Abstract
In this protocol, PAH is induced by combining a 60 mg/kg monocrotalin (MCT) injection with increased pulmonary blood flow through an aorto-caval shunt (MCT+Flow). The shunt is created by inserting an 18-G needle from the abdominal aorta into the adjacent caval vein. Increased pulmonary flow has been demonstrated as an essential trigger for a severe form of PAH with distinct phases of disease progression, characterized by early medial hypertrophy followed by neointimal lesions and the progressive occlusion of the small pulmonary vessels. To measure the right heart and pulmonary hemodynamics in this model, right heart catheterization is performed by inserting a rigid cannula containing a flexible ball-tip catheter via the right jugular vein into the right ventricle. The catheter is then advanced into the main and the more distal pulmonary arteries. The histopathology of the pulmonary vasculature is assessed qualitatively, by scoring the pre- and intra-acinar vessels on the degree of muscularization and the presence of a neointima, and quantitatively, by measuring the wall thickness, the wall-lumen ratios, and the occlusion score.
Keywords: Medicine, Issue 120, Pulmonary arterial hypertension, rat model, increased pulmonary flow, aorto-caval shunt/fistula, right heart catheterization, vascular morphology
Introduction
The goal of this method is to create a reproducible model for severe, flow-induced pulmonary arterial hypertension in rats and to measure its principle hemodynamic and histopathological end points.
Pulmonary arterial hypertension (PAH) is a clinical syndrome that encompasses a progressive increase in pulmonary vascular resistance leading to right ventricular failure and death. Within the superordinate disease spectrum of pulmonary hypertensive diseases (PH), PAH is the most severe form and one that remains without a cure1. The underlying arteriopathy in PAH is characterized by a typical form of vascular remodeling that occludes the vessel lumen. Muscularization of normal non-muscularized vessels and hypertrophy of the medial vessel layer are regarded as early disease phenomena in PAH, are also seen in other forms of PH2, and are thought to be reversible3. As PAH advances, the intimal layer begins to remodel, eventually forming characteristic neointimal lesions2. Neointimal-type pulmonary vascular remodeling is exclusive to PAH and is currently regarded to be irreversible4.
As PAH is a rare disease, advances in its pathobiological comprehension and development of novel therapies have relied heavily on animal models. The monocrotalin (MCT) model in rats is a simple single hit model that has been, and still is, used frequently. MCT is a toxin that causes injury to the pulmonary arterioles and regional inflammation5. 60 mg/kg MCT leads to an increase in the mean pulmonary artery pressure (mPAP), pulmonary vascular resistance (PVR), and right ventricular hypertrophy (RVH) after 3 - 4 weeks6. The histomorphology is characterized by isolated medial hypertrophy without neointimal lesions5. The MCT rat model thus represents a moderate form of PH, and not PAH, although it is commonly presented as the latter.
In children with PAH associated with a congenital left-to-right shunt (PAH-CHD), increased pulmonary blood flow is regarded as the essential trigger for the development of neointimal lesions7,8,9. In rats, increased pulmonary blood flow can be induced by the creation of a shunt between the abdominal aorta and the vena cava, a technique first described in 199010. Alternatives to create increased pulmonary flow are by unilateral pneumonectomy or by subclavian to pulmonary artery anastomosis11. Conceptual disadvantages of these models consist of potential compensatory growth of the remaining lung and adaptive pathway activation induced by the pneumonectomy, or of iatrogenic injury of the pulmonary vasculature due to pulmonary artery anastomosis, both confounding the effects of increased pulmonary blood flow.
When an aorto-caval shunt is created and increased pulmonary blood flow is induced as a second hit in MCT-treated rats, characteristic neointimal lesions occur, and a severe form of PAH and associated right ventricular failure (RVF) develop 3 weeks after the increased flow12. The hemodynamic progression of PAH in this model can be assessed in vivo by echocardiography and right heart catheterization. The vascular histomorphology, vessel wall thickness, degree of arteriolar occlusion, and parameters for right ventricular failure form the pillars of the ex vivo characterization of PAH.
This method describes detailed protocols for the aorto-caval shunt (AC-shunt) surgery, right heart catheterization, and qualitative and quantitative assessment of vascular histomorphology.
Protocol
Procedures involving animal subjects have been approved by the Dutch Central Committee for Animal Experiments and the Animal Care Committee at University Medical Center Groningen (NL). Both Wistar and Lewis rats with weights between 180 and 300 g were used.
1. Housing and Acclimatization
After arrival at the central animal facility, house rats in groups of 5 per cage. During a 7-day acclimatization period, accustom the rats to human handling, but do not perform any experimental procedures.
2. Preparation and Injection of Sterile Monocrotalin
For 1 mL of 60 mg/mL monocrotalin (MCT) solution, weigh 60 mg of monocrotalin in a 2-mL tube. Add 700 µL of 0.9% NaCl. Add 200 µL of 1 M HCl. Warm the solution in the tube under hot running tap water and vortex it. Use 6 N NaOH to bring the pH towards 7.0. Use sterile technique for preparation of MCT for injection into rodents.
Inject 1 mL of sterile 60 mg/mL MCT solution per kg subcutaneously in the neck (0.3 mL of 60 mg/mL MCT for a 300-g rat). NOTE: We prefer not to use smaller volumes due to the greater chance that the injected dose will not be appropriate.
3. Aorta-caval Shunt Surgery
- Anesthesia.
- Fill the induction chamber with 5% isoflurane/100% O2 (flow: 1 L/min) and place the rat in the chamber. Check for adequate depth of the anesthesia by performing a hind toe pinch. Weigh the rat.
- Shave and clean the abdomen over an area that is approximately 8 cm long and 3 cm wide. Place the rat on its back on a heat mat (37 °C) covered by a sterile mat.
- Place the snout in a ventilation mask/hood with 2 - 3% isoflurane/100% O2 (flow: 1 L/min). Check the depth of anesthesia by performing a hind toe pinch. Apply eye ointment to prevent dryness while under anesthesia.
- Shunt Surgery.
- Scrub the skin with chloride-hexidine for disinfection. Inject 0.01 mg/kg buprenorphine subcutaneously for post-operative analgesia.
- Use sterile instruments for surgery. Make an incision with a #10 scalpel blade in the abdomen on the midline, starting 1 cm below the diaphragm an extending down to just above the genitalia.
- Lift up the intestine with a cotton swab, cover the intestines in a sterile, wet gauze (0.9% NaCl), and place them to the left side of the animal.
- Use cotton swabs to separate the membranes that attach the abdominal aorta and the vena cava inferior to the surrounding tissues. NOTE: Do not dissect the membranes between the aorta and the vena cava.
- Using splinter forceps, remove the perivascular aortic fat just above the bifurcation, only on the right side of the aorta and only on the site where the needle will be inserted.
- Use cotton swabs to separate the aorta and vena cava from 2 mm superior to the site where the needle will be inserted in order to create space for a Biemer clamp.
- At this area, first place a loose ligature (5-0 suture) around the aorta. Create tension on the ligature by placing a Kocher clamp on it, and then place the Kocher superior to the incision (Figure 1A). Place the Biemer clamp just superior to the ligature (Figure 1A).
- Using a cotton swab, compress the vena cava as distally as possible to obstruct the flow (Figure 1A). Bend a needle (18 G in this protocol) into a 45-degree angle, with the orifice pointing towards the outside (Figure 1A).
- At an angle of 90 degrees, insert the needle in the aorta, just above the bifurcation, with the orifice of the needle pointing to the left (Figure 1A). Manipulate the tip of the needle to the left and insert it into the vena cava. NOTE: The needle tip should now be visible in the vena cava (Figure 1B).
- Use a second cotton swab to push the remaining blood in the aorta out of the insertion site to prevent thrombosis. Dry the area around the shunt with a sterile gauze in order for the glue to adequately stick.
- Pull the entire needle out of the aorta and immediately apply a drop of tissue glue onto the puncture site in the aorta. Make sure not to glue the cotton swab to the tissue. Unclamp the aorta.
- Verify the shunt manually by pulling on and releasing the ligature on the aorta proximal to the shunt. Loosening should color the vena cava distal to the shunt in bright red and create turbulence at the shunt site. NOTE: Tightening will turn the blood in the vena cava back to dark red.
- Place the intestines back in the animal. Close the muscle layer and skin with resorbable 4-0 sutures. Ventilate the animal with 100% O2 to recover from anesthesia. NOTE: Do not leave an animal unattended until it has regained sufficient consciousness to maintain sternal recumbency.
- Sham Surgery.
- Perform all of the above procedures except for the insertion of the needle into the aorta.
- Post-surgical care.
- Place the rat in a single cage and into an incubator at 37 °C until the next morning.
- Around 6 h after surgery, inject 0.01 mg/kg buprenorphine subcutaneously for post-operative analgesia. Repeat the next morning if the rat shows signs of discomfort. NOTE: The first 3 days after surgery, rats tend to eat and drink less (this is particularly important when chow or drinking water are mixed with drugs). Most rats show normal behavior 3 days after surgery. If not, monitor closely. Weight loss exceeding 15% in 1 week is considered abnormal, and such rats should be euthanized by the extraction of the circulating blood volume while under anesthesia.
4. Development of PAH
NOTE: In this protocol, the animal is euthanized by the extraction of the circulating blood volume while under anesthesia.
Sacrifice 1 day after the surgery (MF8) for the early cellular and functional responses to increased pulmonary blood flow (e.g., gene up-regulation or early transcription factors).
Sacrifice 1 week after the surgery (MF14) for an early-stage PAH vascular phenotype (medial hypertrophy without neointimal lesions).
Sacrifice 2 weeks after the surgery (MF21) for an advanced-stage PAH vascular phenotype (marked medial hypertrophy and neointimal formation) with mild elevation in RVP and mPAP.
Sacrifice 3 weeks after the surgery (MF28) for an end-stage PAH vascular phenotype (marked neointimal occlusion) and strong elevation in RVP and mPAP. Clinical signs of right ventricular failure are common in this stage.
Sacrifice after day 28 (MF-RVF) for PAH-associated right ventricular failure (RVF), clinically defined as dyspnea, severe lethargy, and weight loss (< 10% in 1 week). Terminate rats when one of these signs is present. Frequently, rats develop these symptoms between days 28 and 35 and, if left unguarded, die spontaneously during this time interval.
5. Right Heart Catheterization
- Anesthesia.
- Fill the induction chamber with 5% isoflurane/100% O2 (flow: 1 L/min) and place the rat in the box. Check for adequate depth of the anesthesia by performing a hind toe pinch. Weigh the rat.
- Shave and clean the neck at the right-ventral side of the rat and, for the echocardiography protocol, the thorax and upper abdomen.
- Place the rat on its back on a heat mat (37 °C) and place the snout in a ventilation mask/hood with 2 - 3% isoflurane/100% O2 (flow: 1 L/min). The snout should be faced towards the researcher.
- Check depth of anesthesia. Be careful with rats with severe PH. If the heart rate decreases, reduce the depth of anesthesia. Preferable, perform all measurements within 20 min. Apply eye ointment to prevent dryness while under anesthesia.
- Echocardiography protocol.
- Perform the echocardiography according to the protocol described by Brittain et al. in JoVE13.
- Catheterization protocol. NOTE: This protocol uses a rigid cannula with a preformed tip bent 20 degrees to guide the 15-cm silicon catheter with a ball 2 mm from the tip. A 20-G needle with its orifice slightly bent to the inside is used to insert the cannula into the right jugular vein (see the list of materials). Rats in any phase of PAH progression and control can be used in this protocol.
- Disinfect the neck with chloride-hexidine. Make a 1.5-cm incision with a #10 scalpel blade in the right-ventral side of the neck, from the right collar bone to the mandibular bone.
- Spread the tissue using scissors. Using tweezers, gently pull the tissue apart until the jugular vein appears. Dissect the membranes around the jugular vein using splinter forceps.
- Put tension on the jugular vein by placing a loose ligature (5-0 suture) around the vessel. Increase the tension and tape the ligature onto the ventilation mask (Figure 2A).
- Downstream of the insertion site, place a loose ligature around the vessel to tighten after the cannula is in situ in order to prevent leakage and loss of pressure.
- Using the handles of a forceps, slightly bend the tip of a 20-G needle with the orifice to the inside to conduct the cannula with the catheter.
- Introduce the tip of the 20-G needle into the vein and quickly place the cannula containing the catheter inside the vessel. Pull out the needle, and then close the ligature that was prepared in step 5.3.4.
- Conduct the cannula containing the catheter into the jugular vein. The tip of the cannula is at a 20-degree curve (see step 5.3.5). Maneuver the cannula under the collar bone and advance a little to enter the right atrium (Figure 2C).
- To enter the right ventricle, point the tip of the cannula to the left, towards the heart (Figure 2D). On the bedside monitor, an RV pressure curve should appear, matching Figure 2D.
- When the RV pressure curve is constant, write down the systolic and diastolic right ventricular pressure 1 (sRVP1/dRVP1).
- Manipulate the tip of the cannula to the left and upwards. Advance the catheter within the cannula (Figure 2E).
- Advance the catheter into the main pulmonary artery (PA). No resistance should be felt when passing the pulmonary valve. NOTE: When the catheter enters the main pulmonary artery, the diastolic pressure will rise. On the bedside monitor, a PA pressure curve should appear, matching Figure 2E.
- When the PA pressure curve is constant, write down the systolic, diastolic, and mean PA pressure 1 (sPAP1, dPAP1, mPAP1).
- Further advance the catheter within the cannula until the ball at the tip of the catheter gets wedged in a pulmonary artery. Observe the pressure curve on the bedside monitor drop and match the wedge pressure curve in Figure 2F.
- When the wedge pressure curve is constant, write down the systolic, diastolic, and mean wedge pressure.
- Pull back the catheter slowly and subsequently measure and write down the values for sPAP2, dPAP2, mPAP2, sRVP2, and dRVP2, as displayed on the bedside monitor.
- When in the RV, slightly pull back the cannula and catheter to measure the mean right atrial pressure (RAP). The curve should match the RAP curve in Figure 2A. NOTE: In this protocol, the rats are euthanized after the catheterization protocol by the extraction of the circulating blood volume while under anesthesia.
6. Morphology Assessment and Morphometry
NOTE: In this protocol, the animal is euthanized by the extraction of the circulating blood volume while under anesthesia. Rats in any phase of PAH progression and control can be used in this protocol.
After sacrifice, take out the lungs by cutting the trachea about 5 mm above the bronchial bifurcation and the vessels that connect the lungs to the heart. Put the lungs in cold saline. Dissect the left lung. Cut the left main bronchus at the bifurcation.
Fill a 50-mL syringe with 4% paraformaldehyde, attach a tube with a cannula to the syringe, and hang the syringe about a meter above the work table. Fit the cannula in the left main bronchus to passively fill the lung with paraformaldehyde. Handle paraformaldehyde with caution.
Incubate the left lung in paraformaldehyde for 48 h.
Dehydrate the left lung by incubating it consecutively in 70% ethanol (1 h), 80% ethanol (1 h), 90% ethanol (1 h), 100% ethanol (3 h), xylol (2 h), and paraffin (2 h).
Embed the left lung in paraffin, with the hilum of the lung facing the cassette.
Stain the paraffin-embedded, 4-µm lung sections using a Verhoeff or Elastica-van Gieson staining, as per manufacturer's instructions29. Make sure the elastic laminae are well differentiated (as in Figure 3). Scan the stained sections at 40X magnification.
Divide the lung into 4 quadrants. In each quadrant, find 10 vessels with an outer diameter < 50 µm (intra-acinar) and 10 vessels with an outer diameter > 50 µm (pre-acinar). Take a picture (2 x 40 pictures per lung). Zoom in randomly up to 20x magnification and photograph every vessel in this field of view to minimize selection bias.
Exclude vessels that have a longest/shortest diameter ratio of > 2, an incomplete circular shape, or a collapse of more than one quarter of the vessel wall. NOTE: An example of an excluded vessel is shown in Figure 3b Make each picture on the same magnification (40X) and include a scale bar.
Open ImageJ and the first picture. Draw a straight line on the scale bar in the picture to set the scale via "Analyse"and "Set scale." For "known distance," use the value on the picture's scale bar. Use micrometers (µm) as the unit of length. Set the scale to global.
Using "freehand selections,"draw a line on the inner border of the luminal area (Figure 3),and use "measure"(crtl m) to measure this area. Then, draw a line around the outer elastic lamina (Figure 3) to measure the total vessel area.
Calculate the luminal and outer diameter (
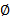 ) using
) using 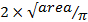 .
.Calculate wall thickness using
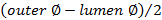 .
.Calculate the wall/lumen ratio using
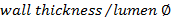 .
.Calculate the occlusion score using
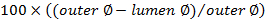 .
.Score the vessel on muscularization (no, partial, or total muscularization) (Figure 3B). NOTE: Vessels with a double elastic lamina for more than half the circumference are defined as totally muscularized. Vessels with a double elastic lamina less than half the circumference are defined as partially muscularized.
Score the vessel on the presence of a neointima (yes or no) (Figure 3C). NOTE: Vessels without a clearly-defined internal elastic lamina combined with (often eccentric) luminal occlusion are defined as neointimal lesions.
Representative Results
Representative results are presented in Figure 4. The presented results show characteristics of MCT+FLOW in Lewis rats in the following groups: Control (n = 3), MF8 (n = 5), MF14 (n = 5), MF28 (n = 5), and MF-RVF (n = 10). Statistical analyses were performed using the one-way ANOVA with Bonferroni correction.
60 mg/kg MCT and increased pulmonary blood flow lead to a mean rise in systolic right ventricular pressure (sRVP) (23 ± 6 to 56 ± 11 mmHg), systolic pulmonary artery pressure (sPAP) (20 ± 4 to 54.0 ± 10 mmHg), and mean pulmonary artery pressure (mPAP) (16 ± 3 to 36 ± 4 mmHg) at 28 days (MF28). They remain equally high up to the stage when right ventricular failure develops (MF-RVF) (Figure 4). At the early PAH stages (MF8 and MF14), no rise in sRVP, sPAP, and mPAP is observed. Diastolic PAP and right atrial pressure increase in the late phases, but not significantly. Wedge pressures do not change significantly during disease progression.
The right ventricular-to-left ventricular and septal weight ratio increases significantly from MF14 to MF-RVF, indicating right ventricular hypertrophy. The liver's wet-to-dry weight ratio is significantly increased at the MF-RVF stage, indicating liver edema and congestive right ventricular failure.
Muscularization of intra-acinar vessels < 50 µm increases progressively during PAH progression. Vessels of this size normally do not have a muscular medial layer in control rats. At MF14, almost half of these vessels (43 ± 17%) has a total muscular media (as in Figure 3B). At MF28 and MF-RVF, nearly every arteriole is muscularized (98.7 ± 2.5% and 100 ± 0%). Neointimal lesions first occur at MF21, while at MF28 and MF-RVF, around 65% of all arterioles have a neointimal layer (as in Figure 3C). The arteriolar wall-to-lumen ratio and occlusion scores both increase significantly from MF14 to MF28 (respectively, 10.4 ± 3.9 to 71.5 ± 30 (con: 7.1 ± 0.2) and 20.0 ± 2.8 to 54.7 ± 10.6 (con: 12.2 ± 0.3)). The hemodynamic and histomorphological characteristics of PAH progression in MCT+FLOW in Wistar rats are similar14.
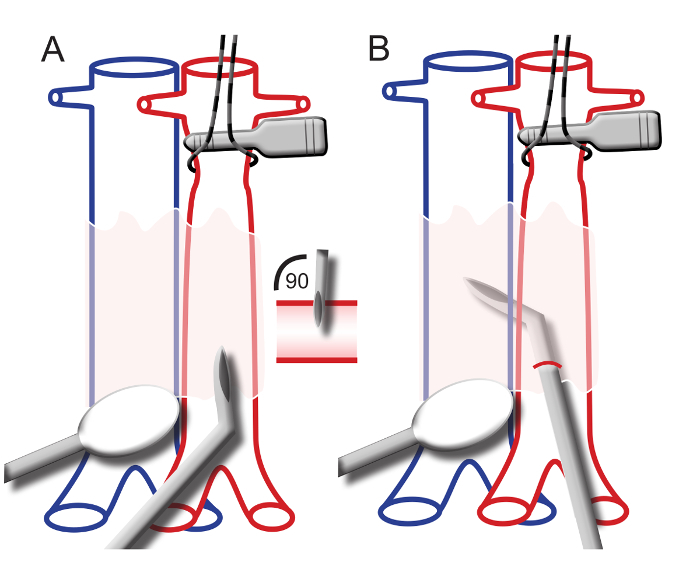 Figure 1. Schematic Representation of the Aorto-caval Shunt Surgery. A) The aorta is tensioned and clamped superior to the insertion site. The vena cava is compressed inferior to the insertion site. The needle, bent at 45° and with the orifice to the outside, is inserted into the aorta at a 90° angle. B) The needle is positioned in the aorta with the tip inserted into the vena cava. Please click here to view a larger version of this figure.
Figure 1. Schematic Representation of the Aorto-caval Shunt Surgery. A) The aorta is tensioned and clamped superior to the insertion site. The vena cava is compressed inferior to the insertion site. The needle, bent at 45° and with the orifice to the outside, is inserted into the aorta at a 90° angle. B) The needle is positioned in the aorta with the tip inserted into the vena cava. Please click here to view a larger version of this figure.
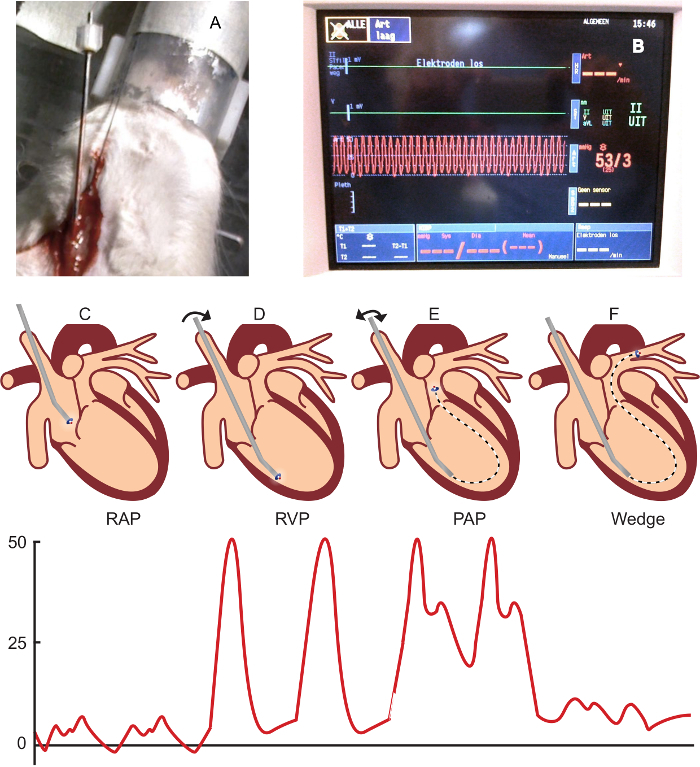 Figure 2. Right Heart Catheterization Procedure and Representative Pressure Curves. A) The right jugular vein is tensioned with a ligature and taped onto the ventilation mask. The catheter is placed into the jugular vein. B) A bedside monitor displaying a right ventricular pressure wave. C) The catheter within the cannula placed in the right atrium after introduction into the right jugular vein. Below: a typical right atrial pressure wave. D) The catheter within the cannula placed in the right ventricle. Below: a typical right ventricular pressure wave in end-stage PAH. E) The catheter is advanced in the cannula to enter the main pulmonary artery. Below: a typical pulmonary arterial pressure wave. F) The catheter is advanced into the pulmonary arteries until a wedge pressure wave is displayed on the monitor. Below: a typical pulmonary wedge pressure wave. Please click here to view a larger version of this figure.
Figure 2. Right Heart Catheterization Procedure and Representative Pressure Curves. A) The right jugular vein is tensioned with a ligature and taped onto the ventilation mask. The catheter is placed into the jugular vein. B) A bedside monitor displaying a right ventricular pressure wave. C) The catheter within the cannula placed in the right atrium after introduction into the right jugular vein. Below: a typical right atrial pressure wave. D) The catheter within the cannula placed in the right ventricle. Below: a typical right ventricular pressure wave in end-stage PAH. E) The catheter is advanced in the cannula to enter the main pulmonary artery. Below: a typical pulmonary arterial pressure wave. F) The catheter is advanced into the pulmonary arteries until a wedge pressure wave is displayed on the monitor. Below: a typical pulmonary wedge pressure wave. Please click here to view a larger version of this figure.
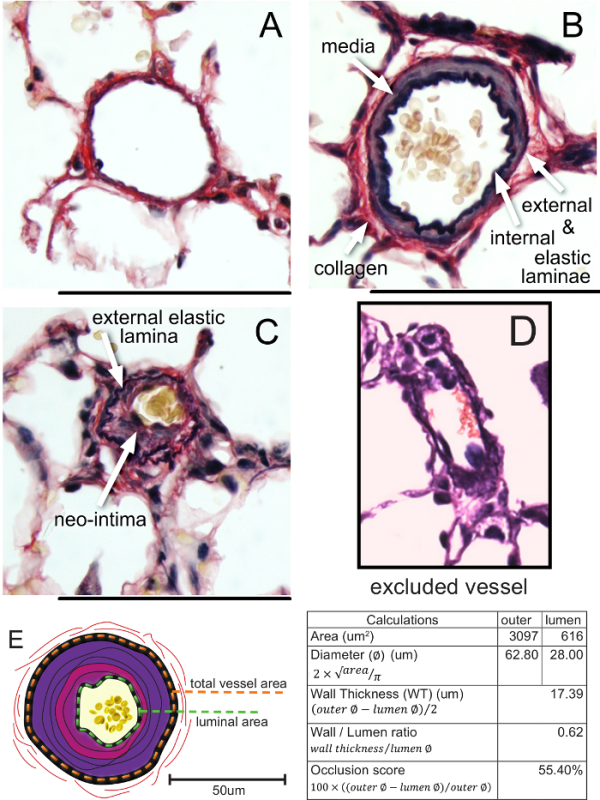 Figure 3. Vascular Morphology and Morphometry in Control and PAH Rats. A) A normal, non-muscularized vessel with an occlusion score of 3.7%. B) A totally muscularized arteriole with an occlusion score of 24.3%. C). A neointimal lesion with an occlusion score of 54.1%. D) An excluded vessel (longest/shortest diameter ratio of > 2 and an incomplete circular shape). E) The measurement of the total vessel and luminal area (in a vessel with a schematic representation of a neointimal lesion), including calculations. The bars represent 50 µm. Please click here to view a larger version of this figure.
Figure 3. Vascular Morphology and Morphometry in Control and PAH Rats. A) A normal, non-muscularized vessel with an occlusion score of 3.7%. B) A totally muscularized arteriole with an occlusion score of 24.3%. C). A neointimal lesion with an occlusion score of 54.1%. D) An excluded vessel (longest/shortest diameter ratio of > 2 and an incomplete circular shape). E) The measurement of the total vessel and luminal area (in a vessel with a schematic representation of a neointimal lesion), including calculations. The bars represent 50 µm. Please click here to view a larger version of this figure.
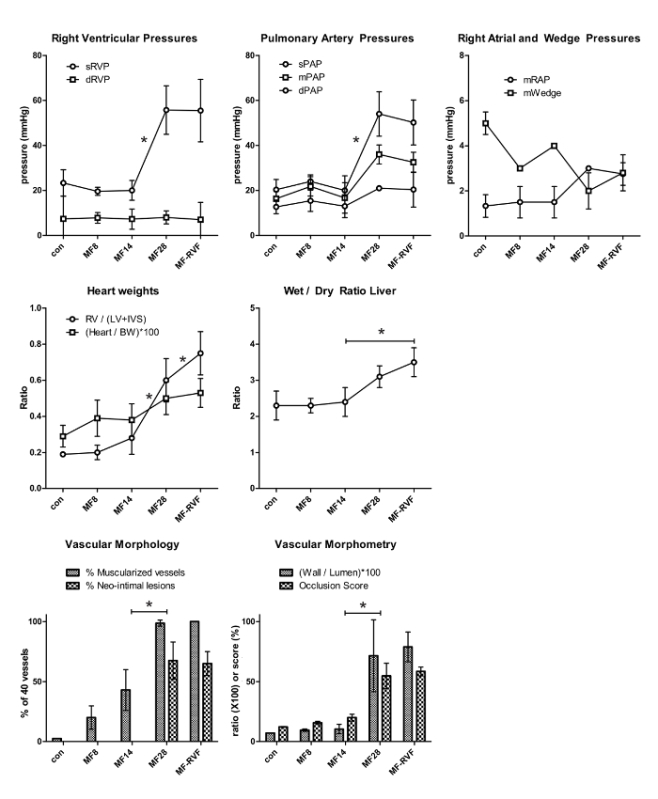 Figure 4. Representative Results of Pulmonary Hemodynamics and Vascular Morphology/Morphometry. The statistical analyses were performed using the one-way ANOVA with Bonferroni corrections. Values are represented as the mean ± SEM. con: control; MF (monocrotalin + flow); RVF: right ventricular failure; s: systolic; d: diastolic; m: mean; RVP: right ventricular pressure; PAP: pulmonary arterial pressure; RAP: right atrial pressure. RV: right ventricle; LV: left ventricle; IVS: interventricular septum; BW: body weight. Please click here to view a larger version of this figure.
Figure 4. Representative Results of Pulmonary Hemodynamics and Vascular Morphology/Morphometry. The statistical analyses were performed using the one-way ANOVA with Bonferroni corrections. Values are represented as the mean ± SEM. con: control; MF (monocrotalin + flow); RVF: right ventricular failure; s: systolic; d: diastolic; m: mean; RVP: right ventricular pressure; PAP: pulmonary arterial pressure; RAP: right atrial pressure. RV: right ventricle; LV: left ventricle; IVS: interventricular septum; BW: body weight. Please click here to view a larger version of this figure.
Discussion
This method describes the surgical procedure of an aorto-caval shunt in rats pre-treated with MCT to create flow-induced PAH and the techniques to assess the principle hemodynamic and histopathological end points that characterize PAH and this model.
Critical Steps within the Protocol and Troubleshooting
Surgery and post-surgery. During the aorto-caval shunt surgery, the most critical step is the dissection of the aorta and vena cava. The membranes that enclose the aorta and vena cava should be dissected enough to create 1) good visibility of the aortic area, where the needle will be inserted, and of the position of the needle in the vena cava after insertion and 2) sufficient space to clamp the aorta above the insertion site. The same membranes, however, are also used to conduct the aortic blood through the puncture site between both vessels (Figure 1). Dissecting the membranes too much will cause the shunt to leak. Tissue glue may solve the leakage, but it may then also seep into the shunt, compromising its size. When the glue has restricted flow through the shunt or either of the vessels, the glue can be removed gently, but rupture of the vena cava or of the membranes that conduct the shunt may occur. The size or adequacy of the shunt can be estimated by comparing the color difference and the degree of turbulence of the blood in the vena cava during compression and decompression of the proximal aorta with a cotton swab.
An 18-G needle has been shown to create an adequate shunt that results in a consistent and reproducible form of PAH progression in Lewis (this article) and Wistar (see Reference 14) rats and of right ventricular volume overload15. An 18-G needle created the most well-balanced shunt, with significantly increased pulmonary flow on the one hand and a low post-operative complication rate on the other hand.
The most common post-surgical problem is weight loss. Weight loss up to 10% in 1 week occurs in all rats after surgery, presumably due to lower intake the first few days after surgery. Rats are euthanized when the weight loss exceeds 15% in 1 week, as this is considered a sign of being unwell. Liquid chow can improve feeding in the first week after surgery. Rare postoperative complications are hind leg paralysis and bowel ischemia, which also result in euthanasia. In total, less than 5% of the rats had to be euthanized postoperatively.
Catheterization. Critical steps during the catheterization protocol start with the regulation of anesthesia. The depth of the anesthesia should be as minimal as possible (1.5 - 2% isoflurane in this protocol), as an increase in anesthetic depth appears to decrease right ventricular and pulmonary artery pressures, especially in rats with right ventricular failure. Measurements have a tendency to become unreliable when the protocol exceeds 20 min in duration.
The next critical step is the manipulation of the catheter in the RV and in the main pulmonary artery. This can be challenging. Flushing the catheter can help to curve the catheter in the outflow tract when the tip is stuck in the RV's trabeculae. The manipulation itself can cause RV dyskinesia, which shows irregular pressure curves on the bedside monitor. The introduction of the catheter into the right ventricle and the pulmonary artery should run smoothly. When the tip gets stuck at the pulmonary valve, a resistance is felt. Pressing through this resistance may cause the pulmonary valve to rupture, which limits the reliability of subsequent measurements.
In the present protocol, rats are sacrificed after the catheterization procedure. In theory, however, the jugular vein and surgical wound can be closed after the catheter is pulled out, as animals can live with only the remaining left jugular vein.
Morphometry. In the assessment of vascular wall thickness and occlusion scores, the most critical step is to identify the elastic laminae. From experience, the likelihood of success to this end is the greatest with a well-differentiated Verhoeff or Elastica-van Gieson staining. While the lumen can usually be discerned easily from the intima (to measure internal vascular area), distinction of the media from the adventitia may require a closer look (to measure the external vascular area). Some protocols measure intimal and medial thickness separately, defining the intima as the layer between the lumen and the internal elastic lamina, and the media as the layer between the internal and outer elastic lamina. This is usually possible in early-stage MCT+FLOW PAH. However, arterioles in advanced disease, particularly neointimal lesions, may display multiple elastic laminae and often lose the integrity of the elastic laminae (Figure 3C). In advanced lesions, the internal lamina elastica is therefore often difficult to identify, hindering the distinction between the media and the (neo-) intima. This reticulation of the intimal and medial layer prohibits separate layer thickness assessments. Therefore, the use of the total vessel wall thickness (to lumen ratio) and occlusion scores are preferred as an end point, instead of separate intimal and medial thicknesses.
Advantages and Limitations of Adding the Flow as a Trigger
The use of increased pulmonary blood flow to create PAH in rats has several advantages, the most prominent being that it is a known (patho-)physiological trigger for the disease, which favors translation to human PAH-CHD (Eisenmenger physiology), but also to other forms of PAH9. The model allows for the regulation of the flow by varying the size of the needle when creating the AC-shunt.
In human PAH-CHD, closure of the shunt will lead to the reversal of PAH in the early phase of disease, but to progression of PAH in advanced disease stages. Closure of the shunt in vivo would allow one to investigate the effect of removal of the trigger at different time points of disease progression and thus to investigate the mechanisms of (non-)reversal of PAH. Unfortunately, at present, shunt closure is not feasible in the current model. The effects of hemodynamic normalization (e.g., the removal of excess flow and the normalization of pulmonary artery pressure) in rats with flow-associated PAH can be investigated by transplantation of the affected left lung into a recipient rat with normal circulation. It has been shown previously that hemodynamic normalization, in rats by lung transplantation and in human PAH-CHD by closure of a cardiac shunt, leads to the regression of medial hypertrophy in early-stage PAH21. The effects of hemodynamic normalization in the advanced stages of experimental flow-PAH are currently unknown.
Significance with Respect to Alternative Models
The single-hit MCT model. A subcutaneous injection of 60 mg/kg MCT is a simple and effective way to create a model for pulmonary hypertension in rats. MCT induces pulmonary arterial endothelial cell injury, followed by hypertrophy of the muscular layer of the pulmonary arteries5. Although the exact mechanisms remain unclear, various pathways and growth factors have been identified that participate in medial hypertrophy following MCT. Pharmaceutical intervention upon these pathways has often successfully reduced medial hypertrophy and mPAP in MCT-rats. However, since medial hypertrophy is known to have a natural tendency to reverse in humans3 and has also been described to reverse spontaneously in MCT-rats16, the effect of these treatments should be appraised critically.
The double-hit MCT+FLOW model. The addition of increased pulmonary blood flow 7 days after MCT injection critically alters the (vascular) phenotype in a characteristic time-dependent fashion. At MF14 (7 days after the induction of increased flow), normally non-muscularized vessels start to develop a muscular medial layer. At MF21, the medial thickness increases and the first neointimal lesions occur. At MF28, a neointimal layer has developed in the majority of vessels. Between MF28 and MF35, most rats develop right heart failure and die of its sequellae. Previous studies in MCT+Flow rats have shown that the addition of flow to MCT leads to the activation of specific clusters of genes. In some clusters, flow opposed the effects induced by MCT; in others, flow enhanced these effects, and one cluster contained genes that were specifically up-regulated after flow17. One of those flow-specific genes is the early growth response-1 gene14 (Egr-1). Early inhibition of Egr-1 resulted in the attenuation of PAH and neointimal formation in MCT+Flow rats18. Egr-1 was also associated with neointimal remodeling in human PAH (PAH-CHD and idiopathic PAH)19. These observations add to the evidence that increased or disturbed pulmonary blood flow is an essential trigger for neointimal formation.
The single-hit flow-only model. In rats with an aorto-caval shunt without MCT-injection, pulmonary hypertension (mPAP > 25 mmHg) develops between 10 and 20 weeks after shunt induction20. At 20 weeks, the pulmonary vascular histology is dominated by medial hypertrophy of the pre-acinar arteries and neo-muscularization of the intra-acinar arterioles. Although some neointimal lesions have also been described in this model20, the development of these lesions needs to be confirmed and quantified.
The Sugen-Hypoxia model. Another common model for PAH with neointimal lesions is the Sugen5416-Hypoxia (SuHx) rat. Sugen5416 blocks the vascular endothelial growth factor (VEGF) receptor. This induces endothelial cell damage and a signaling cascade that, in combination with hypoxia, evokes endothelial apoptosis and proliferation22. After Sugen5416 injection, the rat is placed in a hypoxic chamber for 4 weeks, upon which PAH develops. The rat is then re-exposed to normoxia for 4 weeks. Pharmacological compounds that target endothelial apoptosis-resistance or the signaling cascades of TGF-B and BMP have shown the potential to reverse the neointimal lesions in this model23,24,25. A new variant of the SuHx model is the Sugen-pneumectomy model, which also results in severe PAH with neointimal lesions26. However, this model has not been fully characterized yet. A novel genetic method to induce PH in rats involves a mutation in the BMP-receptor-2 gene, which results in significant muscularization (PH) but no neointimal formation (PAH)27.
Comparable results have been reported regarding the number of neointimal lesions and the degree of luminal occlusion in the end-stage of untreated SuHx and MCT+Flow rats28. The main differences between both models are 1) that the mPAP in MCT+Flow progressively increases, whereas in SuHx, the mPAP has been shown to decrease gradually after re-exposure to normoxia28; 2) that the MCT+Flow model knows an early disease stage, characterized by medial hypertrophy and endothelial dysfunction; 3) that the time it takes both models to reach an end-stage where right ventricular failure begins to develop (4 weeks in MCT+Flow, 8 weeks in SuHx)28 is different; and 4) that Sugen5416 interferes in a molecular pathway (VEGF) whose role in the pathogenesis of PAH is still unclear. This may hinder the translation to human PAH.
Future Applications or Directions
The distinct disease phases of the MCT+Flow model allow one 1) to test the mechanisms of disease progression (human tissue in general is only available from post-mortem or explant procedures) and 2) to test interventions in different strategies. A preventive strategy could be initiated at the construction of the shunt (MF7). An early intervention can be initiated at MF14. This may be relevant as a treatment strategy prior to shunt closure in children with a congenital cardiac shunt and associated PAH that has progressed into the grey zone between reversible and irreversible disease. Reversal strategies can be initiated at MF21 or MF28. Later stages both show neointimal lesions, a manifestation of end-stage PAH.
In conclusion, the addition of increased pulmonary flow to MCT in rats creates a model of progressive and severe PAH that mimics human disease development. Right heart catheterization and the qualitative and quantitative assessment of the vascular histopathology form the cornerstones of the disease characterization in this and other models for PAH.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This study was supported by the Netherlands Cardiovascular Research Initiative, the Dutch Heart Foundation, the Dutch Federation of University Medical Centers, the Netherlands Organization for Health Research and Development, and the Royal Netherlands Academy of Sciences (CVON nr. 2012-08, PHAEDRA, The Sebald fund, Stichting Hartekind).
References
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- Stacher E, Graham BB, Hunt JM, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186(3):261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Maurey C, Celermajer DS, et al. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol. 2007;49(7):803–810. doi: 10.1016/j.jacc.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Sakao S, Tatsumi K, Voelkel NF. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2010;43(6):629–634. doi: 10.1165/rcmb.2009-0389TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Arroyo JG, Farkas L, Alhussaini AA, et al. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012;302(4):L363–L369. doi: 10.1152/ajplung.00212.2011. [DOI] [PubMed] [Google Scholar]
- Jones JE. Serial noninvasive assessment of progressive pulmonary hypertension in a rat model. Am J Physiol - Heart Circ Physiol. 2002;283(1):364–371. doi: 10.1152/ajpheart.00979.2001. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, Rudolph AM, Heymann MA. Pulmonary vascular disease with congenital heart lesions: Pathologic features and causes. Circulation. 1981;64(5):873–877. doi: 10.1161/01.cir.64.5.873. [DOI] [PubMed] [Google Scholar]
- van Albada ME, Berger RM. Pulmonary arterial hypertension in congenital cardiac disease--the need for refinement of the evian-venice classification. Cardiol Young. 2008;18(1):10–17. doi: 10.1017/S1047951107001849. [DOI] [PubMed] [Google Scholar]
- Dickinson MG, Bartelds B, Borgdorff MA, Berger RM. The role of disturbed blood flow in the development of pulmonary arterial hypertension: Lessons from preclinical animal models. Am J Physiol Lung Cell Mol Physiol. 2013;305(1):L1–L14. doi: 10.1152/ajplung.00031.2013. [DOI] [PubMed] [Google Scholar]
- Garcia R, Diebold S. Simple, rapid, and effective method of producing aortocaval shunts in the rat. Cardiovasc Res. 1990;24(5):430–432. doi: 10.1093/cvr/24.5.430. [DOI] [PubMed] [Google Scholar]
- Okada K, Tanaka Y, Bernstein M, Zhang W, Patterson GA, Botney MD. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol. 1997;151(4):1019–1025. [PMC free article] [PubMed] [Google Scholar]
- van Albada ME, Schoemaker RG, Kemna MS, Cromme-Dijkhuis AH, van Veghel R, Berger RM. The role of increased pulmonary blood flow in pulmonary arterial hypertension. Eur Respir J. 2005;26(3):487–493. doi: 10.1183/09031936.05.00015405. [DOI] [PubMed] [Google Scholar]
- Brittain E. Echocardiographic assessment of the right heart in mice. JVis Exp. 2013. [DOI] [PMC free article] [PubMed]
- Dickinson MG, Bartelds B, Molema G, et al. Egr-1 expression during neointimal development in flow-associated pulmonary hypertension. Am J Pathol. 2011;179(5):2199–2209. doi: 10.1016/j.ajpath.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgdorff MA, Bartelds B, Dickinson MG, Steendijk P, de Vroomen M, Berger RM. Distinct loading conditions reveal various patterns of right ventricular adaptation. Am J Physiol Heart Circ Physiol. 2013;305(3):H354–H364. doi: 10.1152/ajpheart.00180.2013. [DOI] [PubMed] [Google Scholar]
- Ruiter G, de Man FS, Schalij I, et al. Reversibility of the monocrotaline pulmonary hypertension rat model. Eur Respir J. 2013;42(2):553–556. doi: 10.1183/09031936.00012313. [DOI] [PubMed] [Google Scholar]
- van Albada ME, Bartelds B, Wijnberg H, et al. Gene expression profile in flow-associated pulmonary arterial hypertension with neointimal lesions. Am J Physiol Lung Cell Mol Physiol. 2010;298(4):L483–L491. doi: 10.1152/ajplung.00106.2009. [DOI] [PubMed] [Google Scholar]
- Dickinson MG, Kowalski PS, Bartelds B, et al. A critical role for egr-1 during vascular remodelling in pulmonary arterial hypertension. Cardiovasc Res. 2014;103(4):573–584. doi: 10.1093/cvr/cvu169. [DOI] [PubMed] [Google Scholar]
- van der Feen DE, Dickinson MG, Bartelds MG, et al. Egr-1 identifies neointimal remodeling and relates to progression in human pulmonary arterial hypertension. Jheart lung transplant. 2016;35(4):481–490. doi: 10.1016/j.healun.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Rungatscher A. Chronic overcirculation-induced pulmonary arterial hypertension in aorto-caval shunt. Microvasc Res. 2014;94:73–79. doi: 10.1016/j.mvr.2014.05.005. [DOI] [PubMed] [Google Scholar]
- O'Blenes SB, Fischer S, McIntyre B, Keshavjee S, Rabinovitch M. Hemodynamic unloading leads to regression of pulmonary vascular disease in rats. J Thorac Cardiovasc Surg. 2001;121(2):279–289. doi: 10.1067/mtc.2001.111657. [DOI] [PubMed] [Google Scholar]
- Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005;19(9):1178–1180. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter E. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123(8):3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel NP, Spiekerkoetter E, Gu M, et al. Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am J Respir Crit Care Med. 2015;191(11):1273–1286. doi: 10.1164/rccm.201412-2291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche J, Potus F, Vaillancourt M, et al. Bromodomain-containing protein 4: The epigenetic origin of pulmonary arterial hypertension. Circ Res. 2015;117(6):525–535. doi: 10.1161/CIRCRESAHA.115.307004. [DOI] [PubMed] [Google Scholar]
- Happé CM. Pneumonectomy combined with SU5416 induces severe pulmonary hypertension in rats. Am J Physiol Lung Cell Mol Physiol. 2016;310(11):L1088–L1097. doi: 10.1152/ajplung.00023.2016. [DOI] [PubMed] [Google Scholar]
- Ranchoux B, Antigny F, Rucker-Martin C, et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131(11):1006–1018. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- de Raaf MA. SuHx rat model: Partly reversible pulmonary hypertension and progressive intima obstruction. Eur Respy J. 2014;44(1):160–168. doi: 10.1183/09031936.00204813. [DOI] [PubMed] [Google Scholar]
- Elastic (Connective Tissue Stain) Instructions for Use. 1506. Available from: http://www.abcam.com/ps/products/150/ab150667/documents/ab150667-Elastic%20Stain%20Kit%20(website).pdf.


