Abstract
Wound healing is the main problem in the therapy of anal fistula (AF). Daphne genkwa root has been traditionally used as an agent to soak sutures in operation of AF patients, but its function in wound healing remains largely unclear. The aim of the present study was to illuminate mechanisms of D. genkwa root treatment on AF. In the present study, 60 AF patients after surgery were randomly divided into two groups, external applied with or without the D. genkwa extractive. Wound healing times were compared and granulation tissues were collected. In vitro, we constructed damaged human skin fibroblasts (HSFs) with the treatment of TNF-α (10 μg/ml). Cell Count Kit-8 (CCK-8) and flow cytometry analysis were used to determine the effects of D. genkwa root extractive on cell viability, cell cycle and apoptosis of damaged HSFs. Furthermore, protein levels of TGF-β, COL1A1, COL3A1, Timp-1, matrix metalloproteinase (MMP)-3 (MMP-3) and MEK/ERK signalling pathways were investigated both in vivo and in vitro. Results showed that D. genkwa root extractive greatly shortens the wound healing time in AF patients. In granulation tissues and HSFs, treatment with the extractive significantly elevated the expressions of COL1A1, COL3A1, Timp-1, c-fos and Cyclin D1, while reduced the expression of MMP-3. Further detection presented that MEK/ERK signalling was activated after the stimulation of extractive in HSFs. Our study demonstrated that extractive from D. genkwa root could effectively improve wound healing in patients with AF via the up-regulation of fibroblast proliferation and expressions of COL1A1 and COL3A1.
Keywords: Anal fistula, Cell signaling pathways, Daphne genkwa, human skin fibroblasts, Wounds healing
Introduction
Anal fistula (AF) is an anorectum disease characterized as an abnormal communication between the anal glands and the perianal skin [1], which could cause leakage, discomfort and occasional pain in patients [2]. This disease mostly occurs in male patients and can be ascribed to excessive secretion of sex hormone [3]. Currently, surgery is the standard treatment, but it remains controversial to be an effective therapy for some potential risk factors, such as internal open wound, prolonged wound healing and susceptible infection [4–6]. Therefore, it is of great necessity to shorten the wound healing time and repair contaminated wounds after operation.
It has been reported that the basic pathology and physiology of wound healing includes three stages, including local inflammation response, cell proliferation and granulation tissue formation and tissue reconstruction, which determined the time and quality of wound healing [7]. The complex healing process consisted of interaction of structural protein, growth factors and protein kinase, as well as consequence of repaired cell proliferation, differentiation and apoptosis etc. [8]. Among these, granulation tissues played a crucial role in the process of wound healing. As the main cellular constituents of granulation tissues, fibroblast cells could regulate reparative process by secreting a variety of types of cytokines [9].
Daphne genkwa is a medicinal plant widely distributed in Yellow and Yangtze Rivers regions in China, which has already been used as traditional agent for anti-inflammation, tranquilization, analgesia and anticonvulsion [10]. It has been demonstrated that the root of D. genkwa constituted the majority of the secondary metabolites including flavonoids, coumarins and diterpenoids [11]. Previous studies presented that total flavonoids from the root of D. genkwa possessed profiles of anti-inflammatory [10], immunomodulatory [12], analgesic [13] and even antitumour activities [14]. However, the function of D. genkwa root on wound healing after operation in patients with AF remains unclear. Thus, the potential mechanism and activity of D. genkwa root need to be ascertained.
In the present study, we investigated the effects of D. genkwa root on cell proliferation, cell cycle and apoptosis of human skin fibroblasts (HSFs), as well as the mechanism underlying the biological functions. These findings might provide a meaningful basis for clinical application of D. genkwa on tissues repair of contaminated wound healing in patients with AF.
Materials and methods
Plant materials and extraction
D. genkwa was obtained from Nanjing University of Chinese Medicine. The roots of D. genkwa (5 kg) were well air-dried, chopped and extracted with double distilled water, as described previously [15]. The obtained root extractive from D. genkwa was used in the treatment of following tissues and cell lines.
Granulation tissue collection
Total 60 patients with AF (from 2015 to 2016) were enrolled from the Department of Anorectal Surgery in the First People’s Hospital of Lianyungang and randomly divided into two groups: treatment group and control group. All the patients accepted therapy of cutting with thread ligation, but for D. genkwa treated group, the sutures were soaked in D. genkwa root extractive (30 min, 90°C) and after operation, the extractive was smeared on the wound once a day (1 week). PBS was used in control group correspondingly. Informed written consent was taken from every patient. The present study was reviewed and approved by medical ethics committee of Affiliated Hospital of Nanjing University of Chinese Medicine. After 7 days, fresh granulation tissues were obtained from the surface of the wound in these patients. A part of granulation tissues were stored in liquid nitrogen for quantitative real-time PCR (qRT-PCR) and Western blotting. The other resected specimens were fixed in 10% formalin solution and embedded in paraffin for immunohistochemistry assay.
Cell lines and cell treatment
Cheloid HSFs (c-HSFs) and HSFs were purchased from the cell bank of Chinese Academy of Science, Shanghai. The c-HSFs were separated from cheloid. All cells were cultured in DMEM medium containing 20% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. Then, c-HSF cells were treated with 1, 5, 10, 25, 50 and 100 μg/ml of root extractive from D. genkwa. HSF cells were added with TNF-α (10 ng/ml) to build damaged cell model and also for the treatment by root extractive from D. genkwa. These cells were incubated at 37°C in humidified atmosphere of 5% CO2.
qRT-PCR assay
Total RNA from tissues and cells after 48 h cultivation was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer’s instructions. cDNA was synthesized from 1 μg of total RNA by using the Bestar qPCR RT Kit (DBI Bioscience, Ludwigshafen, Germany). Real-time PCR was performed on Stratagene Mx3000P Real time PCR platform (Agilent Technologies, New Castle, DE, U.S.A.) using total volume of 20 μl DBI Bestar® SybrGreen qPCR master Mix (DBI Bioscience, Ludwigshafen, Germany). The Β-actin was used as an internal control. Each sample was run in triplicate in three independent experiments. Relative quantification was determined by the method of 2−ΔΔCt. The primer sequences were as follows: COL1A1 (forward: 5′-GACGAAGACATCCCACCAATC-3′ and reverse: 5′-GGAGACCACGAGGACCAGAG-3′), COL3A1 (forward: 5′-GCTGGCATCAAAGGACATCG-3′ and reverse: 5′-CAACACCACCACAGCAAGGA-3′), TIMP-1 (forward: 5′-GGGGACACCAGAAGTCAACC-3′ and reverse: 5′-GCATTCCTCACAGCCAACAG-3′), matrix metalloproteinase (MMP)-3 (MMP-3) (forward: 5′-CCCTGATGTCCTCGTGGTA-3′ and reverse: 5′-GGTCCTGAGAGATTTTCGC-3′), Cyclin D1 (forward: 5′-CCCTCGGTGTCCTACTTC-3′ and reverse: 5′-TTTGCGGATGATCTGTTTGT-3′) and c-fos (forward: 5′-CCGAAGGGAAAGGAATAAGA-3′ and reverse: 5′-TGCTGGGAACAGGAAGTCA-3′).
Western blot assay
Total proteins were extracted from tissues and cells after 72 h cultivation by using lysis buffer (20 mM Tris/HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA) and quantified by Pierce BCA Protein Assay Reagent Kit (Thermo Fisher Scientific, Waltham, MA, U.S.A.) according to the manufacturer’s protocol. Equal amount of protein was separated on denaturing SDS gel and transferred to a PVDF membrane. The membrane was blocked with 10% skim milk and the incubated with primary antibodies overnight at 4°C, followed by incubation in appropriate horseradish peroxidase (HRP)–conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.). The membrane was washed twice with PBS and blots were then visualized by ECL system (Bio–Rad Laboratories, Hercules, CA, U.S.A.). GAPDH was used as loading control. The specific primary antibodies were as follows: anti-COL1A1, COL3A1, Timp-1, MMP-3, Cyclin D1, c-fos (Abcam, U.S.A.), MEK1/2 and p-MEK1/2, ERK1/2 and p-ERK1/2 (Cell Signaling, Beverly, MA, U.S.A.).
Immunohistochemistry
In brief, the sections from paraffin-embedded tissue were deparaffinized in xylene, rehydrated in ethanol, washed in PBS and blocked using 5% goat serum. Then, these sections were incubated with anti-COL1A1, COL3A1 and ERK1/2 (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) at 4°C overnight, followed by incubation with HRP–conjugated secondary antibody for 1 h at room temperature. Immunoreactivity was detected with the DAB kit (Vector Laboratories, Burlingame, CA, U.S.A.) and counterstained slightly with haematoxylin. Section images were captured using a microscope (Nikon, Chiyoda, Japan).
CCK-8 assay
Cell Count Kit-8 (CCK-8, Beyotime, Beijing, China) was used to measure the number of viable cells from different treatments. Briefly, cells were digested, resuspended and reseeded into 96-well plates. Then, 10% CCK-8 solution was added into each well. After 1-h incubation, the absorbance was determined on ELISA reader (Bio–Rad, Hercules, CA, U.S.A.) at a wavelength of 450 nm.
Flow cytometry analysis for cell cycle and apoptosis
Cells from different treatments were collected and seeded on 6-cm dishes at a density of 1 × 105 cells/dish. For cell-cycle analysis, cells were added in 0.7 ml of 70% ethanol and then stained by adding propidium iodide (PI) solution, followed by incubation for 30 min at room temperature. The suspension was filtered through a 50-mm nylon mesh and stained cells were analysed a FACSCalibur flow cytometer (BD, New Jersey, U.S.A.). For cell apoptosis, it was detected by Annexin V/PI apoptosis detection kit (MULTI SCIENCES, Hangzhou, Zhejiang, China) following manufacturer’s instructions.
Statistical analysis
The differences between groups were compared using Student’s t test and quantificative data were expressed as means ± S.D. of three independent experiments. Statistically significant difference was accepted at P<0.05.
Results
D. genkwa promoted wound healing and led to the expression change of COL1A1, COL3A1, TIMP-1, MMP-3, TGF-β, MEK1/2 and ERK1/2
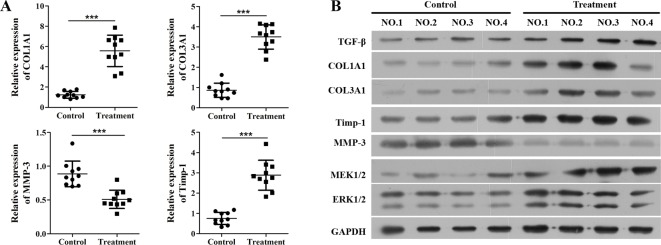
Comparison of curative effect between control group and D. genkwa-treated group revealed that D. genkwa significantly reduced the wound healing time (Table 1). To investigate the mechanism of D. genkwa on wound healing of AF patients, granulation tissues were obtained from the surface of wound for qRT-PCR and Western blot analysis. Since the level of collagen was evaluated for the phase of wound healing, TIMP-1 protein and MMP were associated with the degradation of extracellular collagen, our data determined that as shown in Figure 1A, the expressions of COL1A1, COL3A1, TIMP-1 mRNA were significantly up-regulated in granulation tissues, while the level of MMP-3 was obviously down-regulated after D. genkwa treatment (P<0.001). Similar results were also observed in Western blot analysis (Figure 1B). Furthermore, we detected the expression of TGF-β, MEK1/2 and ERK1/2 using Western blot and found that they were all up-regulated after treatment with D. genkwa (Figure 1B).
Table 1.
Comparation of curative effect between control group and D. genkwa-treated group
| Control | Treatment with D. genkwa | |
|---|---|---|
| Gender | ||
| Male | 22 | 23 |
| Female | 8 | 7 |
| Age (years) | 36.7 ± 3.5 | 35.4 ± 2.1 |
| Type | ||
| High complexity | 11 | 14 |
| Low complexity | 19 | 16 |
| History (years) | 3.1 ± 0.7 | 2.8 ± 0.8 |
| Recent curative effect (recovery) | 30 | 30 |
| Wound healing time (d) | 25.7 ± 3.4 | 18.1 ± 2.8* |
| Anal resting pressure after surgery (kPa) | 10.37 ± 2.23 | 10.88 ± 1.96 |
| Anal maximal contraction pressure after surgery (kPa) | 13.41 ± 3.88 | 15.89 ± 3.22 |
*P<0.05, wound healing time in this group was significantly shorter as compared with control group.
Figure 1. D. genkwa root extractives change extracellular matrix composition of patients’ granulation tissues.
Expression levels of COL1A1, COL3A1, Timp-1, MMP-3, TGF-β, MEK1/2 and ERK1/2 in granulation tissues. (A) Expression levels of COL1A1, COL3A1, TIMP-1 and MMP-3 in 15 paired granulation tissues by qRT-PCR analysis. The levels of COL1A1, COL3A1, Timp-1 were significantly up-regulated and the level of MMP-3 was statistically reduced after the treatment of root extracts. ***P<0.05 compared with controls, n=10. (B) Western blot analysis of COL1A1, COL3A1, Timp-1, MMP-3, TGF-β, MEK1/2 and ERK1/2 protein expression in representative four paired granulation tissues. The expressions of COL1A1, COL3A1, Timp-1, TGF-β, MEK1/2 and ERK1/2 proteins were obviously elevated while the expression of MMP-3 was remarkably decreased.
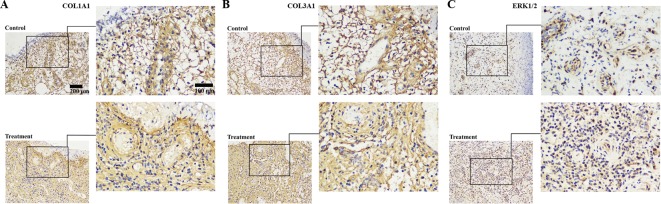
Because direct photos on private body parts were unavailable in practice, the immunohistochemical detection of collagen in the wound tissue was an indirect evaluation of wound healing instead. To verify this observation, we further examined the expression of COL1A1, COL3A1 and ERK1/2 in granulation samples by immunohistochemical staining. The results indicated that most of the granulation tissues showed positive staining of COL1A1, COL3A1 and ERK1/2 proteins after D. genkwa treatment compared with controls (Figure 2).
Figure 2. Analysis of COL1A1, COL3A1 and ERK1/2 proteins in granulation tissues by immunohistochemistry.
COL1A1 (A), COL3A1 (B) and ERK1/2 (C) expression were obviously increased in D. genkwa-treated granulation tissues, when compared with non-treated controls. Positive cells were stained brown. ERK1/2- positive cells were increased in D. genkwa-treated granulation tissues.
D. genkwa root extractives favoured the proliferation of c-HSF cells
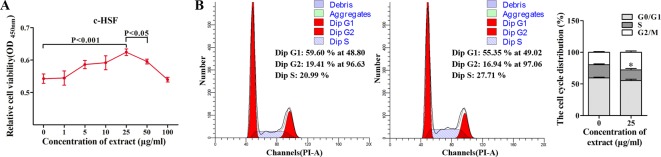
To further analyse the reparative process of D. genkwa root extractives in granulation tissues, c-HSF cells, as main constitutes of granulation tissues, were chosen to experiment in vitro. As shown in Figure 3A, 25 μg/ml D. genkwa root extractives caused remarkable promotion of c-HSF cell growth compared with the other concentration of D. genkwa. Additionally, no obvious side effect on the inflammation was found among patients with the treatment of the extract (25 μg/ml) though expressions of certain proteins were altered, compared with the patients with PBS. Based on this result, we chose 25 μg/ml D. genkwa root extractives as the optimal dose for the following assays. Flow cytometric analysis showed that treatment with 25 μg/ml extractives significantly decreased the arrest of cells at G1/S-phase of the cell cycle from 59.60%/20.99% in controls to 55.35%/27.71% in D. genkwa treatment group, indicating that a promoting role of D. genkwa on cell proliferation (Figure 3B).
Figure 3. Effects of D. genkwa root extractives on c-HSFs.
Effects of D. genkwa on the cell viability and cell cycle of c-HSFs, as determined by CCK-8 (A) and flow cytometry assay (B) respectively (*P<0.05, values are means of three replicates). The concentration of 25 μg/ml was optimal for the promotion of cell viability. The G1/S-phase of the cell cycle was decreased after the treatment of D. genkwa.
D. genkwa root induced the expression of collagen in fibroblasts
To examine the regulatory mechanism of D. genkwa, multiple signalling pathways were analysed in c-HSF cells. Consistent with the results of tissues, 25 μg/ml D. genkwa root extractives obviously elevated the expression of COL1A1, COL3A1, TIMP-1, Cyclin D1 and c-fos mRNAs, whereas reduced the expression of MMP-3 mRNA (Figure 4A, P<0.05). In addition, we also detected the protein levels of these molecules using Western blot. As shown in Figure 4B, except MMP-3, the expression of COL1A1, COL3A1, TIMP-1, Cyclin D1 and c-fos mRNA levels were obviously up-regulated in c-HSF cells after treatment with 25 μg/ml D. genkwa root extractives. As expected, 25 μg/ml D. genkwa root extractives notably promoted the activation of MEK/ERK pathway by up-regulating the phosphorylation of MEK1/2 and ERK1/2, indicating D. genkwa root extractives affected cell proliferation possibly via the activated MEK/ERK pathway (Figure 4C).
Figure 4. D. genkwa root extractives influence extracellular matrix composition of c-HSF cells througe inucing MEK/ERK signaling pathways.
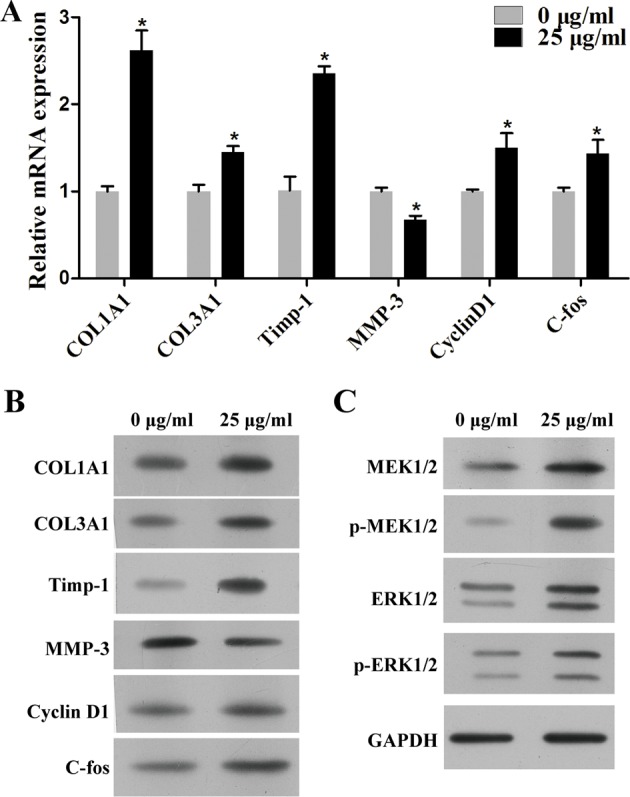
Mechanism study of D. genkwa in c-HSF cells. (A) qRT-PCR analysis of expression levels of COL1A1, COL3A1, Timp-1, MMP-3, Cyclin D1 and c-fos. The levels of COL1A1, COL3A1, Timp-1, Cyclin D1 and c-fos mRNAs were significantly increased while the level of MMP-3 was down-regulated. *P<0.05 compared with controls. Values are means of three replicates. (B) The protein levels of COL1A1, COL3A1, Timp-1, MMP-3, Cyclin D1 and c-fos in c-HSF cells after treatment with D. genkwa. (C) Western blotting analysis of MEK1/2, p-MEK1/2, ERK1/2 and p-ERK1/2. The protein levels of COL1A1, COL3A1, Timp-1, Cyclin D1, MEK1/2, p-MEK1/2, ERK1/2 and p-ERK1/2 were obviously up-regulated while MMP-3 was down-regulated after the treatment of extracts.
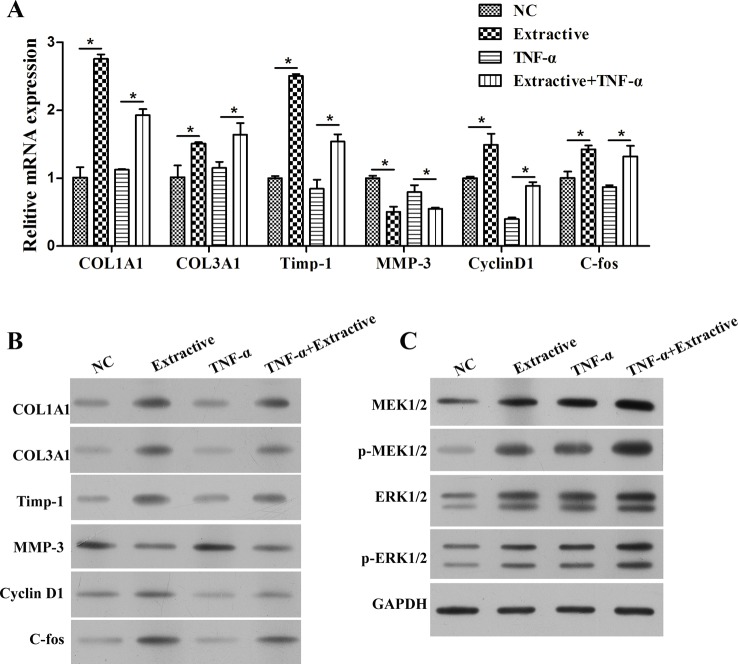
D. genkwa root extractives could protect HSF cells against the damage by TNF-α
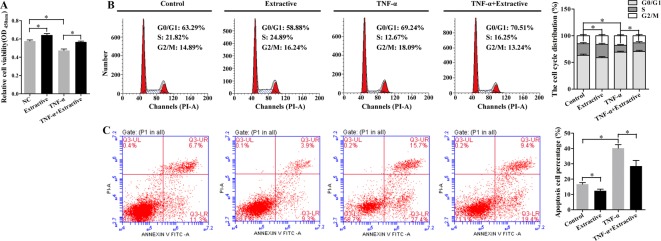
Next we investigated the effects of D. genkwa on impaired HSF cells. We constructed damaged HSF cell model by treating cells with TNF-α. As shown in Figure 5A, TNF-α, as a pro-inflammatory cytokine significantly reduced the cell viability compared with normal HSF cells (P<0.05). Interestingly, D. genkwa root extractives obviously elevated the relative cell viability of impaired HSF cells, suggesting that D. genkwa root extractives might protect impaired cells against TNF-α damage.
Figure 5. D. genkwa root extractives protect cells against TNF-α damage.
Effects of D. genkwa on HSF cells damaged by TNF-α. The cell viability (A), cell cycle (B) and apoptosis (C) of HSF cells treated with D. genkwa extractives, TNF-α, TNF-α + D. genkwa extractives respectively using CCK-8 assay and flow cytometry assay (*P<0.05 compared with control. Values are means of three replicates). The root extractives remarkably alleviated the suppression of cell proliferation, decreased cell early and late apoptosis by TNF-α.
D. genkwa extractives facilitated cell proliferation and suppressed cell apoptosis induced by TNF-α
Subsequently, we determined whether D. genkwa root extractives affected cell-cycle progression and apoptosis in impaired HSF cells. As shown in Figure 5B, TNF-α remarkably induced cell-cycle arrest at S-phase from 63.29%/21.82% in control group to 69.24%/12.67% in TNF-α group, indicating a negative effect of TNF-α on cell proliferation, but this cell-cycle arrest was notably alleviated by addition of D. genkwa extractives. Consistent with the result of cell cycle, TNF-α induced early apoptosis of cell from 11.3 to 27.4% and late apoptosis from 6.7 to 15.7%. Surprisingly, D. genkwa root extractives dramatically decreased cell early apoptosis from 27.4 to 19.4% and late apoptosis from 15.7 to 9.4% in impaired HSF cells (Figure 5C).
D. genkwa root extractives suppressed TNF-α induced wounding by regulating expression of molecules associated with healing
To further detect the molecular mechanism of D. genkwa on proliferation of impaired HSF cells, we also measured the expression of extracellular matrix (ECM) and MEK/ERK signalling pathways. As shown in Figure 6A, soluble collagen (COL1A1 and COL3A1), Timp-1, Cyclin D1 and c-fos were all significantly down-regulated in HSF cells induced by TNF-α, but obviously up-regulated under the existence of D. genkwa root extractives (P<0.05). Similar results were verified using Western blot analysis (Figure 6B). In addition, the activation of MEK/ERK signalling pathways further demonstrated the protective roles of D. genkwa root extractives for the repair of TNF-α-damaged HSF cells, as revealed by the up-regulation of p-MEK1/2 and p-ERK1/2 (Figure 6C).
Figure 6. Mechanism study of D. genkwa extractives in HSF cells after treatment with TNF-α.
(A) qRT-PCR analysis of expression levels of COL1A1, COL3A1, Timp-1, MMP-3, Cyclin D1 and c-fos, *P<0.05 compared with controls or TNF-α group. Values are means of three replicates. (B) The protein levels of COL1A1, COL3A1, Timp-1, MMP-3, Cyclin D1 and c-fos in HSF cells. (C) Western blotting analysis of MEK1/2, p-MEK1/2, ERK1/2 and p-ERK1/2. The root extractives obviously increased the levels of COL1A1, COL3A1, Timp-1, Cyclin D1, c-fos, MEK1/2, p-MEK1/2, ERK1/2 and p-ERK1/2 and decreased MMP-3 level affected by TNF-α.
Discussion
In our study, thread-drawing method was used for the therapy of AF. The operation line in the experimental group was presoaked in the roots extract of D. genkwa (boiled) and the root extract was also applied outside the wound site. The operation line boiled with the extract followed the way of traditional Chinese medicine. D. genkwa root has been reported to possess antitumour and anti-inflammatory activities, but its effects on wound healing remain largely unknown. The key finding of the present study was the root extractives of D. genkwa could promote wounding healing by accelerating granulation tissue formation and facilitating fibroblast cell proliferation via multiple signalling pathways. These results suggested that D. genkwa root functioned as a positive factor during the wound healing process.
Further investigation indicated that the root extractives of D. genkwa elevated the expressions of type I collagen (COL1A1 and COL3A1), TIMP-1, c-fos and Cyclin D1 at mRNA and protein levels in granulation tissues and fibroblast cells. As we all know, ECM was involved in directing epithelial cell functions [16]. As a major structural component of ECM, type I collagen played an important role in remodelling ECM microenvironment [17,18]. In our results, up-regulation of COL1A1 and COL3A1 showed D. genkwa might enhance the function of ECM by regulating type I collagen. Activating protein-1 (AP-1) mainly participated in JNK stress pathway via the regulation of cell growth, differentiation and apoptosis [19]. c-fos has been identified as a major component of AP-1 complex and implicated in signal transduction, cell proliferation and angiogenesis [20,21]. Additionally, mouse fibroblast cell lines deficient in c-fos was found to be more sensitive to radiation by which cell apoptosis was intensified [22,23]. We also observed Cyclin D1 was up-regulated by the root extractives of D. genkwa, which was closely associated with cell cycle at G0/G1-phase [24] in fibroblast cells. As evidence showed that the induction of G1/S-phase arrest was engaged with cell apoptosis, our data validated that D. genkwa promoted the cell proliferation via inhibiting the arrests of cells at G1 /S-phase. It has been demonstrated that MMPs belonged to a family of metal-dependent proteolytic enzymes that degraded the ECM components and participated in bone remodelling, wound healing and apoptosis [25]. Timps were specific inhibitors of MMPs and there was a balance among them [26]. In agreement with the evidence, our result indicated that D. genkwa significantly elevated the expression of Timp-1, but reduced the expression of MMP-3, which had a significant effect on wound healing.
We found D. genkwa elevated phosphorylation levels of MEK/ERK. Previous study showed that the classic MEK/ERK pathway was a key signal transduction component of cell proliferation in many cells, which contains a cascade of protein kinases: MEK and ERK [27]. Activation of MEK/ERK signalling pathway has been shown to contribute to cell growth, invasion and EMT [28,29]. These data further support our results that activation of MEK/ERK pathway was required for D. genkwa stimulated cell proliferation in granulation tissues as well as the growth of fibroblast cells. Interestingly, the total expressions of MEK and ERK were also elevated. For we cannot exclude difference of the total MEK/ERK of the clinical samples detected by WB, we will further analyse the effective components of the extract, and it is more appropriate to determine the mechanism of activation of MEK/ERK pathway with the monomer. However, in the present study, we additionally built damaged cell model of HSF and also determined the effects of D. genkwa on these damaged cells. Consistently, we found D. genkwa could effectively protect cells against the damage of TNF-α and reverse the inhibition of cell growth and induction of apoptosis, via activating MEK/ERK signalling pathways and ECM components. In summary, we demonstrated for the first time that D. genkwa root played a crucial role in wound healing via protecting granulation tissues and fibroblast cells. We also found the function of D. genkwa root in wound healing had a close relation with ECM components and MEK/ERK signalling pathway. The extracts induced the expression of Timp-1 protein and inhibited the degradation of extracellular collagen by MMP. The deposition of collagen itself was also considered as the manifestation of wound healing, along with the activation of MEK/ERK signalling pathway in the promotion of skin fibroblast proliferation, which together facilitated the fibrosis of wound healing and led to wound closure.
As the difference of treatment between AF wound and ordinary wound was the source of infection, the wound healing difficulty of AF was mainly due to contamination of Escherichia coli in faeces. However, the specific bacteriostatic effect of the extracts needs to be further investigated. Besides, the clinical value of these findings requires to be evaluated before D. genkwa root was applied to shorten the period of wound healing in patients with AFs. The exact mechanism of D. genkwa on the regulation of COL1A1 and COL3A1 needs further studies such as using k/o experiments to be clarified. The application of D. genkwa in wound healing still requires the official approval such as Food and Drug Adminisration (FDA).
Taken together, our preliminary data demonstrated the therapeutic effect of D. genkwa on AF and showed that root extractive from D. genkwa benefited the wound healing through up-regulation of collagen genes in HSFs.
Abbreviations
- AF
anal fistula
- AP-1
activating protein-1
- CCK-8
cell count kit-8
- c-HSF
cheloid human skin fibroblast
- COL1A1
collagen type I alpha 1 chain
- COL3A1
collagen type III alpha 1 chain
- ECM
extracellular matrix
- ERK
extracellular regulated protein kinases
- HRP
horseradish peroxidase
- HSF
human skin fibroblast
- MEK
mitogen-activated protein kinase kinase
- MMP
matrix metalloproteinase
- PI
propidium iodide
- qRT-PCR
quantitative real-time PCR
- TNF
tumour necrosis factor
- TGF-β
transforming growth factor beta
- Timp
tissue inhibitor of metalloproteases
Funding
This work was supported by the Science and Technology Project from Jiangsu Provincial Bureau of Traditional Chinese Medicine [grant number Y132015022].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Author contribution
D.Y. and R.-j.S. conceived and designed the paper. D.Y. and J.-h.X. collected and analysed the data. D.Y. drafted and revised the article. All authors read and approved the final manuscript.
References
- 1.Scoglio D., Walker A.S. and Fichera A. (2014) Biomaterials in the treatment of anal fistula: hope or hype? Clin. Colon Rectal Surg. 27, 172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundqvist A., Ahlberg I., Hjalte F. and Ekelund M. (2016) Direct and indirect costs for anal fistula in Sweden. Int. J. Surg. 35, 129–133 [DOI] [PubMed] [Google Scholar]
- 3.Zanotti C., Martinez-Puente C., Pascual I., Pascual M., Herreros D. and Garcia-Olmo D. (2007) An assessment of the incidence of fistula-in-ano in four countries of the European Union. Int. J. Colorectal Dis. 22, 1459–1462 [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Aguilar J., Belmonte C., Wong W.D., Goldberg S.M. and Madoff R.D. (1996) Anal fistula surgery. Factors associated with recurrence and incontinence. Dis. Colon Rectum 39, 723–729 [DOI] [PubMed] [Google Scholar]
- 5.Hanley P.H. (1965) Conservative surgical correction of horseshoe abscess and fistula. Dis. Colon Rectum 8, 364–368 [DOI] [PubMed] [Google Scholar]
- 6.Thompson J.E. Jr, Bennion R.S. and Hilliard G. (1989) Adjustable seton in the management of complex anal fistula. Surg. Gynecol. Obstet. 169, 551–552 [PubMed] [Google Scholar]
- 7.Hall J.F., Bordeianou L., Hyman N., Read T., Bartus C., Schoetz D. et al. (2014) Outcomes after operations for anal fistula: results of a prospective, multicenter, regional study. Dis. Colon Rectum 57, 1304–1308 [DOI] [PubMed] [Google Scholar]
- 8.Singer A.J. and Clark R.A. (1999) Cutaneous wound healing. N. Engl. J. Med. 341, 738–746 [DOI] [PubMed] [Google Scholar]
- 9.Steinbrech D.S., Longaker M.T., Mehrara B.J., Saadeh P.B., Chin G.S., Gerrets R.P. et al. (1999) Fibroblast response to hypoxia: the relationship between angiogenesis and matrix regulation. J. Surg. Res. 84, 127–133 [DOI] [PubMed] [Google Scholar]
- 10.Zheng W.-f., Wang L. and Shi F. (2004) Anti-inflammatory activity of ethanol extracts from root of Daphne genkwa. Chinese Traditional Herbal Drugs 35, 1262–1269 [Google Scholar]
- 11.Zheng W.F. and Shi F. (2005) Three biflavonoids from ethanol extract of the root of Daphne genkwa. Yao Xue Xue Bao 40, 438–442 [PubMed] [Google Scholar]
- 12.Zheng W.-F., Li W., Feng S. and Cui G.-Y. (2004) Modulatory effects on cell immunity of mice by total flavonoids from radix Daphne genkwa. Pharmaceutical J. Chinese Peoples Liberation Army, 4, 1–5 [Google Scholar]
- 13.Li W., Weifa Z., Jianhua W., Guiyou C. and Yanqiu X. (2005) The study on the analgesic effects and mechanism of the total flavonoids from Daphne genkwa Sieb et. Zucc. Ningxia Medical J. 27, 21–23 [Google Scholar]
- 14.Carlo G.D., Mascolo N., Izzo A.A. and Capasso F. (1999) Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 65, 337–353 [DOI] [PubMed] [Google Scholar]
- 15.Zheng W., Gao X., Gu Q., Chen C., Wei Z. and Shi F. (2007) Antitumor activity of daphnodorins from Daphne genkwa roots. Int. Immunopharmacol. 7, 128–134 [DOI] [PubMed] [Google Scholar]
- 16.Lu P., Weaver V.M. and Werb Z. (2012) The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aitken K.J. and Bagli D.J. (2009) The bladder extracellular matrix. Part I: architecture, development and disease. Nat. Rev. Urol. 6, 596–611 [DOI] [PubMed] [Google Scholar]
- 18.Egeblad M., Rasch M.G. and Weaver V.M. (2010) Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 22, 697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis R.J. (2000) Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252 [DOI] [PubMed] [Google Scholar]
- 20.Milde-Langosch K. (2005) The Fos family of transcription factors and their role in tumourigenesis. Eur. J. Cancer 41, 2449–2461 [DOI] [PubMed] [Google Scholar]
- 21.Akagi J., Egami H., Kurizaki T., Ohmachi H. and Ogawa M. (1997) Signal transduction pathway of the induction of cell motility in hamster pancreatic ductal adenocarcinoma cell. Invasion Metastasis 17, 16–25 [PubMed] [Google Scholar]
- 22.Lackinger D., Eichhorn U. and Kaina B. (2001) Effect of ultraviolet light, methyl methanesulfonate and ionizing radiation on the genotoxic response and apoptosis of mouse fibroblasts lacking c-Fos, p53 or both. Mutagenesis 16, 233–241 [DOI] [PubMed] [Google Scholar]
- 23.Lackinger D. and Kaina B. (2000) Primary mouse fibroblasts deficient for c-Fos, p53 or for both proteins are hypersensitive to UV light and alkylating agent-induced chromosomal breakage and apoptosis. Mutat. Res. 457, 113–123 [DOI] [PubMed] [Google Scholar]
- 24.Pham H.H., Seong Y.A., Oh C.W. and Kim G.D. (2016) The herbal medicine Cyperus amuricus inhibits proliferation of human hepatocellular carcinoma Hep3B cells by inducing apoptosis and arrest at the G0/G1 cell cycle phase. Int. J. Oncol. 49, 2046–2054 [DOI] [PubMed] [Google Scholar]
- 25.Nagase H. and Woessner J.F. Jr (1999) Matrix metalloproteinases. J. Biol. Chem. 274, 21491–21494 [DOI] [PubMed] [Google Scholar]
- 26.Balli U., Cetinkaya B.O., Keles G.C., Keles Z.P., Guler S., Sogut M.U. et al. (2016) Assessment of MMP-1, MMP-8 and TIMP-2 in experimental periodontitis treated with kaempferol. J. Periodontal Implant Sci. 46, 84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roovers K. and Assoian R.K. (2000) Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays 22, 818–826 [DOI] [PubMed] [Google Scholar]
- 28.Jiang L., Dong P., Zhang Z., Li C., Li Y., Liao Y. et al. (2015) Akt phosphorylates Prohibitin 1 to mediate its mitochondrial localization and promote proliferation of bladder cancer cells. Cell Death Dis. 6, e1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J., Qin G., Luo M., Chen J., Zhang Q., Li L. et al. (2015) Reciprocal positive regulation between Cx26 and PI3K/Akt pathway confers acquired gefitinib resistance in NSCLC cells via GJIC-independent induction of EMT. Cell Death Dis. 6, e1829. [DOI] [PMC free article] [PubMed] [Google Scholar]