Abstract
Survivors of acute lymphoblastic leukemia (ALL), the most common cancer in children, are at increased risk of developing late cardiometabolic conditions. However, the mechanisms are not fully understood. This study aimed to characterize the plasma lipid profile, Apo distribution, and lipoprotein composition of 80 childhood ALL survivors compared with 22 healthy controls. Our results show that, despite their young age, 50% of the ALL survivors displayed dyslipidemia, characterized by increased plasma triglyceride (TG) and LDL-cholesterol, as well as decreased HDL-cholesterol. ALL survivors exhibited lower plasma Apo A-I and higher Apo B-100 and C-II levels, along with elevated Apo C-II/C-III and B-100/A-I ratios. VLDL fractions of dyslipidemic ALL survivors contained more TG, free cholesterol, and phospholipid moieties, but less protein. Differences in Apo content were found between ALL survivors and controls for all lipoprotein fractions except HDL3. HDL2, especially, showed reduced Apo A-I and raised Apo A-II, leading to a depressed Apo A-I/A-II ratio. Analysis of VLDL-Apo Cs disclosed a trend for higher Apo C-III1 content in dyslipidemic ALL survivors. In conclusion, this thorough investigation demonstrates a high prevalence of dyslipidemia in ALL survivors, while highlighting significant abnormalities in their plasma lipid profile and lipoprotein composition. Special attention must, therefore, be paid to these subjects given the atherosclerotic potency of lipid and lipoprotein disorders.
Keywords: dyslipidemia, lipid and lipoprotein metabolism, apolipoproteins, atherosclerosis, cancer, clinical studies, cardiovascular diseases, metabolic syndrome
Acute lymphoblastic leukemia (ALL) accounts for 25% of all childhood malignancies and represents the most common form of leukemia in children. Cure rates for ALL now exceed 85%, allowing a growing number of childhood survivors to live into adulthood (1). However, survivors face severe, even life-threatening, long-term sequelae decades after the end of treatments (2, 3). Of interest, ALL survivors are at increased risk of developing cardiovascular conditions, including congestive heart failure, coronary artery disease, myocardial infarction, cardiac arrest, and cerebrovascular accidents (4–6). Studies on pediatric ALL survivors have reported a high prevalence of the typical components of the metabolic syndrome (MetS), such as obesity (7), hypertension (8), glucose tolerance (9), or dyslipidemia (10), and clustering of the three surrogates of MetS was also described (11). While chemo- and radiotherapy have often been associated with the development of these disorders in childhood cancer survivors (12–15), the precise etiology and the mechanisms of these late complications are not fully understood.
In view of the presence of metabolic disorders, lipid abnormalities were examined to appraise the cardiovascular risk in populations of long-term ALL survivors. Various research groups have focused on absolute LDL-cholesterol (LDL-C) and HDL-cholesterol (HDL-C) levels after treatment with cranial radiotherapy and chemotherapy. Compared with the general population, ALL survivors were found to be at higher risk of high LDL-C [relative risk (RR) = 1.25], elevated triglyceride (TG) (RR = 1.32), and low HDL-C (RR = 1.78) levels. These increased risks were particularly notable in those who were exposed to cranial radiotherapy (11). Similarly, another smaller study found that childhood ALL (cALL) survivors treated with cranial radiotherapy and chemotherapy had higher LDL-C and lower HDL-C values than did controls (16). Other studies did not find differences between ALL survivors and noncancer subjects (17, 18), apart from HDL-C, which was decreased in women exposed to cranial radiotherapy (18). In addition, despite LDL-C concentrations in the normal range, an atherogenic LDL profile was identified in young adult ALL survivors (10). LDL-C and HDL-C measurements are important, but clearly not sufficient, to identify cardiometabolic risk, as it appears that lipoprotein composition rather than concentration predicts atherosclerosis (19, 20). For example, HDL particles are highly heterogeneous and growing evidence suggests that their atheroprotective potential depends on their component composition and unique functional properties rather than their cholesterol concentrations (21–24). This was corroborated by studies that showed that higher levels of HDL-C are not associated with reduced risk of cardiovascular events in patients with advanced CVD (25, 26). In addition, a stronger correlation was found between CVD and small sized-LDL rather than with LDL-C concentrations (27). Accordingly, cholesterol of small sized-LDL was associated with an increased risk of subclinical atherosclerosis (28), CVDs (29), and future disadvantageous outcomes (30). Apo composition of lipoproteins is also considered a predictor of cardiovascular risk, as the Apo B-100/A-I ratio constitutes one of the strongest predictors of coronary heart disease (31).
The lipid disorders in the present study are reported for the first time in a French-Canadian population of cALL survivors, who are part of a unique population from the province of Quebec (Canada). Historically, this settler group expanded rapidly in relative isolation due to linguistic, religious, and geographic barriers, while reinforcing the strong genetic founder effect (32, 33). Although available literature generally reports an abnormal plasma lipid profile of ALL survivors, Apo distribution and lipoprotein core and surface composition have not been characterized in populations of childhood cancer survivors. Given the increased risk of CVDs suspected in these subjects, the present study was designed to examine these important issues in a cohort of dyslipidemic and normolipidemic pediatric ALL survivors compared with healthy controls.
MATERIALS AND METHODS
Study population
The 80 ALL survivors included in this study were recruited between January 2012 and September 2014 as part of the PETALE program at the Sainte-Justine University Hospital Center in Montreal. The study design was described elsewhere (34). Briefly, it aimed to characterize long-term effects in cALL survivors, namely, cardiotoxicity, cardiometabolic complications, neurocognitive problems, bone morbidity, quality of life issues, and genomic determinants. The PETALE cohort is comprised almost exclusively of cALL survivors of European-descent from the province of Québec. Participants enrolled in the PETALE study were unrelated, under age 19 at diagnosis, treated using Dana Farber Cancer Institute protocols (35), without hematopoietic stem cell transplantation, and more than 5 years event-free post diagnosis. For the present study, we included 40 adults and 40 adolescents (50% male) in order to create four groups: 20 men, 20 women, 20 boys, and 20 girls. Recruitment started with the first PETALE participant and ended when a total of 20 subjects had been reached for each group. The anthropometric and clinical features of the participants are outlined in Table 1. For comparison purposes, 22 unrelated age- and gender-matched healthy controls, who suffered from minor trauma (e.g., ankle sprains, mild strain injuries, broken leg), were recruited at the Orthopedics Clinic (adolescents) and within the Research Center (adults) of the Sainte-Justine Hospital. The exclusion criteria for their recruitment included an abnormal lipid profile, a chronic metabolic disease, and/or a chronic inflammatory disease. Adults were defined as being ≥18 years of age and children <18 years old. The Institutional Review Board of Sainte-Justine Hospital approved the study and investigations were carried out in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from study participants and/or parents/guardians.
TABLE 1.
Clinical and biochemical characteristics of ALL survivors compared with age- and gender-matched controls
| Healthy Controls | ALL Survivors | |
| Total | n = 22 | n = 80 |
| Age at visit (y) | 21.23 ± 1.44 | 21.10 ± 0.83 |
| Age at cancer diagnosis (y) | N/A | 6.61 ± 0.53 |
| Survival time (y) | N/A | 12.38 ± 0.71 |
| Gender (male, %) | 50 | 50 |
| Children (%) | 45 | 50 |
| BMI (kg/m2) | 22.53 ± 0.51 | 24.45 ± 0.64 |
| Glucose (mmol/l) | 5.11 ± 0.05 | 5.08 ± 0.06 |
| Insulin (pmol/l) | 55.32 ± 4.6 | 59.12 ± 4.34 |
| HOMA-IR | 1.82 ± 0.15 | 1.95 ± 0.15 |
| Children | n = 10 | n = 40 |
| Age at visit (y) | 15.60 ± 0.31 | 15.28 ± 0.31 |
| Age at cancer diagnosis (y) | N/A | 4.36 ± 0.39 |
| Survival time (y) | N/A | 8.74 ± 0.42 |
| Gender (male, %) | 50 | 50 |
| BMI (kg/m2) | 22.32 ± 0.87 | 22.08 ± 0.60 |
| Glucose (mmol/l) | 5.14 ± 0.09 | 4.90 ± 0.07 |
| Insulin (pmol/l) | 71.51 ± 5.5 | 60.51 ± 4.47 |
| HOMA-IR | 2.34 ± 0.16 | 1.92 ± 0.15 |
| Adults | n = 12 | n = 40 |
| Age at visit (y) | 25.92 ± 1.66 | 26.92 ± 0.61 |
| Age at cancer diagnosis (y) | N/A | 8.87 ± 0.84 |
| Survival time (y) | N/A | 16.02 ± 1.08 |
| Gender (male, %) | 50 | 50 |
| BMI (kg/m2) | 22.70 ± 0.61 | 26.82 ± 1.01a |
| Glucose (mmol/l) | 5.08 ± 0.06 | 5.25 ± 0.08 |
| Insulin (pmol/l) | 41.83 ± 4.1 | 57.77 ± 7.44 |
| HOMA-IR | 1.36 ± 0.14 | 1.98 ± 0.27 |
Data are expressed as percentage or as mean ± SEM. Subjects were stratified according to age (adults: ≥18 years old; children: <18 years old). HOMA-IR was calculated using the formula: [fasting insulin (mU/ml) × fasting glucose (mmol/l)]/22.5. N/A, not applicable.
P < 0.05 versus healthy controls.
Biochemical analyses and LDL particle size
Overnight fasting blood samples were collected in tubes containing 1 g/l EDTA and were kept on ice until centrifugation. Plasma was separated within 45 min of collection and stored at −80°C until analysis. Fasting insulin, glucose, total cholesterol (TC), TG, and HDL-C concentrations were measured as described previously (36). LDL-C was calculated using the Friedewald formula (37). Plasma Apos (A-I, A-IV, B-100, C-II, C-III, and E) were determined by commercial ELISA kits. BMI was computed as weight in kilograms divided by height in square meters. The homeostasis model assessment-insulin resistance (HOMA-IR) index was calculated using the formula: [fasting insulin (mU/ml) × fasting glucose (mmol/l)]/22.5. Nondenaturing 2–16% PAGE was used to assess LDL particle size, as previously described (38, 39). LDL particle size was determined on the basis of the relative migration of plasma standards of known diameter and LDL peak particle size was computed as the estimated diameter for the major peak of each scan (38). An integrated (or mean) LDL size, corresponding to the weighed mean size of all LDL subclasses in each individual, was also assessed (40).
Lipoprotein isolation
Lipoprotein separation was carried out as previously described (41–43). Briefly, plasma was separated by low speed centrifugation (2,200 g, 20 min) at 4°C and the lipoprotein fractions were isolated by sequential ultracentrifugation with a Ti-50 rotor in a Beckman model LE-80 ultracentrifuge. After removal of chylomicrons by preliminary centrifugation (25,000 g, 30 min), VLDL (1.006 g/ml), IDL (1.019 g/ml), and LDL (1.063 g/ml) were isolated by centrifugation at 40,000 g for 18 h at 4°C. Isolation of HDL2 (1.125 g/ml) and HDL3 (1.21 g/ml) was carried out at 40,000 g for 48 h at 4°C.
Characterization of lipoprotein composition
The total protein content of each lipoprotein was determined by the Bradford method with BSA as a standard, whereas phospholipids (PLs) were measured using the Bartlett method (44). Commercial kits were used to quantify TG (Randox TRIGS, UK), TC, and free cholesterol (FC) (Wako Diagnostics) by enzymatic colorimetric methods. Esterified cholesterol (EC) was calculated as the difference between TC and FC. Lipoprotein composition was determined as the percentage of TG, EC, FC, PL, and total protein. The lipoprotein distribution of Apos was examined by electrophoresis on 4–20% gradient SDS-PAGE (41–43) and by tetramethylurea (TMU) gel electrophoresis (43). Electrophoretic analyses were performed in 22 controls and in 38 survivors selected according to their lipid profile (dyslipidemic and nondyslipidemic: n = 26 for SDS-PAGE and n = 12 for TMU). Apo densitometric distribution was assessed using the ChemiDoc MP and Imaging System (Bio-Rad) for band visualization followed by densitometric evaluation with the UN-SCAN-IT gel 6.1 software. Percent distribution of Apos was calculated as percent of total Apos.
Assessment of dyslipidemia
Dyslipidemia was determined by presenting at least one of three factors: high LDL-C, high TG, and/or low HDL-C. In adults, TG levels <1.3 mmol/l were classified as optimal, borderline (≥1.3 and <1.7 mmol/l) (45) and high (≥1.7 mmol/l) (46). Values of LDL-C <2.6 mmol/l were considered optimal, borderline between ≥2.6 and <3.4 mmol/l, and elevated when ≥3.4 mmol/l (46). Values of HDL-C <1.03 in men and <1.3 in women were considered low (46). In children, LDL-C, TG, and HDL-C values were compared with those of the National Heart, Lung, and Blood Institute guidelines according to gender and age group and were classified as optimal, borderline, or abnormal (45).
Whole-exome sequencing and analysis
APOE, LDL receptor (LDLR), and LPL gene variants were identified using whole-exome sequencing for the ALL survivor cohort. Sequencing data were obtained from the Sainte-Justine Hospital and Génome Québec Integrated Clinical Genomic Center using the SOLiD (Thermo Fisher Scientific) or Illumina HiSeq 2500 platforms and then aligned on the Hg19 reference genome. Rare and common variants with a predicted functional impact on protein were identified using the functional annotation from ANNOVAR (47). Only variants with a PolyPhen-2 score ≥0.85 (48) or a SIFT score ≤0.1 (49) were labeled as “potentially damaging” and used for further analyses. Following this analysis, two polymorphisms were identified (rs7412 in APOE and rs118204057 in LPL). For validation, these two SNPs were genotyped in the survivor and control cohorts, as described previously (50). Primers used were: rs7412, forward: GCCGATGACCTGCAGAAG, reverse: CTGCCCATCTCCTCCATC, allele specific probes: GCAGAAGCGCCTGGC, GCAGAAGTGCCTGGC; and rs118204057, forward: CGAGTCGTCTTTCTCCTGAT, reverse: CTGGCTGAAAAGTACCTCCA, allele specific probes: CCAGAGGGTCCCCTG, CCAGAGAGTCCCCTG (specific allele is shown in bold). Potential genotyping errors were assessed using Chi-square tests to evaluate the deviation from the Hardy-Weinberg equilibrium.
Statistical analyses
ALL survivors were categorized according to their lipid status (dyslipidemic/nondyslipidemic). Differences in plasma lipids, lipoproteins, Apos, and in lipoprotein composition between groups were assessed using unpaired Student’s t-tests or Mann-Whitney U tests depending on the normality of the distribution. Continuous variables are expressed as mean ± SEM unless otherwise specified. P < 0.05 was considered statistically significant. Statistical analyses were performed with GraphPad Prism version 6.0.
RESULTS
Anthropometric and clinical characteristics of participants
The clinical characteristics of ALL survivors and healthy controls are shown in Table 1. The mean age of ALL survivors was 21.2 years and 50% were male. The mean of their survival time was 12.4 years and age at diagnosis was 6.6 years. Analysis of the entire cohorts revealed no significant differences between ALL survivors and controls in terms of age, gender distribution, BMI, glucose, insulin, and HOMA-IR. Similarly, no differences between groups were noted after stratification by age (i.e., children vs. adults) with the exception of higher BMI in adult survivors compared with healthy adult controls.
Dyslipidemia in survivors of ALL
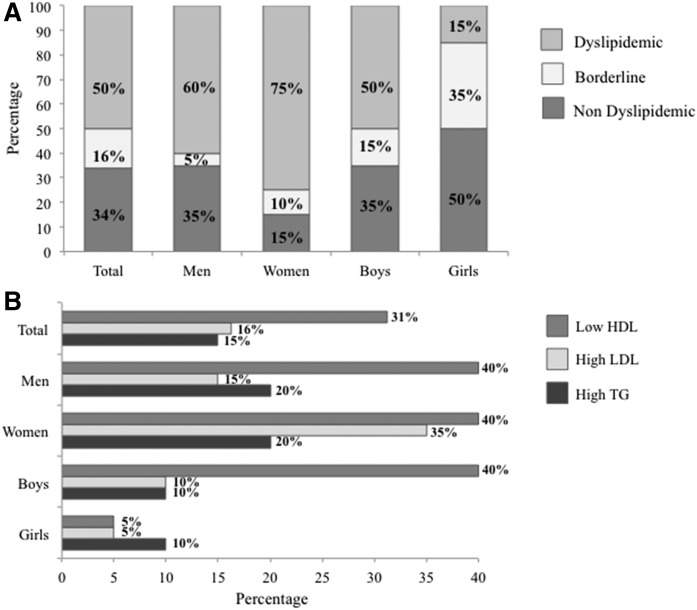
As outlined in Fig. 1A, analysis of the entire cohort of survivors revealed dyslipidemia in 50% of subjects. Stratification of data according to age and gender demonstrated a lower prevalence of dyslipidemia in girls (15%) than in boys (50%). This trend was reversed in adulthood with a predominance of dyslipidemia in women (75%) compared with men (60%). It should be pointed out that 35% of girls were characterized with borderline lipid levels, a condition that may deteriorate in adulthood. In contrast, a difference of only 10% was observed between boys and men.
Fig. 1.
Prevalence of dyslipidemia in n = 80 survivors of cALL. Data were analyzed in the entire cohort and stratified according to age (adults ≥18 years old and children <18 years old) and gender (n = 20/group). Levels of LDL-C, TGs, and HDL-C were used to classify subjects according to their lipid profile, as described in the Materials and Methods. Subjects with at least one abnormal lipid value were defined as dyslipidemic; those with at least one borderline lipid value were defined as borderline; and subjects without abnormal and/or borderline values were classified as nondyslipidemic (A). The prevalence of high LDL-C, high TGs, and low HDL-C was assessed in the entire group and in men, women, boys, and girls (B). Data are expressed as a percentage. Prevalence of dyslipidemia in the control group is not represented due to the exclusion criteria described in the Materials and Methods.
Next, we scrutinized the types of disturbances that define dyslipidemia (Fig. 1B). Among ALL survivors, 15% had elevated TG, 16% disclosed high LDL-C, and 31% displayed low HDL-C levels. While the type of dyslipidemia varied among groups, the most frequent abnormalities observed were low HDL-C and high TG. Specifically, 20% of women and men had high TG and 40% of men, women, and boys presented with low HDL-C. High levels of LDL-C were mainly found in adults (15% of men and 35% of women).
We then compared fasting lipid values between the whole group of ALL survivors and age- and gender-matched healthy controls (Table 2). Results reveal a trend of increased LDL-C along with significantly decreased HDL-C values, which resulted in significantly higher ratios of LDL-C/HDL-C and TC/HDL-C than those of controls. Similarly, when we segregated the ALL survivor group according to their normal and abnormal lipid profile, the dyslipidemic ALL subgroup disclosed higher concentrations of TG and LDL-C, lower concentrations of HDL-C, as well as more elevated ratios of LDL-C/HDL-C and TC/HDL-C than did controls. The same significant trend was observed when ALL children were analyzed separately from ALL adults. Only TG levels were found to be significantly different between nondyslipidemic survivors and controls. In addition, neither the mean LDL-C diameter nor the proportions of small dense LDL-C (diameter <255 Å) showed any significant divergences among the groups (Table 2).
TABLE 2.
Plasma lipid and lipoprotein values in child and adult survivors of ALL and controls
| Healthy Controls | ALL Survivors | |||
| Entire Group | Subgroup without Dyslipidemia | Subgroup with Dyslipidemia | ||
| Total | n = 22 | n = 80 | n = 40 | n = 40 |
| TG (mmol/l) | 0.91 ± 0.06 | 1.08 ± 0.07 | 0.75 ± 0.04a | 1.42 ± 0.11b,c |
| TC (mmol/l) | 4.23 ± 0.14 | 4.41 ± 0.08 | N/A | N/A |
| LDL-C (mmol/l) | 2.40 ± 0.12 | 2.63 ± 0.07 | 2.33 ± 0.08 | 2.92 ± 0.10b,c |
| HDL-C (mmol/l) | 1.42 ± 0.05 | 1.29 ± 0.03a | 1.40 ± 0.04 | 1.18 ± 0.05c,d |
| LDL mean diameter (Å) | 254.4 ± 0.29 | 254.7 ± 0.18 | 254.8 ± 0.26 | 254.6 ± 0.25 |
| Small dense LDL (%) | 57.61 ± 2.65 | 53.60 ± 1.45 | 53.89 ± 2.29 | 53.30 ± 1.81 |
| LDL/HDL-C | 1.73 ± 0.10 | 2.17 ± 0.08a | 1.74 ± 0.08 | 2.61 ± 0.11c,d |
| TC/HDL-C | 3.03 ± 0.11 | 3.58 ± 0.10b | 2.99 ± 0.09 | 4.18 ± 0.13c,d |
| Children | n = 10 | n = 40 | n = 27 | n = 13 |
| TG (mmol/l) | 0.85 ± 0.07 | 0.94 ± 0.08 | 0.71 ± 0.05 | 1.42 ± 0.15b,c |
| TC (mmol/l) | 3.96 ± 0.22 | 4.20 ± 0.09 | N/A | N/A |
| LDL-C (mmol/l) | 2.24 ± 0.17 | 2.49 ± 0.09 | 2.40 ± 0.10 | 2.70 ± 0.16 |
| HDL-C (mmol/l) | 1.34 ± 0.06 | 1.28 ± 0.04 | 1.394 ± 0.05 | 1.03 ± 0.05c,d |
| LDL mean diameter (Å) | 254.2 ± 0.42 | 254.8 ± 0.25 | 254.9 ± 0.29 | 254.6 ± 0.48 |
| Small dense LDL (%) | 60.73 ± 3.36 | 53.16 ± 1.87 | 52.28 ± 2.30 | 54.99 ± 3.24 |
| LDL/HDL-C | 1.69 ± 0.14 | 2.08 ± 0.12 | 1.79 ± 0.10 | 2.70 ± 0.22b,c |
| TC/HDL-C | 2.97 ± 0.15 | 3.45 ± 0.15 | 3.03 ± 0.11 | 4.33 ± 0.26c,d |
| Adults | n = 12 | n = 40 | n = 13 | n = 27 |
| TG (mmol/l) | 0.95 ± 0.08 | 1.22 ± 0.11 | 0.82 ± 0.09 | 1.42 ± 0.14e |
| TC (mmol/l) | 4.45 ± 0.15 | 4.61 ± 0.14 | N/A | N/A |
| LDL-C (mmol/l) | 2.53 ± 0.15 | 2.76 ± 0.11 | 2.20 ± 0.12 | 3.03 ± 0.12a,c |
| HDL-C (mmol/l) | 1.49 ± 0.08 | 1.30 ± 0.05 | 1.40 ± 0.06 | 1.25 ± 0.07a,f |
| LDL mean diameter (Å) | 254.6 ± 0.4 | 254.7 ± 0.25 | 254.5 ± 0.54 | 254.7 ± 0.28 |
| Small dense LDL (%) | 55.02 ± 3.93 | 54.03 ± 2.24 | 57.22 ± 5.21 | 52.49 ± 2.20 |
| LDL/HDL | 1.77 ± 0.16 | 2.27 ± 0.12a | 1.64 ± 0.14 | 2.57 ± 0.13c,d |
| TC/HDL-C | 3.06 ± 0.17 | 3.71 ± 0.14a | 2.91 ± 0.14 | 4.10 ± 0.14c,d |
Data are expressed as mean ± SEM. Dyslipidemia was defined when subjects presented at least one of three factors: high LDL-C, high TG, and/or low HDL-C. In adults, LDL-C <3.4 mmol/l was considered normal and ≥3.4 mmol/l high. TGs <1.7 and ≥1.7 mmol/l were classified as normal and high, respectively. HDL-C <1.03 mmol/l in men and <1.3 mmol/l in women were considered low. In children, cut-off values were defined according to the guidelines of the National Heart, Lung, and Blood Institute with consideration of gender and age (45). N/A, not applicable.
P < 0.05 versus healthy controls.
P < 0.01 versus healthy controls.
P < 0.001 versus ALL survivors without dyslipidemia.
P < 0.001 versus healthy controls.
P < 0.01 versus ALL survivors without dyslipidemia.
P < 0.05 versus ALL survivors without dyslipidemia.
Plasma Apo profile
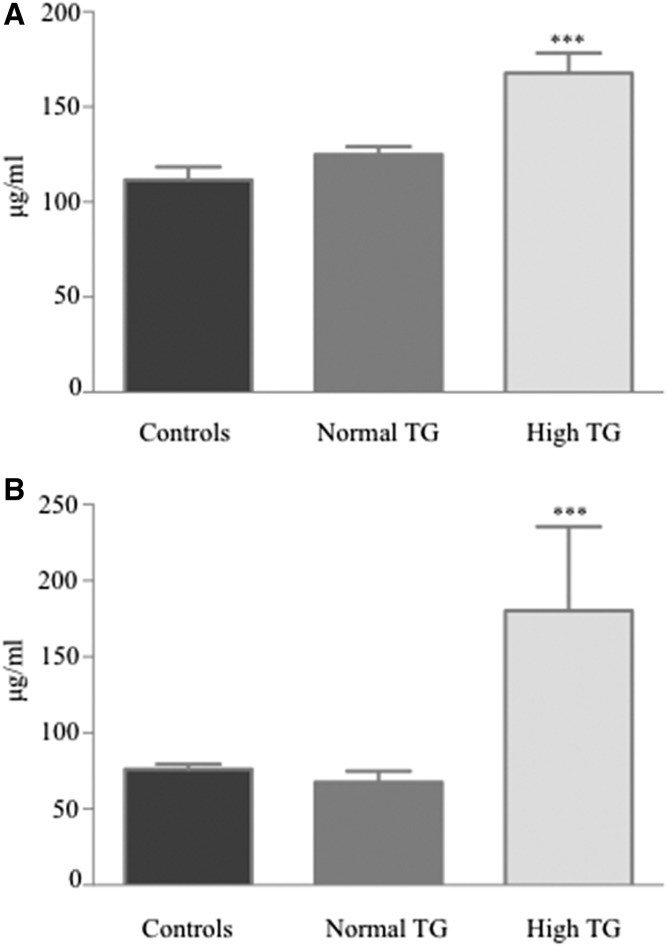
Several differences were observed between the plasma Apo profile of ALL survivors and that of healthy controls (Table 3). ALL survivors had significantly lower Apo A-I concentrations along with higher levels of Apo B-100 and Apo C-II. No significant divergences were seen in Apo A-IV, C-III, and E concentrations. Calculation of important ratios revealed higher Apo C-II/C-III and Apo B-100/A-I in the ALL survivor group. Stratification of the ALL survivor cohort according to age showed that both adults and children contributed to the observed differences (Table 3). Interestingly, Apos C-II and C-III were significantly higher in ALL survivors with hypertriglyceridemia (Fig. 2). Compared with healthy controls, differences in Apos A-I, B-100, and the Apo B100/A-I ratio were observed for ALL survivors with and without dyslipidemia (Table 3). However, compared with nondyslipidemic ALL survivors, those presenting abnormal lipid profiles had lower Apo A-I concentrations, as well as Apo C-II/C-III and Apo B-100/TG ratios, but a higher plasma content in Apos C-II, C-III, and E (Table 3). Similar trends were observed in the child and adult subgroups.
TABLE 3.
Plasma Apo profile of ALL survivors and controls
| Healthy Controls | ALL Survivors | |||
| Entire Group | Subgroup without Dyslipidemia | Subgroup with Dyslipidemia | ||
| Total | ||||
| Apos | ||||
| A-I (g/l) | 2.64 ± 0.09 (22) | 2.28 ± 0.06 (79)a | 2.35 ± 0.07 (40)b | 2.22 ± 0.09 (39)a |
| A-IV (μg/ml) | 1.22 ± 0.12 (22) | 1.28 ± 0.06 (53) | 1.22 ± 0.09 (27) | 1.34 ± 0.08 (26) |
| B-100 (g/l) | 0.69 ± 0.02 (22) | 0.85 ± 0.02 (78)c | 0.86 ± 0.03 (39)c | 0.83 ± .03 (39)a |
| C-II (μg/ml) | 111.6 ± 6.82 (21) | 131.9 ± 4.26 (80)b | 123.3 ± 5.27 (40) | 140.2 ± 6.51 (40)b,d |
| C-III (μg/ml) | 76.05 ± 3.47 (22) | 86.22 ± 11.62 (79) | 73.15 ± 11.60 (39)b | 98.95 ± 19.93 (40) |
| E (μg/ml) | 34.60 ± 0.91 (22) | 31.70 ± 0.91 (80) | 29.82 ± 0.98 (40)a | 33.59 ± 1.48 (40)d |
| Ratios | ||||
| C-II/C-III | 1.50 ± 0.83 (21) | 2.53 ± 0.24 (79)a | 2.81 ± 0.42 (39)a | 2.25 ± 0.22 (40)b |
| B-100/TG | 0.82 ± 0.06 (22) | 0.99 ± 0.06 (78) | 1.30 ± 0.10 (39)c | 0.69 ± 0.05 (39)b,e |
| B-100/A-I | 0.27 ± 0.01 (22) | 0.38 ± 0.01 (78)c | 0.37 ± 0.01 (39)c | 0.39 ± 0.02 (39)c |
| Children | ||||
| Apos | ||||
| A-I (g/l) | 2.41 ± 0.12 (10) | 2.19 ± 0.07 (40) | 2.26 ± 0.07 (27) | 2.03 ± 0.14 (13)b,d |
| A-IV (μg/ml) | 1.34 ± 0.24 (10) | 1.22 ± 0.09 (27) | 1.23 ± 0.12 (21) | 1.15 ± 0.10 (6) |
| B-100 (g/l) | 0.73 ± 0.03 (10) | 0.85 ± 0.03 (40)b | 0.84 ± 0.03 (27) | 0.87 ± 0.05 (13)b |
| C-II (μg/ml) | 109.3 ± 6.75 (9) | 131.2 ± 5.55 (40)b | 124.1 ± 6.84 (27) | 146.1 ± 8.37 (13)a |
| C-III (μg/ml) | 81.60 ± 3.67 (10) | 78.03 ± 11.33 (39) | 77.62 ± 16.43 (26)b | 78.85 ± 9.73 (13) |
| E (μg/ml) | 34.19 ± 1.14 (10) | 29.79 ± 1.20 (40) | 28.56 ± 1.22 (27)b | 32.35 ± 2.62 (13) |
| Ratios | ||||
| C-II/C-III | 1.36 ± 0.06 (9) | 2.55 ± 0.36 (39)a | 2.61 ± 0.49 (26)a | 2.44 ± 0.47 (13)b |
| B-100/TG | 0.91 ± 0.09 (10) | 1.12 ± 0.10 (40) | 1.34 ± 0.12 (27)a | 0.67 ± 0.07 (13)b |
| B-100/A-I | 0.31 ± 0.02 (10) | 0.40 ± 0.02 (40)b | 0.38 ± 0.02 (27)b | 0.45 ± 0.04 (13)a |
| Adults | ||||
| Apos | ||||
| A-I (g/l) | 2.84 ± 0.11 (12) | 2.39 ± 0.09 (39)b | 2.53 ± 0.13 (13) | 2.32 ± 0.12 (26)a |
| A-IV (μg/ml) | 1.13 ± 0.10 (12) | 1.34 ± 0.09 (26) | 1.21 ± 0.14 (2) | 1.27 ± 0.13 (10) |
| B-100 (g/l) | 0.65 ± 0.03 (12) | 0.85 ± 0.03 (38)a | 0.92 ± 0.04 (12)c | 0.82 ± 0.04 (26)b |
| C-II (μg/ml) | 113.3 ± 11.07 (12) | 132.6 ± 6.54 (40) | 122.5 ± 8.15 (13) | 137.4 ± 8.81 (27) |
| C-III (μg/ml) | 71.42 ± 5.12 (12) | 94.20 ± 20.21 (40) | 64.23 ± 12.01 (13) | 108.6 ± 29.17 (27) |
| E (μg/ml) | 34.94 ± 1.40 (12) | 33.62 ± 1.31 (40) | 32.44 ± 1.44 (13) | 34.18 ± 1.81 (27) |
| Ratios | ||||
| C-II/C-III | 1.61 ± 0.13 (12) | 2.50 ± 0.31 (40) | 3.22 ± 0.80 (13) | 2.16 ± 0.23 (27) |
| B-100/TG | 0.75 ± 0.07 (12) | 0.88 ± 0.08 (38) | 1.23 ± 0.14 (12)a | 0.71 ± 0.07 (26)f |
| B-100/A-I | 0.23 ± 0.02 (12) | 0.37 ± 0.02 (38)c | 0.38 ± 0.03 (12)c | 0.37 ± 0.03 (26)a |
Data are expressed as mean ± SEM (n).
P < 0.01 versus healthy controls.
P < 0.05 versus healthy controls.
P < 0.001 versus healthy controls.
P < 0.05 versus ALL survivors without dyslipidemia.
P < 0.001 versus ALL survivors without dyslipidemia.
P < 0.01 versus ALL survivors without dyslipidemia.
Fig. 2.
Plasma levels of Apos (C-II and C-III) in controls and in ALL survivors with and without hypertriglyceridemia. Concentrations of Apo C-II (A) and Apo C-III (B) were measured by commercial ELISA kits in n = 22 healthy controls, n = 67 in ALL survivors with normal TGs, and n = 13 in ALL survivors with high TGs. ***P < 0.001 versus controls.
Composition of lipid moieties in lipoprotein classes
Appraising the composition of lipoproteins in lipids and total proteins did not reveal significant differences in the relative content of VLDL and LDL between the entire cohorts of ALL survivors and controls (Table 4). However, lower PL percentages were noticed in IDL, HDL3, and HDL2 for both dyslipidemic and nondyslipidemic survivors when compared with controls. Moreover, an increased proportion of FC characterized survivor HDL2 particles. We calculated the weight ratio that estimates the size of lipoproteins by evaluating the mass ratio of core constituents (TG+EC) to surface constituents (FC+PR+PL), as lighter and larger particles are relatively enriched with TG and EC (41). Our results showed a significant increase in the weight ratio of HDL2 in ALL survivors (both subgroups) when compared with controls. Comparing dyslipidemic with normolipidemic survivors revealed that the VLDL fraction of dyslipidemic subjects contained more TG, FC, and PL, but dramatically less protein (Table 4). These alterations led to a higher weight ratio, indicating larger VLDL particles. Moreover, IDL content in FC was higher in ALL survivors with dyslipidemia. While no differences were noted in LDL fraction composition among subgroups of ALL survivors and healthy controls, a higher content of TG in HDL3 and of TG and FC in HDL2 characterized dyslipidemic survivors.
TABLE 4.
Lipoprotein composition of ALL survivors compared with age- and gender-matched controls
| Composition | ||||||
| Lipoprotein | TG | FC | EC | PL | PR | Weight Ratio [(TG+EC):(FC+PL+PR)] |
| Percentage of total content | ||||||
| VLDL (1.006 g/ml) | ||||||
| Controls | 41.69 ± 2.05 | 4.65 ± 0.34 | 6.36 ± 0.67 | 18.53 ± 0.80 | 28.77 ± 2.79 | 1.00 ± 0.08 |
| ALL survivors | 44.90 ± 1.27 | 5.14 ± 0.17 | 5.66 ± 0.25 | 16.74 ± 0.39 | 27.56 ± 1.76 | 1.13 ± 0.05 |
| No dyslipidemia | 40.27 ± 1.89 | 4.68 ± 0.28 | 5.21 ± 0.40 | 15.36 ± 0.61a | 34.48 ± 2.62 | 0.93 ± 0.07 |
| Dyslipidemia | 49.53 ± 1.36a,b | 5.60 ± 0.19a,c | 6.11 ± 0.31 | 18.13 ± 0.38b | 20.63 ± 1.78a,b | 1.33 ± 0.06a,b |
| Hypertriglyceridemia | 55.04 ± 1.44d | 5.63 ± 0.37e | 6.01 ± 0.62 | 18.73 ± 0.54 | 14.59 ± 1.55a | 1.60 ± 0.08d |
| IDL (1.019 g/ml) | ||||||
| Controls | 27.65 ± 1.83 | 6.93 ± 0.32 | 13.94 ± 0.98 | 29.13 ± 1.94 | 22.35 ± 2.02 | 0,75 ± 0.05 |
| ALL survivors | 27.68 ± 0.83 | 7.92 ± 0.27 | 14.46 ± 0.72 | 24.02 ± 0.64a | 25.93 ± 1.55 | 0.78 ± 0.03 |
| No dyslipidemia | 27.66 ± 1.21 | 7.44 ± 0.40 | 12.58 ± 0.99 | 23.27 ± 1.02a | 29.06 ± 2.22e | 0.73 ± 0.05 |
| Dyslipidemia | 27.69 ± 1.17 | 8.40 ± 0.36a | 16.34 ± 0.97b | 24.76 ± 0.78e | 22.80 ± 2.07c | 0.84 ± 0.04 |
| LDL (1.063 g/ml) | ||||||
| Controls | 6.51 ± 0.45 | 11.02 ± 0.31 | 40.32 ± 1.09 | 27.73 ± 1.06 | 14.43 ± 0.50 | 0.89 ± 0.04 |
| ALL survivors | 7.15 ± 0.30 | 10.76 ± 0.20 | 40.64 ± 0.71 | 27.33 ± 0.41 | 14.11 ± 0.53 | 0.93 ± 0.02 |
| No dyslipidemia | 7.19 ± 0.45 | 10.62 ± 0.25 | 40.59 ± 0.91 | 27.28 ± 0.51 | 14.31 ± 0.59 | 0.91 ± 0.03 |
| Dyslipidemia | 7.11 ± 0.38 | 10.90 ± 0.30 | 40.70 ± 1.10 | 27.39 ± 0.64 | 13.91 ± 0.88 | 0.94 ± 0.03 |
| HDL3 (1.210 g/ml) | ||||||
| Controls | 2.24 ± 0.22 | 2.06 ± 0.09 | 15.49 ± 0.76 | 31.89 ± 3.00 | 49.58 ± 2.53 | 0.20 ± 0.01 |
| ALL survivors | 2.17 ± 0.09 | 2.26 ± .07 | 16.35 ± 0.38 | 25.41 ± 0.48a | 53.77 ± 0.69 | 0.23 ± 0.005 |
| No dyslipidemia | 1.92 ± 0.12 | 2.32 ± 0.08 | 16.66 ± 0.58 | 25.15 ± 0.74a | 53.96 ± 1.00 | 0.23 ± 0.008 |
| Dyslipidemia | 2.41 ± 0.13b,c | 2.21 ± 0.11 | 16.05 ± 0.51 | 25.67 ± 0.60e | 53.52 ± 0.96 | 0.23 ± 0.007 |
| HDL2 (1.125 g/ml) | ||||||
| Controls | 3.14 ± 0.27 | 5.09 ± 0.23 | 20.14 ± 1.02 | 35.04 ± 1.28 | 36.60 ± 1.63 | 0.31 ± 0.02 |
| ALL survivors | 3.70 ± 0.16 | 6.29 ± 0.23e | 22.36 ± 0.61 | 32.30 ± 0.40e | 35.35 ± 0.64 | 0.36 ± 0.01e |
| No dyslipidemia | 3.24 ± 0.20 | 5.93 ± 0.24e | 22.56 ± 0.82 | 32.56 ± 0.43e | 35.71 ± 0.86 | 0.36 ± 0.02e |
| Dyslipidemia | 4.16 ± 0.24a,c | 6.66 ± 0.39e | 22.16 ± 0.91 | 32.04 ± 0.67e | 34.98 ± 0.97 | 0.37 ± 0.02e |
| Low HDL-C | 4.01 ± 0.24e | 6.91 ± 0.53e | 21.91 ± 1.24 | 31.33 ± 0.72a | 35.79 ± 1.32 | 0.36 ± 0.02 |
Data are expressed as percentage of total lipoprotein content ± SEM. VLDL, IDL LDL, HDL3, and HDL2 of n = 80 ALL survivors and n = 22 gender- and age-matched healthy controls were characterized as described in the Materials and Methods. ALL survivors were stratified in two groups according to their dyslipidemia status, as described in the Materials and Methods (n = 40/group). Two additional subgroups were stratified among dyslipidemic survivors: hypertriglyceridemic ALL survivors (n = 12) and ALL survivors with low HDL (n = 25). PR, protein.
P < 0.01 versus healthy controls.
P < 0.001 versus ALL survivors without dyslipidemia.
P < 0.01 versus ALL survivors without dyslipidemia.
P < 0.001 versus healthy controls.
P < 0.05 versus healthy controls.
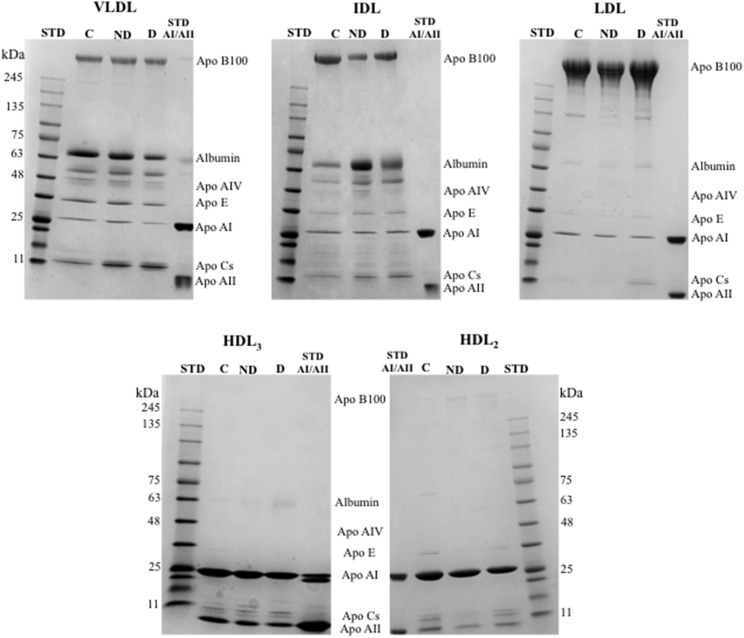
Composition of Apo moieties in lipoprotein classes
Table 5 summarizes the Apo composition of VLDL, IDL, LDL, HDL3, and HDL2 in ALL survivors and controls. Representative gels are shown in Fig. 3. Differences in Apo distributions were detected between groups for all lipoprotein fractions. In contrast, with higher Apo B-100 plasma concentrations in ALL survivors compared with controls (Table 3), the proportion of Apo B-100 among groups was similar in VLDL and reduced in LDL (Table 5, Fig. 3). However, the percentage of Apo E was increased in the VLDL and IDL of both subgroups of ALL survivors compared with controls. While all the differences were not significant, a tendency for an elevated percentage of Apo Cs was noted in VLDL and IDL for both subgroups compared with controls and in the LDL of dyslipidemic survivors. Furthermore, the HDL2 fraction showed reduced Apo A-I and augmented Apo A-II proportions, thereby resulting in a higher Apo A-I/A-II ratio in ALL survivors versus controls (Table 5). Similar to what was observed in VLDL fractions, Apo E was higher in the HDL2 of dyslipidemic ALL survivors. No significant differences in the HDL3 fraction were found between survivors and controls.
TABLE 5.
Composition of Apo moieties in lipoproteins of ALL survivors compared with age- and gender-matched controls using SDS-PAGE
| Composition in Apos | |||||||
| Lipoprotein | B-100 | A-IV | E | A-I | Cs | A-II | Ratio (A-I/A-II) |
| Percentage of total content | |||||||
| VLDL (1.006 g/ml) | |||||||
| Controls | 49.55 ± 3.12 | 16.41 ± 1.91 | 10.09 ± 1.16 | 10.82 ± 1.59 | 13.23 ± 1.61 | — | — |
| ALL survivors | 42.88 ± 1.92 | 13.88 ± 1.11 | 14.46 ± 0.83a | 11.65 ± 1.00 | 17.00 ± 1.33 | — | — |
| No dyslipidemia | 44.08 ± 2.51 | 14.77 ± 1.07 | 13.69 ± 1.25 | 11.00 ± 1.21 | 16.38 ± 1.71 | — | — |
| Dyslipidemia | 41.69 ± 2.98 | 13.00 ± 1.97 | 15.23 ± 1.09a | 12.31 ± 1.61 | 17.62 ± 2.09 | — | — |
| IDL (1.019 g/ml) | |||||||
| Controls | 79.18 ± 2.30 | 4.68 ± 0.51 | 4.75 ± 0.60 | 4.69 ± 0.82 | 6.54 ± 0.86 | — | — |
| ALL survivors | 57.81 ± 3.68b | 9.96 ± 0.68b | 8.35 ± 0.59b | 8.15 ± 0.94c | 15.81 ± 2.25a | — | — |
| No dyslipidemia | 59.08 ± 4.41b | 10.69 ± 0.92b | 8.00 ± 0.88a | 6.85 ± 1.08 | 15.31 ± 3.26 | — | — |
| Dyslipidemia | 56.54 ± 6.07b | 9.23 ± 0.99b | 8.69 ± 0.83b | 9.46 ± 1.49c | 16.31 ± 3.22a | — | — |
| LDL (1.063 g/ml) | |||||||
| Controls | 65.77 ± 1.53 | 3.27 ± 0.21 | 4.32 ± 0.31 | 23.23 ± 1.64 | 3.55 ± 0.30 | — | — |
| ALL survivors | 68.73 ± 2.26 | 3.88 ± 0.36 | 4.50 ± 0.26 | 19.08 ± 2.13 | 3.89 ± 0.49 | — | — |
| No dyslipidemia | 67.69 ± 2.97 | 3.46 ± 0.51 | 4.23 ± 0.23 | 23.69 ± 2.79 | 2.85 ± 0.52 | — | — |
| Dyslipidemia | 71.62 ± 2.94c | 4.31 ± 0.49 | 4.77 ± 0.47 | 14.46 ± 2.74a,d | 4.92 ± 0.75e | — | — |
| HDL3 (1.210 g/ml) | |||||||
| Controls | — | 1.10 ± 0.07 | 1.58 ± 0.10 | 83.29 ± 0.81 | 4.67 ± 0.35 | 9.52 ± 0.82 | 10.10 ± 0.76 |
| ALL survivors | — | 1.26 ± 0.09 | 1.78 ± 0.09 | 83.04 ± 0.63 | 5.54 ± 0.43 | 8.58 ± 0.49 | 10.58 ± 0.63 |
| No dyslipidemia | — | 1.44 ± 0.14c | 1.78 ± 0.16 | 83.15 ± 0.84 | 5.31 ± 0.63 | 8.39 ± 0.63 | 10.54 ± 0.77 |
| Dyslipidemia | — | 1.09 ± 0.09e | 1.79 ± 0.10 | 82.92 ± 0.96 | 5.77 ± 0.59 | 8.77 ± 0.78 | 10.62 ± 1.03 |
| HDL2 (1.125 g/ml) | |||||||
| Controls | — | 1.27 ± 0.14 | 3.33 ± 0.33 | 72.23 ± 0.97 | 13.09 ± 0.92 | 10.14 ± 0.85 | 7.31 ± 0.65 |
| ALL survivors | — | 0.89 ± 0.033a | 3.85 ± 0.41 | 68.50 ± 1.11c | 11.88 ± 0.74 | 14.54 ± 1.21c | 5.59 ± 0.47a |
| No dyslipidemia | — | 0.80 ± 0.04 | 2.64 ± 0.24 | 69.31 ± 1.76 | 10.46 ± 0.67 | 16.31 ± 1.88a | 4.99 ± 0.63a |
| Dyslipidemia | — | 0.97 ± 0.04d | 4.73 ± 0.59c,d | 67.69 ± 1.40c | 13.31 ± 1.22 | 12.77 ± 1.44 | 6.19 ± 0.67 |
Data are expressed as percentage of total Apo content ± SEM. Composition in Apos was assessed in VLDL, IDL, LDL, HDL3, and HDL2 of n = 26 ALL survivors and n = 22 gender- and age-matched controls. ALL survivors were stratified in two groups according to their dyslipidemia status as described in the Materials and Methods (n = 13/group). Apo distribution was analyzed using SDS-PAGE (4–20% gradient).
P < 0.01 versus controls.
P < 0.001 versus controls.
P < 0.05 versus controls.
P < 0.01 versus ALL survivors without dyslipidemia.
P < 0.05 versus ALL survivors without dyslipidemia.
Fig. 3.
VLDL, LDL, IDL, HDL3, and HDL2 composition in Apos. Content of each lipoprotein fraction was examined in n = 22 controls and n = 26 ALL survivors with or without dyslipidemia, gradient SDS-PAGE (4–20%). A molecular mass marker and Apo A-I and A-II standards were used to identify Apos. A representative blot is shown. STD, molecular mass marker standard; STD AI/AII, Apos A-I and A-II standard; C, controls; ND, nondyslipidemic ALL survivors; D, dyslipidemic ALL survivors.
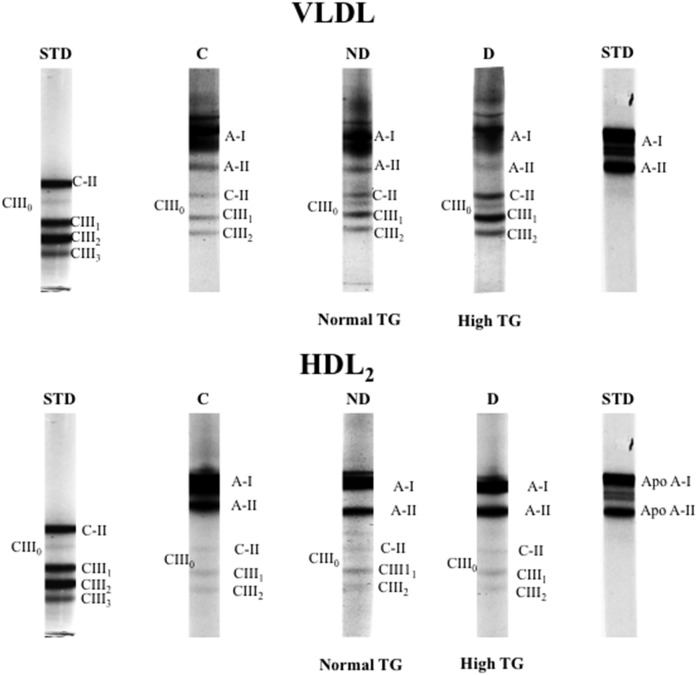
We further analyzed the distributions of Apo C isoforms in VLDL and HDL2 fractions using TMU gels. Representative profiles are illustrated in Fig. 4 and Apo distribution is presented in Table 6. The separation of VLDL-Apo Cs revealed a tendency for higher content in the Apo C-III1 isoform in dyslipidemic ALL survivors compared with healthy controls and to nondyslipidemic ALL survivors. This trend was not observed in VLDL Apo C-III2 or Apo C-II. Similar to what was observed on SDS-PAGE, no differences were noted in HDL2 Apo content using the TMU gels.
Fig. 4.
VLDL and HDL2 composition in Apos (A-I, A-II, C-II, CIII0, CIII1, and CIII2). Lipoprotein composition examined in n = 6 controls and n = 12 ALL survivors with or without dyslipidemia using 12.5% polyacrylamide gels containing TMU. Human purified Apos C-II and C-III and purified Apos A-I and A-II were used as standards to identify Apos. A representative blot is shown. STD, standard; C, controls; ND, nondyslipidemic ALL survivors; D, dyslipidemic ALL survivors.
TABLE 6.
Distribution of Apos A-I, A-II, C-II, C-III and Apo C-III isoforms (C-III1, C-III2) in VLDL and HDL2 of ALL survivors compared with age- and gender-matched controls using TMU gels
| Composition in Apos | |||||||
| Lipoprotein | A-I | A-II | C-II | C-III | C-III1 | C-III2 | Ratio (C-II/CIII) |
| Percentage of total content | |||||||
| VLDL (1.006 g/ml) | |||||||
| Controls | 57.48 ± 7.36 | 12.89 ± 2.74 | 6.92 ± 1.24 | 22.40 ± 4.31 | 12.02 ± 2.30 | 10.42 ± 2.13 | 0.32 ± 0.04 |
| ALL survivors | 67.56 ± 7.47 | 3.66 ± 0.97a | 7.80 ± 1.86 | 22.05 ± 4.94 | 13.81 ± 3.17 | 8.08 ± 1.88 | 0.35 ± 0.04 |
| No dyslipidemia | 78.95 ± 9.30 | 4.08 ± 1.78 | 5.24 ± 1.97 | 13.10 ± 5.84 | 7.54 ± 3.48 | 5.54 ± 2.53 | 0.39 ± 0.07 |
| Dyslipidemia | 56.18 ± 10.36 | 3.24 ± 0.93b | 9.93 ± 2.84 | 29.52 ± 6.50 | 19.03 ± 4.11 | 10.20 ± 2.60 | 0.31 ± 0.04 |
| HDL2 (1.125 g/ml) | |||||||
| Controls | 78.97 ± 2.65 | 17.05 ± 2.34 | 0.67 ± 0.15 | 3.30 ± 0.62 | 1.97 ± 0.41 | 1.35 ± 0.30 | 0.20 ± 0.02 |
| ALL survivors | 76.13 ± 2.32 | 18.80 ± 2.12 | 1.08 ± 0.15 | 4.03 ± 0.47 | 2.40 ± 0.42 | 1.62 ± 0.18 | 0.32 ± 0.07 |
| No dyslipidemia | 80.02 ± 1.75 | 15.20 ± 1.77 | 1.00 ± 0.13 | 3.82 ± 0.35 | 2.02 ± 0.31 | 1.80 ± 0.22 | 0.25 ± 0.02 |
| Dyslipidemia | 72.23 ± 3.82 | 22.40 ± 3.39 | 1.15 ± 0.27 | 4.23 ± 0.91 | 2.78 ± 0.79 | 1.43 ± 0.28 | 0.38 ± 0.15 |
Data are expressed as percentage of total Apo content ± SEM. VLDL, IDL, LDL, HDL3, and HDL2 relative composition in Apos was characterized using polyacrylamide gels containing TMU in n = 12 survivors of ALL and n = 6 gender- and age-matched controls. ALL survivors were stratified in two groups according to their dyslipidemia status as described in the Materials and Methods (n = 6/group).
P < 0.05 versus controls.
P < 0.01 versus controls.
APOE, LDLR, and LPL gene polymorphisms
To determine whether SNPs could account for the observed lipid alterations, we focused on the analysis of three major lipid-associated genes, APOE, LDLR, and LPL. The genetic characterization of the cohort revealed that rs7412 (APOE) minor allele frequency was not different between ALL survivors and controls (0.125 vs. 0.136, respectively; odds ratio: 0.905; 95% confidence interval: 0.338–2.425). In the ALL survivor cohort, we found 59 carriers of the APOE ε3/ε3 genotype (77.6%), 15 carriers (19.7%) of APOE ε3/ε2, and 2 carriers (2.6%) of APOE ε2/ε2 (supplemental Table S1). In the control group, 16 participants (72.7%) were APOE ε3/ε3 carriers, six (27.3%) had APOE ε3/ε2, and none had the APOE ε2/ε2 genotype. In the LPL gene, rs118204057 was identified in one survivor (1.3%) and none in healthy controls, while no SNP was identified in the LDLR gene in ALL survivors. Moreover, our analyses revealed that the metabolic and lipid profiles were not different between survivor and control carriers of the same genotype. However, in both groups, APOE ε3/ε2 carriers had reduced LDL-C compared with APOE ε3/ε3 (supplemental Table S1).
DISCUSSION
The aim of this study was to scrutinize the lipid, Apo, and lipoprotein abnormalities in pediatric ALL survivors in order to better understand their late cardiovascular risks. Our study focused on the PETALE cohort, which is unique due to its origin and its established genetic founder effect (51, 52). The homogenous ethnic background of the participants provided us with a significant advantage for association studies by reducing the number of confounding variables (51, 52). Overall, this study has identified important alterations in lipid and lipoprotein profiles. In particular, dyslipidemia was highly prevalent in ALL survivors of both genders and age groups. Of note, the proportion of women ALL survivors presenting with high TG (20%) was twice that of the one reported in the general Canadian population of women between 18 and 39 years old (53). Low HDL-C affected 40% of men in our cohort, while a general prevalence of 29% was reported for the same Canadian age group (53). Using the same Canadian Health Measure Survey, MacPherson, de Groh, and Loukine (54) reported that 19.1% of boys had low HDL-C compared with 40% in our cohort. In both studies, cut-off values to classify dyslipidemia were identical to ours.
Our study also revealed differences in plasma Apo concentrations between ALL survivors with and without dyslipidemia compared with healthy controls. Predominantly, plasma Apo B-100 levels were significantly elevated, while Apo A-I concentrations were decreased, leading to an increase in the Apo B-100/A-I ratio in ALL survivors with and without dyslipidemia. This unbalanced ratio between potentially atherogenic Apo B-100 and anti-atherogenic Apo A-I particles is a predictor of cardiovascular risk (20, 31). It is noticeable that, for both plasma Apo A-I and B-100, similar alterations were observed in dyslipidemic and nondyslipidemic survivors. To a lesser extent, alterations in plasma Apo Cs were identified in ALL subjects with dyslipidemia.
Dyslipidemia is a significant component of the MetS definition, as stated by the National Cholesterol Education Program-Adult Treatment Panel III and the International Diabetes Federation (55). The alterations in TG and HDL-C levels found in our study correspond to risk factors that define dyslipidemia in MetS. Because the presence of MetS increases the risk for type 2 diabetes and CVD in the general population (56), it is reasonable to include lipid profile analysis in the long-term follow-up of ALL survivors. In line with our results, a high prevalence of dyslipidemia was reported in a cohort from the St. Jude Lifetime Cohort Study composed of older ALL survivors (median age of 31.7 years) (11).
To our knowledge, prior to our study, no information was available on lipid and Apo composition of lipoproteins in the survivorship context. Our findings disclose variations in the content of various lipoproteins. In subjects with dyslipidemia and particularly with hypertriglyceridemia, we found VLDL with a reduced protein moiety and an increased TG and FC content that resulted in a higher weight ratio and that reflected a larger particle size. Although the precise mechanism leading to hypertriglyceridemia in our population is unclear, it may indicate increased production of large-sized VLDL rather than an enhanced biogenesis of the number of VLDL particles by the liver, as reflected by the lower ratio of Apo B-100/TG in dyslipidemic ALL survivors. Derangements in VLDL-TG degradation are also possible in view of the concomitantly high Apo C-II and C-III concentrations noted in the plasma and also observed using SDS-PAGE. In addition, the tendency of increased Apo C-III1 in VLDL observed by TMU gels might impact on VLDL-TG degradation (57). If Apo C-II at moderate concentrations represents an activator of LPL for VLDL lipolysis, its excess (as we observed in ALL survivors) may impede LPL activity and VLDL clearance, thereby leading to hypertriglyceridemia (58). Moreover, because Apo C-III is a well-known inhibitor of LPL, higher levels suggest a delay in TG-rich lipoprotein clearance from circulation (59, 60). Altogether, these alterations in VLDL composition may affect VLDL catabolism and explain hypertriglyceridemia in ALL survivors.
Polymorphisms in APOE, LPL, or LDLR could impact lipoprotein composition and lipid and Apo profile and, thus, contribute to CVD risk (61–64). The frequencies of APOE ε3/ε2 and ε2/ε2 genotypes found in our ALL survivor cohort were slightly higher than those reported in adult Caucasian populations (65). This is in line with the previously reported higher ε2 allele frequency in the French-Canadian population of Quebec than in other Caucasian populations (66). It was found that the ε2 allele potentially has a nil effect on CVD risk: while it may reduce LDL-C levels, it may also increase the accumulation of large VLDL and remnant TG-rich lipoproteins and, accordingly, we found lower LDL-C in APOE ε2/ε2 carriers (61). However APOE genotypes did not appear to impact the differences observed between the two cohorts.
Furthermore, LPL G188E (rs118204057) is one of three missense mutations that account for >97% of complete LPL deficiency in the homozygous French-Canadian population (62). It could result in a very important increase of plasma TG levels (63). However, due to the complexity of interactions between genetic and environmental factors, G188E heterozygote carriers may develop hypertriglyceridemia generally later in adulthood (62, 67). In our cohort, the detection of only one heterozygote carrier of this allele prevented us from going further. Note that in the French-Canadian population of Quebec, the carrier frequency for familial LPL deficiency disorder is 1:40 (68), which is above the general population average (one per million) (69) and is attributed to a founder effect (68). To our knowledge, none of the study participants showed symptoms of this disorder, which usually manifests in childhood and includes abdominal pain, failure to thrive, hepatosplenomegaly, lipemia retinalis, or eruptive xanthomas (70). Apparently, the increased plasma TG levels and proportions in VLDL fractions observed in our study are, therefore, not related to LPL deficiency, but this assumption should be confirmed with the determination of LPL activity in view of the association of specific variants with mild hypertriglyceridemia (71).
Our data also documented important alterations in HDL2 composition and Apo distribution and size in dyslipidemic and nondyslipidemic ALL survivors. The HDL2 subfraction was enriched in TG and poorer in PL, indicators of increased particle size. The Apo A-I/A-II ratio was significantly reduced (given the high proportion of Apo A-II), but only a rising trend was noted in the Apo C-II/C-III ratio. All these modifications may strongly compromise the protective role of HDL against CVD. Higher Apo A-II levels displayed pro-atherogenic potentials (72) and predicted the incidence of MetS and type 2 diabetes (73). In support of these assertions, some investigators stressed the opposite functions of Apos A-I and A-II: the former was more effective in enhancing reverse cholesterol transport and activating LCAT, whereas the latter exhibited its pernicious inhibitory effects (74–76).
In conclusion, this biochemical investigation highlights significant abnormalities in the plasma concentration and composition of lipids, Apos, and lipoproteins of ALL survivors. Several of these alterations were more prominent in survivors defined as dyslipidemic, although they were observed in nondyslipidemic survivors as well. Therefore, special attention must be paid to these subjects, given the atherosclerotic potency of lipid and lipoprotein disorders.
Supplementary Material
Acknowledgments
The authors thank Mrs. Schohraya Spahis and Anita Franco for their technical assistance.
Footnotes
Abbreviations:
- ALL
- acute lymphoblastic leukemia
- cALL
- childhood acute lymphoblastic leukemia
- EC
- esterified cholesterol
- FC
- free cholesterol
- HDL-C
- HDL-cholesterol
- HOMA-IR
- homeostasis model assessment-insulin resistance
- LDL-C
- LDL-cholesterol
- LDLR
- LDL receptor
- MetS
- metabolic syndrome
- PL
- phospholipid
- RR
- relative risk
- TC
- total cholesterol
- TG
- triglyceride
- TMU
- tetramethylurea
This work was supported by a grants from the Institute of Cancer Research of the Canadian Institutes of Health Research, in collaboration with C17 Council, Canadian Cancer Society, Cancer Research Society, Garron Family Cancer Centre at the Hospital for Sick Children, Ontario Institute for Cancer Research, and J.A. DeSeve Research Chair in Nutrition; the Fonds de Recherche du Québec – Santé; and the Cole Foundation.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Pui C. H., Mullighan C. G., Evans W. E., and Relling M. V.. 2012. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. 120: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Essig S., Li Q., Chen Y., Hitzler J., Leisenring W., Greenberg M., Sklar C., Hudson M. M., Armstrong G. T., Krull K. R., et al. . 2014. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 15: 841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mody R., Li S., Dover D. C., Sallan S., Leisenring W., Oeffinger K. C., Yasui Y., Robison L. L., and Neglia J. P.. 2008. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood. 111: 5515–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M. H., Colan S. D., and Diller L.. 2011. Cardiovascular disease: cause of morbidity and mortality in adult survivors of childhood cancers. Circ. Res. 108: 619–628. [DOI] [PubMed] [Google Scholar]
- 5.Gurney J. G., Ojha R. P., Ness K. K., Huang S., Sharma S., Robison L. L., Hudson M. M., and Kaste S. C.. 2012. Abdominal aortic calcification in young adult survivors of childhood acute lymphoblastic leukemia: results from the St. Jude Lifetime Cohort Study. Pediatr. Blood Cancer. 59: 1307–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertens A. C., Liu Q., Neglia J. P., Wasilewski K., Leisenring W., Armstrong G. T., Robison L. L., and Yasui Y.. 2008. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 100: 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janiszewski P. M., Oeffinger K. C., Church T. S., Dunn A. L., Eshelman D. A., Victor R. G., Brooks S., Turoff A. J., Sinclair E., Murray J. C., et al. . 2007. Abdominal obesity, liver fat, and muscle composition in survivors of childhood acute lymphoblastic leukemia. J. Clin. Endocrinol. Metab. 92: 3816–3821. [DOI] [PubMed] [Google Scholar]
- 8.Chow E. J., Pihoker C., Hunt K., Wilkinson K., and Friedman D. L.. 2007. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 110: 2313–2320. [DOI] [PubMed] [Google Scholar]
- 9.Neville K. A., Cohn R. J., Steinbeck K. S., Johnston K., and Walker J. L.. 2006. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J. Clin. Endocrinol. Metab. 91: 4401–4407. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra J., Tonorezos E. S., Rozenberg M., Vega G. L., Sklar C. A., Chou J., Moskowitz C. S., Eshelman-Kent D. A., Janiszewski P., Ross R., et al. . 2012. Atherogenic low density lipoprotein phenotype in long-term survivors of childhood acute lymphoblastic leukemia. J. Lipid Res. 53: 2747–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nottage K. A., Ness K. K., Li C., Srivastava D., Robison L. L., and Hudson M. M.. 2014. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia - from the St. Jude Lifetime Cohort. Br. J. Haematol. 165: 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armenian S. H., Gelehrter S. K., and Chow E. J.. 2012. Strategies to prevent anthracycline-related congestive heart failure in survivors of childhood cancer. Cardiol. Res. Pract. 2012: 713294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harake D., Franco V. I., Henkel J. M., Miller T. L., and Lipshultz S. E.. 2012. Cardiotoxicity in childhood cancer survivors: strategies for prevention and management. Future Cardiol. 8: 647–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipshultz S. E., Lipsitz S. R., Mone S. M., Goorin A. M., Sallan S. E., Sanders S. P., Orav E. J., Gelber R. D., and Colan S. D.. 1995. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N. Engl. J. Med. 332: 1738–1743. [DOI] [PubMed] [Google Scholar]
- 15.Silverman L. B. 2014. Balancing cure and long-term risks in acute lymphoblastic leukemia. Hematology Am. Soc. Hematol. Educ. Program. 2014: 190–197. [DOI] [PubMed] [Google Scholar]
- 16.Link K., Moell C., Garwicz S., Cavallin-Stahl E., Bjork J., Thilen U., Ahren B., and Erfurth E. M.. 2004. Growth hormone deficiency predicts cardiovascular risk in young adults treated for acute lymphoblastic leukemia in childhood. J. Clin. Endocrinol. Metab. 89: 5003–5012. [DOI] [PubMed] [Google Scholar]
- 17.Geenen M. M., Bakker P. J. M., Kremer L. C. M., Kastelein J. J. P., and van Leeuwen F. E.. 2010. Increased prevalence of risk factors for cardiovascular disease in long-term survivors of acute lymphoblastic leukemia and Wilms tumor treated with radiotherapy. Pediatr. Blood Cancer. 55: 690–697. [DOI] [PubMed] [Google Scholar]
- 18.Oeffinger K. C., Adams-Huet B., Victor R. G., Church T. S., Snell P. G., Dunn A. L., Eshelman-Kent D. A., Ross R., Janiszewski P. M., Turoff A. J., et al. . 2009. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J. Clin. Oncol. 27: 3698–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parish S., Peto R., Palmer A., Clarke R., Lewington S., Offer A., Whitlock G., Clark S., Youngman L., Sleight P., et al. . 2009. The joint effects of apolipoprotein B, apolipoprotein A1, LDL cholesterol, and HDL cholesterol on risk: 3510 cases of acute myocardial infarction and 9805 controls. Eur. Heart J. 30: 2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walldius G., and Jungner I.. 2004. Apolipoprotein B and apolipoprotein A-I: risk indicators of coronary heart disease and targets for lipid-modifying therapy. J. Intern. Med. 255: 188–205. [DOI] [PubMed] [Google Scholar]
- 21.Maranhão R. C., and Freitas F. R.. 2014. HDL metabolism and atheroprotection: predictive value of lipid transfers. Adv. Clin. Chem. 65: 1–41. [PubMed] [Google Scholar]
- 22.Schwendeman A., Sviridov D. O., Yuan W., Guo Y., Morin E. E., Yuan Y., Stonik J., Freeman L., Ossoli A., Thacker S., et al. . 2015. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J. Lipid Res. 56: 1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gursky O. 2015. Structural stability and functional remodeling of high-density lipoproteins. FEBS Lett. 589: 2627–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guha M., Gao X., Jayaraman S., and Gursky O.. 2008. Correlation of structural stability with functional remodeling of high-density lipoproteins: the importance of being disordered. Biochemistry. 47: 11393–11397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angeloni E., Paneni F., Landmesser U., Benedetto U., Melina G., Lüscher T. F., Volpe M., Sinatra R., and Cosentino F.. 2013. Lack of protective role of HDL-C in patients with coronary artery disease undergoing elective coronary artery bypass grafting. Eur. Heart J. 34: 3557–3562. [DOI] [PubMed] [Google Scholar]
- 26.Heinecke J. 2011. HDL and cardiovascular-disease risk–time for a new approach? N. Engl. J. Med. 364: 170–171. [DOI] [PubMed] [Google Scholar]
- 27.Koba S., Yokota Y., Hirano T., Ito Y., Ban Y., Tsunoda F., Sato T., Shoji M., Suzuki H., Geshi E., et al. . 2008. Small LDL-cholesterol is superior to LDL-cholesterol for determining severe coronary atherosclerosis. J. Atheroscler. Thromb. 15: 250–260. [DOI] [PubMed] [Google Scholar]
- 28.Aoki T., Yagi H., Sumino H., Tsunekawa K., Araki O., Kimura T., Nara M., Ogiwara T., Nakajima K., and Murakami M.. 2015. Relationship between carotid artery intima-media thickness and small dense low-density lipoprotein cholesterol concentrations measured by homogenous assay in Japanese subjects. Clin. Chim. Acta. 442: 110–114. [DOI] [PubMed] [Google Scholar]
- 29.Hoogeveen R. C., Gaubatz J. W., Sun W., Dodge R. C., Crosby J. R., Jiang J., Couper D., Virani S. S., Kathiresan S., Boerwinkle E., et al. . 2014. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 34: 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arsenault B. J., Lemieux I., Despres J. P., Wareham N. J., Luben R., Kastelein J. J., Khaw K. T., and Boekholdt S. M.. 2007. Cholesterol levels in small LDL particles predict the risk of coronary heart disease in the EPIC-Norfolk prospective population study. Eur. Heart J. 28: 2770–2777. [DOI] [PubMed] [Google Scholar]
- 31.Walldius G., Jungner I., Holme I., Aastveit A. H., Kolar W., and Steiner E.. 2001. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 358: 2026–2033. [DOI] [PubMed] [Google Scholar]
- 32.Bherer C., Labuda D., Roy-Gagnon M. H., Houde L., Tremblay M., and Vezina H.. 2011. Admixed ancestry and stratification of Quebec regional populations. Am. J. Phys. Anthropol. 144: 432–441. [DOI] [PubMed] [Google Scholar]
- 33.Heyer E., Tremblay M., and Desjardins B.. 1997. Seventeenth-century European origins of hereditary diseases in the Saguenay population (Quebec, Canada). Hum. Biol. 69: 209–225. [PubMed] [Google Scholar]
- 34.Marcoux S., Drouin S., Laverdiere C., Alos N., Andelfinger G. U., Bertout L., Curnier D., Friedrich M. G., Kritikou E. A., Lefebvre G., et al. . The PETALE study: late adverse effects and biomarkers in childhood acute lymphoblastic leukemia survivors. Pediatr. Blood Cancer. Epub ahead of print. December 4, 2016; doi:10.1002/pbc.26361. [DOI] [PubMed] [Google Scholar]
- 35.Vrooman L. M., Neuberg D. S., Stevenson K. E., Asselin B. L., Athale U. H., Clavell L., Cole P. D., Kelly K. M., Larsen E. C., Laverdiere C., et al. . 2011. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. Eur. J. Cancer. 47: 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert M., Paradis G., O’Loughlin J., Delvin E. E., Hanley J. A., and Levy E.. 2004. Insulin resistance syndrome in a representative sample of children and adolescents from Quebec, Canada. Int. J. Obes. Relat. Metab. Disord. 28: 833–841. [DOI] [PubMed] [Google Scholar]
- 37.Friedewald W. T., Levy R. I., and Fredickson D. S.. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 38.St-Pierre A. C., Ruel I. L., Cantin B., Dagenais G. R., Bernard P-M., Després J-P., and Lamarche B.. 2001. Comparison of various electrophoretic charateristics of LDL particles and their relationship to the risk of ischemic heart disease. Circulation. 104: 2295–2299. [DOI] [PubMed] [Google Scholar]
- 39.Stan S., Levy E., Delvin E. E., Hanley J. A., Lamarche B., O’Loughlin J., Paradis G., and Lambert M.. 2005. Distribution of LDL particle size in a population-based sample of children and adolescents and relationship with other cardiovascular risk factors. Clin. Chem. 51: 1192–1200. [DOI] [PubMed] [Google Scholar]
- 40.Tchernof A., Lamarche B., Prud’Homme D., Nadeau A., Moorjani S., Labrie F., Lupien P. J., and Després J. P.. 1996. The dense LDL phenotype. Association with plasma lipoprotein levels, visceral obesity, and hyperinsulinemia in men. Diabetes Care. 19: 629–637. [DOI] [PubMed] [Google Scholar]
- 41.Levy E., Lepage G., Bendayan M., Ronco N., Thibault L., Galéano N., Smith L., and Roy C. C.. 1989. Relationship of decreased hepatic lipase activity and lipoprotein abnormalities to essential fatty acid deficiency in cystic fibrosis patients. J. Lipid Res. 30: 1197–1209. [PubMed] [Google Scholar]
- 42.Levy E., Roy C. C., Thibault L., Bonin A., Brochu P., and Seidman E. G.. 1994. Variable expression of familial heterozygous hypobetalipoproteinemia: transient malabsorption during infancy. J. Lipid Res. 35: 2170–2177. [PubMed] [Google Scholar]
- 43.Levy E., Thibault A., Roy C. C., Bendayan M., Lepage G., and Letarte J.. 1988. Circulation lipids and lipoproteins in glycogen storage disease type I with nocturnal intragastric feeding. J. Lipid Res. 29: 215–226. [PubMed] [Google Scholar]
- 44.Bartlett G. R. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234: 466–468. [PubMed] [Google Scholar]
- 45.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents and National Heart, Lung, and Blood Institute. 2011. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 128(Suppl 5): S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genest J., McPherson R., Frohlich J., Anderson T., Campbell N., Carpentier A., Couture P., Dufour R., Fodor G., and Francis G. A.. 2009. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Can. J. Cardiol. 25: 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang K., Li M., and Hakonarson H.. 2010. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38: e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adzhubei I. A., Schmidt S., Peshkin L., Ramensky V. E., Gerasimova A., Bork P., Kondrashov A. S., and Sunyaev S. R.. 2010. A method and server for predicting damaging missense mutations. Nat. Methods. 7: 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng P. C., and Henikoff S.. 2003. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 31: 3812–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ansari M., Sauty G., Labuda M., Gagne V., Rousseau J., Moghrabi A., Laverdiere C., Sinnett D., and Krajinovic M.. 2012. Polymorphism in multidrug resistance-associated protein gene 3 is associated with outcomes in childhood acute lymphoblastic leukemia. Pharmacogenomics J. 12: 386–394. [DOI] [PubMed] [Google Scholar]
- 51.Krajinovic M., Labuda D., Richer C., Karimi S., and Sinnett D.. 1999. Susceptibility to childhood acute lymphoblastic leukemia: influence of CYP1A1, CYP2D6, GSTM1, and GSTT1 genetic polymorphisms. Blood. 93: 1496–1501. [PubMed] [Google Scholar]
- 52.Sinnett D., Krajinovic M., and Labuda D.. 2000. Genetic susceptibility to childhood acute lymphoblastic leukemia. Leuk. Lymphoma. 38: 447–462. [DOI] [PubMed] [Google Scholar]
- 53.Riediger N. D., and Clara I.. 2011. Prevalence of metabolic syndrome in the Canadian adult population. CMAJ. 183: E1127–E1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacPherson M., de Groh M., Loukine L., Prud’homme D., and Dubois L.. 2016. Prevalence of metabolic syndrome and its risk factors in Canadian children and adolescents: Canadian Health Measures Survey Cycle 1 (2007-2009) and Cycle 2 (2009-2011). Health Promot. Chronic Dis. Prev. Can. 36: 32–40. [Erratum. 2016. Health Promot. Chronic Dis. Prev. Can.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorenzo C., Williams K., Hunt K. J., and Haffner S. M.. 2007. The National Cholesterol Education Program - Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 30: 8–13. [DOI] [PubMed] [Google Scholar]
- 56.Grundy S. M., Cleeman J. I., Daniels S. R., Donato K. A., Eckel R. H., Franklin B. A., Gordon D. J., Krauss R. M., Savage P. J., Smith S. C. Jr., et al. . 2005. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 112: 2735–2752. [Erratum. 2005. Circulation. 112: e297–e298.] [DOI] [PubMed] [Google Scholar]
- 57.Mauger J. F., Couture P., Bergeron N., and Lamarche B.. 2006. Apolipoprotein C-III isoforms: kinetics and relative implication in lipid metabolism. J. Lipid Res. 47: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 58.Kei A. A., Filippatos T. D., Tsimihodimos V., and Elisaf M. S.. 2012. A review of the role of apolipoprotein C–II in lipoprotein metabolism and cardiovascular disease. Metabolism. 61: 906–921. [DOI] [PubMed] [Google Scholar]
- 59.Ito Y., Asrolan N., O’Connell A., Walsh A., and Breslow J. L.. 1990. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 249: 790–793. [DOI] [PubMed] [Google Scholar]
- 60.Bobik A. 2008. Apolipoprotein CIII and atherosclerosis: beyond effects on lipid metabolism. Circulation. 118: 702–704. [DOI] [PubMed] [Google Scholar]
- 61.Song Y., Stampfer M. J., and Liu S.. 2004. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann. Intern. Med. 141: 137–147. [DOI] [PubMed] [Google Scholar]
- 62.Minnich A., Kessling A., Roy M., Giry C., DeLangavant G., Lavigne J., Lussier-Cacan S., and Davignon J.. 1995. Prevalence of alleles encoding defective lipoprotein lipase in hypertriglyceridemic patients of French Canadian descent. J. Lipid Res. 36: 117–124. [PubMed] [Google Scholar]
- 63.Merkel M. 2002. Lipoprotein lipase: genetics, lipid uptake, and regulation. J. Lipid Res. 43: 1997–2006. [DOI] [PubMed] [Google Scholar]
- 64.Franceschini N., Muallem H., Rose K. M., Boerwinkle E., and Maeda N.. 2009. Low density lipoprotein receptor polymorphisms and the risk of coronary heart disease: the Atherosclerosis Risk in Communities study. J. Thromb. Haemost. 7: 496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eichner J. E., Dunn S. T., Perveen G., Thompson D. M., Stewart K. E., and Stroehla C.. 2002. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am. J. Epidemiol. 155: 487–495. [DOI] [PubMed] [Google Scholar]
- 66.Robitaille N., Cormier G., Couture R., Bouthillier D., Davignon J., and Perusse L.. 1996. Apolipoprotein E polymorphism in a French Canadian population of northeastern Quebec: allele frequencies and effects on blood lipid and lipoprotein levels. Hum. Biol. 68: 357–370. [PubMed] [Google Scholar]
- 67.Wilson D. E., Emi M., Iverius P. H., Hat A., Wu L. L., Hillas E., Williams R. R., and Lalouel J. M.. 1990. Phenotypic expression of heterozygous lipoprotein lipase deficiency in the extended pedigree of a proband homozygous for a missense mutation. J. Clin. Invest. 86: 735–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gagné C., Brun L. D., Julien P., Moorjani S., and Lupien P. J.. 1989. Primary lipoprotein-lipase-activity deficiency: clinical investigation of a French Canadian population. CMAJ. 140: 405–411. [PMC free article] [PubMed] [Google Scholar]
- 69.Rahalkar A. R., Giffen F., Har B., Ho J., Morrison K. M., Hill J., Wang J., Hegele R. A., and Joy T.. 2009. Novel LPL mutations associated with lipoprotein lipase deficiency: two case reports and a literature review. Can. J. Physiol. Pharmacol. 87: 151–160. [DOI] [PubMed] [Google Scholar]
- 70.Kolářová H., Tesařová M., Švecová Š., Stránecký V., Přistoupilová A., Zima T., Uhrová J., Volgina S. Y., Zeman J., and Honzík T.. 2014. Lipoprotein lipase deficiency: clinical, biochemical and molecular characteristics in three patients with novel mutations in the LPL gene. Folia Biol. (Praha). 60: 235–243. [PubMed] [Google Scholar]
- 71.Hegele R. A. 2016. Multidimensional regulation of lipoprotein lipase: impact on biochemical and cardiovascular phenotypes. J. Lipid Res. 57: 1601–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alaupovic P., Mack W. J., Knight-Gibson C., and Hodis H. N.. 1997. The role of triglyceride-rich lipoprotein families in the progression of atherosclerotic lesions as determined by sequential coronary angiography from a controlled clinical trial. Arterioscler. Thromb. Vasc. Biol. 17: 715–722. [DOI] [PubMed] [Google Scholar]
- 73.Onat A., Hergenc G., Ayhan E., Ugur M., and Can G.. 2009. Impaired anti-inflammatory function of apolipoprotein A-II concentrations predicts metabolic syndrome and diabetes at 4 years follow-up in elderly Turks. Clin. Chem. Lab. Med. 47: 1389–1394. [DOI] [PubMed] [Google Scholar]
- 74.Barbaras R., Puchois P., Fruchart J. C., and Ailhaud G.. 1987. Cholesterol efflux from cultured adipose cells is mediated by LpAI particles but not by LpAI:AII particles. Biochem. Biophys. Res. Commun. 142: 63–69. [DOI] [PubMed] [Google Scholar]
- 75.Durbin D. M., and Jonas A.. 1999. Lipid-free apolipoproteins A-I and A-II promote remodeling of reconstituted high density lipoproteins and alter their reactivity with lecithin:cholesterol acyltransferase. J. Lipid Res. 40: 2293–2302. [PubMed] [Google Scholar]
- 76.Lagrost L., Dengremont C., Athias A., de Geitere C., Fruchart J. C., Lallemant C., Gambert P., and Castro G.. 1995. Modulation of cholesterol efflux from Fu5AH hepatoma cells by the apolipoprotein content of high density lipoprotein particles. Particles containing various proportions of apolipoproteins A-I and A-II. J. Biol. Chem. 270: 13004–13009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.