Abstract
Consumption of the tomato carotenoid, lycopene, has been associated with favorable health benefits. Some of lycopene’s biological activity may be due to metabolites resulting from cleavage of the lycopene molecule. Because of their structural similarity to the retinoic acid receptor (RAR) antagonist, β-apo-13-carotenone, the “first half” putative oxidative cleavage products of the symmetrical lycopene have been synthesized. All transformations proceed in moderate to good yield and some with high stereochemical integrity allowing ready access to these otherwise difficult to obtain terpenoids. In particular, the methods described allow ready access to the trans isomers of citral (geranial) and pseudoionone, important flavor and fragrance compounds that are not readily available isomerically pure and are building blocks for many of the longer apolycopenoids. In addition, all of the apo-11, apo-13, and apo-15 lycopenals/lycopenones/lycopenoic acids have been prepared. These compounds have been evaluated for their effect on RAR-induced genes in cultured hepatoma cells and, much like β-apo-13-carotenone, the comparable apo-13-lycopenone and the apo-15-lycopenal behave as RAR antagonists. Furthermore, molecular modeling studies demonstrate that the apo-13-lycopenone efficiently docked into the ligand binding site of RARα. Finally, isothermal titration calorimetry studies reveal that apo-13-lycopenone acts as an antagonist of RAR by inhibiting coactivator recruitment to the receptor.
Keywords: chemical synthesis, diet and dietary lipids, gene expression, nuclear receptors, retinoids/vitamin A, apolycopenoids, apolipoprotein-13-lycopenone, retinoic acid receptor antagonist
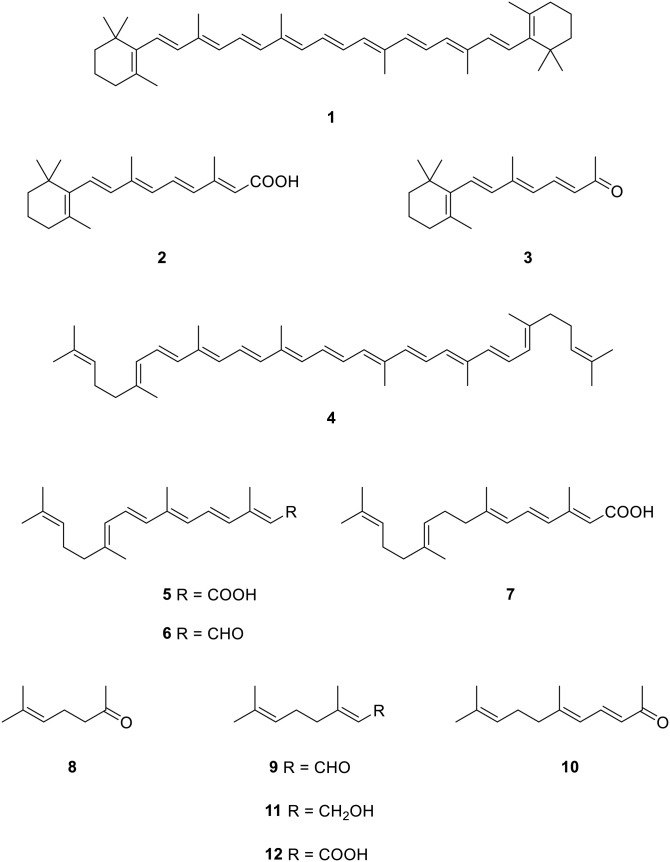
Recently, we have been interested in the synthesis and biological actions of β-apocarotenoids, which derive from oxidative cleavage of the plant pigment, β-carotene (1) (1, 2). Central cleavage of the symmetrical 1 (Fig. 1) mediated by the enzyme β,β-carotene-15,15’-dioxygenase (BCO1) provides the important vitamin A compounds in animals (3). The retinoic acid (RA; 2), which ultimately results from this central cleavage, is essential for the growth of mammals and for epithelial tissue differentiation and these biological effects of 2 are mainly mediated by interaction with nuclear retinoid receptor proteins, which regulate gene expression as ligand-activated transcription factors (4). Surprisingly, while we found that few of the oxidative cleavage products of 1 that could result from excentric cleavage had any RA receptor (RAR) activating effects, the side-chain shortened product, β-apo-13-carotenone (3), has high affinity for the RARs as well as the retinoid X receptors and is a potent retinoid receptor antagonist (2, 5, 6).
Fig. 1.
Structures of carotenoids and apocarotenoids (1, β-carotene; 2, RA; 3, apo-13-carotenone; 4, lycopene; 5, apo-15-lycopenoic acid; 6, apo-15-lycopenal; 9, geranial; 10, pseudoionone; 11, geraniol; 12, geranic acid).
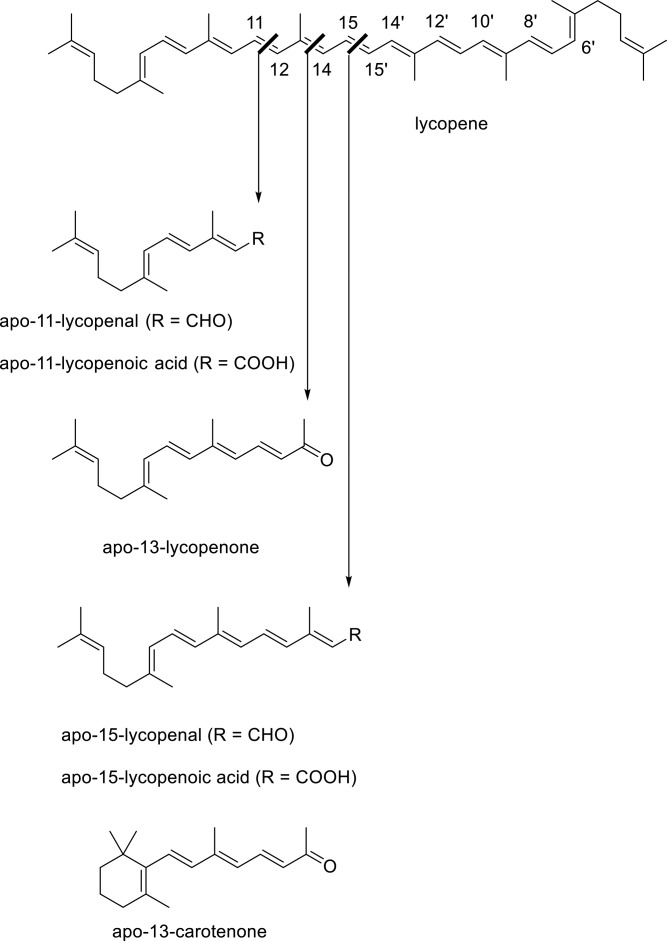
In the course of this work, we have also begun some chemical explorations of the similar apolycopenoids that could derive from the tomato pigment, lycopene (4) (7). For many years, it has been suggested that lycopene consumption has beneficial effects on human health and disease (8), particularly in the areas of cancer, especially of the prostate gland (9), and cardiovascular disease (10). It has also been proposed that oxidative cleavage products of lycopene may also be bioactive (11). For example, a possible product of central cleavage of lycopene, apo-15-lycopenoic acid (5), has been shown to increase gap junction communication in fibroblasts (12). It has recently been shown that lycopene can be cleaved by BCO1 to yield apo-15-lycopenal (6) (13), which could then be oxidized to 5. The very similar synthetic analog, called acyclo-retinoid (7), has also been extensively studied as an inhibitor of hepatic carcinogenesis and is used clinically in Japan to treat recurrent hepatocellular carcinoma (14). A second mammalian carotenoid cleavage enzyme, β,β-carotene-9’,10’-oxygenase (BCO2), has also been found to cleave cis isomers of lycopene excentrically at the 9’-10’ double bond, and the apo-10’-lycopenoic acid that could result from this cleavage has been found to inhibit lung cancer cell growth in vitro and lung carcinogenesis in vivo (15). Thus, there may be value in the further study of the possible actions of the putative oxidative cleavage products of lycopene, as is being done for the β-apocarotenoids.
Because of the surprising RA-antagonistic activity of 3 and the possibility that similar “short” cleavage products derived from lycopene might behave likewise, we targeted for synthesis and preliminary study the aldehyde, ketone, and carboxylic acid species that could arise because of oxidative cleavage of the olefin bonds of the “first half” of lycopene (see Fig. 2). Excepting the shortest possible product, acetone, the next longer cleavage compound, 6-methyl-5-hepten-2-one (8), is commercially available and has no double bond stereochemistry issues, but is likely to be too short to bind to the RARs. While they are also unlikely to mimic 3, the two and four carbon atoms longer geranial (9) and pseudoionone (10) are important flavor and fragrance compounds. Geranial is widely available as a 2:1 trans to cis double bond mixture called “citral”. Pseudoionone is mainly available as a similar 2:1 isomer mixture, but as the pure trans isomer it has been a useful building block for preparation of lycopene and longer apolycopenoids (16–18). We report herein our preparation of the short apolycopenoids up to the apo-15 first half compounds with reasonable control of double bond stereochemistry. The procedures now make these compounds available for study of their biological effects, as well as providing standards for assay of their presence in biological matrices. We then demonstrate the effects of selected apolycopenoids on RAR activation and subsequent RA-induced gene expression, their inhibition of nuclear coactivator binding to the receptor, and the docking of apo-13-lycopenone to the ligand binding site of RARα.
Fig. 2.
Lycopene (4) and key cleavage products showing similarity to apo-13-carotenone (3).
MATERIALS AND METHODS
Chemicals and analyses
The 6-methyl-5-hepten-2-one, geraniol, and ethyl trans-3-methyl-4-oxocrotonate (19) were from Sigma-Aldrich (St. Louis, MO); tetrapropylammonium perruthenate (TPAP) was from Alfa Aesar (Ward Hill, MA); and N-methylmorpholine N-oxide (NMO) was from Acros Organics (Morris Plains, NJ). The RAR antagonist, BMS-195614, was from Tocris Bioscience (Bristol, UK). All other agents and reagent chemicals for synthesis were from Sigma-Aldrich. Brine equals saturated NaCl solution. All chemical transformations were conducted under low-intensity gold fluorescent lighting. Preparative TLC was performed on 20 cm × 20 cm × 1 mm silica gel plates from Analtech (Newark, DE). 1H and 13C NMR spectra were recorded at the appropriate digital resolution in CDCl3 at 400 and 100 MHz frequencies, respectively, on a Bruker DRX400 instrument (Billerica, MA). UV absorption spectra were recorded on a Beckman Instruments DU-40 spectrophotometer (Brea, CA). Analytical HPLC analysis was done on a Beckman unit (model 127 pump, model 166 detector) using 1 ml/min of 80% methanol-water through a Polaris C18, 4.6 × 250 mm column with monitoring at 265 nm for 9 and 10. High resolution electrospray mass spectra were measured on a Micromass QTOF mass spectrometer in the Ohio State University Campus Chemical Instrument Center.
Synthesis of geranial [(2E)-3,7-dimethyl-2,6-octadienal] (9)
A mixture of geraniol (11; 5.7 mmol), NMO (29 mmol), and 1.5 g of powdered 3 Å molecular sieves were suspended in dry CH2Cl2 (15 ml) and cooled to 0°C. The TPAP (0.43 mmol) was added and the suspension stirred under Ar for 5 min and then at room temperature for 1 h at which time TLC (20% ethyl acetate/hexane) showed all 11 was consumed. The reaction mixture was vacuum filtered through 30 g of a 2:1 mixture of silica gel (40–63 μm)/diatomaceous earth with copious rinsing by CH2Cl2. Evaporation of solvent provided 766 mg of 9 (88%) as a clear oil: UV (CH3OH) λmax 234 nm; HPLC: tR = 5 min (>99%); 1H NMR: δ 1.50 (s, 3, CH3), 1.57 (s, 3, CH3), 2.08 (s, 3, CH3), 2.10 (m, 4, CH2CH2), 4.96 (br s, 1, vinyl), 5.76 (d, 1, vinyl, J = 8.0 Hz), 9.88 (d, 1, CHO, J = 8.0 Hz); 13C NMR: δ 18.18, 18.34, 26.28, 26.43, 41.26, 123.32, 128.07, 133.45, 164.29, 191.75; HRMS (ESI) m/z [M + Na]+ calculated for C10H16O + Na: 175.1099; measured: 175.1102.
Synthesis of pseudoionone [(3E,5E)-6,10-dimethyl-3,5,9-undecatrien-2-one] (10)
A mixture of geranial (9; 1.4 mmol), acetone (15 ml), and 10% NaOH(aq) (500 μl) was stirred in a closed vial in a 65°C oil bath. Reaction progress was monitored by HPLC and at 22 h the reaction mixture was partitioned between water and ether and the ether layer washed with brine, dried (Na2SO4), filtered, and concentrated to give crude oil, which was purified by preparative TLC (20% ethyl acetate/hexane) to give 134 mg (51%) of 10 as a clear oil: UV (CH3OH) λmax 296 nm; HPLC: tR = 7.4 min (>98%); 1H NMR: δ 1.56 (s, 3, CH3), 1.63 (s, 3, CH3), 1.86 (s, 3, CH3), 2.12 (m, 4, CH2CH2), 2.22 (s, 3, CH3) 5.02 (br s, 1, vinyl), 5.97 (d, 1, vinyl, J = 11.4 Hz), 6.05 (d, 1, vinyl, J = 15.3 Hz) 7.38 (dd, 1, vinyl, J = 11.4 and 15.3 Hz); 13C NMR: δ 18.24, 18.42, 26.39, 27.05, 28.21, 41.16, 123.99, 124.47, 129.19, 133.01, 140.31, 151.81, 199.45; HRMS (ESI) m/z [M + Na]+ calculated for C13H20O + Na: 215.1412; measured: 215.1402.
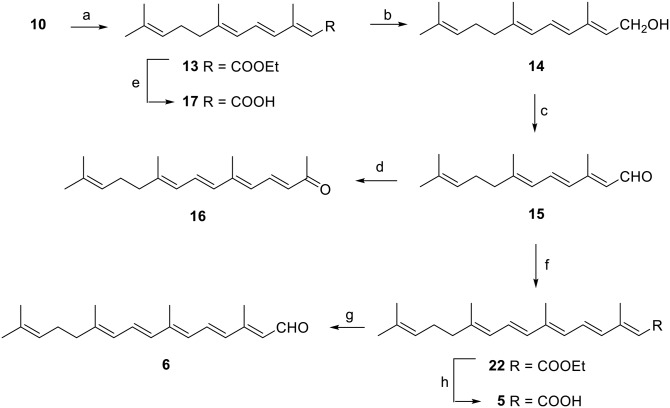
Synthesis of ethyl apo-11-lycopenoate [ethyl (2E and 2Z,4E,6E)-3,7,11-trimethyldodeca-2,4,6,10-tetraenoate] (13)
A solution of triethylphosphonoacetate (9.7 ml, 49 mmol) in dry tetrahydrofuran (THF) (5 ml) was added dropwise to a slurry of dry THF (30 ml) and NaH (1.15 g, 48 mmol) at 0°C. After complete evolution of hydrogen, 10 (2.09 ml, 9.8 mmol) was added dropwise over 1 h. The reaction mixture was stirred for 30 h at room temperature and then quenched with 25 ml of saturated aqueous NH4Cl solution. The product was extracted with ether (3 × 10 ml). The combined organic layers were washed with brine (3 × 10 ml), dried (Na2SO4), filtered, and concentrated. Silica gel column chromatography (2% ethyl acetate/hexane) afforded 2.1 g of 13 as a light yellow oil (82%): 1H NMR: δ 1.32 (t, 3, J = 8.0 Hz, CH2CH3), 1.65 (s, 3, CH3), 1.72 (s, 3, CH3), 1.88 (s, 3, CH3), 2.16 (m, 4, CH2CH2), 2.36 (s, 3, CH3), 4.17 (q, 2, J = 8.0 Hz, CH2CH3), 5.12 (m, 1, vinyl), 5.64 (s, 1, 2Z-vinyl), 5.77 (s, 1, 2E-vinyl), 6.09 (d, 1, J = 11.5 Hz, vinyl) 6.20 (d, 1, J = 11.5 Hz, vinyl), 6.87 (1H, m, vinyl); 13C NMR: δ 13.72, 14.31, 17.06, 17.63, 25.62, 26.49, 40.22, 59.47, 116.10 (2Z-vinyl), 118.05 (2E-vinyl), 123.64, 124.94, 130.93, 132.21, 133.46, 143.67, 152.90, 167.11; HRMS (ESI) m/z [M + Na]+ calculated for C17H26O2 + Na: 285.1825; measured: 285.1835.
Synthesis of apo-11-lycopenol [(2E,4E,6E)-3,7,11-trimethyldodeca-2,4,6,10-tetraen-1-ol] (14)
A solution of diisobutylaluminum hydride (DIBAL-H) (1.9 mmol) in hexane (1.9 ml) was carefully added over 1 h to a solution of 13 (197 mg, 0.75 mmol) in dry THF (5 ml) at 0°C and the resulting mixture stirred for 3 h at room temperature. Then, careful addition of 50% CH3OH/water (5 ml) at 0°C resulted in a gel that was dissociated by addition of aqueous 2 M potassium sodium tartrate (20 ml) and vigorous stirring for 10 h. The solution was then extracted with ether (3 × 10 ml) and the combined organic phases dried (Na2SO4), filtered, and concentrated. The residue was purified and diastereomers separated by silica gel column chromatography (20% ethyl acetate/hexane). Alcohol 14 was obtained as a white solid (145 mg, 66%): 1H NMR: δ 1.63 (s, 3, CH3), 1.69 (s, 3, CH3), 1.81 (s, 3, CH3), 1.84 (s, 3, CH3), 2.12 (m, 4, CH2CH2), 4.28 (d, 2, J = 7.0 Hz, CH2OH), 5.11 (m, 1, vinyl), 5.65 (t, 1, J = 7.0 Hz, vinyl), 5.90 (d, 1, J = 10.8 Hz, vinyl), 6.17 (d, 1, J = 15.2 Hz, vinyl), 6.47 (dd, 1, J = 10.8 and 15.2 Hz, vinyl); 13C NMR: δ 12.57, 16.84, 17.67, 25.67, 26.62, 40.10, 59.39, 123.91, 125.08, 125.18, 129.06, 131.72, 134.35, 136.86, 139.62; HRMS (ESI) m/z [M + Na]+ calculated for C15H24O + Na: 243.1823; measured: 243.1831.
Synthesis of apo-11-lycopenal [(2E,4E,6E)-3,7,11-trimethyldodeca-2,4,6,10-tetraenal] (15)
MnO2 (1.4 g, 14 mmol) was added to a solution of a diastereomeric mixture of 14 (137 mg, 0.62 mmol) in ethyl acetate (10 ml). The heterogeneous mixture was vigorously stirred at room temperature for 3 h, filtered through diatomaceous earth, dried (Na2SO4), filtered, and concentrated. The residue was purified and diastereomers separated by preparative TLC (10% ethyl acetate/hexane). Aldehyde 15 was obtained as light-yellow oil (64 mg, 47%): 1H NMR: δ 1.58 (s, 3, CH3), 1.66 (s, 3, CH3), 1.85 (s, 3, CH3), 2.11 (m, 4, CH2CH2), 2.27 (s, 3, CH3), 5.06 (m, 1, vinyl), 5.91 (d, 1, J = 8.2 Hz, vinyl), 5.99 (d, 1, J = 11.2 Hz, vinyl), 6.24 (d, 1, J = 15.3 Hz, vinyl), 6.96 (dd, 1, J = 11.2 and 15.3 Hz, vinyl), 10.07 (d, 1, J = 8.2 Hz, CHO); 13C NMR: δ 13.07, 17.31, 17.69, 25.66, 26.44, 40.60, 123.44, 124.97, 128.66, 132.70, 132.89, 133.48, 146.14, 155.31, 191.19; HRMS (ESI) m/z [M + Na]+ calculated for C15H22O + Na: 241.1568; measured: 241.1574.
Synthesis of apo-13-lycopenone [(3E,5E,7E,9E)-6,10,14-trimethylpentadeca-3,5,7,9,13-pentaen-2-one] (16)
To a solution of 15 (0.22 g, 1 mmol) in acetone (10 ml), 10% aqueous NaOH solution (0.4 ml) was added and the mixture stirred at 45°C for 90 min. After completion of the reaction (by TLC), the mixture was partitioned between water and ether and the ether layer washed with brine, dried (Na2SO4), filtered, and concentrated to give crude yellow oil that was purified by preparative TLC (12% ethyl acetate/hexane) to give 134 mg (52%) of 16 as a clear yellow oil: 1H NMR: δ 1.59 (s, 3, CH3), 1.66 (s, 3, CH3), 1.82 (s, 3, CH3), 2.04 (s, 3, CH3), 2.11 (s, 4, CH2CH2), 2.26 (m, 3, CH3), 5.07 (br s, 1, vinyl), 5.93 (d, 1, J = 11.1 Hz, vinyl), 6.12-6.25 (m, 3, vinyls), 6.65 (dd, 1, J = 11.1 and 15.1 Hz, vinyl), 7.49 (dd, 1, J = 11.8 and 15.1 Hz, vinyl); 13C NMR: δ 13.02, 18.21, 19.72, 24.5, 26.34, 27.38, 39.80, 123.64, 124.53, 125.30, 127.88, 130.24, 132.45, 137.56, 138.22, 139.40, 143.80, 187.71; HRMS (ESI) m/z [M + Na]+ calculated for C18H26O + Na: 281.1881; measured: 281.1875.
Synthesis of apo-11-lycopenoic acid [(3,7,11-trimethyldodeca-2,4,6,10-tetraenoic acid] (17)
Ester 13 (92 mg, 0.35 mmol) was dissolved in 1:1 ethanol/benzene (10 ml) and aqueous 5 N KOH was added to the solution. The reaction was stirred overnight and monitored by TLC. When complete, the solution was concentrated, acidified using 6 N HCl, and extracted twice with ethyl acetate. The combined organic layers were dried (Na2SO4), filtered, and concentrated to yield 17 as a yellow solid (75 mg, 91%): 1H NMR: δ 1.59 (s, 3, CH3), 1.66 (s, 3, CH3), 1.84 (s, 3, CH3), 2.11 (m, 4, CH2CH2), 2.32 (s, 3, CH3), 5.07 (m, 1, vinyl), 5.74 (s, 1, vinyl), 5.94 (d, 1, J = 10.9 Hz, vinyl), 6.17 (d, 1, J = 15.1 Hz, vinyl), 6.86 (dd, 1, J = 10.9 and 15.1 Hz, vinyl); 13C NMR: δ 14.02, 17.21, 17.70, 25.68, 26.49, 40.27, 116.94, 123.58, 124.88, 131.99, 132.22, 133.4, 144.80, 155.66, 171.71; HRMS (ESI) m/z [M + Na]+ calculated for C15H22O2 + Na: 257.1512; measured: 257.1515.
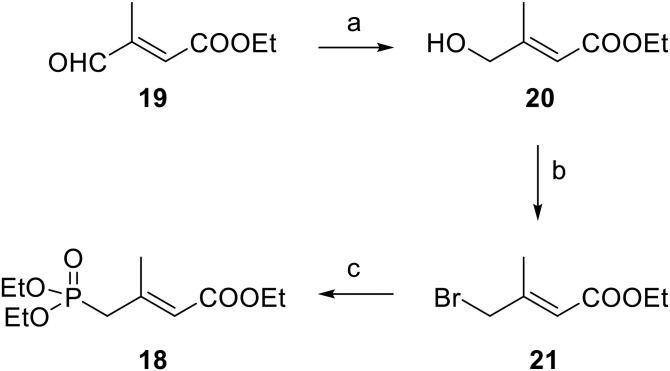
Synthesis of ethyl E-4-hydroxy-3-methyl-2-butenoate (20)
To a solution of now commercially available 19 (19) (1.5 g, 10.6 mmol) in dry ethanol (20 ml), 800 mg (21.1 mmol) of NaBH4 was carefully added and the mixture stirred at room temperature for 1 h. Excess NaBH4 was quenched with dropwise addition of saturated NH4Cl solution and the resulting mixture diluted with water and extracted with ethyl acetate. The ethyl acetate layer was washed with brine, dried (MgSO4), filtered, and concentrated to give 762 mg (50%) of 20 as a light yellow oil that was used as obtained for the preparation of 18 via 21 by the methods of Magoulas et al. (20): 1H NMR: δ 1.26 (t, 3, J = 7.2 Hz, CH2CH3), 1.75 (br s, 1, CH2OH), 2.07 (s, 3, CH3), 4.12 (br s, 2, CH2OH), 4.15 (q, 2, J = 7.2 Hz, CH2CH3), 5.96 (br s, 1, vinyl).
Synthesis of ethyl apo-15-lycopenoate [ethyl (2E,4E,6E,8E,10E)-3,7,11,15-tetramethylhexadeca-2,4,6,8,10,14-hexaenoate) (22)
To a solution of trans-triethyl-3-methyl-4-phosphono-2-butenoate (18) (20) (0.38 g, 1.46 mmol) in dry THF (40 ml), N,N’-dimethylpropylene urea (DMPU) (0.35 ml, 2.75 mmol) and n-butyllithium (1.6 M in hexanes, 1 ml) was added and the resulting mixture was stirred at 0°C for 40 min and then cooled to −78°C. A solution of 15 (0.16 g, 0.73 mmol) in dry THF (10 ml) was added dropwise over 20 min and the mixture stirred for an additional 2 h at −78°C and 1 h at 0°C. The reaction was monitored by TLC and quenched with 5 ml of saturated NH4Cl solution after completion. The organic layer was separated, dried (Na2SO4), filtered, and concentrated. The resulting residue was purified by silica gel column chromatography (10% ethyl acetate/hexane) to give 0.13 g (54%) of 22 as a light yellow gum: 1H NMR: δ 1.26 (t, 3, J = 7.1 Hz, CH2CH3), 1,59 (s, 3, CH3), 1,66(s, 3, CH3), 1.81 (s, 3, CH3), 1.95 (s, 3, CH3), 2.04 (m, 4, CH2CH2), 2.32 (s, 3, CH3), 4.14 (q, 2, J = 7.1 Hz, CH2CH3), 5.12 (m, 1, vinyl), 5.74 (s, 1, vinyl), 5.92 (d, 1, J = 11.0 Hz, vinyl), 6.17-6.27 (m, 3, vinyls), 6.52 (dd, 1, J = 11.0 and 14.3 Hz, vinyl), 6.94 (dd, 1, J = 11.8 and 14.8 Hz, vinyl); 13C NMR: δ 13.42, 14.20, 14.75, 17.41, 18.04, 26.08, 27.03, 40.66, 60.01, 118.99, 119.04, 124.18, 125.97, 126.80, 128.85, 130.45, 131.30, 134.95, 138.70, 140.09, 141.51, 167.57; HRMS (ESI) m/z [M + Na]+ calculated for C22H32O2 + Na: 351.2300; measured: 351.2301.
Synthesis of apo-15-lycopenal [(2E,4E,6E,8E,10E)-3,7,11,15- tetramethylhexadeca-2,4,6,8,10,14-hexaenal] (6)
A solution of DIBAL-H (1M, 1.9 mmol) in hexane (1.9 ml) was carefully added over 1 h to a solution of 22 (246 mg, 0.75 mmol) in dry THF (5 ml) at 0°C and the resulting mixture stirred for 3 h at room temperature. Careful addition of 1:1 CH3OH/water (5 ml) at 0°C then resulted in a gel that was dissociated by addition of aqueous 2 M potassium sodium tartrate (20 ml) and vigorous stirring for 10 h. The mixture was extracted with ether (3 × 10 ml) and the combined organic phases dried (Na2SO4), filtered, and concentrated to the dark red lycopenol residue that was used without further purification. To a cooled (0°C) solution of apo-15-lycopenol (35 mg, 0.123 mmol) in dry THF (2.4 ml), MnO2 (0.2 g, 2.22 mmol) and Na2CO3 (0.235 g, 2.22 mmol) were added. The reaction mixture was stirred at room temperature for 30 min and then filtered through diatomaceous earth. The solvent was evaporated and the residue purified by preparative TLC (12% ethyl acetate/hexane) to afford 7 mg (20%) of 6 as a pale yellow thick oil: 1H NMR: δ 1.54 (br s, 6, 2 CH3), 1.59 (s, 3, CH3), 1.82 (s, 3, CH3), 2.00 (m, 2, CH2), 2.03 (m, 2, CH2), 2.30 (s, 3, CH3), 5.08 (m, 1, vinyl), 5.94 (br d, 2, J = 8.0 Hz, vinyl), 6.17 (m, 2, vinyls), 6.32 (d, 1, J = 15.1 Hz, vinyl), 6.61 (dd, 1, J = 11.2 and 14.9 Hz, vinyl), 7.06 (dd, 1, J = 11.7 and 14.8 Hz, vinyl), 10.07 (d, 1, J = 8.0 Hz, CHO); 13C NMR: δ 13.50, 13.54, 17.48, 18.10, 26.08, 27.01, 27.26, 40.67, 124.17, 125.89, 127.91, 129.39, 130.27, 132.29, 132.89, 134.95, 141.83, 142.22, 155.11, 191.43; HRMS (ESI) m/z [M + Na]+ calculated for C20H28O + Na: 307.2038; measured: 307.2032.
Synthesis of apo-15-lycopenoic acid [(2E,4E,6E,8E,10E)-3,7,11,15-tetramethylhexadeca-2,4,6,8,10,14-hexaenoic acid] (5)
To a solution of 22 (120 mg, 0.37 mmol) in 2-propanol (2 ml), KOH (47 mg) was added and the reaction mixture was refluxed for 15 min. The solution was then poured into water, acidified by addition of 5 N HCl and extracted with ether. The ether layer was washed with water, dried (MgSO4), filtered, and concentrated. The residue was dissolved in 10 ml of hexane and cooled to −20°C to precipitate 5 as pale yellow powder (68 mg, 61%): 1H NMR: δ 1.58 (s, 3, CH3), 1.65 (s, 3, CH3), 1.79 (s, 3, CH3), 1.96 (s, 3, CH3), 2.09 (br s, 4, CH2CH2), 2.33 (s, 3, CH3), 5.07 (m, 1, vinyl), 5.77 (s, 1, vinyl), 5.92 (d, 1, J = 11.0 Hz, vinyl), 6.14-6.30 (m, 3, vinyls), 6.53 (dd, 1, J = 11.0 and 15.1 Hz, vinyl), 6.96 (dd, 1, J = 11.6 and 14.9 Hz, vinyl); 13C NMR: δ 12.73, 13.61, 18.52, 19.71, 23.81, 25.39, 38.63, 116.89, 123.83, 124.59, 124.78, 126.43, 130.64, 131.41, 132.89, 136.29, 137.01, 139.78, 155.43, 171.31; HRMS (ESI) m/z [M + Na]+ calculated for C20H28O2 + Na: 323.2089; measured: 323.2079.
Gene expression assays
HepG2 cells were cultured (2) in MEM supplemented with 10% FBS. Cells were maintained at 37°C with 5% CO2 in air. The cells were treated with test compounds in triplicate wells for 4 or 20 h in several independent experiments and total RNA was isolated from each well and subjected to reverse transcription. Quantitative PCR was carried out using TaqMan chemistry for human cytochrome P450 26A1 (CYP26A1) (Hs01084852_g1) and human RARB (Hs00977137_m1) as target genes and human glyceraldehyde 3-phosphate dehydrogenase (Hs02786624_g1) as a housekeeping gene. Analyses were carried out at the Nucleic Acids Shared Resource at The Ohio State University Center for Clinical and Translational Research using a QuantStudio PCR machine (Thermo Fisher Scientific). The comparative Ct method (ΔΔCt) was used to quantify the results obtained by real-time RT-PCR.
Computational docking
Given the structural similarity of the compounds of interest to RA, the RA-bound crystal structure of human RARα (21) was used for docking experiments (Protein Data Bank ID: 3A9E), which were performed using Glide XP (22). To prepare the receptor for docking, the Protein Preparation Wizard of the Schrödinger Suite (Schrödinger Inc.) was used to place hydrogens while optimizing hydrogen bonds followed by complete model optimization with the OPLS force field. The general AMBER force field was used to describe the bond, angle, and dihedral parameters to be used for the ligands to be docked (23). Partial atomic charges were calculated using the restrained electrostatic potential method (24). To derive the charges for RA, initial coordinates were extracted from the RAR bound crystal structure of RA with the coordinates for apo-13-lycopenone obtained by modification of RA using Maestro (Schrödinger Inc.).
Coactivator binding assays
Isothermal titration calorimetry (ITC) experiments were performed to measure the influence of ligand binding on the ability of RARα to interact with the coactivator steroid receptor coactivator-1 (SRC-1). The ligand binding domain (LBD) of human RARα (residues 182-421) was expressed from the pET28a vector obtained as a gift from Dr. Noa Noy, Case Western Reserve University. The plasmid was transformed into BL21-Gold(DE3) cells and grown to an optical density of 0.6 in Luria broth with 0.05 mg/ml kanamycin. Protein expression was then induced with 0.5 mM isopropyl β-D-1-thiogalactopyranoside for 5 h at 30°C. The cells were then pelleted and resuspended in 20 mM Tris-HCl, 500 mM NaCl, 5 mM imidazole, and one tablet of Mini, EDTA-free Roche cOmplete protease inhibitor cocktail, pH 8.0 prior to freezing. After thawing, lysozyme and DNase were added to final concentrations of 0.1 and 0.001 mg/ml, respectively, prior to lysis via sonication. The protein was then purified by nickel affinity chromatography and gel filtration during which the buffer was exchanged to 10 mM Tris, 150 mM NaCl, and 1 mM tris(2-carboxyethyl)phosphine, pH 7.5.
For ITC experiments, the RARα (50–100 μM; Bradford assay) was pretreated with a 2- to 3-fold molar excess of the ligand and was then dialyzed overnight in 4 l of the protein buffer. The dialysate was then used to solubilize the ligand for the ITC experiment; SRC-1 NR2 peptide (686-Ac-RHKILHRLLQEGS-NH2-698), which was ordered from EZBiolab (Carmel, IN), was added. The heat evolved during SRC-1 binding to ligand-bound RARα was measured with a VP-ITC isothermal titration calorimeter from MicroCal using an injection size of 10 μl at 25°C while stirring at 307 revolutions/minute using a reference power of 12 μcal/s. Integrating the recorded heat evolved over time results in the ΔH of each injection. Fitting models to the resulting curve yields the binding constant, Kb (= 1/Kd), and the stoichiometry of binding (n). Given the relationship ΔGbind = RTln Kd and using the relationship ΔG = ΔH – TΔS, the entropic change attributed to the binding event can also be calculated (25).
RESULTS
Apolycopenoid syntheses
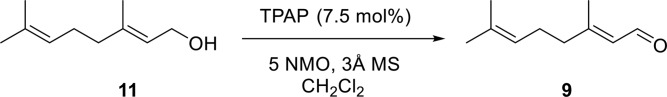
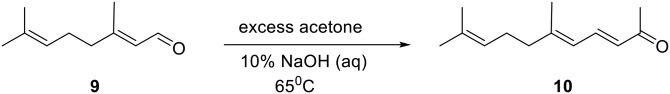
Geraniol (11) is available as essentially pure trans isomer, as judged by 1H NMR spectroscopy, and was smoothly oxidized to geranial (9) in high yield (88%) using TPAP and NMO as shown in Fig. 3 (26). Crude 9 contained less than 0.5–1% of cis isomer, as judged by HPLC analysis as well as integration of the aldehyde proton resonance(s) in the 1H NMR spectrum. However, silica gel column or preparative thin-layer chromatographic purification of the aldehyde induced formation of significant amounts (up to 30%) of cis isomer. Fortunately, vacuum filtration of crude product through 2:1 silica gel/diatomaceous earth, using dichloromethane as eluent, yielded high quality 9 in which no change in isomeric composition had occurred. We used this 9 directly to prepare pseudoionone (10) with no problems. Base-catalyzed Claisen-Schmidt condensation of 9 with acetone at 65°C (Fig. 4) provided 10 in moderate yield (51%). This reaction was monitored for completion by HPLC because, as expected, the rate was dependent on the ratio of acetone to 9, although the amount of cis isomer formed (<1–3% as judged by HPLC analysis) did not appear to differ much with reaction rate. Chromatographic purification of 10 also did not appear to change isomeric composition. Aldehyde 9 was air oxidized under mild conditions to give geranic acid (12) (data not shown).
Fig. 3.
Oxidation of geraniol (11) to geranial (9).
Fig. 4.
Claisen-Schmidt condensation of geranial (9) with acetone to give pseudoionone (10).
As has been observed by others (27), we found that homologation of 10 to ethyl apo-11-lycopenoate (13) by Wadsworth-Emmons reaction (Fig. 5) resulted in an approximately 3:1 isomeric mixture of the 2E/Z products. Fortunately, reduction of the ester to apo-11-lycopenol (14) and oxidation to apo-11-lycopenal (15) resulted in apolycopenoids from which the trans isomer was readily purified. As a result, Claisen-Schmidt condensation of 15 with acetone provided trans-apo-13-lycopenone (16). The more difficultly separated isomer mixture of 13 was saponified and the resulting apo-11-lycopenoic acid isomer mixture (17) was used as obtained.
Fig. 5.
Synthesis of apolycopenoids from pseudoionone (10). Reagents and conditions: triethyl phosphonoacetate, NaH, THF, rt (a); DIBAL-H, THF, rt (b); MnO2, ethyl acetate, rt (c); acetone, 3% aq. NaOH, 40°C (d); NaOH (aq), ethanol, benzene, rt (e); compound 18, n-butyllithium, DMPU, THF, 0 to −70°C (f); i) DIBAL-H, petroleum ether, rt, ii) MnO2, petroleum ether, rt (g); KOH (aq), isopropanol, reflux (h) (17, apo-11-lycopenoic acid; 14, apo-11-lycopenol; 15, apo-11-lycopenal; 16, apo-13-lycpopenone; 5, apo-15-lycopenoic acid; 6, apo-15-lycopenal).
Four-carbon Wadsworth-Emmons homologation of aldehyde 15 to give the apo-15-lycopenoids was done with phosphonate 18, which is commercially available as a 4:3 trans/cis mixture. However, Magoulas et al. (20) have recently shown that 18 can be prepared as the trans isomer and use of it under appropriate conditions suppresses the amount of cis Wadsworth-Emmons product formed. We developed an alternative approach to trans-18 using now commercially available aldehyde 19 (Fig. 6) (19), and otherwise followed the Magoulas method. Thus, reduction of aldehyde 19 to alcohol 20 proceeded smoothly and conversion of alcohol to the bromide 21 occurred readily under Appel conditions (28). Arbuzov reaction of 21 with triethyl phosphite gave trans-18 readily for the Wadsworth-Emmons reaction. Base-catalyzed reaction of 18 with 15 at reduced temperature and in the presence of DMPU provided ethyl apo-15-lycopenoate (22) with much less cis isomer (<10%) than is obtained when commercially available 18 is employed. Once again, reduction of 22 and allylic oxidation afforded apo-15-lycopenal (6), which was readily purified to remove any cis isomer. However, as in the case of the apo-11-lycopenoids, even with reduced amounts of cis isomer, saponification of ester 22 resulted in difficultly purified apo-15-lycopenoic acid (5), which was >95% trans isomer and was used as obtained.
Fig. 6.
Synthesis of phosphonate 18. Reagents and conditions: NaBH4, ethanol (a); PPh3, CBr4, CH3CN (b); triethyl phosphite, 120°C (c).
Effects of apolycopenoids on gene expression
Preliminary experiments demonstrated that short first half cleavage products of lycopene (namely, citral, geranic acid, and pseudoionone) had no effect on the expression of target genes induced by RA interaction with the RAR. We then tested the effects of the apo-11-lycopenoids, the apo-15-lycopenoids, and apo-13-lycopenone. In these experiments, HepG2 cells were treated with 10 nM RA alone, 10 nM of the compounds alone, or with equimolar concentrations of the test compounds and RA (10 nM each) and the levels of mRNA for CYP26A1 or RARβ were determined. None of the test compounds alone induced the expression of CYP26A1 or RARβ (at up to 100 nM; data not shown). Table 1 shows the results of experiments assessing the possible antagonist effects of the lycopenoids on RA induction of CYP26A1 and RARβ gene expression. For CYP26A1, the apo-11-lycopenoids had little to no effect; however, apo-13-lycopenone- and apo-15-lycopenal-inhibited RA induced CYP26A1 expression by 50–78%. Similar results were observed for RARβ, although the effects were slightly weaker. The apo-11-lycopenoids also had little or no effect on RA induction of this gene. However, apo-13-lycopenone and the apo-15-lycopenal inhibited RA induction of RARβ by 30–60%. Limited quantities of apo-15-lycopenoic acid allowed us to evaluate its antagonistic effect on RA-induced gene expression only once for each gene. However, based on this single measurement, it appears that this acid is less effective than the apo-13 ketone or apo-15 aldehyde. The results show that some of these first half cleavage products of lycopene, like some of the β-apocarotenoids, can function as RAR antagonists.
TABLE 1.
Inhibition of RA-induced transactivation of the RARβ and CYP26A1 genes by apolycopenoids
| Lycopenoid | RARβ (% of RA Induction) | CYP26A1 (% of RA Induction) |
| apo-11-lycopenal | 75, 90, 93, 99 | 92, 105, 111 |
| apo-11-lycopenoic acid | 69, 95, 104 | 79, 91, 97 |
| apo-13-lycopenone | 50, 66, 67, 68 | 22, 35, 50 |
| apo-15-lycopenal | 40, 62, 63 | 37, 48 |
| apo-15-lycopenoic acid | 64 | 63 |
HepG2 cells were incubated as described in Materials and Methods in the presence of 10 nM RA and 10 nM of the indicated lycopene cleavage products. The levels of mRNA for RARβ and CYP26A1 were determined by RT-PCR. Results are expressed as the percent of the fold induction observed with RA alone that is defined as 100%. The absolute fold induction with 10 nM RA alone for RARβ varied from 11- to 26-fold and for CYP26A1 varied from 68- to 135-fold in independent experiments.
Computational docking of apo-13-lycopenone
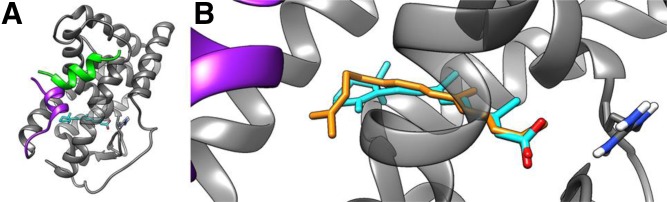
Docking of apo-13-lycopenone to the RAR LBD indicated that the compound could fit into the ligand binding site of the receptor in a similar way to RA, which redocked into the receptor with a root mean square deviation of 1.07 Å from the binding conformation observed in the crystal structure. As shown in Fig. 7, the polar ends of apo-13-lycopenone and RA superimpose well in the binding site, suggesting that binding of the lycopenone to the receptor is stabilized by the conserved Arg276, as it does for RA. The hydrophobic tail of the lycopenone extends across the receptor to make contact with residues that form helix 12, a region important for forming the coactivator binding site. Given the conformational flexibility of apo-13-lycopenone compared with RA, it seemed likely that binding of the former shifts the conformational ensemble of the receptor away from conformations suited for coactivator binding, thereby acting as an RA antagonist.
Fig. 7.
Docking mode of apo-13-lycopenone to RAR LBD. A: Binding mode of RA (cyan) to the LBD, highlighting helix 12 (purple) and coactivator binding domain (green). B: Docking mode of apo-13-lycopenone (yellow) superimposed over RA (cyan) binding mode, Arg 267 is highlighted in blue.
ITC studies of coactivator binding
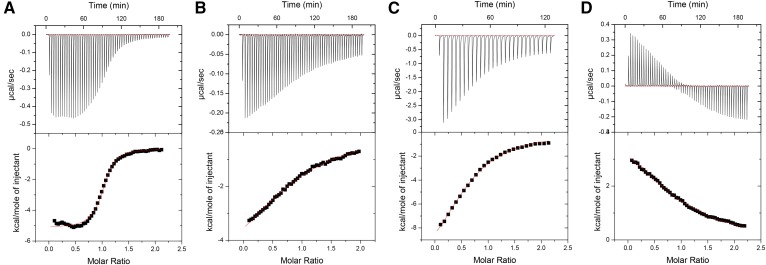
To further investigate the mode of RAR antagonism exhibited by the apolycopenoids, ITC experiments were performed to determine the effect of apo-13-lycopenone on the ability of RARα to recruit the coactivator, SRC-1. RA was used as a control agonist and the neutral antagonist, BMS-195614 (29), which has no intrinsic activity but blocks the actions of agonist/inverse agonists, was used as a control antagonist. Representative thermograms for all ligands investigated are shown in Fig. 8. When compared with the binding affinity of SRC-1 to the apo receptor, prebinding of RA increased the affinity by 9.1-fold, while BMS 195614 decreased the peptide affinity by ∼20-fold (Table 2). In line with the behavior of an antagonist, apo-13-lycopenone binding was found to decrease the affinity of the receptor for SRC-1 6- to 7-fold relative to the apo receptor. These results indicate that apo-13-lycopenone acts as an RAR antagonist by inhibiting the coactivator binding required for gene transcription.
Fig. 8.
Representative thermograms of the coactivator peptide (SRC-1) titrated into RAR LBD presaturated with RA (A), no ligand (apo) (B), apo-13-lycopenone (C), and BMS-195614 (D). The top panels show the raw data while the bottom panels show the binding isotherm created by plotting the integrated heat peaks against the molar ratio of the SRC-1 peptide to the RAR-LBD; note the data in (D) are indicative of essentially no coactivator binding in the case of the potent neutral antagonist.
TABLE 2.
Summary of ITC experiments of ligand-bound hRARα association with SRC-1
| Ligand | Kd (μM)a |
| ATRA | 0.65 ± 0.01 |
| apo-receptor | 5.97 ± 0.94 |
| apo-13-lycopenone | 39.67 ± 10.0 |
| BMS-195614 | 115.7 ± 61.6 |
The Kd of SRC-1 peptide binding to purified apo hRARα-LBD or liganded receptor; average data ± 1 SD from n = 3. Ligand-bound receptors were compared with apo-receptor using pair-wise t-tests for the Kds of the SRC-1 coactivator peptide. ATRA-bound receptor showed a significantly decreased (P < 0.001) Kd indicating increased affinity of coactivator binding (i.e., agonist behavior). Significant increases in SRC-1 Kd were observed for receptors with bound apo-13-lycopenone (P < 0.005) or the known antagonist BMS-195614 (P < 0.05).
DISCUSSION
In 2012, we reported the synthesis and/or purification of all of the possible eccentric cleavage products of β-carotene (i.e., the β-apocarotenals and β-apocarotenones and the carboxylic acid derivatives of the carotenals, namely, the β-apocarotenoic acids) (2). We found that some of these compounds functioned as RAR antagonists in transactivation assays and in RARβ-dependent RA induced expression of RA target genes in HepG2 cells in culture. Among all the compounds tested, the most potent antagonist was β-apo-13-carotenone. In the present work, we have evaluated the effects of the acyclic analogs of some of these β-apocarotenals, β-apocarotenoic acids, and β-apocarotenones on RA-induced target genes in HepG2 cells, with a particular focus on those near the molecular size of β-apo-13-carotenone.
For the preparation of these apolycopenoids, we started with geranial (9), which is readily available as an approximately 2:1 mixture with its cis isomer, neral. As mentioned above, this mixture of geranial and neral is referred to as citral. Pseudoionone (10) is likewise available at reasonable cost as a comparable 2:1 isomer mixture; however, purchasing either compound as the trans isomer is considerably more expensive with 9 costing about $250 per gram and 10 commanding a price of about $350 per gram. Both 9 and 10 have generally been obtained as trans isomers by steam distillation or fractional distillation using packed or spinning band distillation columns (30–32). In our hands, these procedures proved tedious and met with limited success. Starting with the inexpensive ($0.50 per gram) commercially available trans alcohol, geraniol (11), we have smoothly prepared 9. A somewhat related approach to 10 starting from the more expensive geranyl chloride (approximately $12 per gram) and utilizing pyrolysis of α-sulfinyl carbonyl compounds has been reported (33). More importantly, the procedure described here proceeds in fewer operationally easier steps (one vs. four), with comparable or better yields. With isomerically pure 9 and 10 in hand, relatively straightforward organic chemical transformations, including homologation, reduction, and oxidation, produced the longer apo-11, apo-13, and apo-15 lycopenoids. These compounds were obtained in moderate to good yields and generally good control of stereochemistry about newly formed double bonds. In those cases where isomer mixtures were obtained, except in the case of the apo-11-lycopenoate, the desired trans isomers could readily be purified from the minor contaminating isomers.
The binding of RA to the nuclear RARs induces the expression of a number of genes, including that for CYP26A1 as well as RARβ. None of the apolycopenoids synthesized and evaluated here showed any ability to mimic this RA-mediated gene expression at up to 100 nM concentrations. However, while co-treatment of the apo-11-lycopenoids was generally ineffective at lower equimolar concentrations to RA at antagonizing the RA-induced gene expression, apo-15-lycopenal, perhaps apo-15-lycopenoic acid, and most interestingly apo-13-lycopenone were effective as RA antagonists. It is particularly noteworthy that, like β-apo-13-carotenone, these compounds are effective in nanomolar concentrations. Our previous work (2) demonstrated that endogenous levels of β-apo-13-carotenone in human plasma ranged from 3 to 5 nM, near that of the natural RAR agonist, RA. We do not currently have information on the levels of apo-13-lycopenone or the apo-15-lycopenoids in human plasma or tissues, although our previous work has shown that the “longer” apolycopenals (namely, apo-6’-, apo-8’-, apo-10’-, apo-12’-, and apo-14’-lycopenals) are present endogenously in lycopene-containing foods and are present in human plasma in concentrations of 0.12–0.73 nM (7).
In order to gain some insight into the possible mechanism of this antagonistic effect, computational docking experiments with the most effective antagonist, apo-13-lycopenone, were conducted. Despite the lack of the trimethylcyclohexene (or β-ionylidene) ring present in the apocarotenoids and RA, we found that apo-13-lycopenone docked quite readily into the RA-binding site in a manner similar to RA. Inspection of the docked structure(s), however, suggested that because of its greater conformational flexibility, the binding of apo-13-lycopenone might be causing subtle changes to the position of the RAR helix 12, which is essential for coactivator binding to the RAR. It has long been suggested that the bulk appended to the analog of the trimethylcyclohexene ring of RA (such as the bicyclic quinoline ring system of BMS-195614) projects out from the RA binding site preventing proper positioning of helix 12 and creating the classical neutral RAR antagonists (34). The docking experiment shown in Fig. 7 suggests that the flexible acyclic “tail” of apo-13-lycopenone may perform a similar function for this antagonist. The antagonistic activity of apo-13-lycopenone suggested that it would be feasible to conduct ITC studies of coactivator binding to RAR prebound with these ligands. As shown in Table 2, like the classical neutral RAR antagonist, BMS-195614, apo-13-lycopenone reduces coactivator binding to the RAR relative to the apo receptor, an effect opposite to that of RA and in support of the hypothesis developed based on the docking experiments. It should be noted that the range of binding affinities determined here is similar to previous reports of coactivator binding to nuclear receptors by ITC (35), as well as those measured by fluorescence anisotropy (36).
In summary, we have reported facile and frequently novel syntheses for the first half cleavage products of the tomato carotenoid, lycopene. If found in a food matrix or in plasma, these apolycopenoids may be relatively stable. However, we have found that when synthesized as purified compounds, particularly the apo-11-, apo-13-, and apo-15-lycopenoids are somewhat unstable compared with the comparable apocarotenoids. As we have done in the present case, we recommend that these compounds be purified, characterized, and bioassayed in a timely manner to ensure that they remain intact. Having these compounds in hand, we have been able to show that some of these acyclic apolycopenoids can function as antagonists of the hormone, RA, like some of their cyclized analogs, the β-apocarotenoids that derive from the cleavage of β-carotene. Given this heretofore unrecognized biological activity of the apolycopenoids, it will be important to explore other possible activities and these efforts are now possible given the availability of the pure compounds. Finally, having access to the compounds as standards will allow the development of sensitive and specific mass spectrometric methods for the quantitative analyses of these apolycopenoids in foods and biological samples.
Footnotes
Abbreviations:
- BCO1
- β,β-carotene-15,15’-dioxygenase
- BCO2
- β,β-carotene-9’,10’-oxygenase
- CYP26A1
- cytochrome P450 26A1
- DIBAL-H
- diisobutylaluminum hydride
- DMPU
- N,N’-dimethylpropylene urea
- ITC
- isothermal titration calorimetry
- LBD
- ligand binding domain
- NMO
- N-methylmorpholine N-oxide
- RA
- all-trans retinoic acid
- RAR
- retinoic acid receptor
- SRC-1
- steroid receptor coactivator-1
- THF
- tetrahydrofuran
- TPAP
- tetrapropylammonium perruthenate
This work was supported by National Institutes of Health Grant R01-HL49879. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Marsh R. S., Yan Y., Reed V. M., Hruszkewycz D. P., Curley R. W., and Harrison E. H.. 2010. β-Apocarotenoids do not significantly activate retinoic acid receptors α and β. Exp. Biol. Med. (Maywood). 235: 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eroglu A., Hruszkewycz D. P., dela Sena C., Narayanasamy S., Riedl K. M., Kopec R. E., Schwartz S. J., Curley R. W. Jr., and Harrison E. H.. 2012. Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 287: 15886–15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paik J., During A., Harrison E. H., Mendelsohn C. L., Lai K., and Blaner W. S.. 2001. Expression and characterization of a murine enzyme able to cleave β-carotene. The formation of retinoids. J. Biol. Chem. 276: 32160–32168. [DOI] [PubMed] [Google Scholar]
- 4.Germain P., Chambon P., Eichele G., Evans R. M., Lazar M. A., Leid M., De Lera A. R., Lotan R., Mangelsdorf D. J., and Gronemeyer H.. 2006. International union of pharmacology. LX. Retinoic acid receptors. Pharmacol. Rev. 58: 712–725. [DOI] [PubMed] [Google Scholar]
- 5.Eroglu A., Hruszkewycz D. P., Curley R. W. Jr., and Harrison E. H.. 2010. The eccentric cleavage product of β-carotene, β-apo-13-carotenone, functions as an antagonist of RXRα. Arch. Biochem. Biophys. 504: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J., Narayanasamy S., Curley R. W. Jr., and Harrison E. H.. 2014. β-Apo-13-carotenone regulates retinoid X receptor transcriptional activity through tetramerization of the receptor. J. Biol. Chem. 289: 33118–33124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopec R. E., Riedl K. M., Harrison E. H., Curley R. W. Jr., Hruszkewycz D. P., Clinton S. K., and Schwartz S. J.. 2010. Identification and quantitation of apo-lycopenals in fruits, vegetables, and human plasma. J. Agric. Food Chem. 58: 3290–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinton S. K. 1998. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr. Rev. 56: 35–51. [DOI] [PubMed] [Google Scholar]
- 9.Campbell J. K., Canene-Adams K., Lindshield B. L., Boileau T. W-M., Clinton S. K., and Erdman J. W. Jr. 2004. Tomato phytochemicals and prostate cancer risk. J. Nutr. 134: 3486S–3492S. [DOI] [PubMed] [Google Scholar]
- 10.Böhm V. 2012. Lycopene and heart health. Mol. Nutr. Food Res. 56: 296–303. [DOI] [PubMed] [Google Scholar]
- 11.Lindshield B. L., Canene-Adams K., and Erdman J. W. Jr. 2007. Lycopenoids: are lycopene metabolites bioactive? Arch. Biochem. Biophys. 458: 136–140. [DOI] [PubMed] [Google Scholar]
- 12.Stahl W., von Laar J., Martin H-D., Emmerich T., and Sies H.. 2000. Stimulation of gap junctional communication: comparison of acyclo-retinoic acid and lycopene. Arch. Biochem. Biophys. 373: 271–274. [DOI] [PubMed] [Google Scholar]
- 13.dela Seña C., Narayanasamy S., Riedl K. M., Curley R. W. Jr., Schwartz S. J., and Harrison E. H.. 2013. Substrate specificity of purified recombinant human β-carotene 15,15′-oxygenase (BCO1). J. Biol. Chem. 288: 37094–37103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komi Y., Sogabe Y., Ishibashi N., Sato Y., Moriwaki H., Shimokado K., and Kojima S.. 2010. Acyclic retinoid inhibits angiogenesis by suppressing the MAPK pathway. Lab. Invest. 90: 52–60. [DOI] [PubMed] [Google Scholar]
- 15.Lian F., Smith D. E., Ernst H., Russell R. M., and Wang X. D.. 2007. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 28: 1567–1574. [DOI] [PubMed] [Google Scholar]
- 16.Hengartner U., Bernhard K., Meyer K. , G. Englert, and E. Glinz. 1992. Synthesis, isolation, and NMR-spectroscopic characterization of fourteen (Z)-isomers of lycopene and of some acetylenic didehydro- and tetradehydrolycopenes. Helv. Chim. Acta. 75: 1848–1865. [Google Scholar]
- 17.Ernst H. 2002. Recent advances in industrial carotenoid synthesis. Pure Appl. Chem. 74: 2213–2226. [Google Scholar]
- 18.Reynaud E., Aydemir G., Ruehl R., Dangles O., and Caris-Veyrat C.. 2011. Organic synthesis of new putative lycopene metabolites and preliminary investigation of their cell-signalling effects. J. Agric. Food Chem. 59: 1457–1463. [DOI] [PubMed] [Google Scholar]
- 19.Curley R. W. Jr., and Ticoras C. J.. 1986. A convenient synthesis of the important retinoid synthon ethyl trans-3-formyl-2-butenoate. Synth. Commun. 16: 627–631. [Google Scholar]
- 20.Magoulas G. E., Bariamis S. E., Athanassopoulos C. M., Haskopoulos A., Dedes P. G., Krokidis M. G., Karamanos N. K., Kletsas D., Papaioannou D., and Maroulis G.. 2011. Synthesis, antiproliferative activity and theoretical characterization of acitretin-type retinoids with changes in the lipophilic part. Eur. J. Med. Chem. 46: 721–737. [DOI] [PubMed] [Google Scholar]
- 21.Sato Y., Ramalanjaona N., Potier N., Osz J., Antony P., Peluso-Iltis C., Poussin-Courmontagne P., Ennifar E., Mely Y., Dejaegere A., et al. 2010. The “phantom effect” of the rexinoid LG100754: structural and functional insights. PLoS One. 5: e15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friesner R. A., Murphy R. B., Repasky M. P., Frye L. L., Greenwood J. R., Halgren T. A., Sanschagrin P. C., and Mainz D. T.. 2006. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 49: 6177–6196. [DOI] [PubMed] [Google Scholar]
- 23.Wang J., Wolf R. M., Caldwell J. W., Kollman P. A., and Case D. A.. 2004. Development and testing of a general amber force field. J. Comput. Chem. 25: 1157–1174. [DOI] [PubMed] [Google Scholar]
- 24.Bayly C. L., Cieplak P., Cornell W. D., and Kollman P. A.. 1993. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem. 97: 10269–10280. [Google Scholar]
- 25.Freyer M. W., and Lewis E. A.. 2008. Isothermal titration calorimetry: experimental design, data analysis, and probing macromolecule/ligand binding and kinetic interactions. Methods Cell Biol. 84: 79–113. [DOI] [PubMed] [Google Scholar]
- 26.Benhaddou R., Czernecki S., Farid W., Ville G., Xie J., and Zegar A.. 1994. Tetra-n-propylammonium terta-oxoruthenate (VII): a reagent of choice for the oxidation of diversely protected glycopyranoses and glycofuranoses. Carbohydr. Res. 260: 243–250. [Google Scholar]
- 27.Goupy P., Reynaud E., Dangles O., and Caris-Veyrat C.. 2012. Antioxidant activity of (all-E)-lycopene and synthetic apo-lycopenoids in a chemical model of oxidative stress in the gastro-intestinal tract. New J. Chem. 36: 575–587. [Google Scholar]
- 28.Appel R. 1975. Tertiary phosphane/tetrachloromethane, a versatile reagent for chlorination, dehydration, and P-N linkage. Angew. Chem. Int. Ed. Engl. 14: 801–811. [Google Scholar]
- 29.Germain P., Gaudon C., Pogenberg V., Sanglier S., Van Dorsselaer A., Royer C. A., Lazar M. A., Bourguet W., and Gronemeyer H.. 2009. Differential action on coregulatory interaction defines inverse retinoid agonists and neutral antagonists. Chem. Biol. 16: 479–489. [DOI] [PubMed] [Google Scholar]
- 30.Venuto P. B., and Day A. R.. 1964. The preparation of allylic alcohols from citral a and citral b. A study of their dehydration reactions. J. Org. Chem. 29: 2735–2739. [Google Scholar]
- 31.Sacks J., Greenley E., Leo G., Willey P., Gallis D., and Mangravite J. A.. 1983. Separation and analysis of citral isomers. J. Chem. Educ. 60: 434–436. [Google Scholar]
- 32.Chen J., and Pi S.. 2011. Method for preparing all-trans-pseudoionone. Chinese Patent 102070424; Chem. Abstr. 155: 14862..
- 33.Boulin B., Arreguy-San Miguel B., and Delmond B.. 2003. New access to pseudo-ionones and pseudo-iso-methyl ionones from geranyl derivatives. Synth. Commun. 33: 1047–1055. [Google Scholar]
- 34.Rochel N., and Moras D.. 2014. Architecture of DNA bound RAR heterodimers. In The Biochemistry of Retinoic Acid Receptors I: Structure, Activation and Function at the Molecular Level. M. A. Asson-Batres and C. Rochette-Egly, editors. Springer, Dordrecht, NL. 21–36. [DOI] [PubMed] [Google Scholar]
- 35.Wright E., Vincent J., and Fernandez E. J.. 2007. Thermodynamic characterization of the interaction between CAR-RXR and SRC-1 peptide by isothermal titration calorimetry. Biochemistry. 46: 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pogenberg V., Guichou J. F., Vivat-Hannah V., Kammerer S., Perez E., Germain P., de Lera A. R., Gronemeyer H., Royer C. A., and Bourguet W.. 2005. Characterization of the interaction between retinoic acid receptor/retinoid X receptor (RAR/RXR) heterodimers and transcriptional coactivators through structural and fluorescence anisotropy studies. J. Biol. Chem. 280: 1625–1633. [DOI] [PubMed] [Google Scholar]