Abstract
The aim of this study was to evaluate the vasoprotective effects of HDL isolated from carriers of LCAT deficiency, which are characterized by a selective depletion of LpA-I:A-II particles and predominance of preβ migrating HDL. HDLs were isolated from LCAT-deficient carriers and tested in vitro for their capacity to promote NO production and to inhibit vascular cell adhesion molecule-1 (VCAM-1) expression in cultured endothelial cells. HDLs from carriers were more effective than control HDLs in promoting eNOS activation with a gene-dose-dependent effect (PTrend = 0.048). As a consequence, NO production induced by HDL from carriers was significantly higher than that promoted by control HDL (1.63 ± 0.24-fold vs. 1.34 ± 0.07-fold, P = 0.031). HDLs from carriers were also more effective than control HDLs in inhibiting the expression of VCAM-1 (homozygotes, 65.0 ± 8.6%; heterozygotes, 53.1 ± 7.2%; controls, 44.4 ± 4.1%; PTrend = 0.0003). The increased efficiency of carrier HDL was likely due to the depletion in LpA-I:A-II particles. The in vitro findings might explain why carriers of LCAT deficiency showed flow-mediated vasodilation and plasma-soluble cell adhesion molecule concentrations comparable to controls, despite low HDL-cholesterol levels. These results indicate that selective depletion of apoA-II-containing HDL, as observed in carriers of LCAT deficiency, leads to an increased capacity of HDL to stimulate endothelial NO production, suggesting that changes in HDL apolipoprotein composition may be the target of therapeutic interventions designed to improve HDL functionality.
Keywords: endothelium, nitric oxide, apolipoprotein A-II, high density lipoprotein, lecithin:cholesterol acyltransferase
LCAT deficiency is a rare disorder of lipoprotein metabolism due to loss-of-function mutations in the human LCAT gene. LCAT deficiency is inherited in an autosomal codominant fashion with respect to the obligate biochemical phenotypes characterized by reduced plasma cholesterol esterification, LCAT activity, HDL-cholesterol (HDL-C) and apoA-I levels, and enhanced unesterified/total cholesterol ratio (1, 2). LCAT deficiency leads to the development of two biochemically and clinically distinct syndromes, classical familial LCAT deficiency (FLD) and fish-eye disease (FED). In FLD cases, the lack of LCAT activity is complete, and the enzyme loses its ability to esterify cholesterol in both HDL and LDL particles with severe clinical manifestations that include corneal opacity, anemia, and renal disease (3). In FED cases, the enzyme loses its capacity to esterify cholesterol in HDL, but retains the activity in LDL, thus leading to less severe clinical manifestations, normally limited to corneal opacity (3). The severe deficiency of atheroprotective HDL in carriers of LCAT deficiency should increase their risk of developing coronary heart disease; however, imaging studies evaluating carotid intima-media thickness, a validated surrogate marker for atherosclerotic coronary heart disease (4), suggest that LCAT deficiency does not remarkably increase preclinical atherosclerosis (5, 6), or could even be protective for human arteries (7). The mechanism behind this observation may be linked to an effect of LCAT in raising the plasma content of atherogenic LDL cholesteryl esters, or it may be consequent to the accumulation in LCAT-deficient plasma of highly efficient HDL particles. Indeed, we have shown that sera and isolated HDL from carriers of LCAT deficiency have increased capacity to promote cell cholesterol efflux (8), the most relevant function of HDL (9). Besides their role in reverse cholesterol transport, HDLs can contribute to the maintenance of vascular endothelium homeostasis through a variety of effects on vascular tone, inflammation, and endothelial cell integrity (10, 11). In the last years, it has become evident that the vasoprotective effects of HDL are altered in common pathological conditions, such as acute coronary syndrome (12, 13), diabetes (14), and chronic kidney disease (15, 16), as well as in rare genetic HDL disorders (17). The reason for the impaired endothelial protective activity of HDL in such conditions likely resides in structural HDL properties. In the present study, we evaluated the vasoprotective effects of HDLs isolated from carriers of LCAT deficiency, which are characterized by a selective depletion of large LpA-I:A-II particles and predominance of small preβ migrating HDL (1, 2).
MATERIALS AND METHODS
Subjects
Seventy-five carriers of LCAT gene mutations and 32 family controls, all belonging to the Italian LCAT-deficient families (1, 2), volunteered for the study. The carriers’ group was comprised of nine homozygotes and six compound heterozygotes, defined as homozygotes throughout this study, and 60 heterozygotes. The majority of the carriers included in the present study had FLD (10 homozygotes and 48 heterozygotes); the remaining had FED. HDLs for the in vitro studies were isolated from FLD subjects.
All subjects were fully informed of the modalities and end points of the study and signed an informed consent, and the procedures were approved by the Institutional Review Board.
Biochemical analyses
Blood samples were collected after an overnight fast. Plasma total cholesterol, HDL-C, triglyceride, and apolipoprotein levels were determined with certified methods by using a Roche Diagnostics c311 autoanalyzer. LDL-cholesterol (LDL-C) was calculated using Friedewald’s formula.
The plasma concentration of HDL particles containing only apoA-I (LpA-I) and of particles containing both apoA-I and apoA-II (LpA-I:A-II) was determined by electroimmunodiffusion in agarose gel (Sebia Italia). HDL size was analyzed by nondenaturing gradient gel electrophoresis of the d < 1.21 g/ml plasma total lipoprotein fraction, using precast 4–30% gels (18). Serum preβ-HDL content was assessed after separation by 2D electrophoresis followed by immunodetection against human apoA-I and expressed as a percentage of total apoA-I (18).
Plasma levels of the soluble forms of intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin were measured by commercial ELISA kits (R&D Systems) (19).
HDL purification
HDLs (d = 1.063–1.21 g/ml) were purified by sequential ultracentrifugation from the plasma of six homozygotes carrying four different LCAT mutations (Thr274-Ile, Arg147-Trp, Lys218-Asn, and Leu372-Arg), 10 heterozygotes carrying six different LCAT mutations (Thr274-Ile, Arg147-Trp, Lys218-Asn, delG stop 16, Val309-Met, and delG Thr13-Met), and 10 family controls. Due to the very low plasma HDL concentrations detected in homozygotes, plasma samples from three carriers were pooled before ultracentrifugation in order to obtain sufficient amounts of HDL to perform the in vitro experiments; two pools of HDL were thus prepared. HDLs were instead purified by individual plasma samples from heterozygotes and controls.
Purified lipoproteins were dialyzed against sterilized saline immediately before use. HDL content of sphingosine-1-phosphate (S1P) was measured by a commercial competitive ELISA assay and expressed as picomoles per milligram of HDL protein (13). The inter-assay coefficient of variation was 10.4%.
Reconstituted HDL preparation
apoA-I and apoA-II were purified from human plasma, as previously described (20). Discoidal reconstituted HDLs (rHDLs) containing apoA-I and POPC (rLpA-I) were prepared by the cholate dialysis technique, as previously described (21). rHDLs containing both apoA-I and apoA-II (rLpA-I:A-II) were obtained by incubating rLpA-I with lipid-free apoA-II (apoA-II:apoA-I = 1:2, w:w), as previously described (20). rHDLs were then isolated by ultracentrifugation (d < 1.21 g/ml) and the presence of apoA-I and apoA-II was verified by SDS-PAGE followed by Coomassie blue R250 staining. The size of the particles was estimated by gradient gel electrophoresis (21) using the Pharmacia Phast system. Phospholipid content of rHDL was determined by an enzymatic method (22), and proteins were measured by the method of Lowry et al. (23).
Studies on endothelial cells
Experiments were performed on primary cultures of human umbilical vein endothelial cells purchased from Clonetics (Lonza), in M199 with 0.75% BSA and 1% FCS. Plasma HDLs were used at the protein concentration of 1.0 mg/ml in all experiments. rLpA-I and rLpA-I:A-II were used at increasing concentrations (0.125–1.0 mg/ml).
The inhibition of VCAM-1 expression induced by TNFα was assessed as previously described (24). Briefly, endothelial cells were incubated overnight with HDL, washed with PBS, and then stimulated with TNFα (10 ng/ml) for 8 h; VCAM-1 concentration in conditioned medium was evaluated by a commercial matched antibody pairs ELISA kit (BioSource) and normalized by the protein concentration of the total cell lysate.
NO production and eNOS expression and activation in endothelial cells were evaluated as previously described (13). Expression of eNOS was evaluated by SDS-PAGE and immunoblotting after overnight incubation with HDL. Membranes were developed against total eNOS (BD Biosciences), stripped, and reprobed with an antibody against β-actin (Sigma-Aldrich Chemie). eNOS activation by phosphorylation was evaluated by SDS-PAGE and immunoblotting after 10 min of incubation with HDL. Membranes were developed against phosphorylated eNOS (Ser1177; Cell Signaling Technology), stripped, and reprobed with an antibody against total eNOS. Bands were visualized by enhanced chemiluminescence (GE Healthcare Biosciences) and analyzed with a GS-690 imaging densitometer and Multi-Analyst software (Bio-Rad Laboratories). NO production was evaluated after 30 min incubation with HDL or rHDL by fluorescence using diacetate 4,5-diaminofluorescein (DAF-2 DA, Sigma-Aldrich Chemie). Fluorescence intensity was detected with a Synergy multi-mode microplate reader equipped with the GEN5 software (BioTek). For each sample, fluorescence was normalized by the protein concentration of the total cell lysate.
Assessment of flow-mediated vasodilation
Flow-mediated vasodilation (FMD) was assessed in 15 of the enrolled carriers (five FLD homozygotes, seven FLD heterozygotes, and three FED heterozygotes), as previously described (25). Because of the small number of available nonaffected family members, a group of 35 control subjects matched for gender and age with the carriers was recruited among blood donors attending the Servizio Immunoematologico Trasfusionale of the Niguarda Hospital in Milano. Evaluations were performed at fasting, between 8:30 AM and 10:30 AM to exclude circadian variations, abstaining from physical activity and smoking since midnight. Ultrasonic scans were performed in a quiet temperature-controlled (22 ± 2°C) room by using a B-mode ultrasound device (ESAOTE Technos MP) equipped with a 5.0–10.0 MHz linear array transducer (ESAOTE LA-523), held throughout the scan at the same point of the brachial artery (BA) of the nondominant arm using a stereotactic device. The ultrasonic device was gated to the peak R-wave on ECG and images were collected during the end-diastolic phase of each cardiac cycle and recorded on S-VHS videotapes for off-line measurements.
Images of the BA were continuously recorded: i) for 1 min at rest; ii) during 5 min of low-flow obtained by inflating a pneumatic tourniquet placed in the forearm to a pressure 30–50 mmHg above the individual systolic blood pressure; and iii) for 3 min after cuff deflation (i.e., during the reactive hyperemic phase). In each patient, the BA diameter (BAD) was measured using dedicated software, which allowed the automatic and continuous detection of the distance between the media-adventitia interfaces of the near and far wall of the vessel. BAD at rest was the average of the 60 BAD values obtained throughout the preischemic phase. BAD max was the highest BAD value in the hyperemic phase. FMD was calculated as the percent change between BAD at rest and BAD max.
Statistical analysis
Results are reported as mean ± SD, if not otherwise stated. Variables with non-Gaussian distribution are presented as median and interquartile ranges and were log-transformed before analysis. The association of biochemical parameters with LCAT genotype was assessed as the linear trend versus the number of mutant LCAT alleles (0, 1, or 2), and was adjusted for age, sex, and family by covariance analysis. The association of HDL functions with LCAT genotype was assessed as the linear trend versus the number of mutant LCAT alleles (0, 1, or 2). Independent predictors of HDL functionality were identified by multiple regression with stepwise selection of variables. All tests were two-sided and P < 0.05 was considered as significant. All analyses were performed by using the SAS statistical package v. 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Plasma lipids and lipoproteins
Plasma lipid levels in the examined subjects are reported in Table 1. Plasma total cholesterol and HDL-C, apoA-I, apoA-II, and apoB levels were significantly lower and plasma triglyceride levels were significantly increased, in a gene-dose-dependent manner, in carriers of LCAT mutations compared to controls.
TABLE 1.
Plasma lipid/lipoprotein levels and HDL subpopulations in carriers of LCAT gene mutations
| Homozygotes | Heterozygotes | Controls | PTrenda | PAnova | |
| n | 15 | 60 | 32 | ||
| Age (years) | 44.1 ± 4.9 | 48.7 ± 2.7 | 46.6 ± 3.6 | 0.86 | 0.73 |
| Gender (male/female) | 14/1 | 39/21 | 11/21 | <0.0001 | 0.0003 |
| Total cholesterol (mg/dl) | 159.6 ± 22.9 | 166.5 ± 5.5 | 193.0 ± 6.5 | 0.015 | 0.03 |
| LDL-C (mg/dl) | 98.5 ± 17.6 | 97.2 ± 5.0 | 111.4 ± 5.5 | 0.21 | 0.30 |
| HDL-C (mg/dl) | 12.1 ± 2.0 | 41.9 ± 2.1 | 59.8 ± 2.3 | <0.0001 | <0.0001 |
| Triglycerides (mg/dl)b | 180 (114–387) | 110.5 (81–154.5) | 77.5 (62–112.5) | 0.0002 | 0.0004 |
| apoA-I (mg/dl) | 50.5 ± 5.7 | 102.1 ± 3.0 | 136.3 ± 4.6 | <0.0001 | <0.0001 |
| apoA-II (mg/dl) | 9.6 ± 1.5 | 29.0 ± 0.8 | 33.9 ± 1.3 | <0.0001 | <0.0001 |
| apoB (mg/dl) | 67.8 ± 13.0 | 92.2 ± 3.5 | 88.6 ± 3.9 | 0.16 | 0.03 |
| LpA-I (mg/dl) | 28.0 ± 4.2 | 46.3 ± 1.7 | 56.4 ± 2.5 | <0.0001 | <0.0001 |
| LpA-I:A-II (mg/dl) | 22.5 ± 4.3 | 55.8 ± 2.4 | 79.8 ± 3.8 | <0.0001 | <0.0001 |
| Preβ HDL (% of apoA-I)b | 40.0 (23.0–49.0) | 16.0 (11.8–22.0) | 12.8 (10.9–14.1) | <0.0001 | <0.0001 |
| HDL2 size (nm) | N.D. | 11.2 ± 0.1 | 11.1 ± 0.1 | 0.15 | 0.35 |
| HDL3 size (nm)b | 7.4 (7.2–7.7) | 9.0 (8.8–9.3) | 9.0 (8.8–9.0) | <0.0001 | <0.0001 |
Data are expressed as mean ± SEM. N.D., not detectable.
Adjusted for age, gender, and family, except for age, adjusted for gender and family, and gender, adjusted for age and family.
Expressed as median and interquartile range.
HDL2 was undetectable and HDL3 was smaller in homozygotes, while heterozygotes had normally sized HDL particles (Table 1). Plasma LpA-I and LpA-I:A-II concentrations were significantly reduced in carriers compared with controls, but the decrease in the levels of LpA-I:A-II particles was much greater (−72% in homozygotes and −30% in heterozygotes) than the decrease in the levels of LpA-I (−50% in homozygotes and −18% in heterozygotes) (Table 1). The plasma content of preβ HDL was significantly higher in carriers than in controls (Table 1).
HDL content of S1P in homozygotes was comparable to that of controls’ HDL (195.0 ± 55.2 pmol/mg protein vs. 221.2 ± 28.5 pmol/mg protein, respectively), while HDL from heterozygotes showed a slightly higher S1P content (333.8 ± 162.5 pmol/mg protein, PTrend = 0.49).
Ability of HDL to modulate eNOS activity and NO release in endothelial cells
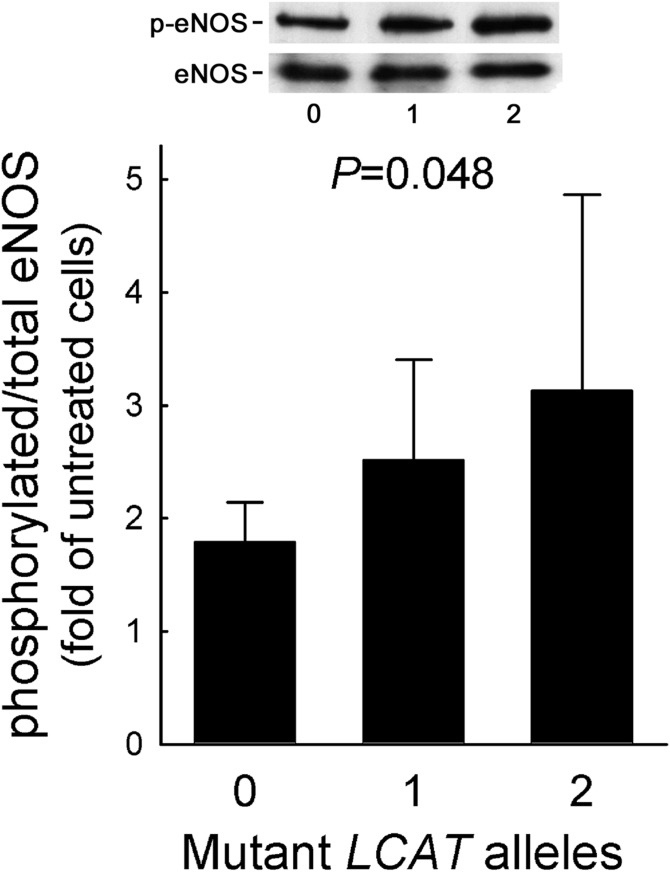
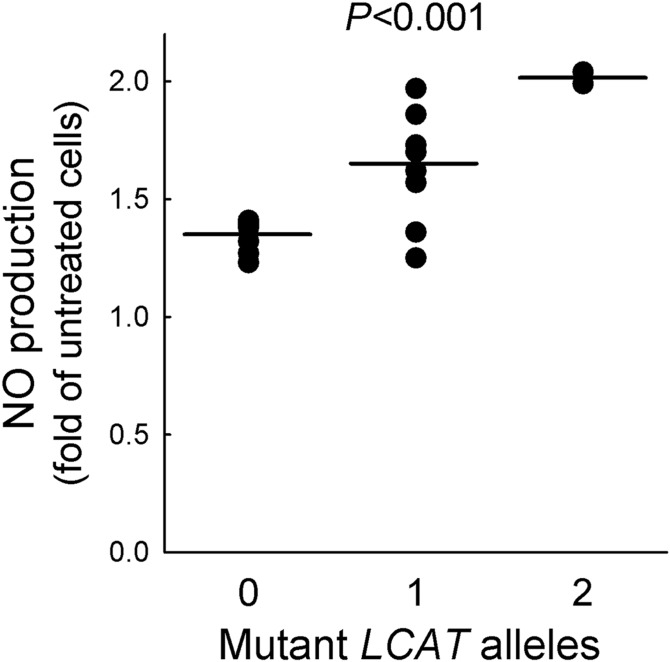
HDLs isolated from carriers and controls displayed a comparable capacity to increase eNOS protein abundance (PAnova = 0.115) (supplemental Fig. S1). However, HDLs from LCAT-deficient subjects proved to be more effective than HDLs from controls in promoting eNOS activation by phosphorylation with a gene-dose-dependent effect; when compared with HDLs from controls, eNOS activation induced by HDLs from heterozygous and homozygous carriers was 40.8% and 75.4% higher, respectively (PTrend = 0.048) (Fig. 1). As a consequence, NO production induced by HDLs from heterozygous and homozygous carriers was significantly higher than that promoted by control HDLs, with a gene-dose-dependent effect (Fig. 2). Interestingly, among the HDL-related parameters, only LpA-I:A-II levels were independent predictors of HDL-induced eNOS activation (β = −0.017 ± 0.006, P = 0.007).
Fig. 1.
eNOS activation by HDL. HDLs from carriers of LCAT deficiency and controls were tested for their ability to induce eNOS activation by phosphorylation. Data are expressed as fold of increase in HDL-treated versus untreated cells; n = 6 for homozygotes (pooled in two preparations), n = 10 for heterozygotes and controls. PTrend is reported. Representative blots are shown on the top.
Fig. 2.
NO production by HDL. HDLs from carriers of LCAT deficiency and controls were tested for their ability to induce NO production in endothelial cells. Data are expressed as fold of increase in HDL-treated versus untreated cells; n = 6 for homozygotes (pooled in two preparations), n = 10 for heterozygotes and controls; single data points are shown. PTrend is reported.
The evaluation of FMD in a small group of carriers of LCAT deficiency, comprised of 5 homozygotes and 10 heterozygotes, in comparison with age-gender matched control subjects indicated that carriers do not show impaired FMD despite the low HDL-C levels (Table 2).
TABLE 2.
FMD in carriers of LCAT gene mutations
| Carriersa | Controls | P | |
| n | 15 | 35 | |
| Age (years) | 43.9 ± 3.0 | 44.1 ± 1.9 | 0.97 |
| Gender (male/female) | 8/7 | 19/16 | 0.95 |
| BMI (kg/m2) | 23.4 ± 0.8 | 23.7 ± 0.5 | 0.75 |
| Total cholesterol (mg/dl) | 160.8 ± 12.0 | 199.1 ± 6.8 | 0.005 |
| LDL-C (mg/dl) | 105.2 ± 9.8 | 124.0 ± 5.3 | 0.07 |
| HDL-C (mg/dl) | 32.9 ± 5.4 | 56.1 ± 2.2 | <0.0001 |
| Triglycerides (mg/dl) | 113.1 ± 12.4 | 95.1 ± 9.2 | 0.27 |
| FMD | 0.237 ± 0.025 | 0.304 ± 0.030 | 0.18b |
Data are expressed as mean ± SEM.
Comprising 5 homozygotes and 10 heterozygotes, pooled for statistical analysis.
P adjusted for HDL-C = 0.22.
Ability of HDL to inhibit VCAM-1 expression in endothelial cells
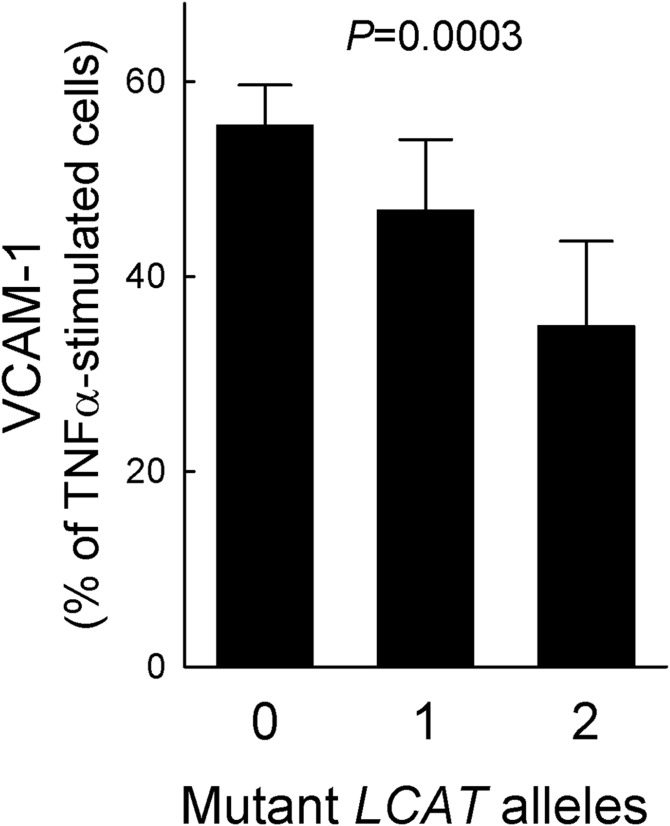
As it has been shown that the stimulatory effect of HDLs on endothelial NO production is critical for their endothelial anti-inflammatory action (12), we further investigated the in vitro effect of carriers’ HDL on the expression of endothelial adhesion molecules. HDLs isolated from LCAT-deficient subjects were more effective than HDLs from controls in inhibiting the expression of VCAM-1. HDLs from homozygotes reduced VCAM-1 expression by 65.0 ± 8.6%, HDLs from heterozygotes by 53.1 ± 7.2%, and control HDLs by 44.4 ± 4.1% (Fig. 3).
Fig. 3.
Inhibition of VCAM-1 expression by HDL. HDLs from carriers of LCAT deficiency and controls were tested for their ability to reduce VCAM-1 expression induced by TNFα stimulation in endothelial cells. Data are expressed as percentage of VCAM-1 concentration in the conditioned media of TNFα-stimulated cells; n = 6 for homozygotes (pooled in two preparations), n = 10 for heterozygotes and controls. PTrend is reported.
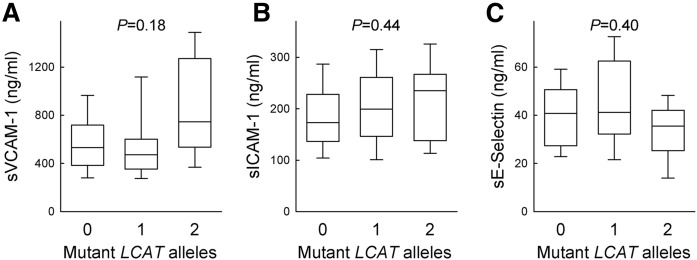
No significant differences in soluble VCAM-1, ICAM-1, and E-selectin plasma levels were detected when values of carriers of two, one, or zero mutant alleles were tested for trend (Fig. 4). Levels of sVCAM-1 showed a marked degree of variability in homozygotes and were significantly higher than those of heterozygotes (P = 0.017) and controls (P = 0.046); this may be due to the presence of renal disease in 9 out of 15 homozygotes (26), as suggested by a trend for elevated VCAM-1 levels in carriers with renal disease compared with those with no sign of renal disease (943.5 ± 395.7 ng/ml vs. 761.1 ± 394.5 ng/ml, respectively, P = 0.397). On the contrary, homozygotes did not show a significant increase of sICAM-1 levels, while plasma levels of sE-selectin were even lower than those of heterozygotes (P = 0.026).
Fig. 4.
Plasma levels of soluble adhesion molecules. Levels of soluble VCAM-1 (A), ICAM-1 (B), and E-selectin (C) were measured by ELISA in the plasma of carriers of LCAT deficiency and controls. Boxes indicate the median and 25th to 75th percentiles, capped bars the 10th to 90th percentiles; n = 15 for homozygotes, n = 60 for heterozygotes, and n = 32 for controls. PTrend adjusted for age and gender are reported.
Ability of rLpA-I and rLpA-I:A-II to promote NO production in endothelial cells
To test the hypothesis that the depletion in LpA-I:A-II particles typically observed in LCAT-deficient subjects was responsible for the increased endothelial protective capacity of HDL from carriers, rHDLs containing apoA-I only or apoA-I and apoA-II were prepared and characterized. rLpA-I consisted of a single population of particles with a diameter of 9.7 nm, containing 148 POPC and two apoA-I molecules per particle. rLpA-I:A-II consisted of a single population of particles with a diameter of 9.8 nm, containing 140 POPC, one apoA-I, and two apoA-II molecules per particle (supplemental Fig. S2, supplemental Table S1).
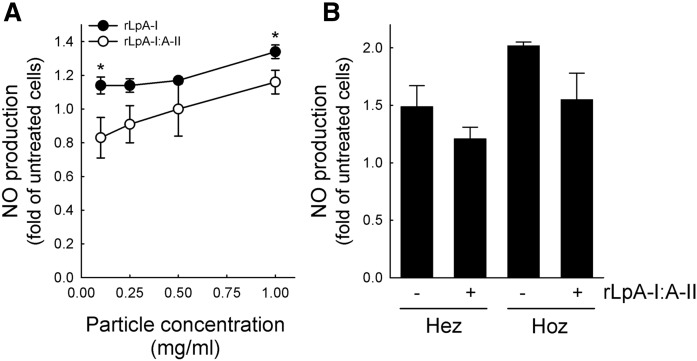
When endothelial cells were incubated with rLpA-I particles, the promotion of NO production was higher than that obtained with rLpA-I:A-II particles in the whole range of concentrations tested (Fig. 5A). Accordingly, rLpA-I particles were more effective than rLpA-I:A-II particles in promoting eNOS activation by phosphorylation (supplemental Fig. S3A), while eNOS protein abundance was comparable between cells incubated with rLpA-I or rLpA-I:A-II particles (supplemental Fig. S3B). rLpA-I and rLpA-I:A-II particles were equally effective in reducing VCAM-1 expression in endothelial cells (supplemental Fig. S3C).
Fig. 5.
NO production by rLpA-I and rLpA-I:A-II. A: Reconstituted particles containing only apoA-I or apoA-I and apoA-II were tested for their ability to induce NO production in endothelial cells. Data are expressed as fold of increase in treated versus untreated cells, n = 3. *P < 0.05 versus rLpA-I:A-II at the same concentration. B: NO production in endothelial cells incubated with HDLs from heterozygous and homozygous patients before and after supplementation with rLpA-I:A-II. Data are expressed as fold of increase in HDL-treated versus untreated cells; n = 3 for heterozygotes and n = 6 for homozygotes (pooled in two preparations).
In additional experiments, HDLs from heterozygous and homozygous carriers were supplemented with an amount of rLpA-I:A-II to restore the LpA-I/LpA-I:A-II ratio observed in controls (HDL to rLpA-I:A-II weight ratio 3:1). After the addition of rLpA-I:A-II, HDLs from heterozygotes and homozygotes displayed an impaired ability to promote NO production by endothelial cells (Fig. 5B).
DISCUSSION
In the present study, we took advantage of the availability of a large group of Italian carriers of LCAT gene mutations (1, 2) and showed that: i) HDLs from carriers of LCAT deficiency had an increased capacity, with a gene-dose-dependent effect, to promote the release of NO and to inhibit the expression of adhesion molecules in cultured endothelial cells, likely due to the reduced content in LpA-I:A-II particles; and ii) carriers had normal FMD and plasma soluble adhesion molecule levels despite the low HDL-C levels.
Over the past two decades a number of studies have shown direct protective effects of HDL on endothelial cells; these effects include the ability of HDL to promote the production of vasoactive molecules, such as NO, and to downregulate CAM expression (10, 11). The various HDL subpopulations are differently efficient in maintaining endothelial cell homeostasis (27) and, in some conditions, HDL can lose its protective properties and even become detrimental (28). Genetic HDL disorders represent a unique tool to understand the relationship between HDL quantity and quality and HDL function, because carriers of inherited HDL disorders not only have extremely low or high HDL-C levels, but also have abnormal HDL subclass distributions (29).
HDL particle distribution is largely altered in carriers of LCAT gene mutations, being characterized by a reduced content of large HDL2 particles, an accumulation of small HDL3 particles, particularly preβ migrating HDL (1, 30), and a selective depletion of apoA-II-containing particles (31). While the role of apoA-II containing HDL in mediating cell cholesterol efflux has been evaluated in a number of studies (20, 32–35), no data are available on the effect of apoA-II in modulating endothelial protective HDL functions. The best characterized endothelial protective HDL function is the capacity to enhance NO production, due to the ability of HDL to promote eNOS expression and activation (24, 36, 37). This effect requires the binding of apoA-I to SR-BI, which leads to the activation of the PI3K/Akt signaling pathway and the subsequent phosphorylation of eNOS (38, 39). The major role of apoA-I is demonstrated by the ability of anti-apoA-I antibody to block eNOS activation by HDL in cultured endothelial cells (38). On the contrary, anti-apoA-II antibody further enhances eNOS stimulation by HDL (38), and the level of apoA-II in HDL is inversely correlated with HDL binding to SR-BI (40), indicating that apoA-II is not necessary and suggesting that it could even be detrimental. In line with this hypothesis, HDLs from carriers of genetic CETP deficiency are enriched in LpA-I:A-II particles and have reduced capacity to stimulate endothelial NO production (17). In addition, an elevated plasma concentration of LpA-I:A-II particles is an independent predictor of a more severe inflammatory response, and is associated with a reduced capacity of HDL to promote endothelial NO production in patients with acute coronary syndrome (13, 41).
The defective ability of LpA-I:A-II particles in promoting endothelial NO release might be explained by the presence of apoA-II itself or by the different proteins carried by the two HDL subclasses. In the present study, we have shown that synthetic HDLs containing apoA-II in addition to apoA-I are less efficient than particles containing only apoA-I in promoting endothelial NO production, likely due to the conformational changes of apoA-I induced by apoA-II (42). It is thus clear that apoA-I is not only needed, but its conformation is also relevant in defining apoA-I-containing HDL capacity to protect the endothelium. HDL-associated PON1 has been suggested to have a major impact on endothelial function, and PON1 inactivation in HDL results in decreased eNOS-Ser1177 phosphorylation and consequent attenuated NO production (12). Furthermore, HDL from PON1-deficient mice fails to stimulate endothelial NO production, and the supplementation of HDL isolated from these mice with purified PON1 restores the protective function (12). PON1 is mostly found in HDL particles containing apoA-I, but not apoA-II, and apoA-I appears to be of major importance in determining serum PON1 activity and stability, likely establishing the architecture of the HDL particle that optimally binds PON1 (43). Moreover, apoA-II enrichment of HDLs can displace PON1, thus impairing their antioxidant properties (44). Notably, carriers of LCAT deficiency have normal PON1 activity despite the low HDL-C levels (45), differently from what is observed in other genetic low HDL disorders (45, 46).
The impact on arterial function of increased efficiency of HDL isolated from carriers of LCAT deficiency is difficult to determine. However, it is noteworthy that carriers of LCAT deficiency show normal FMD despite the low/very low HDL-C levels. Therefore, one could speculate that the increased ability of carriers’ HDL in protecting endothelial function might overcome the markedly reduced concentration of HDL in these subjects.
It must be underlined that the majority of carriers included in the present study had FLD and, thus, the results may not apply to FED. Indeed, a previous study examined pulse wave velocity in Dutch carriers of LCAT mutations, who mainly had FED, and found an increased arterial stiffness in carriers (47). Interestingly, FLD subjects have a more pronounced reduction in LpA-I:A-II particles, particularly evident in homozygotes (2).
In conclusion, the present results indicate that the selective depletion of apoA-II-containing HDL, as observed in carriers of LCAT deficiency, leads to an increased capacity of HDL to stimulate endothelial NO production. These results place the attention on the apolipoprotein composition of HDL as a target of therapeutic interventions designed to improve HDL functionality.
Supplementary Material
Footnotes
Abbreviations:
- BA
- brachial artery
- BAD
- brachial artery diameter
- FED
- fish-eye disease
- FLD
- familial LCAT deficiency
- FMD
- flow-mediated vasodilation
- HDL-C
- HDL-cholesterol
- ICAM-1
- intracellular adhesion molecule-1
- LDL-C
- LDL-cholesterol
- LpA-I
- lipoproteins containing apoA-I
- LpA-I:A-II
- lipoproteins containing apoA-I and apoA-II
- rHDL
- reconstituted HDL
- S1P
- sphingosine-1-phosphate
- VCAM-1
- vascular cell adhesion molecule-1
This work was supported by Telethon Italy Grant GGP08052 (to M.G.) and Fondazione Cariplo Grants 2009-2576 (to M.G.) and 2011-0628 (to L.C.).
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Calabresi L., Pisciotta L., Costantin A., Frigerio I., Eberini I., Alessandrini P., Arca M., Bon G. B., Boscutti G., Busnach G., et al. 2005. The molecular basis of lecithin:cholesterol acyltransferase deficiency syndromes: a comprehensive study of molecular and biochemical findings in 13 unrelated Italian families. Arterioscler. Thromb. Vasc. Biol. 25: 1972–1978. [DOI] [PubMed] [Google Scholar]
- 2.Calabresi L., Simonelli S., Gomaraschi M., and Franceschini G.. 2012. Genetic lecithin:cholesterol acyltransferase deficiency and cardiovascular disease. Atherosclerosis. 222: 299–306. [DOI] [PubMed] [Google Scholar]
- 3.Santamarina-Fojo S., Hoeg J. M., Assmann G. , and. Brewer H. B. Jr. 2001. Lecithin cholesterol acyltransferase deficiency and fish eye disease. In Scriver C. R., Beaudet A. L., Sly W. S., et al. , editors. McGraw-Hill, New York: 2817–2833. [Google Scholar]
- 4.O’Leary D. H., Polak J. F., Kronmal R. A., Manolio T. A., Burke G. L., and Wolfson S. K. Jr. 1999. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N. Engl. J. Med. 340: 14–22. [DOI] [PubMed] [Google Scholar]
- 5.Ayyobi A. F., McGladdery S. H., Chan S., John Mancini G. B., Hill J. S., and Frohlich J. J.. 2004. Lecithin:cholesterol acyltransferase (LCAT) deficiency and risk of vascular disease: 25 year follow-up. Atherosclerosis. 177: 361–366. [DOI] [PubMed] [Google Scholar]
- 6.Hovingh G. K., Hutten B. A., Holleboom A. G., Petersen W., Rol P., Stalenhoef A., Zwinderman A. H., de Groot E., Kastelein J. J., and Kuivenhoven J. A.. 2005. Compromised LCAT function is associated with increased atherosclerosis. Circulation. 112: 879–884. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi L., Baldassarre D., Castelnuovo S., Conca P., Bocchi L., Candini C., Frigerio B., Amato M., Sirtori C. R., Alessandrini P., et al. 2009. Functional lecithin:cholesterol acyltransferase is not required for efficient atheroprotection in humans. Circulation. 120: 628–635. [DOI] [PubMed] [Google Scholar]
- 8.Favari E., Lee M., Calabresi L., Franceschini G., Zimetti F., Bernini F., and Kovanen P. T.. 2004. Depletion of pre-beta-high density lipoprotein by human chymase impairs ATP-binding cassette transporter A1- but not scavenger receptor class B type I-mediated lipid efflux to high density lipoprotein. J. Biol. Chem. 279: 9930–9936. [DOI] [PubMed] [Google Scholar]
- 9.Cuchel M., and Rader D. J.. 2006. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 113: 2548–2555. [DOI] [PubMed] [Google Scholar]
- 10.Calabresi L., Gomaraschi M., and Franceschini G.. 2003. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler. Thromb. Vasc. Biol. 23: 1724–1731. [DOI] [PubMed] [Google Scholar]
- 11.Mineo C., Deguchi H., Griffin J. H., and Shaul P. W.. 2006. Endothelial and antithrombotic actions of HDL. Circ. Res. 98: 1352–1364. [DOI] [PubMed] [Google Scholar]
- 12.Besler C., Heinrich K., Rohrer L., Doerries C., Riwanto M., Shih D. M., Chroni A., Yonekawa K., Stein S., Schaefer N., et al. 2011. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Invest. 121: 2693–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomaraschi M., Ossoli A., Favari E., Adorni M. P., Sinagra G., Cattin L., Veglia F., Bernini F., Franceschini G., and Calabresi L.. 2013. Inflammation impairs eNOS activation by HDL in patients with acute coronary syndrome. Cardiovasc. Res. 100: 36–43. [DOI] [PubMed] [Google Scholar]
- 14.Sorrentino S. A., Besler C., Rohrer L., Meyer M., Heinrich K., Bahlmann F. H., Mueller M., Horvath T., Doerries C., Heinemann M., et al. 2010. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 121: 110–122. [DOI] [PubMed] [Google Scholar]
- 15.Speer T., Rohrer L., Blyszczuk P., Shroff R., Kuschnerus K., Krankel N., Kania G., Zewinger S., Akhmedov A., Shi Y., et al. 2013. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of toll-like receptor-2. Immunity. 38: 754–768. [DOI] [PubMed] [Google Scholar]
- 16.Shroff R., Speer T., Colin S., Charakida M., Zewinger S., Staels B., Chinetti-Gbaguidi G., Hettrich I., Rohrer L., O’Neill F., et al. 2014. HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J. Am. Soc. Nephrol. 25: 2658–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomaraschi M., Ossoli A., Pozzi S., Nilsson P., Cefalu A. B., Averna M., Kuivenhoven J. A., Hovingh G. K., Veglia F., Franceschini G., et al. 2014. eNOS activation by HDL is impaired in genetic CETP deficiency. PLoS One. 9: e95925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franceschini G., Favari E., Calabresi L., Simonelli S., Bondioli A., Adorni M. P., Zimetti F., Gomaraschi M., Coutant K., Rossomanno S., et al. 2013. Differential effects of fenofibrate and extended-release niacin on high-density lipoprotein particle size distribution and cholesterol efflux capacity in dyslipidemic patients. J. Clin. Lipidol. 7: 414–422. [DOI] [PubMed] [Google Scholar]
- 19.Calabresi L., Gomaraschi M., Villa B., Omoboni L., Dmitrieff C., and Franceschini G.. 2002. Elevated soluble cellular adhesion molecules in subjects with low HDL-cholesterol. Arterioscler. Thromb. Vasc. Biol. 22: 656–661. [DOI] [PubMed] [Google Scholar]
- 20.Bernini F., Calabresi L., Bonfadini G., and Franceschini G.. 1996. The molecular structure of apolipoprotein A-II modulates the capacity of HDL to promote cell cholesterol efflux. Biochim. Biophys. Acta. 1299: 103–109. [DOI] [PubMed] [Google Scholar]
- 21.Calabresi L., Vecchio G., Frigerio F., Vavassori L., Sirtori C. R., and Franceschini G.. 1997. Reconstituted high-density lipoproteins with a disulfide-linked apolipoprotein A-I dimer: evidence for restricted particle size heterogeneity. Biochemistry. 36: 12428–12433. [DOI] [PubMed] [Google Scholar]
- 22.Takayama M., Itoh S., Nagasaki T., and Tanimizu I.. 1977. A new enzymatic method for determination of serum choline- containing phospholipids. Clin. Chim. Acta. 79: 93–98. [DOI] [PubMed] [Google Scholar]
- 23.Lowry O. H., Rosebrough N. J., Farr A. L., and Randall R. J.. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 24.Gomaraschi M., Baldassarre D., Amato M., Eligini S., Conca P., Sirtori C. R., Franceschini G., and Calabresi L.. 2007. Normal vascular function despite low levels of high-density lipoprotein cholesterol in carriers of the apolipoprotein A-I(Milano) mutant. Circulation. 116: 2165–2172. [DOI] [PubMed] [Google Scholar]
- 25.Amato M., Frigerio B., Castelnuovo S., Ravani A., Sansaro D., Tremoli E., Squellerio I., Cavalca V., Veglia F., Sirtori C. R., et al. 2013. Effects of smoking regular or light cigarettes on brachial artery flow-mediated dilation. Atherosclerosis. 228: 153–160. [DOI] [PubMed] [Google Scholar]
- 26.Rabelink T. J., de Boer H. C., and van Zonneveld A. J.. 2010. Endothelial activation and circulating markers of endothelial activation in kidney disease. Nat. Rev. Nephrol. 6: 404–414. [DOI] [PubMed] [Google Scholar]
- 27.Calabresi L., Gomaraschi M., and Franceschini G.. 2010. High-density lipoprotein quantity or quality for cardiovascular prevention? Curr. Pharm. Des. 16: 1494–1503. [DOI] [PubMed] [Google Scholar]
- 28.Riwanto M., Rohrer L., Von E. A., and Landmesser U.. 2015. Dysfunctional HDL: from structure-function-relationships to biomarkers. Handb. Exp. Pharmacol. 224: 337–366. [DOI] [PubMed] [Google Scholar]
- 29.Oldoni F., Sinke R. J., and Kuivenhoven J. A.. 2014. Mendelian disorders of high-density lipoprotein metabolism. Circ. Res. 114: 124–142. [DOI] [PubMed] [Google Scholar]
- 30.Asztalos B. F., Schaefer E. J., Horvath K. V., Yamashita S., Miller M., Franceschini G., and Calabresi L.. 2007. Role of LCAT in HDL remodeling: investigation of LCAT deficiency states. J. Lipid Res. 48: 592–599. [DOI] [PubMed] [Google Scholar]
- 31.Rader D. J., Ikewaki K., Duverger N., Schmidt H., Pritchard H., Frohlich J. J., Clerc M., Dumon M. F., Fairwell T., Zech L., et al. 1994. Markedly accelerated catabolism of apolipoprotein A-II (ApoA-II) and high density lipoproteins containing ApoA-II in classic lecithin:cholesterol acyltransferase deficiency and fish-eye disease. J. Clin. Invest. 93: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbaras R., Puchois P., and Fruchart J. C.. 1987. Cholesterol efflux from cultured adipose cells is mediated by LpA-I particles but not by LpA-I:A-II particles. Biochem. Biophys. Res. Commun. 142: 63–69. [DOI] [PubMed] [Google Scholar]
- 33.Lagrost L., Dengremont C., Athias A., De Geitere C., Fruchart J. C., Lallemant C., Gambert P., and Castro G. R.. 1995. Modulation of cholesterol efflux from Fu5AH hepatoma cells by the apolipoprotein content of high density lipoprotein particles. Particles containing various proportions of apolipoproteins A-I and A-II. J. Biol. Chem. 270: 13004–13009. [DOI] [PubMed] [Google Scholar]
- 34.Oikawa S., Mendez A. J., Oram J. F., Bierman E. L., and Cheung M. C.. 1993. Effects of high-density lipoprotein particles containing apo A-I, with or without apo A-II, on intracellular cholesterol efflux. Biochim. Biophys. Acta. 1165: 327–334. [DOI] [PubMed] [Google Scholar]
- 35.Ohta T., Saku K., Takata K., Nakamura R., Ikeda Y., and Matsuda I.. 1995. Different effects of subclasses of HDL containing apoA-I but not apoA-II (LpA-I) on cholesterol esterification in plasma and net cholesterol efflux from foam cells. Arterioscler. Thromb. Vasc. Biol. 15: 956–962. [DOI] [PubMed] [Google Scholar]
- 36.Kuvin J. T., Ramet M. E., Patel A. R., Pandian N. G., Mendelsohn M. E., and Karas R. H.. 2002. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am. Heart J. 144: 165–172. [DOI] [PubMed] [Google Scholar]
- 37.Rämet M. E., Ramet M., Lu Q., Nickerson M., Savolainen M. J., Malzone A., and Karas R. H.. 2003. High-density lipoprotein increases the abundance of eNOS protein in human vascular endothelial cells by increasing its half-life. J. Am. Coll. Cardiol. 41: 2288–2297. [DOI] [PubMed] [Google Scholar]
- 38.Yuhanna I. S., Zhu Y., Cox B. E., Hahner L. D., Osborne-Lawrence S., Lu P., Marcel Y. L., Anderson R. G., Mendelsohn M. E., Hobbs H. H., et al. 2001. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 7: 853–857. [DOI] [PubMed] [Google Scholar]
- 39.Mineo C., Yuhanna I. S., Quon M. J., and Shaul P. W.. 2003. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J. Biol. Chem. 278: 9142–9149. [DOI] [PubMed] [Google Scholar]
- 40.de Beer M. C., Castellani L. W., Cai L., Stromberg A. J., De Beer F. C., and Van der Westhuyzen D. R.. 2004. ApoA-II modulates the association of HDL with class B scavenger receptors SR-BI and CD36. J. Lipid Res. 45: 706–715. [DOI] [PubMed] [Google Scholar]
- 41.Gomaraschi M., Sinagra G., Serdoz L. V., Pitzorno C., Fonda M., Cattin L., Calabresi L., and Franceschini G.. 2009. The plasma concentration of Lpa-I:A-II particles as a predictor of the inflammatory response in patients with ST-elevation myocardial infarction. Atherosclerosis. 202: 304–311. [DOI] [PubMed] [Google Scholar]
- 42.Gauthamadasa K., Vaitinadin N. S., Dressman J. L., Macha S., Homan R., Greis K. D., and Silva R. A.. 2012. Apolipoprotein A-II-mediated conformational changes of apolipoprotein A-I in discoidal high density lipoproteins. J. Biol. Chem. 287: 7615–7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James R. W., and Deakin S. P.. 2010. The contribution of high density lipoprotein apolipoproteins and derivatives to serum paraoxonase-1 activity and function. Adv. Exp. Med. Biol. 660: 173–181. [DOI] [PubMed] [Google Scholar]
- 44.Ribas V., Sanchez-Quesada J. L., Anton R., Camacho M., Julve J., Escola-Gil J. C., Vila L., Ordonez-Llanos J., and Blanco-Vaca F.. 2004. Human apolipoprotein A-II enrichment displaces paraoxonase from HDL and impairs its antioxidant properties: a new mechanism linking HDL protein composition and antiatherogenic potential. Circ. Res. 95: 789–797. [DOI] [PubMed] [Google Scholar]
- 45.Daniil G., Phedonos A. A., Holleboom A. G., Motazacker M. M., Argyri L., Kuivenhoven J. A., and Chroni A.. 2011. Characterization of antioxidant/anti-inflammatory properties and apoA-I-containing subpopulations of HDL from family subjects with monogenic low HDL disorders. Clin. Chim. Acta. 412: 1213–1220. [DOI] [PubMed] [Google Scholar]
- 46.James R. W., Blatter Garin M. C., Calabresi L., Miccoli R., von Eckardstein A., Tilly-Kiesi M., Taskinen M. R., Assmann G., and Franceschini G.. 1998. Modulated serum activities and concentrations of paraoxonase in high density lipoprotein deficiency states. Atherosclerosis. 139: 77–82. [DOI] [PubMed] [Google Scholar]
- 47.van den Bogaard B., Holleboom A. G., Duivenvoorden R., Hutten B. A., Kastelein J. J., Hovingh G. K., Kuivenhoven J. A., Stroes E. S., and van den Born B. J.. 2012. Patients with low HDL-cholesterol caused by mutations in LCAT have increased arterial stiffness. Atherosclerosis. 225: 481–485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.