Abstract
Cerebrotendinous xanthomatosis (CTX) is a treatable neurodegenerative metabolic disorder of bile acid synthesis in which symptoms can be prevented if treatment with chenodeoxycholic acid supplementation is initiated early in life, making CTX an excellent candidate for newborn screening. We developed a new dried blood spot (DBS) screening assay for this disorder on the basis of different ratios between the accumulating cholestanetetrol glucuronide (tetrol) and specific bile acids/bile acid intermediates, without the need for derivatization. A quarter-inch DBS punch was extracted with methanol, internal standards were added, and after concentration the extract was injected into the tandem mass spectrometer using a 2 min flow injection analysis for which specific transitions were measured for cholestanetetrol glucuronide, taurochenodeoxycholic acid (t-CDCA), and taurotrihydroxycholestanoic acid (t-THCA). A proof-of-principle experiment was performed using 217 Guthrie cards from healthy term/preterm newborns, CTX patients, and Zellweger patients. Using two calculated biomarkers, tetrol:t-CDCA and t-THCA:tetrol, this straightforward method achieved an excellent separation between DBSs of CTX patients and those of controls, Zellweger patients, and newborns with cholestasis. The results of this small pilot study indicate that the tetrol:t-CDCA ratio is an excellent derived biomarker for CTX that has the potential to be used in neonatal screening programs.
Keywords: bile acid metabolism, bile acids and salts biosynthesis, inborn errors of metabolism, tandem mass spectrometry, CYP27A1
Cerebrotendinous xanthomatosis (CTX; OMIM# 213700) is a rare autosomal recessive disorder caused by the deficiency of 27-sterol hydroxylase (encoded by the CYP27A1 gene), which plays a crucial role in bile acid synthesis. CTX in adults is characterized by a progressive neurological phenotype. Symptoms during infancy and childhood include neonatal cholestasis, intractable diarrhea (can be the presenting sign and clinically significant shortly after birth), bilateral cataract, and developmental delay. From the second or third decade onward, tendon xanthomas and neuropsychiatric symptoms, including pyramidal and cerebellar signs, peripheral neuropathy, and dementia are observed (1, 2). The metabolic block in bile acid synthesis results in the deficiency of the primary bile acid cholic acid (CA) and particularly chenodeoxycholic acid (CDCA), in addition to the accumulation of 7α-hydroxy-4-cholesten-3-one, which is further converted into different metabolites including cholestanol and characteristic bile alcohols. Cholestanol, together with cholesterol, accumulates in tissues. The pathophysiology of CTX is still poorly understood, but both the cholestanol and cholesterol accumulation likely contribute to the observed pathology. Cholestanol has been shown to induce apoptosis of neuronal cells, and its accumulation is thought to underlie the observed neurodegeneration (3, 4). The development of symptoms and signs can be halted or prevented by supplementation of CDCA, which downregulates both bile acid and cholesterol synthesis through a feedback mechanism that results in the inhibition of cholesterol-7α-hydroxylase and HMG-CoA reductase, respectively, thereby preventing the production and accumulation of cholesterol and cholestanol (5). The prognosis of CTX is good when therapy is started early but is less favorable when initiated at a later age (6–9). An early start to the therapy can prevent the neurological phenotype completely, and patients are expected to remain symptom-free if treatment is started immediately after neonatal diagnosis (7, 10). The incidence of CTX was estimated in different ethnic groups to be as high as 1:36,072 to 1:263,222 (11), when allele frequencies of pathogenic CTX variants in the ExAC database were used. This and other studies suggest that CTX may be underdiagnosed (11, 12). Taken together, this makes CTX an excellent candidate disease for newborn screening. The detection of CTX newborn patients, however, is hampered by the absence of a suitable neonatal screening method in dried blood spots (DBSs). DeBarber and coworkers (13, 14) have published a candidate, high-throughput, newborn screening method for CTX that is based on the quantification of a ketosterol intermediate in DBSs using an LC-ESI/MS/MS method. A potential disadvantage is that a derivatization step is required before analysis, complicating implementation into existing neonatal screening programs and at higher costs.
Here, we report an alternative newborn screening method for CTX that is based on the relative quantification of bile alcohol glucuronides in DBSs using flow injection-MS/MS without prior derivatization. The method is based on the use of different ratios between the accumulating cholestanetetrol glucuronide (the most abundant cholestanetetrol in blood in CTX is 5β-cholestane-3α,7α,12α,25-tetrol glucuronide) and specific bile acids/bile acid intermediates, enabling the detection of CTX patients with high sensitivity and specificity, as demonstrated in a proof-of-principle experiment using 217 (total = 150+50+14+3 = 217) Guthrie cards from healthy term/preterm newborns, CTX patients, and Zellweger patients.
MATERIALS AND METHODS
Patient and control samples
Anonymized DBSs of 150 term [mean gestational age (range): 39.9 (37.0–42.0) weeks] and 50 preterm newborns [mean gestational age (range): 34.5 (26.7–36.9) weeks] were obtained from the biobank of the Dutch newborn screening program (Dutch National Institute for Public Health and the Environment, Bilthoven, The Netherlands). All parents gave informed consent for the anonymized use of DBSs from the Guthrie card of their child at the time of collection (standard procedure in the Dutch newborn screening program; all Guthrie newborn screening cards in the Netherlands are stored for 5 years, after which they are destroyed). Fourteen DBSs, including two original neonatal DBSs, were collected from 14 individual untreated CTX patients. These included DBSs from participants enrolled in studies at Oregon Health and Science University (with institutional review board approval), with written informed consent provided by the participants for use of the DBS. Deidentified diagnostic samples submitted to the Sterol Laboratory for biochemical confirmation of CTX were also used with institutional review board approval. Table 1 lists all relevant details concerning the CTX patients and their DBSs. All patients or their parents/legal representatives gave written informed consent for the use of their DBS/the DBS of their child. Three anonymized Zellweger DBSs were obtained from the biobank at the Academic Medical Center (Amsterdam).
TABLE 1.
DBS and CTX patient information
| DBS | Patienta | DBS Age (years) | Storage Conditions | Patient Age at Sampling (years) | Plasma Cholestanol (mg/dl) | Plasma Cholestanol (µM) | Diagnosisb | Phenotype at Diagnosis |
| 1 | P1 | 0.5 | −20°C | 9 | 1.62 | 42 | G/B | Unknown |
| 2 | P2 | 0.5 | −20°C | 12 | 2.57 | 66 | G/B | Unknown |
| 3 | P3 | 4 | −20°C | 17 | 1.84 | 47 | B | Neonatal jaundice, intractable diarrhea, xanthomas |
| 4 | P4 | 9 | −20°C | 16 | 3.23 | 83 | G/B | Juvenile cataracts, developmental delay, autistiform behavior, cognitive impairment |
| 5 | P5 | 4 | −20°C | 31 | 1.65 | 42 | G/B | Xanthomas, spastic gait, paraparesis |
| 6 | P6 | 0.17 | −20°C | 45 | 3.44 | 88 | B | Cognitive decline, psychiatric disorder, dementia |
| 7 | P7 | 0.17 | −20°C | 46 | 4.79 | 123 | B | Depression, paraparesis, cerebellar ataxia, dystonia |
| 8 | P8 | 7.5 | −20°C | 36 | 4.52 | 116 | G/B | Cataract, xanthomas, seizures, intractable diarrhea |
| 9 | P9 | 0.5 | 4°C | 6.2 | 1.52 | 39 | G | Speech delay, altered stool pattern |
| 10 | P10 | 0.1 | 4°C | 9 | 2.06 | 53 | B | Cataracts |
| 11 | P11 | 0.5 | 4°C | 11 | 4.52 | 116 | G | Cataracts, developmental delay, autistiform behavior, altered stool pattern |
| 12 | P12 | 6 | −20°C | 56 | 0.84 | 22 | G/B | Cataracts, xanthomas |
| 13 | NB1 | 16 | Unknown, last 8 years stored at –20°C | 0.01 | — | — | G/B | Juvenile cataracts, xanthomas, intractable diarrhea |
| 14 | NB2 | 4 | 4°C | 0.01 | 0.86 | 22 | G | None (see Ref. 10) |
NB, original neonatal screening DBS.
B, biochemical; G, genetic.
Materials
Solvents were methanol and acetonitrile purchased from Biosolve. 2H4-g-CDCA, 2H4-g-CA, 2H4-t-CDCA, and 2H4-t-CA were purchased from CDN Isotopes. Water was MilliQ® purified water. 5β-Pregnane-3α,20α-diol glucuronide (Pregnanediol glucuronide) was from Sigma-Aldrich, and filter paper Whatman 903 was purchased from Drukkerij PAL.
Sample preparation
A punch [quarter-inch (6.4 mm) diameter] of a DBS was used for all except for CTX DBS numbers 1–8, 12, and 13, for which two 3.2 mm DBSs were used (for these, the result was corrected by multiplying the result by a factor of two). The DBSs were transferred to a 1.5 ml tube, to which 500 μl methanol was added, and this was incubated for 30 min at room temperature in a sonicator bath (Branson 3510). The filter paper was removed, and the extraction fluid was transferred to a 4 ml glass tube followed by the addition of 20 μl internal standard mixture [1 μM pregnanediol glucuronide, 0.25 μM 2H4-t-CA, 0.25 μM 2H4-t-CDCA, 0.25 μM 2H4-g-CA, 0.25 μM 2H4-g-CDCA dissolved in methanol/water (3:1 v/v)]. The internal standard solution was intentionally not added during the sonication step because this solution contains water, which extracts unwanted salts/interfering compounds that interfere with the MS measurement. After vortex mixing, the sample was taken to dryness under nitrogen flow at 40°C. The residue was reconstituted in 120 μl methanol/water (3:1 v/v), transferred to a sample vial, and capped.
Flow injection MS/MS
We injected 10 μl acetonitrile/water (9:1 v/v) as a mobile phase into a Waters Premier-XE tandem mass spectrometer (MS/MS) (Waters Cooperation) operated in the negative ion electrospray ionization mode. Nitrogen was used as both the nebulizing and the desolvation gas. The collision gas was argon, and the cell pressure was 2.7 × 10−3 mbar. The source temperature was set at 120°C, and the capillary voltage was maintained at −2.5 kV. The detector was used in tandem MS mode with multiple-reaction monitoring to detect the transition of a specific precursor ion to a fragment for each analyte. The transitions, cone voltages, and collision energies established for each compound are listed in Table 2. The analytical run time, from injection to injection, was 2 min. Quantification was performed with Masslynx 4.2 using the NeoLynx™ program. NeoLynx combined and averaged the individual multiple reaction monitoring transition intensities across the flow injection profile and subsequently corrected for background signal by subtracting the average intensity prior to sample infusion. The resulting peak heights of the measured compounds were exported for further calculations in MS Excel (Microsoft).
TABLE 2.
Transitions, cone voltages, and collision energies for measured compounds
| Compound | MRM (m/z) | Cone Voltage (V) | Collision Energy (eV) |
| t-CDCA | 498→80 | 90 | 60 |
| 2H4-t-CDCA | 502→80 | 90 | 60 |
| t-CA | 514→80 | 90 | 60 |
| 2H4-t-CA | 518→80 | 90 | 60 |
| t-THCA | 556→80 | 90 | 60 |
| g-CDCA | 448→74 | 60 | 40 |
| 2H4-g-CDCA | 452→74 | 60 | 40 |
| g-CA | 464→74 | 60 | 40 |
| 2H4-g-CA | 468→74 | 60 | 40 |
| Pregnanediol glucuronide | 495→75 | 45 | 35 |
| Cholestane tetrol glucuronide | 611→75 | 80 | 48 |
MRM, multiple reaction monitoring.
RESULTS
Cholestanetetrol glucuronide
Cholestanetetrol glucuronide (tetrol) is the most abundant bile alcohol glucuronide in plasma of CTX patients and has been shown to predominantly consist of 5β-cholestane-3α,7α,12α,25-tetrol glucuronide (15). We first investigated whether the measurement of the tetrol alone was sufficient to discriminate untreated newborn (and adult) CTX DBSs from control newborn DBSs. No stable isotope-labeled internal standard is available for the tetrol, and several commercially available candidates were tested as internal standards, namely 2H4-t-CDCA, 2H4-g-CDCA, 2H4-t-CA, 2H4-g-CA, and pregnanediol glucuronide (supplemental Fig. S1). Tetrol response was clearly elevated in most patients but was not completely separated from the term/preterm controls. The DBS age and storage conditions appeared to influence the results because the oldest CTX newborn DBS had the lowest tetrol levels. As previously reported, Zellweger patients and patients with cholestatic liver disease also have elevated levels of bile alcohols (16, 17), and in this experiment the Zellweger patients could not be distinguished from CTX by solely measuring the tetrol response, regardless of which internal standard was used (supplemental Fig. S1).
Metabolite ratios
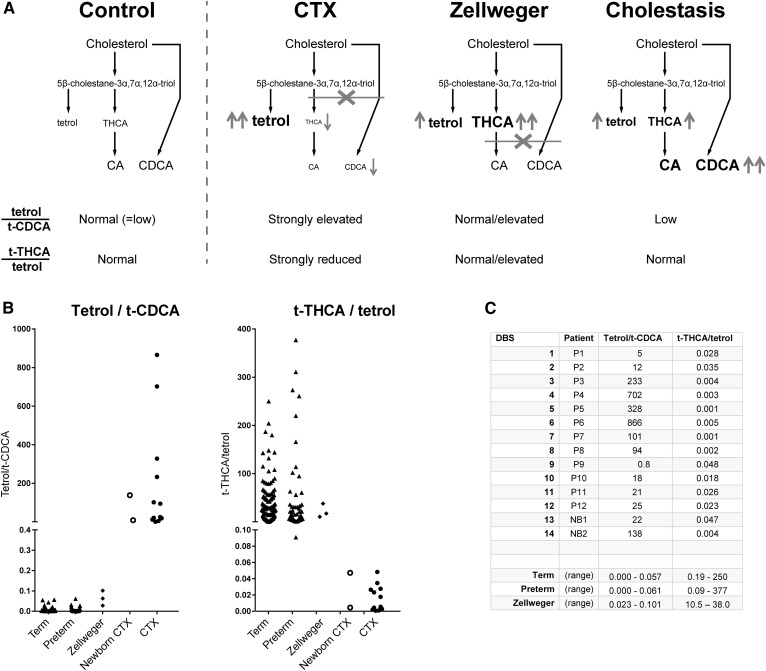
The determination of the concentration of four bile acids (t-CA, g-CA, t-CDCA, g-CDCA) and the taurine conjugate of the bile acid intermediate trihydroxycholestanoic acid (t-THCA) were added to the protocol to investigate whether specific metabolite ratios would enable discrimination of the CTX patients from the Zellweger patients and patients with other causes of cholestatic liver disease. Supplemental Fig. S2 shows that these five metabolites could be measured in DBSs using the corresponding stable isotope-labeled internal standards. As was expected, bile acid and t-THCA concentrations in CTX patients were at the low end of the control range, and t-THCA clearly accumulated in Zellweger patients. To enhance sensitivity, we selected the tetrol over t-CDCA ratio (tetrol:t-CDCA), which is expected to be strongly elevated in CTX. To enhance specificity for CTX and exclude Zellweger patients, we selected the t-THCA over tetrol ratio (t-THCA:tetrol), because this is expected to be strongly reduced in CTX and not in Zellweger syndrome. An additional benefit of this ratio is that it will also be normal in cholestatic liver disease, further enhancing specificity for CTX. Figure 1 shows the rationale of the two ratios (Fig. 1A) as well as the results of the metabolite ratios for term (n = 150) and preterm (n = 50) controls, Zellweger patients (n = 3), and CTX patients (n = 14) (Fig. 1B, C).
Fig. 1.
Ratios to enhance selectivity and specificity of the CTX screening assay. A: Rationale of the two selected ratios. Tetrol:t-CDCA ratio: In CTX, tetrol accumulates and CDCA is deficient, yielding a strongly elevated ratio. In Zellweger, tetrol is elevated but CDCA is normal, resulting in a normal or slightly elevated ratio, whereas during cholestasis, CDCA (and CA) is elevated, keeping the ratio low. t-THCA:tetrol ratio: In CTX this ratio is strongly reduced, whereas it is normal or elevated in Zellweger and cholestasis. B: Tetrol:t-CDCA and t-THCA:tetrol ratios in term/preterm controls (triangles), Zellweger (diamonds), newborn CTX, and untreated CTX patients (open/solid circles). The tetrol:t-CDCA ratio is highly discriminative, as it is for Zellweger and CTX. The t-THCA:tetrol ratio also discriminates Zellweger and CTX. C: Table of individual tetrol:t-CDCA and t-THCA:tetrol ratios for CTX patients and ranges for controls (term/preterm) and Zellweger patients.
With the tetrol:t-CDCA ratio, 100% separation was achieved between the CTX patients, both with DBS from newborns and from older patients, and control (term/preterm) subjects and Zellweger patients. The second ratio, t-THCA:tetrol, could also distinguish CTX DBSs from the three Zellweger syndrome DBSs and control DBSs. Other possible ratios (with other primary bile acid conjugates) are shown in supplemental Fig. S3, but these were less discriminative than the tetrol:t-CDCA or the t-THCA:tetrol ratio.
A series of DBSs of a CTX patient (P9) with a tetrol:t-CDCA ratio at the low end of the spectrum was used to investigate the precision of the metabolite ratios for which all replicates were processed from separate DBSs. For a DBS assay, the interassay precision of the tetrol:t-CDCA ratio was acceptable at 14% (0.64 ± 0.09, n = 10). The interassay precision of the t-THCA:tetrol was higher (63%) because of a low t-THCA concentration in the CTX sample, resulting in low t-THCA:tetrol values (0.07 ± 0.04, n = 10), but this variation was small in comparison with the difference between control and CTX DBSs and is therefore not expected to influence the specificity of the method for CTX.
Cholestasis
To investigate the effects of hypercholanemia, one of the hallmarks of cholestasis, on the metabolite ratios, we calculated total bile acid levels by summation of t-CA, g-CA, t-CDCA, and g-CDCA concentrations, results of which are shown in supplemental Fig. S4. Defining hypercholanemia as a total bile acid level of >100 μM [on the basis of summation of average blood spot bile acid concentrations previously determined in a group of cholestatic newborns (18)], 13 controls (eight term, five preterm) were hypercholanemic. Because all control DBSs were from anonymized term and premature newborns, clinical information was not available as to the etiology of this hypercholanemia.
The hypercholanemic controls could be accurately separated from the CTX patients using both the tetrol:t-CDCA ratio (hypercholanemic range: 0.000–0.018) and the t-THCA:tetrol ratio (hypercholanemic range: 2.5–311), thus enhancing the specificity for CTX by excluding subjects with hypercholanemia.
DISCUSSION
Cerebrotendinous xanthomatosis is a treatable neurodegenerative metabolic disorder that is most likely underdiagnosed (11, 12) and in which symptoms can be prevented if treatment with chenodeoxycholic acid is started early in life (7, 10). Therefore, newborn screening for CTX is warranted, if a suitable screening method is available. On the basis of different ratios between the accumulating cholestanetetrol glucuronide (5β-cholestane-3α,7α,12α,25-tetrol; tetrol) and specific bile acids/bile acid intermediates, we developed a new DBS screening assay for CTX that has the potential to be used for neonatal screening. Using our assay, a good separation was achieved between DBSs of CTX patients and DBSs of controls, Zellweger patients, and newborns with (potential) cholestasis by using ratios between the bile acid t-CDCA and tetrol and the bile acid intermediate t-THCA and tetrol. Initially, we set out to solely measure tetrol because this is the most abundant bile alcohol glucuronide in plasma in untreated CTX patients. However, despite a fairly good separation between the mean response in controls and CTX patients, there was overlap between the responses for the patient and control groups (see supplemental Fig. S1). In addition, other disorders such as Zellweger syndrome (16) and cholestatic liver disease (17) also lead to accumulation of tetrol or similar isobaric sterol alcohol glucuronides, as was also corroborated for the three Zellweger syndrome DBSs in this study, for which tetrol levels were elevated. Thus, because it lacks sensitivity and is not specific enough to detect only CTX patients, quantification of tetrol alone is unsuitable as a neonatal screening test for CTX.
To overcome this problem, we investigated whether calculation of specific metabolite ratios, designed with the biochemistry of CTX, Zellweger syndrome, and cholestatic liver disease in mind, could enhance both sensitivity and specificity of our screening assay. To avoid detecting Zellweger patients and solely identify CTX patients, but also to enhance the specificity of CTX detection with respect to cholestatic liver disease, we calculated the t-THCA:tetrol ratio because t-THCA accumulates in Zellweger syndrome and in cholestatic liver disease, whereas it is decreased in CTX (see Fig. 1A). Indeed, the t-THCA:tetrol ratio separated all CTX cases from the three Zellweger syndrome cases and controls in our relatively small pilot study (Fig. 1C). It should be noted that the Zellweger DBSs were of juvenile and adult patients and therefore may not be predictive of values found in neonatal Zellweger patients. However, neonatal DBSs of Zellweger patients are difficult to obtain because Zellweger spectrum disorders are not included in newborn screening programs because no curative treatment is available. Our goal is to obtain additional Zellweger DBSs to further establish the usefulness of this ratio to avoid detection of Zellweger syndrome or Zellweger spectrum patients.
To further enhance the specificity of our screening assay for CTX, we calculated the tetrol:t-CDCA ratio. It should be noted that as flow injection was used, the transition labeled as t-CDCA could also represent other dihydroxylated C24-tauro-conjugated bile acid species. In CTX, tetrol levels are high and t-CDCA levels are low, resulting in a markedly increased tetrol:t-CDCA ratio in CTX DBSs, whereas this ratio is expected to be (almost) normal in patients with Zellweger syndrome or decreased in patients with cholestatic liver disease. This ratio showed excellent separation between all CTX DBSs and the DBSs of all controls (Fig. 1B), including those with hypercholanemia, and Zellweger patients. The control with the highest tetrol:t-CDCA ratio was 13.1 times lower than the lowest CTX tetrol:t-CDCA, suggesting that this ratio is an excellent candidate biomarker for the detection of CTX patients in DBSs with newborn screening.
The sample workup of our method is straightforward because no derivatization is required, and because a standard methanol extraction is used, the assay can likely be combined with other neonatal screening assays. The measurement itself uses flow injection, which is the preferred measurement mode because it takes only 2 min per run. A possible disadvantage is that many MS newborn screening assays are performed in the positive ion mode, whereas our assay uses the negative ion mode. Because newborn screening programs are constantly being expanded, however, the negative ion mode eventually is going to be needed, and therefore this should not limit the implementation of this method for CTX newborn screening. We used a quarter-inch (6.4 mm) diameter DBS punch (for most CTX DBSs; two eighth-inch DBSs, which equals half the material in one quarter-inch DBS), whereas most neonatal screening laboratories prefer a single eighth-inch (3.2 mm) diameter DBS punch. Because the MS system used in this study was 10 years old, we anticipate that more recent triple quadrupole tandem mass spectrometers will readily detect the three metabolites used in our assay in eighth-inch DBS punches, because detection sensitivity of MS systems has greatly improved over the last decade. Conversely, our study also shows that laboratories that have older machines can still screen for CTX with our method when using a quarter-inch-diameter DBS punch (or two eighth-inch DBSs).
To establish the specificity/false positive rate of our method for the detection of CTX patients in newborn DBSs, it will be necessary to perform a large-scale pilot study on at least 100,000 newborn DBSs. We are currently in the process of setting up such a pilot study in collaboration with the partners of the Dutch neonatal screening program at the Dutch National Institute for Public Health and the Environment and with international partners. The results of this study will show whether our method indeed exhibits the high specificity needed for a newborn screening assay and whether it can be used as a one-tier screening method for the detection of CTX patients in newborn DBSs.
In conclusion, we devised a straightforward MS/MS method for the detection of CTX patients in DBSs without the need for derivatization. The results of this relatively small pilot study indicate that the tetrol:t-CDCA ratio is an excellent derived biomarker for CTX. The t-THCA:tetrol ratio also seems to be a promising marker; however, it may not even be needed because the tetrol:t-CDCA ratio appears to discriminate Zellweger syndrome from CTX and is not sensitive to the occurrence of cholestasis. A large-scale pilot is needed to assess the specificity/false positive rate of our method and will show whether it can be used as a one-tier newborn screening method for CTX.
Supplementary Material
Acknowledgments
The authors would like to thank F. C. C. Klouwer and B. T. Poll at the Academic Medical Center, Amsterdam, The Netherlands, for providing the Zellweger dried blood spots.
Footnotes
Abbreviations:
- CTX
- cerebrotendinous xanthomatosis
- DBS
- dried blood spot
- g-CA
- glycocholic acid
- g-CDCA
- glycochenodeoxycholic acid
- t-CA
- taurocholic acid
- t-CDCA
- taurochenodeoxycholic acid
- tetrol
- cholestanetetrol glucuronide = 5β-cholestane-3α,7α,12α,25-tetrol glucuronide
- t-THCA
- taurotrihydroxycholestanoic acid
This work was financially supported by a research grant of Sigma Tau Rare Disease LTD and a research initiation grant from ESN, a non-profit organization for professionals working in the field of inborn errors of metabolism in the Netherlands and Belgium.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Pierre G., Setchell K., Blyth J., Preece M. A., Chakrapani A., and McKiernan P.. 2008. Prospective treatment of cerebrotendinous xanthomatosis with cholic acid therapy. J. Inherit. Metab. Dis. 30: S241–S241. [DOI] [PubMed] [Google Scholar]
- 2.Verrips A., Hoefsloot L. H., Steenbergen G. C., Theelen J. P., Wevers R. A., Gabreëls F. J., van Engelen B. G., and van den Heuvel L. P.. 2000. Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain. 123: 908–919. [DOI] [PubMed] [Google Scholar]
- 3.Inoue K., Kubota S., and Seyama Y.. 1999. Cholestanol induces apoptosis of cerebellar neuronal cells. Biochem. Biophys. Res. Commun. 256: 198–203. [DOI] [PubMed] [Google Scholar]
- 4.Seyama Y. 2003. Cholestanol metabolism, molecular pathology, and nutritional implications. J. Med. Food. 6: 217–224. [DOI] [PubMed] [Google Scholar]
- 5.Salen G., Meriwether T. W., and Nicolau G.. 1975. Chenodeoxycholic acid inhibits increased cholesterol and cholestanol synthesis in patients with cerebrotendinous xanthomatosis. Biochem. Med. 14: 57–74. [DOI] [PubMed] [Google Scholar]
- 6.Berginer V. M., Salen G., and Shefer S.. 1984. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N. Engl. J. Med. 311: 1649–1652. [DOI] [PubMed] [Google Scholar]
- 7.Berginer V. M., Gross B., Morad K., Kfir N., Morkos S., Aaref S., and Falik-Zaccai T. C.. 2009. Chronic diarrhea and juvenile cataracts: think cerebrotendinous xanthomatosis and treat. Pediatrics. 123: 143–147. [DOI] [PubMed] [Google Scholar]
- 8.van Heijst A. F., Verrips A., Wevers R. A., Cruysberg J. R., Renier W. O., and Tolboom J. J.. 1998. Treatment and follow-up of children with cerebrotendinous xanthomatosis. Eur. J. Pediatr. 157: 313–316. [DOI] [PubMed] [Google Scholar]
- 9.Yahalom G., Tsabari R., Molshatzki N., Ephraty L., Cohen H., and Hassin-Baer S.. 2013. Neurological outcome in cerebrotendinous xanthomatosis treated with chenodeoxycholic acid: early versus late diagnosis. Clin. Neuropharmacol. 36: 78–83. [DOI] [PubMed] [Google Scholar]
- 10.Huidekoper H. H., Vaz F. M., Verrips A., and Bosch A. M.. 2016. Hepatotoxicity due to chenodeoxycholic acid supplementation in an infant with cerebrotendinous xanthomatosis: implications for treatment. Eur. J. Pediatr. 175: 143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appadurai V., DeBarber A., Chiang P-W., Patel S. B., Steiner R. D., Tyler C., and Bonnen P. E.. 2015. Apparent underdiagnosis of cerebrotendinous xanthomatosis revealed by analysis of ∼60,000 human exomes. Mol. Genet. Metab. 116: 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorincz M. T., Rainier S., Thomas D., and Fink J. K.. 2005. Cerebrotendinous xanthomatosis: possible higher prevalence than previously recognized. Arch. Neurol. 62: 1459–1463. [DOI] [PubMed] [Google Scholar]
- 13.Bleyle L., Huidekoper H. H., Vaz F. M., Singh R., Steiner R. D., and DeBarber A. E.. 2016. Update on newborn dried bloodspot testing for cerebrotendinous xanthomatosis: an available high-throughput liquid-chromatography tandem mass spectrometry method. Mol. Genet. Metab. Rep. 7: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBarber A. E., Luo J., Star-Weinstock M., Purkayastha S., Geraghty M. T., Chiang J. P-W., Merkens L. S., Pappu A. S., and Steiner R. D.. 2014. A blood test for cerebrotendinous xanthomatosis with potential for disease detection in newborns. J. Lipid Res. 55: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batta A. K., Salen G., Shefer S., Tint G. S., and Batta M.. 1987. Increased plasma bile alcohol glucuronides in patients with cerebrotendinous xanthomatosis: effect of chenodeoxycholic acid. J. Lipid Res. 28: 1006–1012. [PubMed] [Google Scholar]
- 16.Ferdinandusse S., and Houten S. M.. 2006. Peroxisomes and bile acid biosynthesis. Biochim. Biophys. Acta. 1763: 1427–1440. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa M., Une M., Takenaka S., Tazawa Y., Nozaki S., Imanaka T., and Kuramoto T.. 2001. Urinary bile alcohol profiles in healthy and cholestatic children. Clin. Chim. Acta. 314: 101–106. [DOI] [PubMed] [Google Scholar]
- 18.Mills K. A., Mushtaq I., Johnson A. W., Whitfield P. D., and Clayton P. T.. 1998. A method for the quantitation of conjugated bile acids in dried blood spots using electrospray ionization-mass spectrometry. Pediatr. Res. 43: 361–368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.