Abstract
Plasma apoC-III levels correlate with triglyceride (TG) levels and are a strong predictor of CVD outcomes. ApoC-III elevates TG in part by inhibiting LPL. ApoC-III likely inhibits LPL by competing for lipid binding. To probe this, we used oil-drop tensiometry to characterize binding of six apoC-III variants to lipid/water interfaces. This technique monitors the dependence of lipid binding on surface pressure, which increases during TG hydrolysis by LPL. ApoC-III adsorption increased surface pressure by upward of 18 mN/m at phospholipid/TG/water interfaces. ApoC-III was retained to high pressures at these interfaces, desorbing at 21–25 mN/m. Point mutants, which substituted alanine for aromatic residues, impaired the lipid binding of apoC-III. Adsorption and retention pressures decreased by 1–6 mN/m in point mutants, with the magnitude determined by the location of alanine substitutions. Trp42 was most critical to mediating lipid binding. These results strongly correlate with our previous results, linking apoC-III point mutants to increased LPL binding and activity at lipid surfaces. We propose that aromatic residues in the C-terminal half of apoC-III mediate binding to TG-rich lipoproteins. Increased apoC-III expression in the hypertriglyceridemic state allows apoC-III to accumulate on lipoproteins and inhibit LPL by preventing binding and/or access to substrate.
Keywords: lipoprotein lipase, lipid and lipoprotein metabolism, LDL/metabolism, lipid/emulsions, protein-lipid interaction, surface pressure, drop tensiometry
apoC-III is a small (79 amino acid), O-glycosylated, secretory protein that is synthesized in the liver and intestine (1). ApoC-III is the most abundant apoC in humans and circulates in plasma as a component of HDLs and the triglyceride (TG)-rich lipoproteins (i.e., VLDLs and chylomicrons) (2–4). In normolipidemic individuals, plasma concentrations of apoC-III are 80–100 μg/ml, with approximately 60% of apoC-III associated with HDL, 20% with LDL, 20% with the TG-rich lipoproteins, and very little free in plasma (5). In the hypertriglyceridemic state, plasma apoC3 levels increase to >300 μg/ml, and the percentage on TG-rich lipoproteins increases from 20% to upward of 60% (5–7). In fact, animal and human studies have established a strong, positive correlation between elevated plasma apoC-III levels and TG levels, which are an independent risk factor for atherosclerotic CVD (1, 8). The overexpression of human apoC-III in mice promoted the development of atherosclerosis and was associated with elevated TG levels, but low HDL cholesterol levels, in plasma (9). In contrast, targeted disruption of apoC-III in mice was associated with the rapid catabolism of TG-rich lipoproteins and a 70% decrease in fasting TG levels (10).
Beyond these mouse models, clinical studies established the importance of apoC-III as a predictor of CVD outcomes. Increased serum apoC-III levels were associated with elevated serum TG and, in turn, insulin resistance, CVD, and type II diabetes (11–13). Plasma levels of apoC-III independently predicted risk for coronary heart disease, even after control for blood lipids (12, 14). Further indicating a crucial role of apoC-III in regulating lipid metabolism, subjects with gain-of-function mutations in the apoC-III gene exhibited hypertriglyceridemia that was associated with elevated plasma apoC-III protein levels (15, 16). In contrast, subjects with loss-of-function mutations in the apoC-III gene exhibited reduced TG and apoC-III levels (17–21), which had a cardioprotective effect. Administration of antisense oligonucleotides, which inhibit the synthesis of apoC-III, to hypertriglyceridemic subjects significantly lowered serum apoC-III and TG levels, encouraging their use in treatment to lower risk of cardiovascular events (22).
The hypertriglyceridemic phenotype associated with apoC-III is due to intracellular and extracellular regulation of TG metabolism (1, 8, 23). Intracellularly, apoC-III promotes TG synthesis and utilization for VLDL assembly within the secretory pathway of the endoplasmic reticulum and Golgi under lipid-rich conditions (24–26). In the hypertriglyceridemic state, apoC-III expression is upregulated by glucose and loss of insulin sensitivity (27). VLDL production subsequently increases, because apoC-III promotes assembly of VLDL with atypically low buoyant density and higher TG content (6, 7).
Extracellularly, plasma apoC-III inhibits the hydrolysis of VLDL and chylomicrons by LPL and impairs hepatic lipoprotein clearance (28). An early connection between apoC-III and LPL was established in apoC-III-deficient subjects who exhibit low plasma levels of TG-rich lipoproteins associated with highly efficient TG hydrolysis by LPL (29). In one study, elevated serum levels of apoC-III were more strongly correlated with fasting TG levels in hypertriglyceridemic subjects than elevated levels of apos B, C-II, and E (30). LPL activity negatively correlated with plasma apoC-III levels in these subjects, further suggesting that apoC-III elevates TG levels via LPL inhibition (30). However, apoC-III-targeted antisense oligonucleotides decreased serum apoC-III and TG levels in patients with familial LPL deficiency, suggesting that apoC-III also inhibits lipoprotein uptake independent of its inhibitory effect on LPL (31).
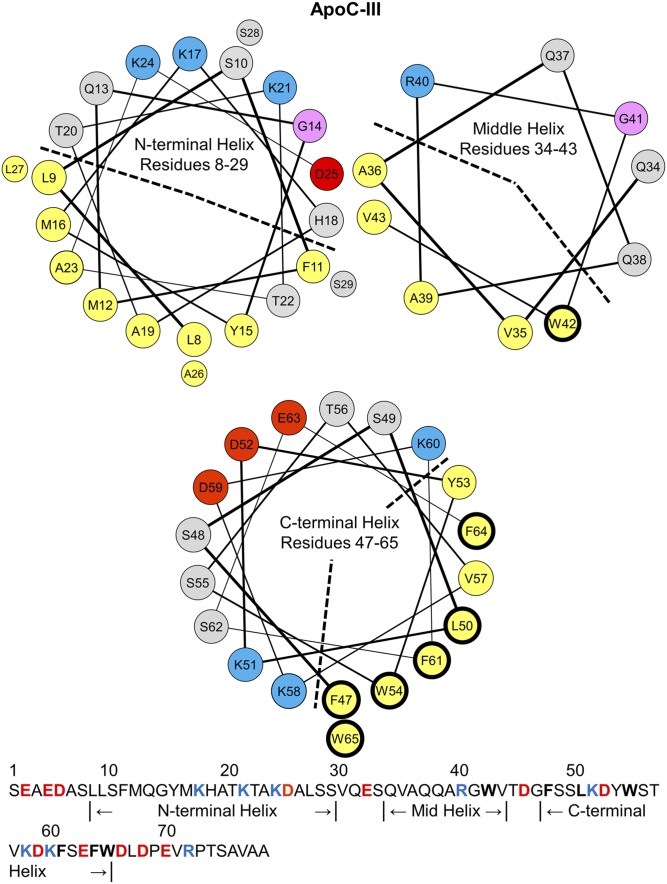
The mechanism of LPL inhibition by apoC-III is unresolved. No LPL:apoC-III interaction has been shown, but evidence suggests that the C-terminal helix of apoC-III mediates LPL inhibition (32, 33). Lipid-free apoC-III has little α-helical content, but apoC-III acquires 50–70% helical content on binding to lipid (33–36). NMR structures of apoC-III:sodium dodecyl sulfate complexes showed helix formation in the protein’s N-terminal (residues 8–29), central (resides 34–43), and C-terminal (resides 47–65) regions (Fig. 1) (36). The C-terminal helix is a class-A amphipathic α-helix, rich in aromatic residues (Fig. 1). This helix contains a large (30–50%) apolar face subtending less than 180°, positively charged residues at its polar/nonpolar interface, and negatively charged residues along its polar face (37, 38). The class-A helix is well suited for lipid binding (37). The other two helices are class-G amphipathic α-helices (Fig. 1), characterized by a random radial distribution of positively and negatively charged residues along the polar face (37).
Fig. 1.
Helix wheel diagrams of the N-terminal, middle, and C-terminal α-helices in lipid-bound apoC-III. The residue assignments for the helices in apoC-III were determined by NMR structures of apoC-III:SDS complexes (36). By convention, apolar residues are colored in yellow, polar in gray, basic in blue, acidic in red, and glycine in pink. The N-terminal and middle helices are amphipathic class-G helices, characterized by a random radial distribution of basic and acidic residues. The apolar faces are marked by a dashed line. The C-terminal helix is a class-A amphipathic helix, featuring an apolar face (marked by a dashed line) of eight hydrophobic residues, basic residues at the polar/apolar lipid/water interface, and acidic residues along the polar face. The sequence of apoC-III (79 amino acids) is shown on the bottom. A total of 78–92% of plasma apoC-III is glycosylated at T74 (59, 60), but we used nonglycosylated recombinant apoC-III for this study. Hydrophobic residues W42, F47, L50, W54, F61, F64, and W65 have thick, black outlines in the wheel diagrams and are bolded in the sequence. These residues were mutated to alanines for the studies described here.
The N-terminal region of apoC-III (i.e., residues 1–40), generated by thrombin cleavage of full-length protein, was unable to bind lipid and inhibit LPL in vitro (32, 39). In contrast, the C-terminal region (i.e., residues 41–79) showed significant binding to lipid vesicles, reflected by changes in near- and far-UV circular dichroism (CD) spectra, and inhibited LPL activity with 73% of the efficacy of full-length apoC-III (32, 39). These results indicate that the N-terminal half of apoC-III binds lipid poorly in the absence of the C-terminal region and that strong lipid binding of apoC-III is essential for LPL inhibition.
A recent study tested the hypothesis that apoC-III inhibits LPL via competition for lipid (33). ApoC-III variants were used that differed in the number or location of alanine substitutions at highly conserved hydrophobic residues: L27, V35, W42, F47, L50, W54, F61, F64, and W65. In vitro assays showed that, at a fixed concentration of LPL, apoC-III inhibited LPL binding to, and activity on, lipid particles in a dose-dependent manner (33). The apoC-III variants showed a decreased ability to inhibit LPL activity, which corresponded to a decrease in unbound LPL. These results suggest that apoC-III competes with LPL for space on lipid surfaces and prevents LPL access to substrate.
In this work, we used several apoC-III variants to determine C-terminal residues critical for mediating the protein:lipid interactions central to LPL inhibition. We used oil-drop tensiometry to characterize the behavior of each peptide at triolein/water (TO/W) and POPC/triolein/water (POPC/TO/W) interfaces (40, 41). The TO/W interface provides a model for protein interactions with a TG core, a component of all TG-rich lipoproteins, whereas POPC/TO/W interfaces more closely mimic the surface of nascent, cholesterol-poor lipoproteins (42). The oil-drop tensiometer measures the surface tension of lipid/water interfaces, which is high at hydrophobic surfaces (40, 41, 43). Peptide adsorption to these interfaces decreases surface tension, and the magnitude of this change reflects the peptide’s ability to remodel the surface (41, 44–46). After peptide adsorption, expansion and compression of the interface mimic local tension changes, such as those induced by LPL activity, and reveal tension values at which peptide desorbs (44–46). By applying this methodology to apoC-III variants, we characterized the quantitative effects of individual mutations on the ability of apoC-III to remodel and remain bound to surfaces mimicking TG-lipoproteins. These results elucidate the role of apoC-III structure on binding to lipoprotein surfaces and reveal residues that are essential for both apoC-III retention at lipid surfaces and LPL inhibition.
MATERIALS AND METHODS
Expression and purification of ApoC-III variants
Full-length cDNA for human apoC-III, including the sequence for a 6× His-tag (preceded by a Leu and Glu) at the C-terminal end, was cloned into pET23b(+) (33, 34, 36). ApoC-III variants were generated by using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). A total of 50 ng of plasmid DNA pET23b/apoC-III was used as a template in a 50 µl reaction. Two oligonucleotide primers, which contained the desired mutation and were complementary to the opposite strands of the vector, were extended by using Pfu Turbo DNA polymerase (34).
The pET-histag-hapoC-III plasmids were transformed into Escherichia coli BL21-condonPlus (DE3)-RIL competent cells (Stratagene), as described previously (33, 34). Transformed bacteria were grown at 37°C until OD600 = 0.6, and expression of apoC-III variants was induced with 1 mM isopropyl β-d-1-thiogalactopyranoside for 1 h. Bacteria were harvested by centrifugation [1,000 g, Beckman J-6B (Beckman, Miami, FL) for 20 min at 4°C] and dissolved in 50 mM sodium phosphate buffer, pH 7.4, containing 0.3 M NaCl, 6 M guanidine CL, and 5 mM imadizole, before freeze/thaw cell lysis at −80°C (33).
Protein was purified on a HisTrapTM FF crude column (GE Healthcare, Piscataway, NJ) by using a gradient of increasing concentrations of imidazole (10–500 mM) in 50 mM sodium phosphate buffer, pH 7.4, containing 0.3 M NaCl and 6 M urea. Fractions that eluted late in the gradient and contained high protein content were dialyzed against 1 mM NH4HCO3 and subsequently frozen at −20°C before lyophilization. Lyophilized protein was divided into small aliquots and stored at −20°C.
Protein was over 95% in purity, as assessed by SDS-PAGE gels, MALDI-TOF mass spectrometry, and HPLC. Before interfacial studies, aliquots of each apoC-III variant were thawed and solubilized with hexafluoro-2-propanol (HFIP) at a concentration of 2.5 or 5.0 mg/ml. For interfacial studies, protein was injected into 7 ml of 5 mM sodium phosphate buffer, pH 7.4, at a concentration of 0.3–14.3 μg/ml. At these protein concentrations, HFIP represented 0.001–0.6% of the total volume. At protein concentrations at which most experiments were conducted (<8 μg/ml), HFIP had no effect on interfacial tension. Protein concentration was determined by the Lowry assay and absorption at 280 nm. Far-UV CD showed that lipid-free apoC-III (at concentrations of 10 and 100 µg/ml in PB with 91% HFIP) had 25 ± 5% α-helix, which is consistent with previous results (34, 47).
Lipids
Triolein (TO; >99% pure) was purchased from NU-CHEK PREP, Inc. (Elysian, MN). The interfacial tension of TO was ∼32 mN/m at 25.0 ± 0.2°C. POPC, dissolved in chloroform at 25.0 mg/ml, was purchased from Avanti Polar Lipids (Alabaster, AL) and stored at −20°C. The purity of both lipids was checked by using high-dose TLC. POPC was dried under nitrogen and resuspended in 5 mM sodium phosphate buffer, pH 7.4, at a concentration of 2.5 mg/ml. POPC in buffer was sonicated for 60 min with a pulsed duty cycle of 30% to form small unilamellar vesicles (SUVs) of d ∼22 nm (48, 49).
Interfacial tension and surface pressure measurements
An oil-drop tensiometer designed by Teclis Instruments (Longessaigne, France) was used to measure the interfacial tension (γ) of lipid/water interfaces (40, 41, 43). γ is the energy required to create one new cm2 of surface (i.e., γ = ergs/cm2 or mN/m) (41, 50). All experiments were conducted at 25.0 ± 0.2°C in a thermostated system and repeated at least three times.
To create TO/W interfaces, TO drops were formed at the tip of a J-needle submerged in 7 ml of bulk buffer. TO drops typically had a volume of 16 μl and surface area of 30 mm2 (diameter = 3.1 mm). These drops are significantly larger than VLDL (diameter = 30–80 nm) and chylomicrons (diameter = 75–1,200 nm) (51), but mimic the TG-rich surface and lack of local curvature of these lipoproteins. Bulk buffer was 5 mM sodium phosphate buffer at pH 7.4. The TO/W interface stabilized at γTO = 32.0 ± 1.0 mN/m. If γTO was outside this range, the drop was discarded, and a new TO drop was created. Adsorption of amphipathic molecules (i.e., phospholipid and apolipoprotein) to this interface shields most of the TO from the aqueous phase and decreases γ. To create POPC/TO/W interfaces, TO drops of 16 μl were formed in bulk buffer containing 0.75–1.25 mg of POPC. After POPC adsorbed to the TO drop, the buffer was exchanged with 250 ml of POPC-free buffer, as described previously (49). This washout removed >99.9% of the original buffer and all POPC SUVs suspended in the bulk phase. After a washout, γ was usually between 23.0 and 26.5 mN/m for POPC/TO/W interfaces.
Varied amounts of apoC-III peptide were added to the bulk phase to obtain different protein concentrations ranging from 0.3 to 14.3 μg/ml. This range is significantly lower than concentrations used in CD studies, such that peptide is monomeric (33, 34). Peptide adsorbed to TO/W and POPC/TO/W interfaces, and γ was monitored continuously as it fell to an equilibrium value (γeq). Surface pressure (Π) was defined as the difference in γ between a pure TO/W interface (γTO = 32.0 mN/m) and the interface with bound POPC and/or protein (Π = γTO − γ). The initial pressures (Πi) of POPC/TO/W interfaces represent the difference between γTO and γ after POPC adsorption (Πi = γTO – γPOPC), while Πi of the TO/W interface was 0 mN/m. After peptide adsorption, the equilibrium pressure of all interfaces was calculated as Πeq = γTO – γeq. The change in pressure (ΔΠ) at these interfaces induced by adsorption was ΔΠ = Πeq − Πi.
Exclusion pressure measurement
Exclusion pressure (ΠEX) for each apoC-III variant is the surface pressure above which that peptide cannot bind and insert into POPC/TO/W interfaces (49, 52). In other words, ΠEX is the pressure of a POPC/TO/W interface at which addition of peptide to the bulk phase leads to no adsorption-induced change in surface pressure (ΔΠ = 0 mN/m).
After POPC adsorption and a 250 ml washout, the initial pressure of POPC/TO/W interfaces was Πi. To achieve a large range of Πi values, TO drop volume was increased or decreased at a rate of 1.2 μl/min, decreasing or increasing Π to new Πi values. For each apoC-III variant, ΔΠ values from peptide adsorption were plotted against Πi. Linear regression was applied to the data, and the x intercept represented ΠEX (i.e., the Πi at which ΔΠ = 0 mN/m). The surface concentration of POPC (ΓPOPC) was also calculated from the Πi of each POPC/TO/W interface, as described previously (49).
Compression and expansion of lipid/water interfaces
Gradual expansion and compression protocol was used to characterize desorption of each apoC-III variant from TO/W and POPC/TO/W interfaces. For interfaces of various Πi, peptide was added to the bulk phase, and protein adsorption was lowered γ to γeq. Buffer was exchanged with 150 ml of protein-free buffer, thereby removing peptide from the bulk phase. Drop volume was increased at a rate of 1.2 μl/min to V≥30 μl. Volume was held constant for >2 min and decreased at 1.2 μl/min to various terminal volumes. Pressure-area (Π-A) isotherms were calculated from the γ and surface area (A) profiles of each compression and expansion, as described previously (45, 49).
The retention pressure of a peptide at a lipid/water interface is the maximum surface pressure peptide molecules withstand before they desorb from that interface. Our laboratory has used envelope pressure (ΠENV) to refer to retention pressure (i.e., the highest pressure at which a peptide is retained at an interface before being ejected) (46, 53).
To determine ΠENV values of apoC-III peptides at TO/W and POPC/TO/W interfaces, the Π-A isotherms from gradual interfacial expansions and compressions were analyzed. A spline fit with a smoothing parameter of 0.38 was applied to each isotherm. The slope of the isotherm was determined by calculating the first derivative of the spline fit, by using MATLAB’s Curve Fitting toolbox (MathWorks, Natick, MA). The slope (dΠ/dA) of compression isotherms to areas smaller than the retention area (AENV) followed this trend: 1) Slope values decreased as the change in pressure (dΠ) became more negative for incremental decreases in area (dA). This occurred because vacant binding sites present on the expanded interface were eliminated, and the density of surface-active amphipathic molecules increased. 2) Slope reached a minimum value. As area continued to decrease, dΠ/dA increased as dΠ became less negative for each dA. This minimum represented a turning point (AP, ΠP), above which bound peptide molecules at least partially desorbed to relax the surface and reduce the dΠ for each dA. 3) Slope values reached a plateau near dΠ/dA = 0. This point represented the envelope point (AENV, ΠENV), above which bound peptide molecules were completely ejected from the surface. Pressure changes were minimal (dΠ ≈ 0 mN/m) as area decreased further, indicating that peptide was partitioning to the bulk phase.
RESULTS
C-terminal tryptophans in ApoC-III mediate protein adsorption to lipid surfaces
We used six apoC-III variants, WT and five point mutants (Fig. 1, Table 1). All of these constructs were expressed with a C-terminal 6× His-tag, as described in Materials and Methods. This tag was essential to solubility and expression of the protein, because expression of untagged constructs was not possible due to their high hydrophobicity. To probe the effect of this His tag on lipid binding, we characterized the lipid binding of plasma apoC-III and compared it with that of the recombinant peptide. Plasma apoC-III bound to lipid surfaces with slower kinetics and induced smaller changes in surface pressure than recombinant apoC-III (supplemental Figs. S1, S2). Likewise, plasma apoC-III desorbed from lipid surfaces at lower retention pressures than recombinant apoC-III (supplemental Fig. S3). This difference in affinity was not due to glycosylation, because deglycosylation of plasma apoC-III did not significantly alter lipid binding of the protein (supplemental Figs. S1–S3). This suggests that the 6× His-tag alters the lipid binding of apoC-III. However, because all apoC-III constructs used in this study contain this tag, any differences in lipid binding are due to the point mutations.
TABLE 1.
ApoC-III variants and their hydrophobic moment
| ApoC-III Variant | <μH> (kcal/mol residue) |
| WT | 0.74 |
| F47A/L50A | 0.70 |
| W42A/F47A | 0.68 |
| W42A/F47A/L50A | 0.64 |
| W42A/F47A/F64A/W65A | 0.61 |
| W54A/F61A/F64A/W65A | 0.63 |
Mean hydrophobic moment (<μH>) of apoC-III variants. Hydrophobic moments (μH) were calculated for the three predicted α-helices within each peptide as previously described (57) by using the Goldman, Engelman, and Stetiz (GES) hydrophobicity scale (58). The GES scale calculates the average free energy change of each amino acid on transition from water to oil. The μH of all three helices were averaged and represent the mean hydrophobic moment (<μH>) for each peptide.
We previously designed a panel of apoC-III mutants that substituted alanine for highly conserved hydrophobic residues predicted to facilitate lipid binding (33). For this study, we selected a subset of these variants with mutations in the C-terminal half of apoC-III because this is the region critical to inhibiting LPL (32). We selected double, triple, and quadruple mutants that had compounding disruptive effects on hydrophobicity (Table 1) and LPL inhibition [shown in (33)]. W54A/F61A/F64A/W65A apoC-III was of particular interest, because these mutations significantly decrease hydrophobicity of the C-terminal helix but do not reduce the ability of apoC-III to inhibit LPL (33).
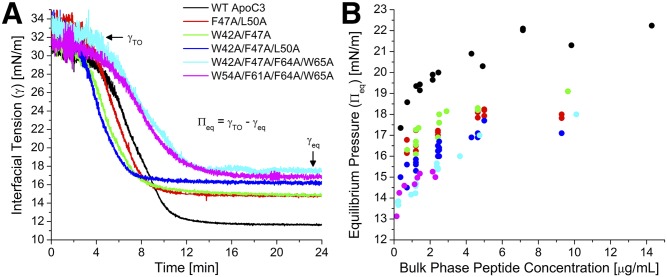
We first characterized the effects of the adsorption of the six apoC-III variants on the surface pressure (П) of lipoprotein-like lipid/water interfaces. These data reflect the ability of apoC-III peptides to bind and insert into the interfaces. Figure 2A shows interfacial tension (γ)-time curves for adsorption of the apoC-III variants to the TO/W interface. Peptide adsorption decreased γ to various equilibrium values (γeq). Adsorption curves showed a sigmoidal time course. The midpoint, t1/2, and transition width, w, were calculated as described in Materials and Methods. This sigmoidal behavior indicates that, once peptide adsorbs to lipid interfaces at adequate concentrations to significantly change γ, γ decreases more rapidly as more peptide adsorbs (i.e., positive cooperativity) until binding reaches an equilibrium. At γeq, lipid-bound peptide is in equilibrium with bulk peptide in the aqueous phase.
Fig. 2.
Loss of C-terminal aromatic residues decreases the ability of apoC-III to remodel the TO/W interface: evidence from equilibrium pressures. A: Representative interfacial tension (γ) versus time curves for the adsorption of the six apoC-III variants to TO/W interfaces. A total of 16 μl TO drops were formed in phosphate buffer, and peptides were added to the bulk phase at concentrations ranging from 1.2 to 1.5 µg/ml. γ decreased from γTO to an equilibrium value (γeq) due to peptide adsorption. This corresponded to an increase in surface pressure (П) from 0 mN/m to an equilibrium pressure (Пeq). Adsorption curves were approximated by sigmoidal functions: γ(t) = γeq + (γi − γeq)/(1 + exp((t − t1/2)/w)), where t1/2 is the midpoint corresponding to 50% decrease in γ, and w is the transition width. B: For each apoC-III point mutant, peptide was injected into the bulk phase at various concentrations in a series of experiments similar to those shown in (A). Пeq values were plotted against bulk phase peptide concentrations for the six apoC-III variants.
The apoC-III variants exhibited different adsorption kinetics. At a TO/W interface (Fig. 2), WT apoC-III and double or triple apoC-III point mutants showed similar adsorption kinetics (t1/2 varied from 4.1 to 7.1 min and w from 1.1 to 1.4 min). In contrast, the quadruple mutants had slower adsorption kinetics, marked by larger values of t1/2 (7.75 ± 0.15 min) and w (1.8 min). This indicates that the binding of these mutants to apolar lipid surfaces is less cooperative than the binding of the other apoC-III variants.
The ability of apoC-III variants to modify the TO/W interface increased with greater bulk phase concentration (Fig. 2B). As peptide concentration increased, equilibrium pressure (Πeq) increased up to a saturation pressure (ΠSAT) at concentrations ≥5 µg/ml. These results indicate that lipid-free and lipid surface-bound forms of apoC-III variants are in a concentration-dependent equilibrium that influences Πeq (44, 54). As the concentration of lipid-free peptide increased, so did that of lipid-bound peptide until, at saturation, no binding sites were available at the lipid surface. All apoC-III mutants saturated the TO/W interface at similar ΠSAT, whereas WT apoC-III had a ΠSAT that was 3–5 mN/m higher (Table 2).
TABLE 2.
Properties of ApoC-III variants at the TO/W interface
| Peptide | ПSAT [mN/m] | ПWO [mN/m] |
| WT ApoC-III | 22.0 ± 0.2 | 17.7 ± 0.4 |
| F47A/L50A | 18.1 ± 0.2 | 15.2 ± 0.4 |
| W42A/F47A | 19.0 ± 0.3 | 15.2 ± 0.3 |
| W42A/F47A/L50A | 17.3 ± 0.4 | 13.8 ± 0.3 |
| W42A/F47A/F64A/W65A | 18.0 ± 0.3 | 12.5 ± 0.2 |
| W54A/F61A/F64A/W65A | 13.0 ± 0.4 |
Saturation pressures (ПSAT) were calculated from the data in Fig. 2, and error bars were derived from n = 2–3 experiments at saturating concentrations of peptide in the bulk phase. Washout pressures (ПWO) were calculated from the data in Fig. 3, and SD for each value was calculated using all the data for each peptide.
Below saturation, the ability of apoC-III to remodel the TO/W interface, as marked by Πeq values at multiple bulk phase concentrations, followed the order WT > F47A/L50A = W42A/F47A > W42A/F47A/L50A > W42A/F47A/F64A/W65A = W54A/F61A/F64A/W65A (Fig. 2B). This order correlates with the number of alanine substitutions, hydrophobicity (Table 1, supplemental Fig. S4A), dimyristoylphosphatidlycholine-bound helical content, and LPL inhibitory potency of the peptides (33).
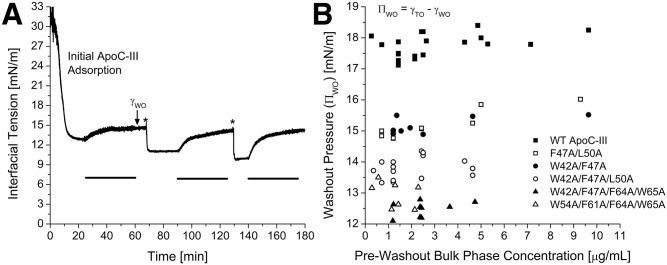
After peptide adsorbed to a TO/W interface, >99% of lipid-free peptide could be removed from the aqueous phase by a washout of 150 ml (Fig. 3A). During this washout, the bulk phase concentration of peptide was reduced to 0 µg/ml, which altered the concentration-dependent equilibrium between the lipid-free and lipid-bound forms of apoC-III. As a result, some lipid-bound peptide desorbed from the apoC-III/TO/W interface, and γ increased as Π decreased (Fig. 3A). The surface pressure after a washout (ΠWO) was lower than Πeq, indicative of a lower surface concentration of lipid-bound protein, but was still very high, indicating that much of the lipid-bound population had been retained at the surface.
Fig. 3.
Loss of C-terminal aromatic residues decreases the ability of apoC-III to remodel the TO/W interface: evidence from washout pressures. A: Changes in γ during three washouts after WT apoC-III adsorbed to a TO/W interface. Peptide was added to the bulk phase at three time points: t = 0 min at a concentration of 1.43 µg/ml, t = 68 min at 4.29 µg/ml (marked by an asterisk), and t = 129.5 min at 7.14 µg/ml (marked by an asterisk). After each addition, apoC-III adsorbed to the TO drop and modified γ (or П) by a different amount, dependent on bulk phase concentration. After each adsorption, a 150 ml washout was conducted (marked by a black bar), at a rate of 4.18 ml/min for 36 min. γ increased to a postwashout tension (γWO), which indicates desorption. B: For each apoC-III variant, the postwashout pressure (ПWO) was a constant, independent of the prewashout, bulk phase peptide concentration, and Пeq. For each apoC-III variant, a series of experiments similar to (A) was conducted that varied in bulk phase concentrations and washout rate and duration. γWO values were converted to ПWO and plotted against prewashout, bulk phase peptide concentration.
All apoC-III variants had ΠWO values that were independent of Πeq (Fig. 3B, Table 2). ΠWO followed the rank order WT apoC-III > F47A/L50A = W42A/F47A > W42A/F47A/L50A > W54A/F61A/F64A/W65A ≥ W42A/F47A/F64A/W65A (Table 2). Together with Πeq data (Fig. 2), this result indicates that, independent of surface concentration, the apoC-III variants show a reduced ability to insert into and modify the TO/W interface. The severity of impaired lipid binding increased with lower hydrophobicity (supplemental Fig. S4A, B) and correlated with a reduction in the ability of these peptides to compete with LPL for binding to TG/PC emulsion particles and chylomicrons (33).
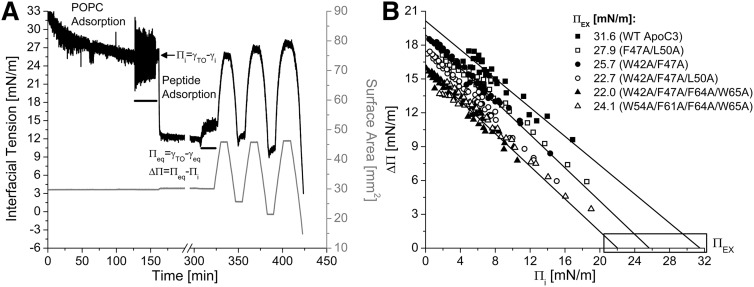
The differences between lipid surface-remodeling of apoC-III variants were independent of lipid composition. The ability of apoC-III variants to remodel POPC/TO/W interfaces was determined for interfaces of various POPC surface concentrations (ΓPOPC). After POPC adsorption to a TO drop and a washout to remove POPC in the bulk phase, the initial pressure (Πi) could be changed by increasing or decreasing drop volume (Fig. 4A). Πi was used to determine ΓPOPC in units of μmol/m2 or as a percentage of TO drop coverage by POPC, as described previously (49). For each apoC-III variant, ΔΠ values were recorded for peptide adsorption to interfaces of various ΓPOPC and plotted as a function of Πi (Fig. 4B). Each dataset was fit linearly, and the x intercept represents the exclusion pressure (ΠEX).
Fig. 4.
Loss of C-terminal aromatic residues decreases the ability of apoC-III to remodel POPC/TO/W interfaces. A: Protocol to monitor apoC-III adsorption to and desorption from a POPC/TO/W interface. POPC adsorbed to a 16 μl TO drop and lowered γ (black line). POPC was removed from the bulk phase by a 250 ml washout (black bar, 8.35 ml/min for 30 min starting at 127.1 min). After the washout, γ = 25.9 mN/m (Πi = 6.1 mN/m). W42A/F47A/L50A apoC-III was added to the bulk phase at a concentration of 2.5 μg/ml, and γ was monitored as it fell to γeq. Peptide was removed from the bulk phase by a 150 ml washout (black bar, 8.35 ml/min for 18 min starting at 306.7 min). The drop volume underwent three sets of gradual expansions and compressions at a rate of 1.2 μl/min (shown as recordings of surface area in mm2, gray line). Volume reached an upper limit of 30.0 μl (area = 45.8 mm2) for expansions and lower limits of 12.9, 9.9, and 5.7 μl (area = 25.7, 21.4, and 14.8 mm2) for compressions. B: ApoC-III mutants have lower exclusion pressures (ΠEX) than WT apoC-III. A series of experiments similar to (A) was conducted for peptide over a range of Πi. ΔΠ was plotted against Πi for all six peptides. Each dataset was fit linearly, as shown for WT, W42A/F47A, and W42A/F47A/F64A/W65A apoC-III. These linear regressions were significant (−0.99 < R < −0.96, P < 0.0001). x intercepts, marked by a box, are ΠEX, the pressure at which a given peptide cannot bind POPC/TO/W interfaces.
At most POPC/TO/W interfaces, ΔΠ followed the same rank order as at a TO/W interface (where ΔΠ = Πeq): WT apoC-III > F47A/L50A = W42A/F47A > W42A/F47A/L50A > W42A/F47A/F64A/W65A = W54A/F61A/F64A/W65A (Fig. 4B). ΠEX followed a similar rank order and strongly correlated with changes in peptide hydrophobicity (supplemental Fig. S4C). At interfaces of higher Πi, the triple and quadruple apoC-III mutants remodeled the surface to a similar extent, as reflected in ΔΠ values. As a result, the differences in ΠEX for triple and quadruple mutants were insignificant.
The ability of ApoC-III to remain bound to lipid at high П is impaired by C-terminal mutations
This study also characterized the effect of increases in surface pressure on the ability of apoC-III variants to remain bound to lipoprotein-like lipid/water interfaces. Gradual compressions of apoC-III/lipid/water interfaces revealed the pressure range in which peptide remained bound to the interfaces and the retention pressures (ΠENV) at which peptide desorbed. These changes in volume were recorded as changes in surface area and mimic LPL activity at the VLDL or chylomicron surface as TG are removed from the core.
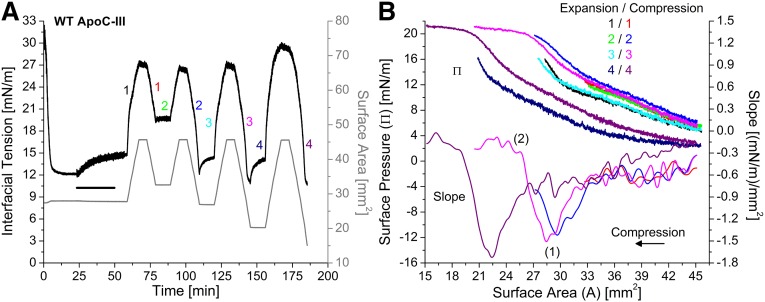
Figure 5A shows γ and area changes during gradual, postwashout expansions and compressions of the WT apoC-III/TO/W interface. Peptide adsorbed to the interface and 150 ml of buffer was exchanged to remove peptide from the bulk phase. After washout, the TO drop underwent a series of gradual (1.2 µl/min) expansions and compressions (expressed as changes in surface area in Fig. 5A). The γ values during expansion and compression phases were converted to Π and plotted against surface area (Fig. 5B).
Fig. 5.
Desorption of WT apoC-III molecules from the TO/W interface is a two-step process. A: Gradual, postwashout expansions and compressions of apoC-III/TO/W interfaces. ApoC-III was added to the bulk phase at 2.14 μg/ml. After adsorption to a TO drop lowered γ to γeq = 12.1 mN/m, apoC-III was removed from the bulk phase by a 150 ml washout (black bar, 6.26 ml/min for 25 min starting at 23.3 min). Drop volume underwent a series of four gradual expansions and compressions, at a rate of 1.2 μl/min (shown as recordings of surface area in mm2, gray line). Each expansion ended at A = 45.6 mm2. Each compression ended at a smaller area than the previous compression(s), ending at A = 32.6, 26.9, 20.2, and 15.0 mm2. B: ApoC-III molecules partially desorb before completely desorbing from a TO/W interface. γ values during expansions and compressions in (A) were converted to Π and plotted against area. Expansions and compressions are labeled by color and number, starting at 1 for the first postwashout set. The compression isotherms were smoothed by using a spline interpolation of smoothing parameter 0.38. The first derivative gave slope values (dП/dA), which were plotted against area. dП/dA plots show one or two inflection points, as marked for the third compression isotherm (magenta).
WT apoC-III and the double and triple mutants showed the ability to adopt multiple lipid-bound conformations during interfacial compressions. In Fig. 5B, the first three sets (i.e., first six) of expansions and compressions were reversible, and the expansion isotherms aligned, as did the compression isotherms. Hysteresis was observed between expansion and compression phases, due to a bend in the expansion isotherms. This bend is near A = 30 mm2, 2 mm2 above the area from which the drop was expanded. The bend in the fourth expansion isotherm is at 22.0 mm2, but it is 2 mm2 higher than the area at the start of the previous compression (Fig. 5B). This bend suggests that apoC-III molecules may expand to occupy larger surface area as it becomes available.
To further probe the ability of apoC-III to adopt multiple lipid-bound conformations, compression Π/A isotherms were smoothed by using a spline interpolation of smoothing parameter 0.38. The first derivative was determined in increments of ΔA = 0.2 mm2. Slope (dΠ/dA) values were plotted as a function of area for the compression isotherms (Fig. 5B). The slope profile can show four phases, depending on the endpoint: 1) Slope is a constant, negative value. For the first three compressions, dΠ/dA ≈ −0.6 (mN/m)/mm2 as area was compressed to A ≈ 33.0 mm2. In addition, the first six expansion and compression isotherms were aligned at A > 33.0 mm2. These two results indicate that vacant binding sites created by expansion of the interface are being eliminated at a constant rate during the first phase of compression. 2) Slope becomes more negative and reaches an absolute minimum (marked as 1 for the slope profiles of the second and third compression isotherms). For each compression, this minimum occurred at areas equal to the area of the drop before the previous expansion. This suggests that, during the second phase of compression, all vacant binding sites are eliminated, and the surface density of apoC-III increases until it is equivalent to the postwashout surface density of apoC-III, relative to surface area. Consistent with this, Π values on the Π/A isotherms were equal to ΠWO at areas corresponding to these minima in dΠ/dA. 3) As area continues to decrease, dΠ/dA increases as dΠ becomes less negative for each dA. This result indicates that the area and pressure corresponding to minimum dΠ/dA is a turning point (AP, ΠP). Beyond this point, bound apoC-III molecules at least partially desorb to relax the surface and reduce dΠ for each dA. 4) The slope reached a constant value near dΠ/dA = 0 (mN/m)/mm2. Pressure changes were minimized as area decreased, indicating that apoC-III was completely desorbing into the bulk phase. The area and pressure at which dΠ/dA reaches 0 (mN/m)/mm2 is the envelope point (AENV, ΠENV), marked as 2 on the slope profile of the third compression. Beyond this point, bound apoC-III molecules irreversibly desorb from the surface.
A comparison of all of the compression isotherms in Fig. 5B indicates that compression of the apoC-III/TO/W interface is reversible between areas AP and AENV. The second compression (blue) ended at A = 26.9 mm2. The slope profile exhibited a minimum at AP ≈ 29.5 mm2. dΠ/dA ≈ −0.8 (mN/m)/mm2 at A = 26.9 mm2, which indicates that this compression ended at A > AENV. The next expansion (cyan) aligned with the first expansion (black), which indicates that apoC-III molecules reversibly (i.e., partially) desorbed from the surface on compression to A < AP. Consistent with this, the second and third (magenta) compression isotherms aligned (Fig. 5B).
In contrast, apoC-III molecules irreversibly (i.e., completely) desorbed from the surface on compression to A < AENV. As shown in Fig. 5B, the third compression (magenta) ended at A = 20.2 mm2, and the slope profile showed two inflection points at AP = 28.5 mm2 and AENV = 25.5 mm2. The isotherms for the subsequent (i.e., fourth) expansion (navy) and compression (purple) exhibited a marked shift to smaller areas (AP = 22.5 mm2, AENV = 19.0 mm2) and hysteresis from previous expansion and compression isotherms (Fig. 5B). This indicates that apoC-III molecules irreversibly desorb at A ≤ AENV, such that a lower surface concentration of apoC-III on subsequent expansions and compressions requires larger changes in surface area to induce similar changes in surface pressure. Together, these results indicate that part of the lipid-bound apoC-III molecule desorbs above the partial pressure (ΠP) (i.e., below AP) before desorption of the entire molecule above the retention pressure (ΠENV) (i.e., below AENV).
ΠP and ΠENV values were determined from compression Π/A isotherms, by using areas from inflection points in the slope profile. Whereas AP and AENV values depended on the surface concentration of protein, ΠP and ΠENV were the same for all compressions of the WT apoC-III/TO/W interface to A ≤ AENV. ΠP = 17.2 ± 0.2 mN/m, approximately equal to ΠWO (Table 2). ΠENV = 20.4 ± 0.3 mN/m. The experiment in Fig. 5 was repeated (n > 5) at different prewashout concentrations of WT apoC-III, and these values were the same.
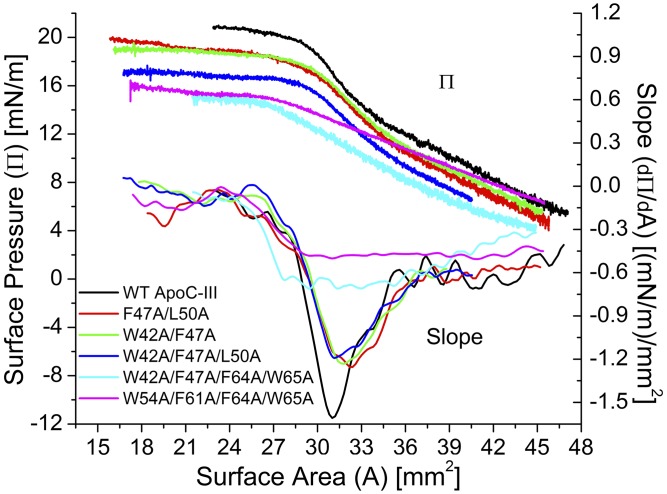
To compare the desorption behavior of the apoC-III variants from lipid surfaces, Π/A isotherms from compressions to A < AENV at TO/W and POPC/TO/W interfaces, and the slope of these isotherms, were plotted in Figs. 6 and 7. At a TO/W interface, ΠENV followed the order WT apoC-III > F47A/L50A = W42A/F47A > W42A/F47A/L50A > W42A/F47A/F64A/W65A = W54A/F61A/F64A/W65A (Fig. 8). In addition, the compression isotherms were significantly different, as reflected in plots of dΠ/dA as a function of area for each peptide (Fig. 6).
Fig. 6.
At the TO/W interface, retention pressures (ПENV) decrease with loss of helical content in apoC-III. In separate experiments, all six apoC-III variants were added to the bulk phase at 2.0 ± 0.5 µg/ml and adsorbed to a TO/W interface. After a washout of 150 ml, the peptide/TO/W interface was gradually expanded and compressed at a rate of 1.2 µl/min, similar to the protocol in Fig. 5A. Compressions ended at areas smaller than the retention area (A ≤ AENV) to ensure desorption of peptide. Shown here are П/A isotherms from the compression phase and their slopes. Slopes were derived as in Fig. 5.
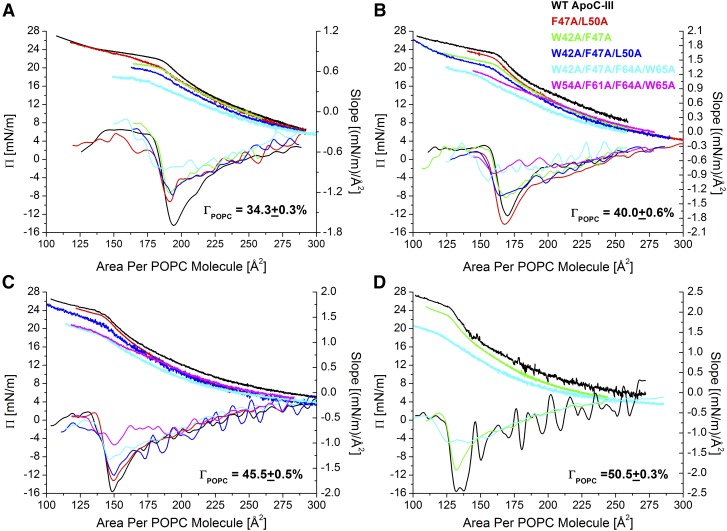
Fig. 7.
At POPC/TO/W interfaces, retention pressures (ΠENV) decrease with loss of helical content in apoC-III. In separate experiments, all six apoC-III variants were added to the bulk phase at 2.0 ± 0.5 µg/ml and adsorbed to POPC/TO/W interfaces of four initial pressures (Πi). These Πi correspond to POPC surface concentrations (ΓPOPC) of 34.3% (A), 40.0% (B), 45.5% (C), and 50.5% (D). After a 150 ml washout, each peptide/POPC/TO/W interface was expanded and compressed at a rate of 1.2 µl/min, similar to the protocol shown in Fig. 4A. Compressions ended at areas smaller than the retention area (A ≤ AENV) to ensure desorption of peptide. Values of γ during the compression phase were converted to Π and plotted against area per POPC molecule (APM). Shown are compression Π/APM isotherms and their slopes.
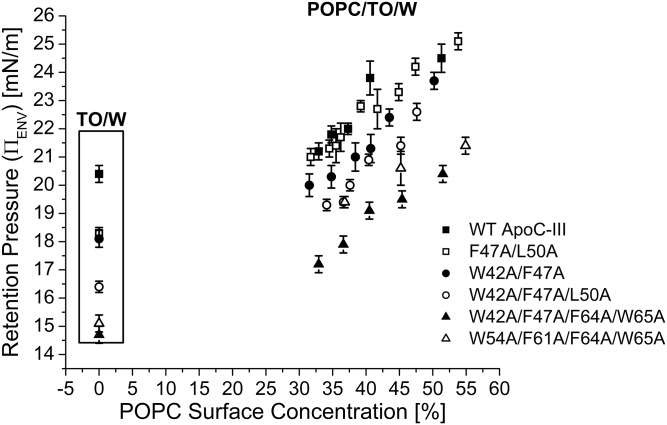
Fig. 8.
Tryptophan 42 enhances apoC-III retention at lipid/water interfaces. Experiments similar to those in Fig. 4A were conducted over a range of Πi values for all six apoC-III variants. Πi values were converted to ΓPOPC, as described previously (49). γ values during the compression phase were converted to Π and plotted against the APM. The retention pressures (ΠENV) of apoC-III peptides at POPC/TO/W interfaces were calculated as described at a TO/W interface in Fig. 5. ΠENV values were plotted as a function of ΓPOPC for each apoC-III variant. x and y error bars are the SD from n = 2–5 experiments.
The structure and presence of aromatic residues was essential for apoC-III to adopt multiple conformations on interfacial compression. The four phases described above were observed in the slope profiles of the compression isotherms of WT, F47A/L50A, W42A/F47A, and W42A/F47A/L50A apoC-III at a TO/W interface (Fig. 6). AP and AENV were very similar for all four peptides. This result indicates that the peptides occupy similar surface areas on the TO drop after adsorption. However, minimum dΠ/dA values were less negative for the double and triple mutants [dΠ/dA ≈ −1.2 (mN/m)/mm2] than for WT apoC-III [dΠ/dA ≈ −1.6 (mN/m)/mm2]. Together with Πeq results (Fig. 2), this result indicates that the mutants do not insert into the surface as well as WT apoC-III, likely due to loss of secondary structure (33).
No pronounced minima (AP) were present in the compression isotherms of the quadruple mutants at the TO/W interface (Fig. 6). Rather, slope was a small, constant value [dΠ/dA ≈ −0.6, −0.45 (mN/m)/mm2] from large areas to A ≈ 28.5 mm2 (W54A/F61A/F64A/W65A) or 27.0 mm2 (W42A/F47A/F64A/W65A). At smaller areas, dΠ/dA increased and approached 0 (mN/m)/mm2. AENV was similar for both peptides, but was 2–3 mm2 smaller than AENV of the other apoC-III variants (Fig. 6). Together with the absence of defined minima in dΠ/dA, this result indicates that denaturation of apoC-III abolishes the multistep desorption of the protein from the surface.
Addition of POPC to the TO/W interface, at various concentrations (ΓPOPC), increased the retention pressures (ΠENV) of each apoC-III variant (Fig. 8), but did not alter desorption behavior of each variant (Fig. 7), with a few notable differences. The compression isotherms of structured apoC-III variants (i.e., WT apoC-III and the double and triple mutants) exhibited four phases, similar to isotherms at a TO/W interface. However, dΠ/dA slowly decreased during the first phase (i.e., was not constant), because POPC contributes to eliminating regions of TG exposed to the surface. In addition, dΠ/dA approached a nonzero, negative constant during the fourth phase of compression. As peptide desorbs from the interface, dΠ/dA ≠ 0 (mN/m)/mm2, because the surface density of PC lipid increases. Consistent with this, |dΠ/dA| during the fourth phase of compression increased for interfaces that contained more POPC (Fig. 7). Together, these results indicate that the presence of POPC at the interface increased the sensitivity of Π response to changes in area. The compression isotherms of variants W42A/F47A/F64A/W65A and W54A/F61A/F64A/W65A did not exhibit minima in slope (Fig. 7), which suggests that intact secondary structure is required for ordered, two-step desorption of apoC-III from lipid surfaces.
A plot of ΠENV as a function of ΓPOPC shows that retention pressure strongly correlates with hydrophobicity of apoC-III variants (Fig. 8, supplemental Fig. S4D). At most POPC/TO/W interfaces, F47A/L50A desorption kinetics (Fig. 7B, C) and ΠENV (Fig. 8) are similar to those of WT apoC-III. Together with the ΠENV differences in the quadruple mutants at POPC/TO/W interfaces, this result indicates that W42 is central to the lipid binding of apoC-III.
DISCUSSION
Previous studies showed that the C terminus of apoC-III (residues 41–78) was responsible for mediating lipid-binding and inhibiting LPL, whereas the N terminus plays a limited, secondary role in these functions (32, 34, 39). To gain novel insight into the unknown mechanism by which apoC-III inhibits LPL, we probed the roles of select C-terminal residues and the structure of apoC-III in lipid binding. This study advanced previous work probing the lipid affinity and LPL inhibitory potential of apoC-III variants in two ways (33). We elucidated the role of apoC-III helical content on binding to interfaces that mimic lipoprotein surfaces. In addition, we determined residues critical to apoC-III retention at these interfaces. Tight lipid binding conferred to apoC-III by these residues is likely critical to preventing LPL access to the surface of apoC-III-rich VLDL.
The loss of helical content, hydrophobicity, and aromatic residues in apoC-III, modeled by double, triple, and quadruple point mutations, impaired the ability of apoC-III to insert into lipid surfaces. ApoC-III variants remodeled TO/W and POPC/TO/W interfaces by different ΔΠ, with a rank order of WT > F47A/L50A = W42A/F47A > W42A/F47A/L50A > W42A/F47A/F64A/W65A = W54A/F61A/F64A/W65A (Figs. 2, 4, supplemental Fig. S4A). These differences in ΔΠ were not due to different amounts of peptide at the interface. The apoC-III variants occupied similar surface areas after adsorption, as reflected in the inflection points occurring at similar areas in compression isotherms (Figs. 6, 7). Likewise, differences in ΔΠ were not attributable to differences in the surface density of peptide at the interface. ApoC-III variants desorb from the TO/W interface during washout, but the postwashout pressures (ΠWO) followed the same order as ΔΠ (Fig. 3, supplemental Fig. S4B, Table 2). These results indicate that loss of secondary structure or select residues are directly responsible for the loss of affinity in the apoC-III variants.
Structure-altering mutations in apoC-III also impaired the protein’s ability to undergo an ordered, two-step desorption from TO/W and POPC/TO/W interfaces (Figs. 5–7). For each apoC-III variant at lipid/water interfaces, the interface could be compressed to retention pressures (ΠENV) that were 2–3 mN/m greater than ΠWO before peptide irreversibly desorbed. During this interval, peptide could partially desorb from, and/or exhibit conformational rearrangement on, the interfaces, as shown for WT apoC-III in Fig. 5. These conformational changes were impaired by alanine substitutions and abolished in the quadruple point mutants (Fig. 6, 7).
Aromatic residues, including phenylalanine and tryptophan, are among the most hydrophobic residues and, within α-helices, are often found along the nonpolar face (37, 38). This nonpolar face inserts into lipid surfaces and associates with the fatty acyl chains of lipids (37, 38). The C-terminal helix of apoC-III is uniquely rich in aromatic residues, with six aromatic residues that comprise 75% of its nonpolar face and likely mediate insertion into lipid surfaces. In this study, we showed that certain aromatic residues were more critical than others to the ability of apoC-III to insert into and desorb from lipid surfaces. At POPC/TO/W interfaces, F47A/L50A adopted similar conformational rearrangements as WT apoC-III and desorbed at similar ΠENV (Figs. 7, 8). These results indicate that F47, perhaps due to its location at the beginning of the C-terminal helix, is not as critical as other apolar residues for apoC-III insertion into more polar lipid surfaces. In contrast, the loss of tryptophans in apoC-III significantly reduced ΠENV and impaired multistep desorption (Figs. 6–8). Of these residues, W42 was most critical for lipid binding. W42A/F47A and W42A/F47A/L50A had significantly lower retention pressures than F47A/L50A and WT apoC-III for POPC/TO/W interfaces (Figs. 7, 8, supplemental Fig. S4D). Likewise, W42A/F47A/F64A/W65A had lower ΠENV than W54A/F61A/F64A/W65A (Fig. 8). Because the quadruple mutants have a similar hydrophobicity (Table 1), these results suggest that W42 promotes folding of the central helix and is critical to apoC-III adsorption and desorption at lipid surfaces.
At interfaces of higher initial pressure (Πi > 9 mN/m) and POPC surface concentration (ΓPOPC > 42%), W42A/F47A/L50A apoC-III remodeled the interface by similar ΔΠ as the quadruple mutants, resulting in similar exclusion pressures (Fig. 4). These results further indicate that aromatic residues closer to the central region of apoC-III (W42, W54, and F61) are most important for inducing proper protein folding, whereas aromatic residues F64 and W65 at the end of the C-terminal helix are not critical for mediating retention at lipid surfaces. Consistent with this minimal contribution of F64 and W65 to lipid binding, elimination of these bulky residues in F64A/W65A apoC-III increased apoC-III helical content from 45% (WT) to 74% (F64A/W65A) in the presence of 2,2,2-trifluoroethanol (34).
Our results show a correlation between the affinity of apoC-III variants for nascent VLDL-like lipid surfaces and the previously characterized ability of these peptides to inhibit LPL (33). In our previous study, WT apoC-III inhibited LPL activity on emulsion particles by 30% at an apolipoprotein concentration of 0.3 µM, 50% at 0.5 µM, and nearly 100% at 1 µM (33). This progressive inhibition corresponds to the range and concentration where LPL is progressively displaced from the lipid particle by WT apoC-III until, at an apolipoprotein concentration of 1 µM, >90% of LPL is unbound (33). In this study, we studied the lipid binding of apoC-III over an overlapping range of apolipoprotein concentrations. At a concentration of 0.1 µM, apoC-III increased the pressure of a TO/W interface to Πeq = 18.5 mN/m. Πeq progressively increased with larger apoC-III concentrations until the surface was saturated with protein, at 0.7 µM and a ΠSAT = 22 mN/m (Fig. 2B, Table 2). These results uniquely indicate that a saturating amount of apoC-III is required to initiate LPL desorption from lipid surfaces, which is responsible for its inhibition.
Over the same range of concentrations, all five apoC-III variants exhibited a reduced ability to inhibit LPL. For example, W42A/F47A/L50A inhibited LPL activity by 15% at an apolipoprotein concentration of 0.5 µM and 25% at 1 µM (33). Although there were insufficient data for W54A/F61A/F64A/W65A, the other four apoC-III variants saturated lipid surfaces at concentrations between 0.7 and 0.9 µM (Fig. 2B). However, these variants saturated the surface at ΠSAT values that were 3.0–4.7 mN/m lower than WT apoC-III (Table 2). These data indicate that, in addition to a saturating concentration of protein, a sufficient increase in surface pressure (>18 mN/m at a triglyceride/water interface) is required for apoC-III to displace prebound LPL from lipid surfaces. Decreases in the ability of apoC-III to not only remodel, but also be retained, at lipid surfaces correlated with compounding alanine substitutions (supplemental Fig. S4) and mirrored decreases in the ability of apoC-III to inhibit LPL (33). Together, these data provide strong evidence that the ability of apoC-III to remodel and be retained to high pressures at lipid surfaces is most critical for its function of displacing LPL from lipid surfaces and thereby inhibiting the enzyme.
Additionally, W42, which is critical for mediating the lipid binding of apoC-III in our studies, is central to mediating LPL inhibition (33, 34). L27A/V35A and F64A/W65A inhibited LPL activity with similar potency as WT apoC-III, but pairing L27/V35 or F64/W65 alanine substitutions in tandem with similar mutations in W42/F47 abolished the ability of apoC-III to inhibit LPL (33, 34). These results indicate that the loss of N- or C-terminal hydrophobic residues do not impair the ability of apoC-III to compete with LPL for lipid binding, unless these mutations are compounded by mutation of W42.
The apoC-III variant W54A/F61A/F64A/W65A was the lone exception to the strong correlation observed between the lipid affinity of apoC-III variants in our study and potency of LPL inhibition in previous studies. W54A/F61A/F64A/W65A adsorbed to and desorbed from PC lipid and TG with significantly lower affinity than WT apoC-III (as discussed above), but inhibited LPL with similar potency as WT apoC-III (33). These noncomplementary results could be explained by differences in experimental conditions. In the present experiments, the loss of lipid affinity in apoC-III variants was observed in experiments with different lipid constituents and conditions than the in vitro assays used to monitor the effect of these proteins on LPL activity (33). Alternatively, these results could indicate that lipid binding is insufficient for apoC-III to inhibit LPL. No direct interactions between apoC-III and LPL have yet been shown, and mechanisms by which apoC-III inhibit LPL that are independent of substrate competition require further study.
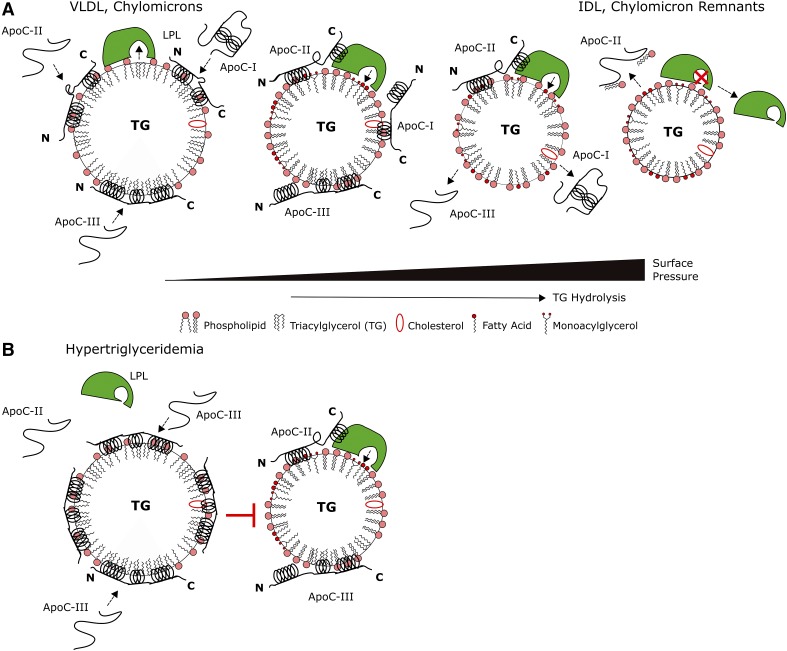
We propose the model in Fig. 9 for LPL regulation by apoC-III. For comparison, this model includes the other apoCs, apoC-I and apoC-II, which we characterized in previous studies (45, 46). ApoC-II, the required cofactor of LPL, was retained to higher pressures than apoC-III and removed phospholipid on desorption from lipid interfaces (45). The apoCs, which have similar sizes and structures, bind to VLDL or chylomicrons after secretion (Fig. 9A) (2, 51). Our results indicate that apoC-III inserts tightly into the lipid surface (Figs. 2–4). In normolipidemic individuals, plasma concentrations of apoC-III are 80–100 μg/ml (2, 30). At these concentrations, most (60%) of plasma apoC-III is localized to HDL, and apoC-III has minor modulatory effects on LPL (5, 30, 55). ApoC-II interaction with LPL results in TG hydrolysis and increases in surface pressure (56). Similar to the other apoCs (45, 46), apoC-III adopts multiple conformations as Π increases (Figs. 5–7). Aromatic residues in the C-terminal helices of apoC-III anchor the protein to the lipid surface, whereas its N-terminal helix may desorb (Figs. 6–8). ApoC-III desorbs at lower retention pressures than apoC-II (Fig. 9A).
Fig. 9.
A model for the regulation of VLDL, chylomicron metabolism by apoC-III. A: ApoC-III does not inhibit LPL under normolipidemic conditions (30). The apoCs (apoC-I, -II, and -III) bind to nascent VLDL and chylomicrons in plasma. Because of similar sizes and structures, the apoCs remodel lipid surfaces to a similar extent (45). As LPL hydrolyzes TG in the particle core, particle size decreases while surface pressure increases. The apoCs exhibit similar desorption behavior from lipid surfaces, indicative of conformational rearrangement (Figs. 5–7) (45, 46). One (or more) helix in each apoC likely desorbs as lipoprotein surface pressure increases. For apoC-II, the C-terminal helix likely desorbs to interact with LPL and promote its activity (45). Tryptophans in the C-terminal helices of apoC-III anchor it to lipid surfaces, whereas the N-terminal helix desorbs. As surface pressure continues to increase, apoC-I and -III desorb at lower retention pressures than apoC-II (45). Desorption of apoC-II:PC complexes likely triggers the inactivation of LPL and release of the enzyme from remnant particles (45). B: In the hypertriglyceridemic state, apoC-III competes with apoC-II and/or LPL for lipid binding to inhibit LPL (33). ApoC-III levels are increased by >5-fold in plasma and by >7-fold on VLDL (5–7). Higher levels of lipid-free and lipid surface-bound apoC-III likely allow the protein to compete with apoC-II and/or LPL for binding to, and prevent LPL from accessing substrate on, TG-rich lipoproteins.
In the hypertriglyceridemic state, apoC-III is enriched at VLDL and chylomicron surfaces (Fig. 9B). The expression of apoC-III is upregulated by glucose and/or loss of insulin sensitivity (1). Plasma apoC-III levels increase to >300 μg/ml and correlate with an enrichment of apoC-III at VLDL and chylomicron surfaces, as the percentage of total apoC-III on TG-rich lipoproteins increases from 20% to upward of 60% (5–7). ApoC-III also accumulates on nascent VLDL intracellularly, because apoC-III promotes VLDL maturation through fusion of lumenal lipid droplets in the Golgi with nascent VLDL in the hypertriglyceridemic state (24, 26). Enriched at the VLDL surface, apoC-III prevents LPL from binding, hydrolyzing TG, and inducing changes in Π (Fig. 9B). ApoC-III may compete with apoC-II via mass effect for binding to VLDL to further inhibit LPL activation, because apoC-III serum levels are 6-fold higher than apoC-II levels in hypertriglyceridemic subjects (Fig. 9B) (6, 7).
In summary, this work characterized the behavior of six apoC-III variants at lipid/water interfaces mimicking the VLDL surface. We determined the role of helical content and select aromatic residues on the binding of apoC-III to these interfaces. We correlated these results with in vitro LPL activity assays with the same variants (33). From these data, we proposed a model for LPL inhibition by apoC-III in vivo (Fig. 9).
Supplementary Material
Acknowledgments
The authors thank Dr. Phillippa Talmud (University College London) for providing the WT plasmid of apoC-III.
Footnotes
Abbreviations:
- CD
- circular dichroism
- HFIP
- hexafluoro-2-propanol
- POPC/TO/W
- POPC/triolein/water
- SUV
- small unilamellar vesicle
- TG
- triglyceride
- TO
- triolein
- TO/W
- triolein/water
This work was supported by American Heart Association Grant 12PRE12060584 and Swedish Science Council Grant 12203.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Yao Z., and Wang Y.. 2012. Apolipoprotein C–III and hepatic triglyceride-rich lipoprotein production. Curr. Opin. Lipidol. 23: 206–212. [DOI] [PubMed] [Google Scholar]
- 2.Jong M. C., Hofker M. H., and Havekes L. M.. 1999. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler. Thromb. Vasc. Biol. 19: 472–484. [DOI] [PubMed] [Google Scholar]
- 3.Saito H., Lund-Katz S., and Phillips M. C.. 2004. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Prog. Lipid Res. 43: 350–380. [DOI] [PubMed] [Google Scholar]
- 4.Narayanaswami V., and Ryan R. O.. 2000. Molecular basis of exchangeable apolipoprotein function. Biochim. Biophys. Acta. 1483: 15–36. [DOI] [PubMed] [Google Scholar]
- 5.Fredenrich A., Giroux L. M., Tremblay M., Krimbou L., Davignon J., and Cohn J. S.. 1997. Plasma lipoprotein distribution of apoC-III in normolipidemic and hypertriglyceridemic subjects: comparison of the apoC-III to apoE ratio in different lipoprotein fractions. J. Lipid Res. 38: 1421–1432. [PubMed] [Google Scholar]
- 6.Cohn J. S., Patterson B. W., Uffelman K. D., Davignon J., and Steiner G.. 2004. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C–III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J. Clin. Endocrinol. Metab. 89: 3949–3955. [DOI] [PubMed] [Google Scholar]
- 7.Huff M. W., Breckenridge W. C., Strong W. L., and Wolfe B. M.. 1988. Metabolism of apolipoproteins C–II, C–III, and B in hypertriglyceridemic men. Changes after heparin-induced lipolysis. Arteriosclerosis. 8: 471–479. [DOI] [PubMed] [Google Scholar]
- 8.Ooi E. M. M., Barrett P. H. R., Chan D. C., and Watts G. F.. 2008. Apolipoprotein C–III: understanding an emerging cardiovascular risk factor. Clin. Sci. (Lond) 114: 611–624. [DOI] [PubMed] [Google Scholar]
- 9.Ito Y., Azrolan N., O’Connell A., Walsh A., and Breslow J. L.. 1990. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 249: 790–793. [DOI] [PubMed] [Google Scholar]
- 10.Maeda N., Li H., Lee D., Oliver P., Quarfordt S. H., and Osada J.. 1994. Targeted disruption of the apolipoprotein C–III gene in mice results in hypotriglyceridemia and protection from postprandial hypertriglyceridemia. J. Biol. Chem. 269: 23610–23616. [PubMed] [Google Scholar]
- 11.Onat A., Hergenç G., Sansoy V., Fobker M., Ceyhan K., Toprak S., and Assmann G.. 2003. Apolipoprotein C–III, a strong discriminant of coronary risk in men and a determinant of the metabolic syndrome in both genders. Atherosclerosis. 168: 81–89. [DOI] [PubMed] [Google Scholar]
- 12.Blankenhorn D. H., Alaupovic P., Wickham E., Chin H. P., and Azen S. P.. 1990. Prediction of angiographic change in native human coronary arteries and aortocoronary bypass grafts. Lipid and nonlipid factors. Circulation. 81: 470–476. [DOI] [PubMed] [Google Scholar]
- 13.Hodis H. N., Mack W. J., Azen S. P., Alaupovic P., Pogoda J. M., LaBree L., Hemphill L. C., Kramsch D. M., and Blankenhorn D. H.. 1994. Triglyceride- and cholesterol-rich lipoproteins have a differential effect on mild/moderate and severe lesion progression as assessed by quantitative coronary angiography in a controlled trial of lovastatin. Circulation. 90: 42–49. [DOI] [PubMed] [Google Scholar]
- 14.Sacks F. M., Alaupovic P., Moye L. A., Cole T. G., Sussex B., Stampfer M. J., Pfeffer M. A., and Braunwald E.. 2000. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 102: 1886–1892. [DOI] [PubMed] [Google Scholar]
- 15.Petersen K. F., Dufour S., Hariri A., Nelson-Williams C., Foo J. N., Zhang X-M., Dziura J., Lifton R. P., and Shulman G. I.. 2010. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N. Engl. J. Med. 362: 1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pullinger C. R., Malloy M. J., Shahidi A. K., Ghassemzadeh M., Duchateau P., Villagomez J., Allaart J., and Kane J. P.. 1997. A novel apolipoprotein C–III variant, apoC-III(Gln38→Lys), associated with moderate hypertriglyceridemia in a large kindred of Mexican origin. J. Lipid Res. 38: 1833–1840. [PubMed] [Google Scholar]
- 17.Bochem A. E., van Capelleveen J. C., Dallinga-Thie G. M., Schimmel A. W. M., Motazacker M. M., Tietjen I., Singaraja R. R., Hayden M. R., Kastelein J. J. P., Stroes E. S. G., et al. 2014. Two novel mutations in apolipoprotein C3 underlie atheroprotective lipid profiles in families. Clin. Genet. 85: 433–440. [DOI] [PubMed] [Google Scholar]
- 18.Jørgensen A. B., Frikke-Schmidt R., Nordestgaard B. G., and Tybjærg-Hansen A.. 2014. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 371: 32–41. [DOI] [PubMed] [Google Scholar]
- 19.Pollin T. I., Damcott C. M., Shen H., Ott S. H., Shelton J., Horenstein R. B., Post W., McLenithan J. C., Bielak L. F., Peyser P. A., et al. 2008. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 322: 1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H., Labeur C., Xu C. F., Ferrell R., Lins L., Brasseur R., Rosseneu M., Weiss K. M., Humphries S. E., and Talmud P. J.. 2000. Characterization of the lipid-binding properties and lipoprotein lipase inhibition of a novel apolipoprotein C–III variant Ala23Thr. J. Lipid Res. 41: 1760–1771. [PubMed] [Google Scholar]
- 21.von Eckardstein A., Holz H., Sandkamp M., Weng W., Funke H., and Assmann G.. 1991. Apolipoprotein C–III(Lys58—-Glu). Identification of an apolipoprotein C–III variant in a family with hyperalphalipoproteinemia. J. Clin. Invest. 87: 1724–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaudet D., Alexander V. J., Baker B. F., Brisson D., Tremblay K., Singleton W., Geary R. S., Hughes S. G., Viney N. J., Graham M. J., et al. 2015. Antisense inhibition of apolipoprotein C–III in patients with hypertriglyceridemia. N. Engl. J. Med. 373: 438–447. [DOI] [PubMed] [Google Scholar]
- 23.Zheng C. 2014. Updates on apolipoprotein CIII: fulfilling promise as a therapeutic target for hypertriglyceridemia and cardiovascular disease. Curr. Opin. Lipidol. 25: 35–39. [DOI] [PubMed] [Google Scholar]
- 24.Sundaram M., Zhong S., Bou Khalil M., Links P. H., Zhao Y., Iqbal J., Hussain M. M., Parks R. J., Wang Y., and Yao Z.. 2010. Expression of apolipoprotein C–III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J. Lipid Res. 51: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundaram M., Zhong S., Bou Khalil M., Zhou H., Jiang Z. G., Zhao Y., Iqbal J., Hussain M. M., Figeys D., Wang Y., et al. 2010. Functional analysis of the missense APOC3 mutation Ala23Thr associated with human hypotriglyceridemia. J. Lipid Res. 51: 1524–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin W., Sundaram M., Wang Y., Zhou H., Zhong S., Chang C-C., Manhas S., Yao E. F., Parks R. J., McFie P. J., et al. 2011. Missense mutation in APOC3 within the C-terminal lipid binding domain of human ApoC-III results in impaired assembly and secretion of triacylglycerol-rich very low density lipoproteins: evidence that ApoC-III plays a major role in the formation of lipid p. J. Biol. Chem. 286: 27769–27780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caron S., Verrijken A., Mertens I., Samanez C. H., Mautino G., Haas J. T., Duran-Sandoval D., Prawitt J., Francque S., Vallez E., et al. 2011. Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 31: 513–519. [DOI] [PubMed] [Google Scholar]
- 28.Ginsberg H. N., and Brown W. V.. 2011. Apolipoprotein CIII: 42 years old and even more interesting. Arterioscler. Thromb. Vasc. Biol. 31: 471–473. [DOI] [PubMed] [Google Scholar]
- 29.Ginsberg H. N., Le N. A., Goldberg I. J., Gibson J. C., Rubinstein A., Wang-Iverson P., Norum R., and Brown W. V.. 1986. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J. Clin. Invest. 78: 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C. S., McConathy W., Kloer H. U., and Alaupovic P.. 1985. Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C–III. J. Clin. Invest. 75: 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaudet D., Brisson D., Tremblay K., Alexander V. J., Singleton W., Hughes S. G., Geary R. S., Baker B. F., Graham M. J., Crooke R. M., et al. 2014. Targeting APOC3 in the familial chylomicronemia syndrome. N. Engl. J. Med. 371: 2200–2206. [DOI] [PubMed] [Google Scholar]
- 32.Sparrow J. T., Pownall H. J., Hsu F. J., Blumenthal L. D., Culwell A. R., and Gotto A. M.. 1977. Lipid binding by fragments of apolipoprotein C–III-1 obtained by thrombin cleavage. Biochemistry. 16: 5427–5431. [DOI] [PubMed] [Google Scholar]
- 33.Larsson M., Vorrsjo E., Talmud P., Lookene A., and Olivecrona G.. 2013. Apolipoproteins C–I and C–III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J. Biol. Chem. 288: 33997–34008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H., Talmud P. J., Lins L., Brasseur R., Olivecrona G., Peelman F., Vandekerckhove J., Rosseneu M., and Labeur C.. 2000. Characterization of recombinant wild type and site-directed mutations of apolipoprotein C–III: lipid binding, displacement of ApoE, and inhibition of lipoprotein lipase. Biochemistry. 39: 9201–9212. [DOI] [PubMed] [Google Scholar]
- 35.Sparrow J. T., Gotto A. M., and Morrisett J. D.. 1973. Chemical synthesis and biochemical properties of peptide fragments of apolipoprotein-alanine. Proc. Natl. Acad. Sci. USA. 70: 2124–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gangabadage C. S., Zdunek J., Tessari M., Nilsson S., Olivecrona G., and Wijmenga S. S.. 2008. Structure and dynamics of human apolipoprotein CIII. J. Biol. Chem. 283: 17416–17427. [DOI] [PubMed] [Google Scholar]
- 37.Segrest J. P., Jones M. K., De Loof H., Brouillette C. G., Venkatachalapathi Y. V., and Anantharamaiah G. M.. 1992. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J. Lipid Res. 33: 141–166. [PubMed] [Google Scholar]
- 38.Segrest J. P., Garber D. W., Brouillette C. G., Harvey S. C., and Anantharamaiah G. M.. 1994. The amphipathic alpha helix: a multifunctional structural motif in plasma apolipoproteins. Adv. Protein Chem. 45: 303–369. [DOI] [PubMed] [Google Scholar]
- 39.Lambert D. A., Catapano A. L., Smith L. C., Sparrow J. T., and Gotto A. M.. 1996. Effect of the apolipoprotein C–II/C-III1 ratio on the capacity of purified milk lipoprotein lipase to hydrolyse triglycerides in monolayer vesicles. Atherosclerosis. 127: 205–212. [DOI] [PubMed] [Google Scholar]
- 40.Labourdenne S., Cagna A., Delorme B., Esposito G., Verger R., and Rivière C.. 1997. Oil-drop tensiometer: applications for studying the kinetics of lipase action. Methods Enzymol. 286: 306–326. [DOI] [PubMed] [Google Scholar]
- 41.Small D. M., Wang L., and Mitsche M. A.. 2009. The adsorption of biological peptides and proteins at the oil/water interface. A potentially important but largely unexplored field. J. Lipid Res. 50(Suppl): S329–S334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller K. W., and Small D. M.. 1983. Surface-to-core and interparticle equilibrium distributions of triglyceride-rich lipoprotein lipids. J. Biol. Chem. 258: 13772–13784. [PubMed] [Google Scholar]
- 43.Labourdenne S., Gaudry-Rolland N., Letellier S., Lin M., Cagna A., Esposito G., Verger R., and Rivière C.. 1994. The oil-drop tensiometer: potential applications for studying the kinetics of (phospho)lipase action. Chem. Phys. Lipids. 71: 163–173. [Google Scholar]
- 44.Mitsche M. A., and Small D. M.. 2013. Surface pressure-dependent conformation change of apolipoprotein-derived amphipathic α-helices. J. Lipid Res. 54: 1578–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyers N. L., Larsson M., Olivecrona G., and Small D. M.. 2015. A pressure-dependent model for the regulation of lipoprotein lipase by apolipoprotein C–II. J. Biol. Chem. 290: 18029–18044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyers N. L., Wang L., Gursky O., and Small D. M.. 2013. Changes in helical content or net charge of apolipoprotein C–I alter its affinity for lipid/water interfaces. J. Lipid Res. 54: 1927–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrisett J. D., David J. S., Pownall H. J., and Gotto A. M.. 1973. Interaction of an apolipoprotein (apoLP-alanine) with phosphatidylcholine. Biochemistry. 12: 1290–1299. [DOI] [PubMed] [Google Scholar]
- 48.Mitsche M. A., Wang L., and Small D. M.. 2010. Adsorption of egg phosphatidylcholine to an air/water and triolein/water bubble interface: use of the 2-dimensional phase rule to estimate the surface composition of a phospholipid/triolein/water surface as a function of surface pressure. J. Phys. Chem. B. 114: 3276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitsche M. A., and Small D. M.. 2011. C-terminus of apolipoprotein A-I removes phospholipids from a triolein/phospholipids/water interface, but the N-terminus does not: a possible mechanism for nascent HDL assembly. Biophys. J. 101: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Small D. M. 1986. The physical chemistry of lipids: from alkanes to phospholipids. Springer, New York: 672. [Google Scholar]
- 51.Cohen D. E., and Fisher E. A.. 2013. Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. Semin. Liver Dis. 33: 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinberg R. B., Anderson R. A., Cook V. R., Emmanuel F., Denèfle P., Tall A. R., and Steinmetz A.. 2002. Interfacial exclusion pressure determines the ability of apolipoprotein A-IV truncation mutants to activate cholesterol ester transfer protein. J. Biol. Chem. 277: 21549–21553. [DOI] [PubMed] [Google Scholar]
- 53.Wang L., Mei X., Atkinson D., and Small D. M.. 2014. Surface behavior of apolipoprotein A-I and its deletion mutants at model lipoprotein interfaces. J. Lipid Res. 55: 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L., Hua N., Atkinson D., and Small D. M.. 2007. The N-terminal (1–44) and C-terminal (198–243) peptides of apolipoprotein A-I behave differently at the triolein/water interface. Biochemistry. 46: 12140–12151. [DOI] [PubMed] [Google Scholar]
- 55.Wang C. S., Bass H. B., Downs D., and Whitmer R. K.. 1981. Modified heparin-sepharose procedure for determination of plasma lipolytic activities of normolipidemic and hyperlipidemic subjects after injection of heparin. Clin. Chem. 27: 663–668. [PubMed] [Google Scholar]
- 56.LaRosa J. C., Levy R. I., Herbert P., Lux S. E., and Fredrickson D. S.. 1970. A specific apoprotein activator for lipoprotein lipase. Biochem. Biophys. Res. Commun. 41: 57–62. [DOI] [PubMed] [Google Scholar]
- 57.Eisenberg D., Weiss R. M., and Terwilliger T. C.. 1982. The helical hydrophobic moment: a measure of the amphiphilicity of a helix. Nature. 299: 371–374. [DOI] [PubMed] [Google Scholar]
- 58.Engelman D. M., Steitz T. A., and Goldman A.. 1986. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu. Rev. Biophys. Biophys. Chem. 15: 321–353. [DOI] [PubMed] [Google Scholar]
- 59.Ito Y., Breslow J. L., and Chait B. T.. 1989. Apolipoprotein C–III0 lacks carbohydrate residues: use of mass spectrometry to study apolipoprotein structure. J. Lipid Res. 30: 1781–1787. [PubMed] [Google Scholar]
- 60.Vaith P., Assmann G., and Uhlenbruck G.. 1978. Characterization of the oligosaccharide side chain of apolipoprotein C–III from human plasma very low density lipoproteins. Biochim. Biophys. Acta. 541: 234–240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.