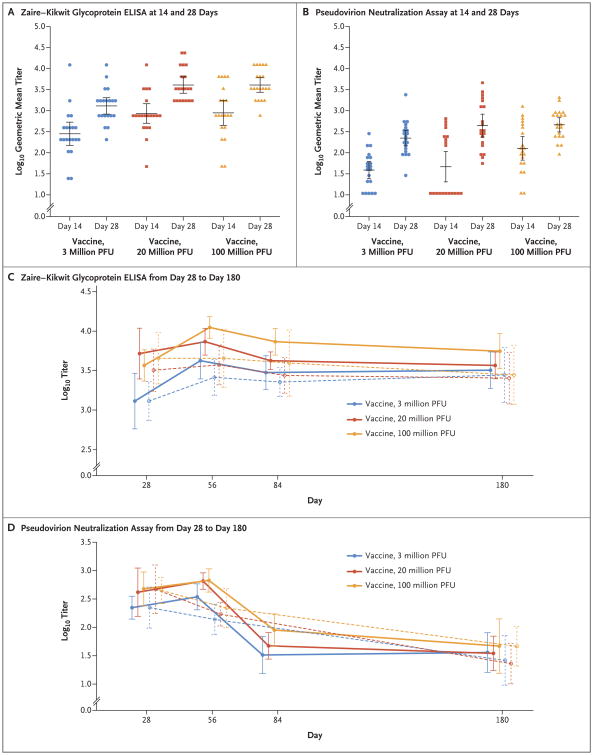
ELISA results are shown in Figure 2 and Table 3, and Tables S1 through S4 in the Supplementary Appendix. After a single dose of vaccine, IgG responses were observed. A total of 16 of 20 volunteers (80%) who received a dose of 3 million PFU, 19 of 20 volunteers (95%) who received a dose of 20 million PFU, and 18 of 20 who received a dose of 100 million PFU had undergone seroconversion by day 14. All 60 vaccinated volunteers (100%) had undergone seroconversion by day 28. The groups that received a dose of 20 million PFU or 100 million PFU had higher geometric titers against the Zaire–Kikwit strain than the group that received a dose of 3 million PFU, both on day 14 (857 and 888 vs. 283; P = 0.008 and P = 0.02, respectively) and on day 28 (4079 and 4079 vs. 1300; P = 0.001 and P<0.001, respectively). All vaccinated cohorts showed increases in titers from day 14 to day 28; titers increased from 283 on day 14 to 1300 on day 28 in the group receiving a dose of 3 million PFU (P<0.001), from 857 to 4079 in the group receiving a dose of 20 million PFU (P<0.001), and from 888 to 4079 in the group receiving a dose of 100 million PFU (P = 0.01)). There was no significant difference in the geometric mean titer between the group that received a dose of 20 million PFU and the group that received a dose of 100 million PFU.
Figure 2. Antibody Responses to Ebola Glycoprotein.
Individual antibody titers as assessed at 14 and 28 days after vaccination are shown according to vaccine dose group, as measured by an enzyme-linked immunosorbent assay (ELISA) against the Zaire–Kikwit strain glycoprotein (Panel A) and a pseudovirion neutralization assay (Panel B). Geometric mean titers (horizontal lines) are shown for each group and time point. Geometric mean titers from 28 days after initial vaccination through 180 days after initial vaccination are shown for the glycoprotein ELISA (Panel C) and the pseudovirion neutralization assay (Panel D). Solid lines indicate groups that received a second dose at day 28, and dashed lines indicate groups that did not receive a second dose at day 28. In all panels, I bars indicate 95% confidence intervals.
Table 3. Geometric Mean Antibody Titers*.
| Study Group | Day 14† | Day 28† | Day 56 | Day 84 | Day 180 | |||
|---|---|---|---|---|---|---|---|---|
| With Second Vaccination‡ | Without Second Vaccination | With Second Vaccination‡ | Without Second Vaccination | With Second Vaccination‡ | Without Second Vaccination | |||
| geometric mean titer (95% CI) | ||||||||
| Zaire–Kikwit glycoprotein ELISA | ||||||||
| Vaccine, 3 million PFU | 283 (150 – 534) | 1300 (831 – 2034) | 4222 (2478 – 7195) | 2599 (1537 – 4395) | 2986 (1823 – 4889) | 2263 (1485 – 3449) | 3200 (1878 – 5452) | 2786 (1248 – 6218) |
| Vaccine, 20 million PFU | 857 (502 – 1465) | 4079 (2601 – 6396) | 7352 (4972 – 10,871) | 3733 (2085 – 6682) | 4222 (3269 – 5455) | 2743 (1634^604) | 3676 (2486 – 5435) | 2540 (1196 – 5396) |
| Vaccine, 100 million PFU | 888 (448 – 1760) | 4079 (2740 – 6070) | 11,143 (8143 – 15,248) | 4525 (1933 – 10,597) | 7352 (4972 – 10,871) | 3940 (1501 – 10,343) | 5572 (3339 – 9298) | 2786 (1169 – 6638) |
| Placebo§ | 29 (23 – 38) | 29 (23 – 38) | 30 (25 – 37) | 27 (23 – 32) | 31 (20 – 47) | |||
| PsVNA | ||||||||
| Vaccine, 3 million PFU | 39 (24 – 62) | 223 (145 – 342) | 344 (203 – 583) | 138 (74 – 256) | 33 (15 – 69) | N/A | 36 (16 – 81) | 26 (10 – 71) |
| Vaccine, 20 million PFU | 47 (20 – 107) | 441 (236 – 825) | 653 (468 – 911) | 170 (106 – 275) | 47 (28 – 81) | N/A | 35 (17 – 70) | 23 (10 – 52) |
| Vaccine, 100 million PFU | 127 (67 – 242) | 461 (312 – 681) | 669 (418 – 1071) | 219 (98 – 486) | 90 (47 – 173) | N/A | 47 (16 – 141) | 46 (21 – 103) |
| Placebo§ | 10 (10 – 10) | 10 (10 – 10) | 10 (10 – 10) | 10 (10 – 10) | 10 (10 – 10) | |||
At day 28, geometric mean titers of antibodies against ZEBOV glycoprotein were higher in the groups that received a vaccine dose of 20 million PFU or 100 million PFU than in the group that received a dose of 3 million PFU, both as assessed by an enzyme-linked immunosorbent assay (ELISA) (4079 and 4079 vs. 1300; P=0.001 and <0.001, respectively) and as assessed by a pseudovirion neutralization assay (PsVNA) (441 and 461 vs. 223; P=0.07 and P=0.01, respectively). For the PsVNA, the day 84 analysis was performed only at the NIH Clinical Center. CI denotes confidence interval, and NA not applicable.
Analyses at day 14 and day 28 include data from volunteers at both study sites (all volunteers with only one vaccination at these time points).
The second vaccination was administered on day 28.
Volunteers in the placebo group received no vaccinations.
At day 28, there were no significant differences in the geometric mean titer between the groups that were to receive a second vaccine dose and those that were not. All three groups that received a second dose had increases in titers from day 28 to day 56; titers increased from 1300 on day 28 to 4222 on day 56 in the group that received a dose of 3 million PFU (P<0.001), from 5198 to 7352 in the group that received a dose of 20 million PFU (P = 0.27), and from 3676 to 11,143 in the group that received a dose of 100 million PFU (P<0.001). Among participants who received a second dose, the geometric mean titer was higher at day 84 than at day 28 in the group that received a dose of 3 million PFU (1300 at day 28 vs. 2986 at day 84 [P = 0.02]) and in the group that received a dose of 100 PFU (3676 at day 28 vs. 7352 at day 84 [P = 0.02]). Among participants who did not receive a second dose, only the group that received a dose of 3 million PFU had significant increases in the geometric mean titer from day 28 through day 84 (1300 at day 28 vs. 2599 at day 56 [P<0.001] and 1300 at day 28 vs. 2263 at day 84 [P = 0.003]). In all three vaccinated groups, participants who received a second vaccination had higher geometric mean titers on day 56 than those who did not (4222 vs. 2599 in the group that received a dose of 3 million PFU [P = 0.16], 7352 vs. 3733 in the group that received a dose of 20 million PFU [P = 0.04], and 11,143 vs. 4525 in the group that received a dose of 100 million PFU [P = 0.04]). At the 180-day follow-up, there was no significant difference in geometric mean titers between the groups that received a second dose of vaccine and the groups that received a single dose.
PSVNA Titers
Results with respect to neutralizing antibody titers against the Zaire–Kikwit strain glycoprotein are shown in Figure 2 and Table 3, and Table S5 through S8 in the Supplementary Appendix. After a single vaccination, all groups had neutralizing antibodies by day 28, in a dose-dependent manner. The geometric mean titer in the group that received a dose of 100 million PFU was significantly higher than in the group that received a dose of 3 million PFU both on day 14 (127 vs. 39 [P = 0.004]) and on day 28 (461 vs. 223 [P = 0.01]). All three dose groups had significant increases in the geometric mean titer from day 14 to day 28; the titer increased from 39 on day 14 to 223 on day 28 in the group that received a dose of 3 million PFU (P<0.001), from 47 to 441 in the group that received a dose of 20 million PFU (P<0.001), and from 127 to 461 in the group that received a dose of 100 million PFU (P<0.001).
At day 28, there were no significant differences in the geometric mean titer between the groups that were to receive a second vaccine dose and those that were not. Groups that received a second dose of vaccine had an initial trend of increased geometric mean titers during the month after revaccination (222 at day 28 vs. 344 at day 56 in the group that received a dose of 3 million PFU [P = 0.08], 415 vs. 653 in the group that received a dose of 20 million PFU [P = 0.33], and 476 vs. 669 in the group that received a dose of 100 million PFU [P = 0.19]). However, this trend was reversed in titers measured 2 months after revaccination (222 at day 28 vs. 33 at day 84 in the group that received a dose of 3 million PFU [P<0.001], 415 vs. 47 in the group that received a dose of 20 million PFU [P = 0.003], and 476 vs. 90 in the group that received a dose of 100 million PFU [P<0.001]). Vaccine groups that did not receive a second dose had a decrease in neutralizing antibody responses from day 28 to 56 (223 vs. 138 in the group that received a dose of 3 million PFU [P = 0.06], 468 vs. 170 in the group that received a dose of 20 million PFU [P = 0.008], and 447 vs. 219 in the group that received a dose of 100 million PFU [P = 0.02]). At the 180-day follow-up, there was no significant difference in neutralizing antibody responses between the groups that received a second dose of vaccine and the groups that received a single dose.
Discussion
Both a single and a second administration of the rVSV-ZEBOV Ebola vaccine candidate elicited an antibody response without any safety concerns being identified. In 60 healthy adults, the vaccine candidate had an acceptable safety profile across all dose concentrations. The most common side effects were injection-site pain, myalgia, fatigue, headache, subjective fever, and chills. Immunogenicity as measured by means of IgG ELISA was concordant with antibody responses measured with the use of a functional (neutralization) assay, and the IgG ELISA results suggested a dose response, especially between the group receiving a dose of 3 million PFU and the groups receiving higher doses, with little difference between the group receiving a dose of 20 million PFU and the group receiving a dose of 100 million PFU. Although transient arthralgia was noted in a minority of volunteers, clinical arthritis, which was reported in another clinical trial of the vaccine candidate, was not observed at the WRAIR or NIH site. These data supported selection of 20 million PFU as the dose for clinical end-point trials (PREVAIL trial, the Guinea study, and STRIVE) in West Africa. In the Guinea study, this dose recently showed high protective efficacy with the use of a ring vaccination strategy.12
Transient rVSV viremia was detected after immunization, recapitulating the experience described previously in nonhuman primates.19 The clinical symptoms associated with this viremia included fever and appeared to peak and then decrease in the 12 to 36 hours after vaccination. Overall, safety laboratory values were subclinical and unremarkable. Moderate asymptomatic declines in leukocyte subsets (e.g., lymphopenia and neutropenia) were noted during the first 3 days after vaccination and resolved rapidly. The data from the clinical trials presented herein are consistent with the preclinical experience and, combined with the established attenuation of the vaccine vector, provide further support for the safety of rVSV vectors.2,13,20
The immunoprotective profile that is required for the prevention of EVD remains largely unknown, and mechanistic correlates of protection remain undefined. Successful protection in the nonhuman primate model has been shown with various vaccine candidates, with imputation of both cellular and humoral immune responses as correlates of protection.21,22 In the nonhuman primate challenge model, antibody response, principally IgG, has been the strongest immune correlate of protection associated with the rVSV-ZEBOV vaccine candidate.10,23,24 Although the Kikwit strain ELISA has become the primary readout, examination of the Mayinga-strain glycoprotein titers (Table S9 in the Supplementary Appendix) suggests that the rVSV-ZEBOV vaccine candidate produces cross-strain glycoprotein-specific antibodies similar to those described for the chimpanzee adenovirus 3 vaccine candidate.3 Neutralizing antibody assays typically have been difficult to correlate with outcomes in studies in animals involving EVD, but the functional assay used for the reported trials showed a strong association with protection of nonhuman primates across multiple vaccine platforms and warrants further investigation as a correlate of protection.24-27
A second dose of vaccine was less reactogenic and induced less viremia than the primary dose. Although a two-dose regimen was associated with a short-term advantage with respect to the magnitude of the humoral response, we did not observe a significant difference in the day 180 titer between the one-dose and two-dose vaccine regimens. The vaccine candidate has already shown efficacy in populations living in regions in which EBV is endemic, but the immunologic profiles presented here suggest that two doses of vaccine administered within a short time frame may provide increased short-term benefit.5 In addition, however, strategies such as longer intervals between doses could be pursued to improve the longer-term immunologic profile. Such work would need to go hand-in-hand with assessment of efficacy in the animal model and validation of the presented immune correlates.
The results reported here support the safety, acceptable side-effect profile, and immunogenicity of up to two doses of the rVSV-ZEBOV vaccine and encourage further investigation of this vaccine candidate. Most promising are the robust immune responses after a single dose of the vaccine and the rapid onset of immunity, which could be particularly useful in outbreak interventions. Although we found short-term increases in humoral immunity after a second dose at the 1-month interval, it remains unknown whether this regimen will translate to improved clinical efficacy. Strategies to better understand and improve immunogenicity, including assessment of dose and regimen alterations, a longer duration of follow-up, and cross-strain protection against other Ebola viruses, could be pursued.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases, NIH; the National Cancer Institute, NIH (contract no. HHSN261200800001E); the Defense Threat Reduction Agency; and the Joint Vaccine Acquisition Program.
Appendix
The authors' full names and academic degrees are as follows: Jason A. Regules, M.D., John H. Beigel, M.D., Kristopher M. Paolino, M.D., Jocelyn Voell, R.N., M.S., Amy R. Castellano, L.P.N., Zonghui Hu, Ph.D., Paula Muñoz, B.S., James E. Moon, M.D., Richard C. Ruck, M.D., Jason W. Bennett, M.D., Patrick S. Twomey, M.D., Ramiro L. Gutiérrez, M.D., Shon A. Remich, M.D., Holly R. Hack, M.S., Meagan L. Wisniewski, Ph.D., Matthew D. Josleyn, M.S., Steven A. Kwilas, Ph.D., Nicole Van Deusen, B.S., Olivier Tshiani Mbaya, M.D., Yan Zhou, Ph.D., Daphne A. Stanley, M.S., Wang Jing, M.S., Kirsten S. Smith, Ph.D., Meng Shi, M.A., Julie E. Ledgerwood, D.O., Barney S. Graham, M.D., Nancy J. Sullivan, Ph.D., Linda L. Jagodzinski, Ph.D., Sheila A. Peel, M.S.P.H., Ph.D., Judie B. Alimonti, Ph.D., Jay W. Hooper, Ph.D., Peter M. Silvera, Ph.D., Brian K. Martin, Ph.D., Thomas P. Monath, M.D., W. Jay Ramsey, M.D., Ph.D., Charles J. Link, M.D., H. Clifford Lane, M.D., Nelson L. Michael, M.D., Ph.D., Richard T. Davey, Jr., M.D., and Stephen J. Thomas, M.D.
The authors' affiliations are as follows: the Walter Reed Army Institute of Research (J.A.R., K.M.P., A.R.C., J.E.M., R.C.R., J.W.B., P.S.T., S.A.R., H.R.H., M.S., L.L.J., S.A.P., N.L.M., S.J.T.) and Naval Medical Research Center (R.L.G.), Silver Spring, Leidos Biomedical Research, Frederick National Laboratory for Cancer Research (J.H.B., W.J.), and the U.S. Army Medical Research Institute of Infectious Diseases (M.L.W., M.D.J., S.A.K., N.V.D., K.S.S., J.W.H., P.M.S.), Frederick, and the National Institute of Allergy and Infectious Diseases (NIAID) (J.V., Z.H., P.M., H.C.L., R.T.D.) and NIAID Vaccine Research Center (O.T.M., Y.Z., D.A.S., J.E.L., B.S.G., N.J.S.), Bethesda — all in Maryland; the Public Health Agency of Canada, Ottawa (J.B.A.); and BioProtection Systems–NewLink Genetics, Ames, IA (B.K.M., T.P.M., W.J.R., C.J.L.).
Footnotes
The views expressed are those of the authors and should not be construed as official or representing the positions of the Departments of the Army, Navy, or Defense or the National Institutes of Health (NIH). The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Ebola situation report — 30 March 2016. Geneva: World Health Organization; 2016. http://apps.who.int/ebola/current-situation/ebola-situation-report-30-march-2016. [Google Scholar]
- 2.Marzi A, Feldmann H. Ebola virus vaccines: an overview of current approaches. Expert Rev Vaccines. 2014;13:521–31. doi: 10.1586/14760584.2014.885841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ledgerwood JE, DeZure AD, Stanley DA, et al. Chimpanzee adenovirus vector Ebola vaccine — preliminary report. N Engl J Med. doi: 10.1056/NEJMoa1410863. [DOI] [PubMed] [Google Scholar]
- 4.Ewer K, Rampling T, Venkatraman N, et al. A monovalent chimpanzee adenovirus Ebola vaccine boosted with MVA. N Engl J Med. 2016;374:1635–46. doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henao-Restrepo AM, Longini IM, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386:857–66. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe S, Takada A, Watanabe T, Ito H, Kida H, Kawaoka Y. Functional importance of the coiled-coil of the Ebola virus glycoprotein. J Virol. 2000;74:10194–201. doi: 10.1128/jvi.74.21.10194-10201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JE, Nasar F, Coleman JW, et al. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology. 2007;360:36–49. doi: 10.1016/j.virol.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geisbert TW, Daddario-Dicaprio KM, Lewis MG, et al. Vesicular stomatitis virus-based Ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog. 2008;4(11):e1000225. doi: 10.1371/journal.ppat.1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisbert TW, Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis. 2011;204(3):S1075–S1081. doi: 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, et al. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008;26:6894–900. doi: 10.1016/j.vaccine.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu X, Fernando L, Alimonti JB, et al. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong Ebola GP-specific immune responses. PLoS One. 2009;4(5):e5547. doi: 10.1371/journal.pone.0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, openlabel, cluster-randomised trial (Ebola Ça Suffit!) Lancet. 2016 Dec 23; doi: 10.1016/S0140-6736(16)32621-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garbutt M, Liebscher R, Wahl-Jensen V, et al. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol. 2004;78:5458–65. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kugelman JR, Sanchez-Lockhart M, Andersen KG, et al. Evaluation of the potential impact of Ebola virus genomic drift on the efficacy of sequence-based candidate therapeutics. MBio. 2015;6:e02227–14. doi: 10.1128/mBio.02227-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Silver Spring, MD; Food and Drug Administration: Sep, 2007. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074775.htm. [Google Scholar]
- 16.Sullivan NJ, Geisbert TW, Geisbert JB, et al. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 2006;3(6):e177. doi: 10.1371/journal.pmed.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agnandji ST, Huttner A, Zinser ME, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med. 2016;374:1647–60. doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huttner A, Dayer JA, Yerly S, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2015;15:1156–66. doi: 10.1016/S1473-3099(15)00154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzi A, Feldmann H, Geisbert TW, Falzarano D. Vesicular stomatitis virus-based vaccines for prophylaxis and treatment of filovirus infections. J Bioterr Biodef. 2011 doi: 10.4172/2157-2526.S1-004. Special Issue 1 ( http://www.omicsonline.org/2157-2526/2157-2526-S1-004.digital/2157-2526-S1-004.html) [DOI] [PMC free article] [PubMed]
- 20.Mire CE, Miller AD, Carville A, et al. Recombinant vesicular stomatitis virus vaccine vectors expressing filovirus glycoproteins lack neurovirulence in nonhuman primates. PLoS Negl Trop Dis. 2012;6(3):e1567. doi: 10.1371/journal.pntd.0001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hensley LE, Mulangu S, Asiedu C, et al. Demonstration of cross-protective vaccine immunity against an emerging pathogenic Ebolavirus species. PLoS Pathog. 2010;6(5):e1000904. doi: 10.1371/journal.ppat.1000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzi A, Engelmann F, Feldmann F, et al. Antibodies are necessary for rVSV/ ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci U S A. 2013;110:1893–8. doi: 10.1073/pnas.1209591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong G, Richardson JS, Pillet S, et al. Immune parameters correlate with protection against Ebola virus infection in rodents and nonhuman primates. Sci Transl Med. 2012;4:158ra146. doi: 10.1126/scitranslmed.3004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones SM, Feldmann H, Ströher U, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–90. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 25.Hooper JW. Neutralization assays. Immunology of protection from Ebola virus infection NIH VideoCasting and Podcasting. http://videocast.nih.gov/launch.asp?18779.
- 26.Feldmann H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog. 2007;3(1):e2. doi: 10.1371/journal.ppat.0030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong G, Audet J, Fernando L, et al. Immunization with vesicular stomatitis virus vaccine expressing the Ebola glycoprotein provides sustained long-term protection in rodents. Vaccine. 2014;32:5722–9. doi: 10.1016/j.vaccine.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.