Abstract
Purpose of review
The incidence of pulmonary infections has declined dramatically with improved access to antiretroviral therapy (ART) and co-trimoxazole prophylaxis, but chronic lung disease (CLD) is an increasingly recognized but poorly understood complication in adolescents with perinatally-acquired HIV.
Recent findings
There is a high prevalence of chronic respiratory symptoms, abnormal spirometry and chest radiographic abnormalities among HIV-infected adolescents in sub-Saharan Africa, where 90% of the world’s HIV-infected children live. The incidence of lymphocytic interstitial pneumonitis, the most common cause of CLD in the pre-ART era, has declined with increased ART access. Small airways disease, particularly constrictive obliterative bronchiolitis and bronchiectasis are emerging as leading causes of CLD among HIV-infected adolescents in low- and middle-income countries. Asthma may be more common in high-income settings. Likely risk factors for CLD include recurrent pulmonary infections, air pollution, HIV-related immune dysfunction and untreated HIV infection, particularly during critical stages of lung development.
Summary
Globally, the importance of HIV-associated CLD as a cause of morbidity and mortality is increasing, especially as survival has improved dramatically with ART and growing numbers of children living with HIV enter adolescence. Further research is urgently needed to elucidate the natural history and pathogenesis of CLD, and to determine optimal screening, diagnostic and treatment strategies.
Keywords: Adolescent, HIV, bronchiectasis, lymphocytic interstitial pneumonitis, obliterative bronchiolitis, chronic lung disease
Introduction
Of the estimated 3.4 million children living with HIV globally, 90% live in sub-Saharan Africa [1]. Although the number of children born with HIV is declining due to scale-up of prevention of mother-to-child transmission (PMTCT) programmes, growing numbers of HIV-infected children are surviving to adolescence due to the widespread roll-out of antiretroviral therapy (ART) [2]. In addition, approximately one-third of HIV-infected infants survive to adolescence in the absence of ART, and children infected a decade ago when PMTCT programmes and early infant diagnosis were not available, are now presenting to health services in large numbers for the first time in older childhood and adolescence [3]. Consequently, the burden of paediatric HIV is shifting toward adolescents [2].
While the incidence of acute pulmonary infections in HIV is declining due to the use of co-trimoxazole prophylaxis and ART [4–6,7**], chronic lung disease (CLD) is emerging as an important, but incompletely understood, complication among older children and adolescents living with HIV [8*]. In particular, adolescents with delayed diagnosis of perinatally-acquired HIV have a disproportionately high burden of chronic respiratory disease [8*,9*,10*,11*]. We review the spectrum of and risk factors for HIV-associated CLD among adolescents living with HIV, as well as considerations in diagnosis and management.
Spectrum of chronic lung disease
Recent studies from sub-Saharan Africa highlight a substantial burden of chronic respiratory symptoms and poor lung function among HIV-infected adolescents. Over half of older children and adolescents reported at least one chronic respiratory symptom in recent studies from Zimbabwe, Malawi and Kenya [8*,10*,11*,12*,13*,14*]. Chronic cough and sputum production were reported by 21–60% and breathlessness by 12–18%, but wheezing was uncommon [8*,10*,11*,12*,13*,14*,15*]. The prevalence of tachypnoea is difficult to quantify as definitions vary between studies, but elevated respiratory rates are common [8*,9*,10*,11*, 15*]. Hypoxia after sub-maximal exercise was a striking finding among 12–38% [10*,11*,12*,13*,15*,16*]. One-third of adolescents had abnormal spirometry: forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were lower among those with HIV, compared to age-, sex-, race- and height-adjusted predicted values, and compared to HIV-uninfected controls [8*,15*,17]. While some studies documented a substantial prevalence of airflow obstruction [10*,11*,12*], others reported a prominence of a restricted pattern [11*,13*,14*]; there was little reversibility with bronchodilators. One study additionally measured lung volumes, compliance and transfer factor for carbon monoxide, finding that each was lower among HIV-infected compared to uninfected participants [18**]. A recent study from the USA also showed a high prevalence of poorly reversible airflow obstruction [19*]. Taken together, these studies suggest that CLD may be more common among HIV-infected adolescents than previously appreciated (Table 1).
Table 1.
| Study, year | Location, participant N | Characteristics of HIV-infected adolescents | Respiratory symptoms/signs | Abnormal spirometry |
|---|---|---|---|---|
| Ferrand, et al. [8*], 2012 | Harare, Zimbabwe N=116 |
|
|
|
| McHugh, et al. [10*], 2016 | Harare, Zimbabwe N=385 |
|
|
|
| Mwalukomo, et al. [11*], 2016 | Blantyre, Malawi N=160 |
|
|
|
| Attia, et al. [12*], 2015 | Nairobi, Kenya N=55 |
|
|
|
| Rylance S, et al. [14*], 2016 | Harare, Zimbabwe N=385 ART-naïve and 202 ART-treated |
ART-naïve compared to ART-treated:
|
ART-naïve compared to ART-treated:
|
|
| Rylance J, et al. [15*], 2016 | Harare, Zimbabwe N=202 HIV-infected and 150 age-matched HIV-uninfected |
HIV-infected compared to HIV-uninfected:
|
HIV-infected compared to HIV-uninfected:
|
|
| Githinji, et al. [18**], 2016 | Cape Town, South Africa N=515 HIV-infected and 110 age-, sex-, ethnically-matched HIV-uninfected controls |
|
Not reported |
HIV-infected compared to HIV-uninfected:
|
| Shearer, et al. [19*], 2015 | United States (multiple sites) N=216 HIV-infected and 151 HIV-exposed uninfected |
Not reported |
HIV-infected compared to HIV-uninfected:
|
The term CLD comprises a spectrum of lung diseases whose prevalence varies between LMICs and high-income countries. The pathogenesis is multifactorial, but HIV-related immune dysfunction is likely important.
Bronchiectasis
Bronchiectasis is a well-recognized and irreversible cause of CLD among HIV-infected children and adolescents [20]. The bronchial architecture is distorted with bronchial diameter enlargement relative to the adjacent pulmonary artery, which appears as a “ring” or “tramline” pattern on chest radiography [21*,22*]. A study from the USA in the pre-ART era reported that 6% of 749 HIV-infected children without pre-existing lung disease developed radiographically or histologically determined bronchiectasis during an average of six years of follow-up [23]. In contrast, in two recent studies conducted in Zimbabwe and Malawi among older children and adolescents with perinatally-acquired HIV, one-half had chest radiographic features consistent with bronchiectasis [8*,11*]. Two-thirds of children in the African studies were on ART for at least a median duration of 20 months and had median CD4 >350 cells/μL. The lower prevalence of bronchiectasis in the USA study despite unavailability of ART was probably due to earlier engagement with healthcare services, prompt treatment of infections and greater attention to maintaining adequate nutrition.
Multiple insults can result in bronchiectasis, including recurrent pulmonary infections, chronic aspiration, and congenital or acquired immunodeficiency syndromes [17,20,21*,22*,23,24]. HIV is associated with an increased risk of tuberculosis, and recurrent viral and bacterial pulmonary infections [7**,17,23,24,25*,26]. However, some data suggest that HIV predisposes to bronchiectasis independently of infection, likely due to HIV-mediated defects in innate immunity and accompanying airway neutrophilic inflammation [17,21*,22*,27]. Bronchiectasis in adolescents can also occur as a late complication of lymphocytic interstitial pneumonitis (LIP) [24]. Bronchiectasis accounts for a substantial proportion of chronic respiratory symptoms, reduced quality of life and risk of premature death [22*].
Constrictive obliterative bronchiolitis
In 2012, Ferrand and colleagues first reported a high prevalence of chronic respiratory symptoms among perinatally HIV-infected children diagnosed in adolescence, nearly three-quarters of whom were taking ART [8*]. This was the first study to perform high-resolution computed tomography (HRCT) in this context. Among the 56 adolescents with chronic respiratory symptoms, the most common HRCT finding was a mosaic pattern of decreased attenuation in 55% (Figure 1). Decreased attenuation was associated with reduced FEV1 and chronic cough [16*]. Together with hypoxia and irreversible airflow obstruction, these findings are consistent with constrictive obliterative bronchiolitis (cOB). This was the first study to demonstrate cOB as a major cause of CLD. To our knowledge, there are no reports of HIV-associated cOB among adolescents in high-income settings, although the findings by Shearer and colleagues could partly be explained by small airways disease [19*]. Due to the cross-sectional nature of these data, however, it is not possible to determine whether findings reflect abnormalities persisting from insults during childhood or recent-onset disease [28*].
Figure 1.
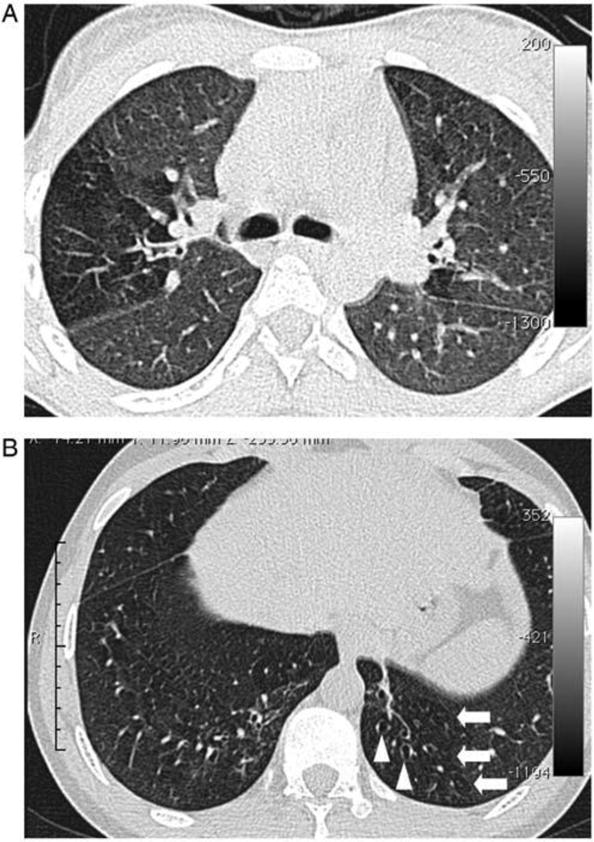
A, Image section at the level of the carina in a 15-year-old female. There is a clear zone of decreased attenuation in the right upper lobe (and, to a lesser extent, the left lung). In regions of decreased attenuation there is reduction in the caliber of pulmonary vessels; there was no bronchiectasis in this patient. B, Image section in a 19-year-old male through the lower zones demonstrating focal areas of decreased attenuation in both lungs (arrows) and bronchiectasis in the left lower lobe (arrowheads).
The pathogenesis and natural history of cOB among HIV-infected adolescents is not well understood. This rare, usually progressive, and fibrotic and inflammatory condition involves the small airways involved in gas exchange, namely the terminal and respiratory bronchioles, but spares the lung parenchyma [29]. These bronchiolar lesions are patchy and may be missed on transbronchial biopsy. Clinically, cOB presents with progressive dyspnea and cough over weeks to months, and hypoxia may be detected in severe cases. The predominant diagnostic features of cOB are poorly reversible airflow obstruction, a mosaic pattern of decreased attenuation on HRCT, and air trapping on pulmonary function testing and/or expiratory HRCT images (Figure 1). Chest radiographs are often normal, or may demonstrate only hyperinflation, until cOB is advanced. It has been mainly described in lung and stem cell transplant patients with chronic rejection and graft-versus-host disease, and as a sequela of community-acquired respiratory viral infections (i.e., influenza, parainfluenza, adenovirus, respiratory syncytial virus) and cytomegalovirus infection. It is very rarely reported among HIV-uninfected children after community-acquired respiratory viral infections. The high incidence of viral infections among children and adolescents with HIV may play an important role in development of cOB, particularly in those with delayed diagnosis and treatment of HIV [25*]. Additionally, decreased attenuation was strongly correlated with bronchiectasis and bronchial wall thickening and dilatation on HRCT [8*], suggesting a continuum of pathological processes that links small and large airways involvement. Although disease course and prognosis of cOB are not known among HIV-infected adolescents, in other populations, cOB responds poorly to therapy and is associated with a high risk of mortality over months to years.
Lymphocytic interstitial pneumonitis
Globally, LIP was the most common cause of CLD among HIV-infected children during the pre-ART era [20,30,31]. It is generally responsive to ART, although severe LIP may require adjunct corticosteroid therapy. The overall incidence of LIP has declined dramatically with the scale-up of ART, and in recent studies, LIP appears to be uncommon among older children and adolescents with HIV. A review of chest radiographic findings in HIV-infected children and adolescents reported that pulmonary tuberculosis and LIP accounted for 70% and 15% of cases, respectively [32*]. However, three-quarters of included studies were conducted during the pre- and early ART era or in ART-naïve individuals. In two recent studies of generally ART-experienced HIV-infected adolescents from Zimbabwe, HRCT was strongly suggestive of LIP in <3% [8*,16*].
Asthma
Conflicting data suggest that asthma risk may be higher among HIV-infected compared with HIV-uninfected children and adolescents, especially in high-income settings [33,34]. Among 1201 HIV-infected adolescents living in the USA, of whom 87% were perinatally-infected and 85% were taking ART, asthma was a common comorbid condition with an incidence of 1.2/100 person-years [35**]. In a study of Thai children with perinatally-acquired HIV, early initiation of ART was associated with higher CD4, and the prevalence of asthma (27%) exceeded that of uninfected Thai children (18%) [36*]. Asthma tended to be more common among individuals with preserved immune function, and may be linked to immune reconstitution [36*,37–39]. However, the prevalence may have been overestimated in these studies, as diagnosis was based on self-report, diagnostic codes or prescription of asthma-related medications. In a study that performed spirometry in 216 perinatally HIV-infected adolescents in the USA, only 30% of those with airflow obstruction had bronchodilator reversibility (overall, 9% of those with interpretable spirometry) [19*]. In Zimbabwe, 3–5% of older children and adolescents with perinatally-acquired HIV reported a history of asthma [13*,15*], similar to the prevalence estimated in the general African paediatric population [40]. Reversibility with bronchodilators was uncommon in African studies reporting airflow obstruction among HIV-infected adolescents, with the exception of one South African study in which 15% had reversibility [10*,11*,12*,13*,18**]. While asthma prevalence may be underestimated in LMICs where few systematic surveys have been completed, it is plausible that incidence is low, but could increase with industrialization, as observed in South Africa.
Pulmonary hypertension
HIV-related pulmonary arterial hypertension is rare with an estimated prevalence of 0.5% among adults in high-income countries; however, small studies from sub-Saharan Africa suggest a prevalence of 5–13% [41,42*]. Pulmonary hypertension is rarely described in HIV-infected children and adolescents [43]. A study in Zimbabwe reported that 4% of perinatally HIV-infected adolescents had elevated estimated pulmonary artery systolic pressure (>30 mmHg) on echocardiography [44]. Overall, 30% had right ventricular dilatation, and 24% had concurrent impaired left ventricular function. In another study, 28% of HIV-infected children and adolescents had right ventricular dilatation, and two-thirds had concomitant left heart abnormalities [45*]. Pulmonary hypertension prevalence, however, remains uncertain as echocardiography may over- or underestimate prevalence [46], and use of pulmonary angiography, the diagnostic gold standard, is limited in LMICs. HIV-related pulmonary hypertension in adolescents may be multifactorial as a sequela of HIV itself or secondary to HIV-associated immune dysfunction and/or cardiopulmonary disease [42,47].
Risk factors for HIV-associated chronic lung disease among adolescents
The burden of CLD among HIV-infected adolescents appears to be greater in LMICs. This may be due to a higher prevalence of risk factors, including pulmonary infections, household air pollution, malnutrition and stunting [1,7**,48]. Additionally, in contrast with high-income settings where the majority of HIV-infected children initiate ART in infancy, a substantial proportion of children are diagnosed with HIV and initiate ART in later childhood. Among adolescents with delayed diagnosis of perinatally-acquired HIV in sub-Saharan Africa, older age and low FEV1 and FVC z-scores appear to be associated [9*,11*,13*,15*]. This might reflect accumulation of insults over time and survivor bias. Multiple risk factors may interact to predispose to CLD (Figure 2).
Figure 2.
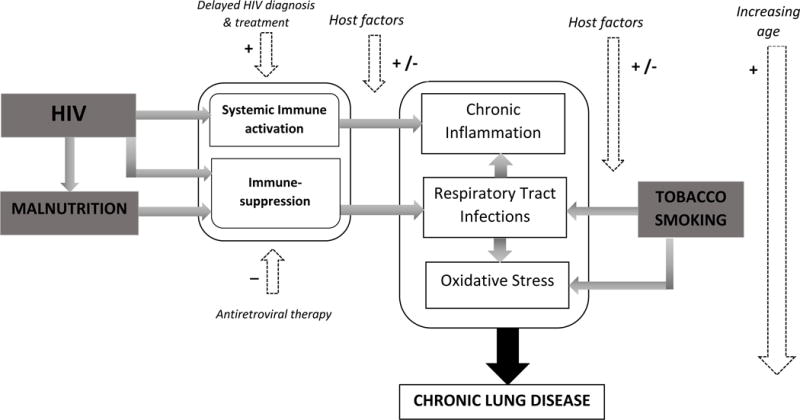
Aetio-pathogenesis of chronic lung disease in HIV-infected adolescents (+ Increases risk; − decreases risk)
Pulmonary infections
Pulmonary pathogens can trigger local inflammation, resulting in scarring and destruction of lung tissue [49]. Pulmonary infections, including Pneumocystis jirovecii pneumonia (PCP), bacterial pneumonia, and tuberculosis are associated with permanent lung function abnormalities among HIV-infected adults [50,51]. Recurrent and severe pulmonary infections early in life are also associated with impaired lung function, and these early impairments may track through later life [52*,53]. Increasing availability of ART and co-trimoxazole have resulted in a decline in the incidence of bacterial pneumonia, pulmonary tuberculosis, and PCP [54,55]. In LMICs, pulmonary infections remain common despite decreases in incidence, and risk of pulmonary infections is particularly high among those with delayed diagnosis and treatment of HIV [26,56*].
HIV infection, systemic immune activation and chronic inflammation
HIV infection is associated with systemic immune activation and chronic inflammation [57–59]. This is driven by translocation of microbial products, including lipopolysaccharide, into the systemic circulation through a gastrointestinal mucosa made “leaky” as a result of HIV HIV-mediated inflammation of gut lymphoid tissue [59–61]. The resultant immune dysregulation is associated with end-organ damage [58,62–64], and may be important mechanistically in CLD development. HIV itself is an independent risk factor for COPD among adults [6,65,66]. Preliminary data suggest that HIV is also independently associated with CLD in children and adolescents [12*,15*,18**]. Infancy and early childhood are critical periods for organ and immune system development. HIV-mediated immune dysregulation may place children at high risk of lung damage, particularly those with untreated HIV, and recurrent pulmonary infections may further accelerate lung function decline. These pathophysiological mechanisms may partly explain why adolescents in LMICs, where HIV diagnosis and ART initiation are often delayed [67*,68], have a higher prevalence of CLD.
While ART reduces markers of inflammation, residual systemic immune activation and chronic inflammation persist [61,69]. The impact of ART on the course of CLD remains unclear. An African study reported a lower prevalence of cough, breathlessness and hypoxia, but no difference in lung function among HIV-infected adolescents who had received ART for a median of five years compared to an age-matched group of ART-naïve adolescents [14*]. Among HIV-infected Kenyan adolescents and adults, perinatally-acquired HIV and nadir CD4 <200 cells/μL were associated with airflow obstruction despite near universal ART use [12*]. Additionally, in Zimbabwe and South Africa, adolescents with HIV had significantly decreased FEV1 and FVC z-scores, lung volumes, and compliance compared to uninfected peers, despite a greater than six-year median duration of ART use [15*,18**].
Tobacco smoke and air pollution
Exposure to tobacco smoke, both prenatally and during infancy, is associated with impaired lung function in one year olds [52*]. Cigarette smoking is a recognized risk factor for chronic obstructive pulmonary disease (COPD) and lung function decline among adults. Levels of cigarette smoking are increasing among adolescents, particularly in LMICs [70]. Therefore, tobacco smoke exposure will likely become an increasingly important contributor to development of CLD.
Exposure to household air pollution during early life has been associated with mortality and pulmonary infections, which in turn may impair lung function [48,71*,72]. Strong evidence also supports the association between household air pollution and CLD, including COPD in adults and wheezing in children [48]. To date, no studies have demonstrated a link between household air pollution and CLD among HIV-infected adolescents, likely due to confounding by other exposures and difficulties in measuring exposures.
Malnutrition and stunted growth
Both malnutrition and stunting are common among children and adolescents living with HIV in LMICs, with prevalence estimated as high as 42% and 73%, respectively [10*,73–76]. Malnutrition during the first year of life is associated with decreased lung function at one year of age [52*]. In a Kenyan study, stunting was associated with tachypnoea among HIV-infected adolescents [9*]. Stunting is a marker of delayed somatic growth; therefore, stunted children are likely to have smaller lungs [77*], which may subsequently predispose to CLD.
Assessment and management of chronic lung disease
Awareness of the burden and spectrum of HIV-associated CLD is limited. Several studies have proposed criteria for defining CLD in children and adolescents [8*,11*,56*], but a sensitive and specific clinical algorithm has not been established. Chest radiographic abnormalities are nonspecific for many CLD subtypes and may be visible only in late stages of disease. Availability of diagnostic modalities such as spirometry and HRCT is limited in LMICs.
In the absence of appropriate diagnostics, chronic respiratory symptoms are frequently empirically treated with repeated courses of antibiotics and anti-tuberculosis therapy in high HIV prevalence settings where tuberculosis is common [8*,15*,17]. The pathogenesis of CLD is poorly understood, and there are no specific management guidelines. However, prevention of pulmonary infections by ensuring routine vaccinations, early ART initiation and continued co-trimoxazole use may mitigate the burden of CLD among HIV-infected adolescents [78].
Future directions
Prospective studies to understand the natural history, pathology and pathogenesis of CLD in HIV-infected adolescents are needed. Standardization of the definition of CLD and of data collection/measurement will allow for comparability across studies and merging of data to achieve greater power to detect associations. Additionally, locally-derived references for defining abnormal lung function in children are needed in LMICs. The 2016 WHO HIV guidelines recommend treatment of all individuals regardless of age or immune status, which should facilitate earlier ART initiation. However, once established, CLD does not appear to be completely reversed by ART. Screening algorithms for CLD for children and adolescents need to be developed, and interventions such as long-term macrolide therapy, prophylactic antibiotics, pulmonary rehabilitation and tobacco smoke avoidance/cessation programmes require evaluation.
Conclusion
Recent studies demonstrate a high burden of CLD and its manifestations among HIV-infected older children and adolescents, particularly in LMICs. CLD represents a spectrum of conditions with overlapping risk factors and pathogenic mechanisms. As greater numbers of HIV-infected children survive to adolescence, the prevalence of CLD in this age-group is likely to increase. Further research is urgently needed to develop optimal diagnostic and therapeutic strategies so as to avoid compromising the substantial gains in mortality accomplished with ART among HIV-infected children.
Key points.
Chronic lung disease is increasingly recognized as a complication among older children and adolescents with perinatally-acquired HIV.
The burden of chronic lung disease disproportionally affects those living in LMICs.
The most common causes of chronic lung disease appear to be large and small airways disease, which may be poorly responsive to antiretroviral therapy once established
The prevalence and incidence of lymphocytic interstitial pneumoniitis appear to have decreased dramatically with the global scale-up of antiretroviral therapy.
Acknowledgments
None
Financial support and sponsorship
EFA was funded by the National Institutes of Health/National Heart Lung & Blood Institute (Grant NIH/NHLBI F32HL125031). RAF is funded by the Wellcome Trust (Grant 095878/Z/11/Z).
Footnotes
Conflicts of interest
Dr Miller is a panel member for Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-infected Adults and Adolescents (Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America) and has received honoraria from Gilead, ViiV, Merck, and Janssen for non-promotional lectures on clinical aspects of HIV infection. For the remaining authors, none were declared.
References
- 1.UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic 2013. Geneva. 2013:1–198. [Google Scholar]
- 2.Ferrand RA, Corbett EL, Wood R, et al. AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. AIDS. 2009;23:2039–2046. doi: 10.1097/QAD.0b013e32833016ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrand RA, Munaiwa L, Matsekete J, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis. 2010;51:844–851. doi: 10.1086/656361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grubb JR, Moorman AC, Baker RK, Masur H. The changing spectrum of pulmonary disease in patients with HIV infection on antiretroviral therapy. AIDS. 2006;20:1095–1107. doi: 10.1097/01.aids.0000226949.64600.f9. [DOI] [PubMed] [Google Scholar]
- 5.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hull MW, Phillips P, Montaner JS. Changing global epidemiology of pulmonary manifestations of HIV/AIDS. Chest. 2008;134:1287–1298. doi: 10.1378/chest.08-0364. [DOI] [PubMed] [Google Scholar]
- 7**.B-Lajoie MR, Drouin O, Bartlett G, et al. Incidence and Prevalence of Opportunistic and Other Infections and the Impact of Antiretroviral Therapy Among HIV-infected Children in Low- and Middle-income Countries: A Systematic Review and Meta-analysis. Clin Infect Dis. 2016;62:1586–1594. doi: 10.1093/cid/ciw139. This systematic review and meta-analysis found a significant reduction in incidence of most opportunistic infections with ART initiation. Nonetheless, bacterial pneumonia and tuberculosis remained the most common infections among ART-naïve and ART-exposed children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Ferrand RA, Desai SR, Hopkins C, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis. 2012;55:145–152. doi: 10.1093/cid/cis271. This is the first study to: 1) report a high prevalence of chronic respiratory symptoms, hypoxia and abnormal spirometry among adolescents with delayed diagnosis of perinatally-acquired HIV; and 2) perform HRCT, finding that obliterative/constrictive bronchiolitis and bronchiectasis are common aetiologies of CLD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Attia EF, Weiss NS, Maleche-Obimbo E, et al. Risk factors for hypoxia and tachypnea among adolescents with vertically-acquired HIV in Nairobi. Pediatr Infect Dis J. 2016 doi: 10.1097/INF.0000000000001453. in press. This study found a high prevalence of hypoxia and tachypnea among adolescents with perinatally-acquired HIV presenting for routine outpatient clinical care. Low CD4, lack of ART use and stunted growth were associated with respiratory abnormalities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.McHugh G, Rylance J, Mujuru H, et al. Chronic morbidity among older children and adolescents at diagnosis of HIV infection. J Acquir Immune Defic Syndr. 2016 May 11; doi: 10.1097/QAI.0000000000001073. [Epub ahead of print] This study examined the prevalence of chronic diseases at the time of delayed HIV diagnosis among children and adolescents with a median age of 11 years and median CD4 of 375 cells/mm. These “slow progressors” had a substantial proportion of stunting, pubertal delay, chronic cough and abnormal lung function despite preserved CD4, supporting the need for early HIV testing and ART initiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Mwalukomo T, Rylance SJ, Webb EL, et al. Clinical Characteristics and Lung Function in Older Children Vertically Infected With Human Immunodeficiency Virus in Malawi. J Pediatric Infect Dis Soc. 2016;5:161–169. doi: 10.1093/jpids/piv045. This study proposed two clinical phenotypes among children and adolescents with perinatally-acquired HIV (median age, 11 years) – one phenotype characterized by cough and the other by hypoxia. These postulated phenotypes might play a role in future diagnostic algorithms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Attia EF, Maleche-Obimbo E, Yatich N, et al. Risk factors for airflow obstruction among HIV+ individuals in Nairobi, Kenya [abstract]. Conference on Retroviruses and Opportunistic Infections; 2015; This is the first study to compare prevalence and risk factors of abnormal spirometry between adolescents with predominantly perinatally-acquired HIV and adults with horizontally-acquired HIV. Perinatally-acquired HIV and cigarette smoking were significantly associated with post-bronchodilator airflow obstruction. [Google Scholar]
- 13*.Rylance J, McHugh G, Desai S, et al. Chronic respiratory ill-health in children with vertically-acquired HIV: clinical features and lung function [abstract]. International AIDS Society Conference; 2015; This study adds evidence that younger age at HIV diagnosis was associated with better lung function, underscoring that early HIV diagnosis and treatment might decrease risk of impaired lung function. [Google Scholar]
- 14*.Rylance S, Rylance J, McHugh G, et al. Chronic respiratory morbidity among HIV-infected children in Zimbabwe: a comparison of ART naive and treated cohorts [abstract] Arch Dis Child. 2016;101(Suppl 1):A156–A157. This study compared ART-experienced (median ART use 6 years) to ART-naïve HIV-infected older children and adolescents (median age 11). Although the ART-experienced had fewer respiratory symptoms, ~25% of both groups had abnormal spirometry, suggesting that delayed HIV diagnosis and initiation of ART in late childhood may not completely reverse sequelae of HIV, including lung function impairment. [Google Scholar]
- 15*.Rylance J, McHugh G, Metcalfe J, Mujuru H, Nathoo K, Wilmore S, Rowland-Jones S, Majonga E, Kranzer K, Ferrand RA. Chronic lung disease in HIV-infected children established on antiretroviral therapy. AIDS. 2016 doi: 10.1097/QAD.0000000000001249. in press. This is one of the first studies to compare ART-experienced older children and adolescents with perinatally-acquired HIV to frequency age-matched HIV-uninfected peers. Despite median CD4 726 cells/μL and HIV diagnosis at a mean age of 5.5 years, respiratory symptoms and lung function impairment were significantly more common among the HIV-infected compared to the uninfected. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Rylance J, Desai SR, Nair A, et al. Obliterative bronchiolitis: a common cause of chronic lung disease in older children and adolescents with vertically acquired HIV [abstract]. 46th Union World Conference on Lung Health; 2015; Respiratory symptoms, spirometry and HRCT were assessed in 39 older children and adolescents who received antiretroviral therapy for ≥6 months. Findings consistent with obliterative/constrictive bronchiolitis and bronchiectasis were common on HRCT and were associated with cough and reduced FEV1; however, an association between HRCT abnormalities and hypoxia or low CD4 was not detected. [Google Scholar]
- 17.Masekela R, Anderson R, Moodley T, et al. HIV-related bronchiectasis in children: an emerging spectre in high tuberculosis burden areas. Int J Tuberc Lung Dis. 2012;16:114–119. doi: 10.5588/ijtld.11.0244. [DOI] [PubMed] [Google Scholar]
- 18**.Githinji L, Gray D, Hlengwa S, et al. Lung function in HIV infected South African adolescents on antiretroviral therapy: the Cape Town adolescent antiretroviral cohort [abstract] Reviews in Infectious Diseases and in Antiviral Therapy: Journal of Abstracts and Conference Reports from International Workshops on Infectious Diseases and Antiviral Therapy. 8th International Workshop on HIV Pediatrics. 2016 abstract: O_21. This is the first study to obtain complete lung function testing among HIV-infected adolescents in a resource-limited setting and compare these findings in HIV-uninfected, age-, sex-, and ethnicity-matched controls. Despite an 8-year median duration of ART and current CD4 of 714 cells/mm3, HIV-infected adolescents had significantly decreased lung volumes, airflow and compliance compared to HIV-uninfected comparators. [Google Scholar]
- 19*.Shearer WT, Leister E, Siberry GK, et al. for the Pediatric HIV/AIDS Cohort Study (PHACS) Pulmonary complications of HIV-1 in youth: the PHACS AMP Study [abstract]. Conference on Retroviruses and Opportunistic Infections; 2015; In this pulmonary substudy of PHACS AMP, pre- and post-bronchodilator spirometry was performed among 216 perinatally HIV-infected adolescents; 22% had pre-bronchodilator airflow obstruction but only 30% of these had bronchodilator reversibility. [Google Scholar]
- 20.Zar HJ. Chronic lung disease in human immunodeficiency virus (HIV) infected children. Pediatr Pulmonol. 2008;43:1–10. doi: 10.1002/ppul.20676. [DOI] [PubMed] [Google Scholar]
- 21*.Milliron B, Henry TS, Veeraraghavan S, Little BP. Bronchiectasis: Mechanisms and Imaging Clues of Associated Common and Uncommon Diseases. Radiographics. 2015;35:1011–1030. doi: 10.1148/rg.2015140214. This article highlights the pathophysiologic features of specific causes of bronchiectasis. It presents important radiographic findings, including spatial distribution, morphologic features, and associated airway or parenchymal abnormalities, that can assist in generating and narrowing the differential diagnosis of the causes of bronchiectasis. [DOI] [PubMed] [Google Scholar]
- 22*.Goyal V, Grimwood K, Marchant J, et al. Pediatric bronchiectasis: No longer an orphan disease. Pediatr Pulmonol. 2016;51:450–469. doi: 10.1002/ppul.23380. This contemporary review summarizes the literature available regarding global epidemiology, risk factors, pathobiology, clinical features and management of non-cystic fibrosis bronchiectasis in children. [DOI] [PubMed] [Google Scholar]
- 23.Berman DM, Mafut D, Djokic B, et al. Risk factors for the development of bronchiectasis in HIV-infected children. Pediatr Pulmonol. 2007;42:871–875. doi: 10.1002/ppul.20668. [DOI] [PubMed] [Google Scholar]
- 24.Sheikh S, Madiraju K, Steiner P, Rao M. Bronchiectasis in pediatric AIDS. Chest. 1997;112:1202–1207. doi: 10.1378/chest.112.5.1202. [DOI] [PubMed] [Google Scholar]
- 25*.Annamalay AA, Abbott S, Sikazwe C, et al. Respiratory viruses in young South African children with acute lower respiratory infections and interactions with HIV. J Clin Virol. 2016;81:58–63. doi: 10.1016/j.jcv.2016.06.002. This study describes the prevalence of respiratory viruses by PCR in South African children less than two years of age, finding that rhinovirus was the most commonly identified virus in lower respiratory tract infection. HIV-infected children were more likely to have pneumonia compared to bronchiolitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theodoratou E, McAllister DA, Reed C, et al. Global, regional, and national estimates of pneumonia burden in HIV-infected children in 2010: a meta-analysis and modelling study. Lancet Infect Dis. 2014;14:1250–1258. doi: 10.1016/S1473-3099(14)70990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masekela R, Anderson R, de Boeck K, et al. Expression of soluble triggering receptor expressed on myeloid cells-1 in childhood CF and non-CF bronchiectasis. Pediatr Pulmonol. 2015;50:333–339. doi: 10.1002/ppul.23121. [DOI] [PubMed] [Google Scholar]
- 28*.Pitcher RD, Lombard CJ, Cotton MF, et al. Chest radiographic abnormalities in HIV-infected African children: a longitudinal study. Thorax. 2015;70:840–846. doi: 10.1136/thoraxjnl-2014-206105. This study prospectively followed chest radiographic findings among 258 children with a median age of 28 months for up to 54 months. Over half of children had severe radiographic abnormalities that persisted for at least 18 months, and 16% had abnormalities that persisted for the entire 54 months of follow-up. [DOI] [PubMed] [Google Scholar]
- 29.Lynch JP, 3rd, Weigt SS, DerHovanessian A, et al. Obliterative (constrictive) bronchiolitis. Semin Respir Crit Care Med. 2012;33:509–532. doi: 10.1055/s-0032-1325161. [DOI] [PubMed] [Google Scholar]
- 30.Jeena PM, Coovadia HM, Thula SA, et al. Persistent and chronic lung disease in HIV-1 infected and uninfected African children. AIDS. 1998;12:1185–1193. doi: 10.1097/00002030-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Sharland M, Gibb DM, Holland F. Respiratory morbidity from lymphocytic interstitial pneumonitis (LIP) in vertically acquired HIV infection. Arch Dis Child. 1997;76:334–336. doi: 10.1136/adc.76.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Pitcher RD, Beningfield SJ, Zar HJ. The chest X-ray features of chronic respiratory disease in HIV-infected children–a review. Paediatr Respir Rev. 2015;16:258–266. doi: 10.1016/j.prrv.2015.01.005. This review summarizes chest radiographic findings among HIV-infected children and adolescents aged 0–14 years with chronic respiratory symptoms; 65% of included individuals were from sub-Saharan Africa and the majority of original research studies were from the pre- and early antiretroviral therapy era. Overall, radiographic findings consistent with pulmonary tuberculosis and lymphocytic interstitial pneumonitis accounted for 70% and 15% of cases, respectively. [DOI] [PubMed] [Google Scholar]
- 33.Siberry GK, Leister E, Jacobson DL, et al. Increased risk of asthma and atopic dermatitis in perinatally HIV-infected children and adolescents. Clin Immunol. 2012;142:201–208. doi: 10.1016/j.clim.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster SB, Paul ME, Kozinetz CA, et al. Prevalence of asthma in children and young adults with HIV infection. J Allergy Clin Immunol. 2007;119:750–752. doi: 10.1016/j.jaci.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 35**.Mirani G, Williams PL, Chernoff M, et al. Changing Trends in Complications and Mortality Rates Among US Youth and Young Adults With HIV Infection in the Era of Combination Antiretroviral Therapy. Clin Infect Dis. 2015;61:1850–1861. doi: 10.1093/cid/civ687. Among 1201 HIV-infected U.S. adolescents and young adults (87% perinatally-infected), asthma and pneumonia are among the most common comorbid conditions, despite the dramatic decline absolute incidence of pneumonia. This study highlights the incidence of pulmonary (and other) complications of HIV among adolescents in a high-income setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Bunupuradah T, Hansudewechakul R, Kosalaraksa P, et al. HLA-DRB1454 and predictors of new-onset asthma in HIV-infected Thai children. Clin Immunol. 2015;157:26–29. doi: 10.1016/j.clim.2014.12.006. This study assessed new onset asthma among children with perinatally-acquired HIV who initiated antiretroviral therapy at different CD4 levels. Investigators found that early initiation of ART was associated with higher CD4 and a prevalence of asthma (27%) exceeding even that of uninfected Thai children (18%) [DOI] [PubMed] [Google Scholar]
- 37.Gutin F, Butt A, Alame W, et al. Asthma in immune-competent children with human immunodeficiency virus. Ann Allergy Asthma Immunol. 2009;102:438. doi: 10.1016/S1081-1206(10)60518-2. [DOI] [PubMed] [Google Scholar]
- 38.Foster SB, Lu M, Thompson B, et al. Association between HLA inheritance and asthma medication use in HIV positive children. AIDS. 2010;24:2133–2135. doi: 10.1097/QAD.0b013e32833cba08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster SB, McIntosh K, Thompson B, et al. Increased incidence of asthma in HIV-infected children treated with highly active antiretroviral therapy in the National Institutes of Health Women and Infants Transmission Study. J Allergy Clin Immunol. 2008;122:159–165. doi: 10.1016/j.jaci.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ait-Khaled N, Odhiambo J, Pearce N, et al. Prevalence of symptoms of asthma, rhinitis and eczema in 13- to 14-year-old children in Africa: the International Study of Asthma and Allergies in Childhood Phase III. Allergy. 2007;62:247–258. doi: 10.1111/j.1398-9995.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 41.Staitieh B, Guidot DM. Noninfectious pulmonary complications of human immunodeficiency virus infection. Am J Med Sci. 2014;348:502–511. doi: 10.1097/MAJ.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Bigna JJ, Sime PS, Koulla-Shiro S. HIV related pulmonary arterial hypertension: epidemiology in Africa, physiopathology, and role of antiretroviral treatment. AIDS Res Ther. 2015;12:36. doi: 10.1186/s12981-015-0078-3. This review highlights the few studies of pulmonary hypertension performed in Africa, reporting that the prevalence of pulmonary hypertension may be as high 5–13% among HIV-infected Africans. This estimate is in stark contrast to the 0.5% prevalence reported among HIV-infected adults living in high-income settings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.L’Huillier AG, Posfay-Barbe KM, Pictet H, Beghetti M. Pulmonary Arterial Hypertension among HIV-Infected Children: Results of a National Survey and Review of the Literature. Front Pediatr. 2015;3:25. doi: 10.3389/fped.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller RF, Kaski JP, Hakim J, et al. Cardiac disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis. 2013;56:576–582. doi: 10.1093/cid/cis911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Majonga ED, Rylance J, Odland JO, et al. Right heart abnormalities in HIV-infected children in Harare, Zimbabwe [abstract]. 21st International AIDS Conference; 2016; This study performed transthoracic echocardiography and spirometry in 201 perinatally HIV-infected children, finding isolated pulmonary hypertension in 2%, and right ventricular dilatation in 28%, of whom 63% had concomitant left heart abnormalities. Right ventricular dilatation was not associated with pulmonary hypertension or abnormal spirometry, suggesting that right ventricular dilatation may be secondary to left heart abnormalities. [Google Scholar]
- 46.Selby VN, Scherzer R, Barnett CF, et al. Doppler echocardiography does not accurately estimate pulmonary artery systolic pressure in HIV-infected patients. AIDS. 2012;26:1967–1969. doi: 10.1097/QAD.0b013e3283579653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Syed FF, Sani MU. Recent advances in HIV-associated cardiovascular diseases in Africa. Heart. 2013;99:1146–1153. doi: 10.1136/heartjnl-2012-303177. [DOI] [PubMed] [Google Scholar]
- 48.Gordon SB, Bruce NG, Grigg J, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2:823–860. doi: 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guillemi S, Staples C, Hogg J, et al. Unexpected lung lesions in high resolution computed tomography (HRTC) among patients with advanced HIV disease. Eur Respir J. 1996;9:33–36. doi: 10.1183/09031936.96.09010033. [DOI] [PubMed] [Google Scholar]
- 50.Morris AM, Huang L, Bacchetti P, et al. Permanent Declines in Pulmonary Function Following Pneumonia in Human Immunodeficiency Virus-Infected Persons. Am J Respir Crit Care Med. 2000;162:612–616. doi: 10.1164/ajrccm.162.2.9912058. [DOI] [PubMed] [Google Scholar]
- 51.Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration. 2013;86:76–85. doi: 10.1159/000350917. [DOI] [PubMed] [Google Scholar]
- 52*.Gray DM, Turkovic L, Willemse L, et al. Lung Function in African Infants in the Drakenstein Child Health Study: Impact of Lower Respiratory Tract Illness. Am J Respir Crit Care Med. 2016 Aug 10; doi: 10.1164/rccm.201601-0188OC. [Epub ahead of print]. This study found that lower respiratory illness within the first year of life impaired lung function at one year of age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stern DA, Morgan WJ, Wright AL, et al. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeena P. The role of HIV infection in acute respiratory infections among children in sub-Saharan Africa. Int J Tuberc Lung Dis. 2005;9:708–715. [PubMed] [Google Scholar]
- 55.Church JA, Fitzgerald F, Walker AS, et al. The expanding role of co-trimoxazole in developing countries. Lancet Infect Dis. 2015;15:327–339. doi: 10.1016/S1473-3099(14)71011-4. [DOI] [PubMed] [Google Scholar]
- 56*.Lloyd S, Taylor NK, Koumans E, et al. Prevalence and risk factors for chronic lung disease among HIV-infected children [abstract]. 8th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; 2015; Abstract WEPEB385. This study reported a 15% prevalence of chronic lung disease based on a clinical definition. Recurrent lower respiratory tract infections and delayed antiretroviral initiation were associated with chronic lung disease. [Google Scholar]
- 57.Armah KA, McGinnis K, Baker J, et al. HIV Status, Burden of Comorbid Disease, and Biomarkers of Inflammation, Altered Coagulation, and Monocyte Activation. Clin Infect Dis. 2012;55:126–136. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and Coagulation Biomarkers and Mortality in Patients with HIV Infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 60.Nazli A, Chan O, Dobson-Belaire WN, et al. Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Somsouk M, Estes JD, Deleage C, et al. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS. 2015;29:43–51. doi: 10.1097/QAD.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ilmarinen P, Tuomisto LE, Niemela O, et al. Comorbidities and elevated IL-6 associate with negative outcome in adult-onset asthma. Eur Respir J. 2016 Aug 18; doi: 10.1183/13993003.02198-2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 63.Attia EF, Akgun KM, Wongtrakool C, et al. Increased Risk of Radiographic Emphysema in HIV Is Associated With Elevated Soluble CD14 and Nadir CD4. Chest. 2014;146:1543–1553. doi: 10.1378/chest.14-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fitzpatrick ME, Nouraie M, Gingo MR, et al. Novel relationships of markers of monocyte activation and endothelial dysfunction with pulmonary dysfunction in HIV-infected persons. AIDS. 2016;30:1327–1339. doi: 10.1097/QAD.0000000000001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crothers K, Thompson BW, Burkhardt K, et al. for the Lung HIV Study HIV-associated lung infections and complications in the era of combination antiretroviral therapy. Proc Am Thorac Soc. 2011;8:275–281. doi: 10.1513/pats.201009-059WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pefura-Yone EW, Fodjeu G, Kengne AP, et al. Prevalence and determinants of chronic obstructive pulmonary disease in HIV infected patients in an African country with low level of tobacco smoking. Respir Med. 2015;109:247–254. doi: 10.1016/j.rmed.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 67*.Sridharan G, Wamalwa D, John-Stewart G, et al. High Viremia and Wasting Before Antiretroviral Therapy Are Associated With Pneumonia in Early-Treated HIV-Infected Kenyan Infants. J Pediatric Infect Dis Soc. 2016 Aug 1; doi: 10.1093/jpids/piw038. [Epub ahead of print]. Among infants with perinatally-acquired HIV with ART initiation at a median age of five months, higher HIV RNA and wasting at ART initiation were associated with pneumonia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siddique MA, Hartman KE, Dragileva E, et al. Low CD4+ T Cell Nadir Is an Independent Predictor of Lower HIV-Specific Immune Responses in Chronically HIV-1–Infected Subjects Receiving Highly Active Antiretroviral Therapy. J Infect Dis. 2006;194:661–665. doi: 10.1086/505913. [DOI] [PubMed] [Google Scholar]
- 69.Neuhaus J, Jacobs DR, Baker JV, et al. Markers of Inflammation, Coagulation, and Renal Function Are Elevated in Adults with HIV Infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization. Tobacco Free Initiative. WHO report of the global tobacco epidemic. 2015 Available at: http://www.who.int/tobacco/global_report/2015/en/. Last accessed: 26 Aug 2016.
- 71*.Gómez L, John-Stewart G, Wamalwa D, et al. IDWeek. New Orleans, LA, USA: 2016. Higher Exposure to Household Air Pollution is Associated with Acute Lower Respiratory Illness in HIV-Infected Kenyan Infants [abstract] This is one of the first studies to evaluate the impact of household air pollution on early childhood pneumonia in HIV-infected children. Investigators found that respirable particulate matter <2.5 micrometers (PM2.5) exposure surpassed safety standards set by the WHO and was associated with higher infant pneumonia incidence. [Google Scholar]
- 72.Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax Mar. 2011;66(3):232–239. doi: 10.1136/thx.2010.147884. [DOI] [PubMed] [Google Scholar]
- 73.Arpadi SM. Growth failure in children with HIV infection. J Acquir Immune Defic Syndr. 2000;1:S37–S42. doi: 10.1097/00042560-200010001-00006. [DOI] [PubMed] [Google Scholar]
- 74.Feucht UD, Van Bruwaene L, Becker PJ, Kruger M. Growth in HIV-infected children on long-term antiretroviral therapy. Trop Med Int Health. 2016;21:619–629. doi: 10.1111/tmi.12685. [DOI] [PubMed] [Google Scholar]
- 75.Jesson J, Masson D, Adonon A, et al. for the Growing Up Working Group Prevalence of malnutrition among HIV-infected children in Central and West-African HIV-care programmes supported by the Growing Up Programme in 2011: a cross-sectional study. BMC Infect Dis. 2015;15:216. doi: 10.1186/s12879-015-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGrath CJ, Diener L, Richardson BA, et al. Growth reconstitution following antiretroviral therapy and nutritional supplementation: systematic review and meta-analysis. AIDS. 2015;29:2009–2023. doi: 10.1097/QAD.0000000000000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77*.Arigliani M, Canciani MC, Mottini G, et al. Evaluation of the Global Lung Initiative 2012 Reference Values for Spirometry in African Children. Am J Respir Crit Care Med. 2016 Aug 26; doi: 10.1164/rccm.201604-0693OC. [Epub ahead of print]. This study of African children documented that malnutrition affects growth, and subsequently lung size, with proportional reductions in FEV1 and FVC. [DOI] [PubMed] [Google Scholar]
- 78.Boettiger DC, Muktiarti D, Kurniati N, et al. Early height and weight changes in children using cotrimoxazole prophylaxis with antiretroviral therapy. Clin Infect Dis. 2016 Jul 28; doi: 10.1093/cid/ciw514. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


