Abstract
Cytokine-induced memory-like natural killer (NK) cells differentiate following short-term pre-activation with interleukin (IL)-12, IL-15, and IL-18 and display enhanced effector function in response to cytokines or tumor targets for weeks following the initial pre-activation. Conventional NK cell function is dependent upon a licensing signal, classically delivered by an inhibitory receptor engaging its cognate MHC class I ligand. How licensing status integrates with cytokine-induced memory-like NK cell responses is unknown. We investigated this interaction using killer immunoglobulin-like receptor (KIR)- and human leukocyte antigen (HLA)-genotyped primary human NK cells. Memory-like differentiation resulted in enhanced IFN-γ production triggered by leukemia targets or FcγRIIIa ligation within licensed NK cells, which exhibited the highest functionality of the NK cell subsets interrogated. IFN-γ production by unlicensed memory-like NK cells was also enhanced, to a level comparable to that of licensed control NK cells. Mechanistically, differences in responses to FcγRIIIa-based triggering were not explained by alterations in key signaling intermediates, indicating that the underlying biology of memory-like NK cells is distinct from that of adaptive NK cells in HCMV+ individuals. Additionally, memory-like NK cells responded robustly to cytokine receptor re-stimulation with no impact of licensing status. These results demonstrate that both licensed and unlicensed memory-like NK cell populations have enhanced functionality, which may be translated to improve leukemia immunotherapy.
Keywords: NK cell, adoptive immunotherapy, cytokines, NK cell education, NK cell memory
Graphical Abstract
Introduction
Natural killer (NK) cells are innate lymphoid cells that mediate anti-tumor immune responses, especially against hematologic malignancies.1,2 Distinct from adaptive T and B lymphocytes, NK cells express a variety of germline DNA-encoded, stochastically-expressed activating and inhibitory receptors that govern functional responses to target cells.3 NK cell activating receptors (e.g. FcγRIIIa, NKG2D, natural cytotoxicity receptors) generally recognize cell stress ligands expressed on virally-infected or malignantly-transformed cells, or antibody-opsonized targets. In contrast, inhibitory receptors [e.g. CD94/NKG2A and killer immunoglobulin-like receptors (KIR)] recognize major histocompatibility complex (MHC) class I-type molecules.
In addition to modulating functional responsiveness, NK cell inhibitory receptors are critical for promoting NK cell tolerance. In order to become functionally competent, maturing NK cells require a signaling event through an inhibitory receptor engaging its cognate MHC class I ligand, a dynamic process termed “licensing” or “education”.4 Unlicensed NK cells that express neither KIR nor CD94/NKG2A, or that only express KIR without a cognate ligand present in that individual, are anergic to activating stimuli,4–8 ensuring NK cell tolerance under normal homeostatic conditions. While licensing can occur through either CD94/NKG2A or KIR, significantly higher effector capacity is achieved when licensing is KIR-mediated.9 Studies have shown that the activation state of murine NK cells can alter unlicensed NK cell responses. Specifically, under inflammatory conditions, traditional licensing rules may not apply.4,8,10 In humans, there have been conflicting reports about the impact of short-term cytokine exposure on licensed or unlicensed NK cell responsiveness.5,6,11,12 Thus, it remains uncertain how specific cytokine receptor signals, such as those induced by interleukin (IL)-12/15/18, may modulate functional response in the context of licensing.
Recent reports have demonstrated that NK cell function is also shaped by prior experience.13 Exposure to specific haptens, viral infection, or combined cytokine pre-activation elicits innate memory or memory-like responses from murine NK cells.14–16 Paralleling findings in model organisms, brief combined pre-activation with IL-12, IL-15, and IL-18 results in the differentiation of human memory-like NK cells.17,18 While the initial pre-activating cytokine stimulus results in potent NK cell activation, within days the NK cells return to their baseline activation state, and memory-like differentiation is detectable after 1 week in vitro with survival supported by low-dose IL-15.17 These memory-like NK cells show enhanced responsiveness upon re-stimulation with diverse stimuli, including cytokines or tumor targets, for weeks to months following the initial pre-activation event.
Human memory-like NK cell recognition of, and functional response against, leukemia targets is also enhanced both in vitro and in vivo.19 As a result, cytokine-induced memory has been translated to the clinic in an ongoing memory-like NK cell adoptive immunotherapy trial in patients with relapsed or refractory acute myeloid leukemia (AML, NCT01898793).19,20 Preliminary results from this trial have demonstrated significant donor memory-like NK cell engraftment of recipient bone marrow, and retention of enhanced memory-like functionality upon ex vivo re-stimulation with tumor targets. In addition, clinical responses were observed in 5 of 9 patients treated with 0.5–10x106 NK cells/kg, demonstrating the immunotherapeutic potential of memory-like NK cells in the setting of AML.19 Based upon results in the hematopoietic cell transplantation setting,21–23 MHC-haploidentical donors are chosen for NK cell-based immunotherapy to facilitate inhibitory KIR to KIR-ligand mismatch, thereby reducing NK cell inhibitory signaling and enhancing anti-tumor activity. However, the impact of donor NK cell licensing status on NK cell adoptive immunotherapy outcomes has not been widely explored.
Here, we investigated how the enhanced function of memory-like NK cells integrates with established licensing rules for NK cell reactivity. We hypothesized that memory-like NK cell differentiation enhances the function of unlicensed NK cells, potentially expanding the pool of tumor-responsive NK cells that could be transferred into a patient. We tested this idea using primary human NK cells with known HLA and KIR expression via three distinct and physiologically-relevant modes of re-stimulation.
Materials and Methods
Determination of NK cell licensing status
NK cells were identified as CD45+CD56+CD3- lymphocytes, and divided into CD56bright and CD56dim subsets based on CD56 and FcγRIIIa (CD16) expression. Analyses were restricted to NKG2A−CD56dim NK cells to eliminate potentially confounding effects of CD94/NKG2A on KIR-based licensing. To establish human NK cell licensing status, non-overlapping NKG2A-CD56dim NK subsets [triple positive (3/3 KIR); double positive (2/3 KIR); single positive (1/3 KIR); triple negative (0/3 KIR)] were gated based on KIR2DL2/2DL3 (ligand human leukocyte antigen (HLA)-C1), KIR2DL1 (ligand HLA-C2), and KIR3DL1 (ligand HLA-Bw4) expression.6,7 Licensed subsets contained at least one self-KIR (KIR with its cognate HLA ligand present in that individual).
Reagents
The following anti-human monoclonal antibodies (mAbs) were used: CD56(N901), CD3(UCHT1), CD159a(Z199), CD158a,h(EB6B), and CD45(J.33; all Beckman Coulter); CD16(3G8), CD158b(CH-L), IFNγ(B27), CD107a(H4A3), Syk(4D10), ZAP-70(1E7.2), pZAP70(pY319)/pSYK(pY352), pERK1/2(pT202/pY204), pAkt(pS473; all BD); CD3z(6B10.2; eBioscience); CD158e1(DX9), CD107a(H4A3), goat anti-mouse IgG(Poly4053; all Biolegend); FcεR1γ (Milli-Mark); and rituximab (Genentech). The following endotoxin-free recombinant human (rh) cytokines were used: rhIL-12, rhIL-18 (Peprotech), rhIL-15 (CellGenix and Miltenyi). The following cell lines were used: K562 (ATCC, CCL-243) and Raji (ATCC, CCL-86). Cells were obtained from ATCC in 2008, viably cryopreserved and stored in LN2, thawed for use in these studies, and maintained for <2 months at a time in continuous culture as per ATCC instructions. K562 cells were authenticated in 2015 by confirming cell growth morphology (lymphoblast), short tandem repeat profile, growth characteristics, and functionally as NK cell sensitive targets. Raji cells were authenticated in 2015 by confirming cell growth morphology (lymphoblast), short tandem repeat profile, growth characteristics, phenotype of uniform expression of human CD20, and functionally as anti-CD20 mAb opsonized targets for ADCC.
NK cell culture
Healthy anonymous human peripheral blood mononuclear cells (PBMC) were obtained by Ficoll centrifugation of cells from leukoreduction filters following platelet apheresis.17 The same KIR- and HLA-genotyped, viably-cryopreserved PBMC from anonymous normal donors were used in licensing experiments (Supplemental Table 1). Additional viably-cryopreserved PBMC and fresh NK cells purified using Rosettesep enrichment (StemCell technologies, Vancouver, Canada) from non KIR- and HLA-genotyped normal donors were used for experiments involving Raji lymphoma targets and intracellular signaling. For naïve NK cells, PBMC were thawed and allowed to recover for 12 hours with low-dose (1ng/mL) rhIL-15 prior to stimulation in functional assays. PBMC or purified NK cells were also pre-activated with rhIL-12 (10ng/mL), rhIL-15 (50ng/mL), and rhIL-18 (50ng/mL) (to generate memory-like NK cells) or low-dose rhIL-15 (to generate control NK cells) for 16 hours, then harvested, washed, and allowed to differentiate for 6 days supported with low-dose rhIL-15 as described.17 Cells were cultured at 3–5x106/mL.
NK cell functional assays
Naïve, control, and memory-like NK cells were stimulated with K562 cells (effector:target, E:T 10:1), cytokines (10ng/mL IL-12 + 100ng/mL IL-15), plate-bound anti-FcγRIIIa, or rituximab-coated Raji cells (E:T 5:1) for 6 hours with brefeldin A and monensin (BD Biosciences) added for the final 5 hours. Cells were then surface stained for NK cell markers, fixed/permeabilized and intracellularly stained for IFN-γ (Cytofix/Cytoperm; BD Biosciences).17,24 For degranulation assays, anti-CD107a mAb was added at the start of the functional assay. During functional assays involving memory-like and control NK cells, 0.5ng/mL rhIL-15 was included in the medium to support NK cell survival.
NK cell signaling assays
For phosphorylation assays, purified anti-FcγRIIIa antibody was added at 10ug/mL to control or memory-like purified NK cells and allowed to bind for 20 minutes at 4°C. Following a wash, purified anti-IgG antibody was added at 20ug/mL and cells were briefly spun down then placed at 37°C for 5 minutes. The reaction was stopped by fixation in 1.7% paraformaldehyde followed by permeabilization in 100% methanol. Cells were stained overnight for NK cell surface markers and phospho-signaling molecules. For signaling molecule expression levels, unstimulated control or memory-like NK cells were surface stained for NK cell markers then fixed/permeabilized (Fixation/Permeabilization Concentrate; eBioscience) and intracellularly stained for FcεRγ, CD3ζ, Syk, and ZAP-70.
Flow-based killing assay (FLoKA)
Freshly-purified (RosetteSep) control or memory-like NK cells were incubated for 4 hours with carboxyfluorescein succinimidyl ester (CFSE)-labelled Raji lymphoma targets or rituximab-coated Raji lymphoma targets. After incubation, cells were stained with a 7-AAD viability dye and analyzed immediately using flow cytometry. Percent specific killing was calculated as the percentage of dead (7-AAD positive) target cells, minus background death (less than 5% in all cases).
Statistical analysis
Statistical comparisons (student paired t-test, one-way repeated measures ANOVA with Newman-Keuls post-hoc analysis, or two-way ANOVA with Bonferroni post-hoc analysis, where appropriate) were performed using GraphPad Prism 5 software (* = p<0.05, ** = p<0.01, *** = p<0.001).
Results
Memory-like licensed and unlicensed NK cells display enhanced IFN-γ responses to tumor targets
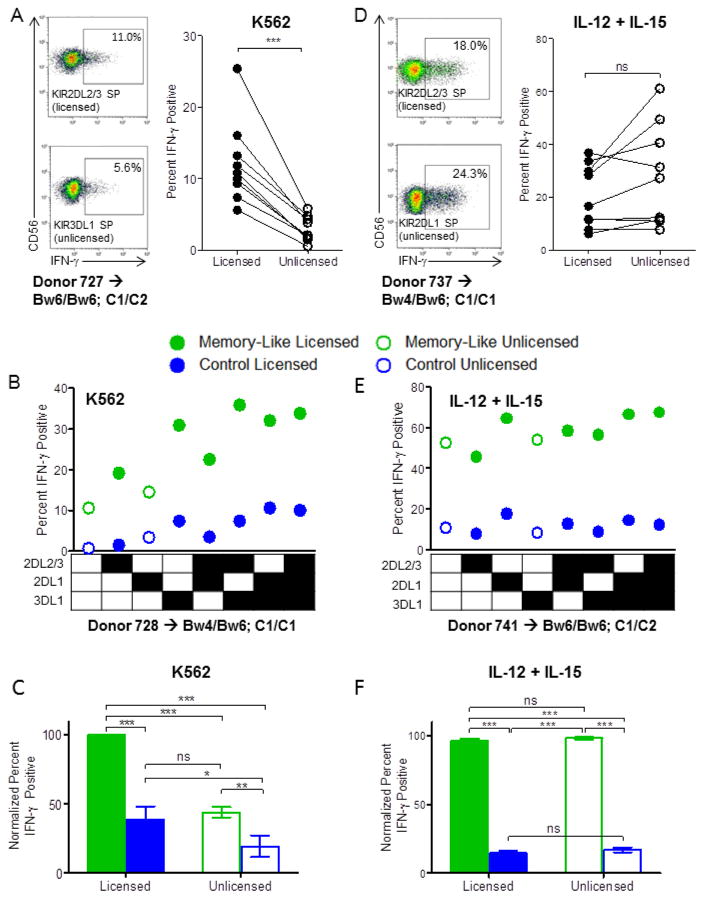
We first confirmed in our cohort of healthy individuals that naïve licensed NK cells produced increased IFN-γ following stimulation with K562 leukemia cells, compared to unlicensed NK cells (Fig. 1A). Next, control and memory-like NK cells were differentiated from the same donors and re-stimulated with K562 leukemia cells while tracking KIR licensing subsets, with a representative individual (Fig. 1B) and summary data from all individuals studied (Fig. 1C) shown. Consistent with a previous report,17 memory-like NK cells responded more robustly than control NK cells following re-stimulation with K562s. Licensed memory-like NK cells exhibited the highest percentage of IFN-γ positive cells when triggered with K562 leukemia targets. Remarkably, unlicensed memory-like NK cells responded similarly to licensed control NK cells, indicating that memory-like differentiation rescued the ability of this hypofunctional population to respond to leukemia targets to the same extent as licensed control NK cells. Since KIR expression amongst NKG2A+ and NKG2A− CD56dim NK cell subsets was unaltered in control compared with memory-like NK cells (not shown), KIR expression changes were not confounding in our experiments.5,12
Figure 1. Cytokine pre-activation enhances the functional response of both licensed and unlicensed NK cells to leukemia targets or cytokine re-stimulation.
(A and D) Naïve PBMC were stimulated with K562 leukemia target cells (A) or IL-12 + IL-15 (D) for 6 hours after which IFN-γ protein levels were assessed via intracellular flow cytometry. Representative data showing the percentage of IFN-γ positive cells from select licensed vs. unlicensed NKG2A−CD56dim NK cell subsets, as well as summary data of all IFN-γ positive licensed vs. unlicensed NKG2A-CD56dim NK cells from n=9 normal donors are shown (n=7 independent experiments). (B, C, E, F) Memory-like or control NK cells were generated from PBMC as described in Methods and re-stimulated with K562 tumor targets (B, C) or IL-12 + IL-15 (E, F) for 6 hours. (B) and (E) show the IFN-γ response of the different NKG2A−CD56dim NK cell subsets according to KIR expression from representative donors in response to K562 targets (B) or cytokines (E). Summary data of the normalized mean ± SEM IFN-γ response of licensed vs. unlicensed memory-like or control NK cells from n=8 normal donors (n=6 independent experiments) to K562 targets (C) or cytokines (F) is also shown. Normalization was performed due to expected inter-individual differences in absolute IFN-γ percentages: for each donor, data were normalized to the NK cell subset (memory-like/licensed, memory-like/unlicensed, control/licensed, control/unlicensed) with the highest IFN-γ response, which was set at 100%. Memory-like NK cells = green, control NK cells = blue. Closed circles and bars = licensed NK cells, open = unlicensed NK cells. HLA-B and -C type is indicated for each representative donor. *p<0.05, **p<0.01, ***p<0.001.
Memory-like NK cells display enhanced IFN-γ responses to cytokine re-stimulation regardless of licensing status
We performed similar experiments comparing licensed/unlicensed and memory-like/control NK cells re-stimulated via cytokine receptors. In contrast to K562 re-stimulation, and different from previous reports that examined only NKG2A−KIR− subpopulations5,6, stimulation via cytokine receptors (IL-12+IL-15) resulted in similar IFN-γ production by licensed and unlicensed naïve NK cells (Fig. 1D), with a trend toward increased IFN-γ in the unlicensed populations (p=0.08). Upon memory-like vs. control NK cell differentiation, memory-like NK cells produced markedly more IFN-γ than controls with no impact of licensing (Fig. 1E, F). This equivalent responsiveness of licensed and unlicensed memory-like NK cells to cytokine re-stimulation was preserved upon serial cytokine dilution, used to elicit sub-optimal responses (not shown). Thus, depending on the re-stimulation approach, licensing status applied (K562 leukemia targets) or was irrelevant (IL-12+IL-15) following memory-like differentiation.
Memory-like NK cell differentiation augments IFN-γ and cytotoxicity responses to FcγRIIIa-based triggering
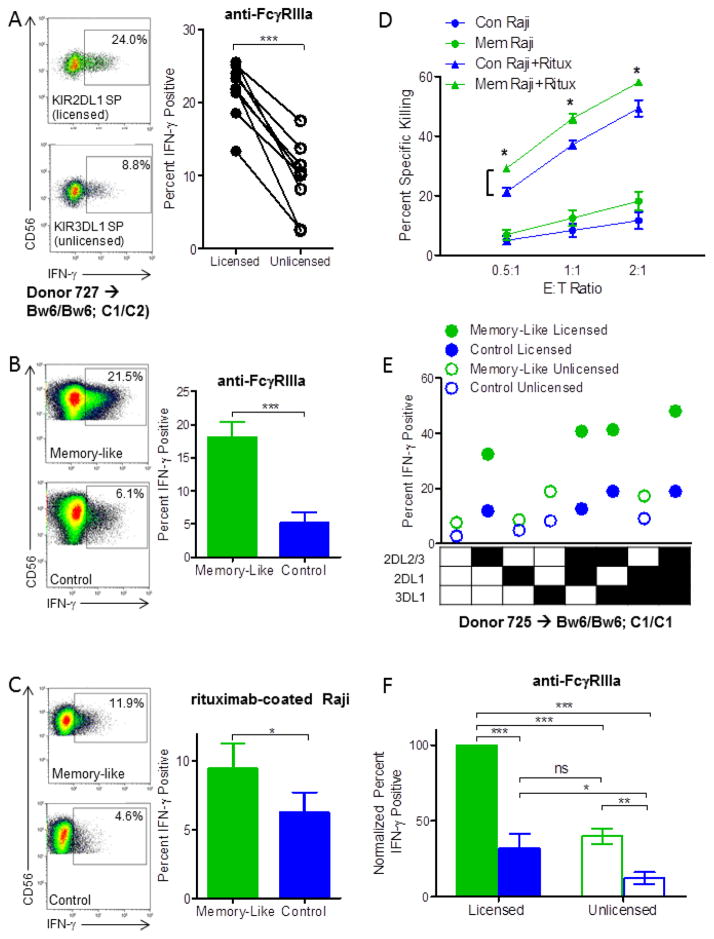
One major activating receptor expressed by NK cells, FcγRIIIa, recognizes the Fc portion of immunoglobulins and triggers antibody-dependent cellular cytotoxicity (ADCC) and cytokine release (ADCR), important mechanisms for therapeutic monoclonal antibody therapy.3 There have been conflicting reports about the responsiveness of licensed vs. unlicensed NK cells to FcγRIIIa ligation.6,7,25 We observed that unlicensed naïve NK cells produced significantly less IFN-γ in response to FcγRIIIa ligation compared to licensed NK cells (Fig. 2A). The ability of memory-like NK cells to respond via FcγRIIIa has not yet been reported. A significantly increased percentage of memory-like NK cells produced IFN–γ in response to stimulation with plate-bound anti-FcγRIIIa (Figure 2B) or rituximab-coated Raji lymphoma targets (Figure 2C) compared to control NK cells. Consistent with K562 re-stimulation,17 degranulation responses to plate-bound anti-FcγRIIIa or rituximab-coated Raji lymphoma targets were similar between memory-like and control NK cells (not shown). To further support these findings, we performed ADCC experiments with rituximab-coated Raji target cells. Memory-like NK cells also displayed enhanced killing of rituximab-coating Raji lymphoma targets compared to control NK cells at all E:T ratios examined (Figure 2D). Thus, NK cell IFN-γ and killing responses to FcγRIIIa-based triggering are enhanced by memory-like differentiation.
Figure 2. Memory-like NK cells show enhanced responsiveness to FcγRIIIa ligation among both licensed and unlicensed NK cells.
(A) Naïve PBMC were stimulated with plate-bound anti-FcγRIIIa antibody for 6 hours and analyzed for intracellular IFN-γ production while gating on licensed vs. unlicensed NKG2A−CD56dim NK cell subsets as described in Figure 1. Flow cytometry plots of IFN-γ positive cells from select licensed vs. unlicensed NKG2A−CD56dim NK subsets from a representative donor and summary data showing the IFN-γ response of all licensed vs. unlicensed NKG2A−CD56dim NK cells from n=9 normal donors (n=7 independent experiments) are shown. (B–F) Memory-like and control NK cells were differentiated as described in Methods and re-stimulated with plate-bound anti-FcγRIIIa antibody (B, E, F) or rituximab-coated Raji lymphoma target cells (C) in a 6 hour functional assay, or rituximab-coated Raji lymphoma targets in a 4 hour FLoKA (D). (B, C) Representative flow cytometry data is shown, as well as summary data of mean ± SEM IFN-γ positive memory-like vs. control NK cells from n=8–10 normal donors (n≥4 independent experiments). (D) Memory-like vs. control NK cell ADCC at different effector to target ratios. Con = control, mem = memory-like, Ritux = rituximab-coated. Raji cells alone are relatively NK cell-resistant, and killing without rituximab did not differ between control and memory-like NK cells. (E) IFN-γ response of the different NKG2A−CD56dim NK cell subsets to plate-bound anti-FcγRIIIa antibody according to KIR expression from a representative donor. (F) Summary data of the normalized mean ± SEM IFN-γ response to plate-bound anti-FcγRIIIa antibody of licensed vs. unlicensed memory-like or control NK cells. Normalization was performed as described in Figure 1. Memory-like NK cells = green, control NK cells = blue. Closed circles and bars = licensed NK cells, open = unlicensed NK cells. HLA-B and -C type is indicated for each representative donor. *p<0.05, **p<0.01, ***p<0.001.
Memory-like licensed and unlicensed NK cells display enhanced IFN-γ responses to FcγRIIIa activating receptor triggering
We next examined responses to FcγRIIIa-based triggering of memory-like vs. control NK cells according to licensing status using the same normal donors examined in Fig. 1. Similar to K562 leukemia targets, memory-like differentiation enhanced the functional responsiveness of both licensed and unlicensed NK cells to plate-bound FcγRIIIa in a pattern similar to that seen with K562 re-stimulation (Fig. 2E, F). These data suggest that both tumor target- and specific ITAM-based activating receptor-triggered memory-like NK cells utilize a common mechanism that results in enhanced functional response.
Intracellular signaling and adaptor molecule expression downstream of FcγRIIIa are similar between memory-like and control NK cells
Currently, the molecular mechanisms responsible for enhanced IFN-γ production by cytokine-induced memory-like NK cells are unknown. We utilized the well-characterized signaling cascade triggered as a result of FcγRIIIa ligation to study potential mechanisms leading to the enhanced IFN-γ response of memory-like NK cells. Adaptive NK cells that arise in human cytomegalovirus (HCMV)-infected individuals are specialized to respond to FcγRIIIa-based triggering via modification of intracellular signaling proteins, including reduced expression of FcεRγ.26–28 We therefore first examined the expression levels of CD3ζ and FcεRγ, the two ITAM-bearing signaling adaptors that couple with FcγRIIIa.29 FcεRγ expression was unchanged between memory-like and control NK cells (Supplemental Fig. 1A). Similarly, CD3ζ expression was comparable between memory-like and control NK cells, with a slight, but statistically significant, decrease in memory-like NK cells (Supplemental Fig. 1A). Reduced expression of Syk was also observed in adaptive NK cells,27,28 and we therefore examined expression of Syk and ZAP-70, related tyrosine kinases that participate in the signaling cascade downstream of FcγRIIIa. Memory-like and control NK cells expressed similar levels of Syk protein, but ZAP70 expression was modestly decreased in memory-like NK cells (Supplemental Fig. 1B). Finally, we assessed key proximal and distal phosphorylation-based signaling intermediates downstream of FcγRIIIa in CD56dim NK cells. We observed no difference in proximal (SYK/ZAP-70) or distal (ERK, Akt) signaling molecule phosphorylation following FcγRIIIa ligation in memory-like vs. control NK cells (Supplemental Fig. 1C). Thus, in contrast to HCMV-induced adaptive NK cells, the enhanced FcγRIIIa-triggered IFN-γ responses of memory-like NK cells do not appear to be the result of differential intracellular signaling. Future studies will examine additional mechanisms, including epigenetic differences between control and memory-like NK cells, to explain the enhanced capacity for IFN-γ production.
Discussion
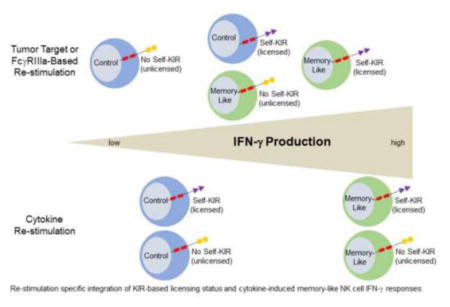
This study reveals mechanistic insights relating to the enhanced functional responses of human cytokine-induced memory-like NK cells.17 Here, we demonstrated that memory-like NK cells follow the general ‘rules’ of inhibitory KIR-based licensing, with licensed NK cells producing significantly more IFN-γ than unlicensed NK cells in response to K562 leukemia targets or FcγRIIIa ligation. However, memory-like differentiation also augmented the IFN-γ response of unlicensed NK cells to leukemia targets or activating receptor stimulation, rescuing their functional response to that of a licensed control NK cell. Thus, one cellular mechanism contributing to the enhanced effector function of memory-like NK cells at the population level is the recruitment of archetypally anergic unlicensed NK cells.
Few reports have examined the molecular mechanisms behind cytokine-induced memory-like NK cell differentiation and function. We observed only modest differences in the expression levels of key intracellular proteins (CD3ζ, Syk) that signal downstream of activating receptors, and a comprehensive analysis of proximal and distal phospho-signaling downstream of FcγRIIIa revealed no differences between control and memory-like NK cells. Thus, enhanced memory-like NK cell responses to activating receptor ligation likely arise from alternate mechanisms, not a difference in secondary signals. This is distinct from HCMV-induced adaptive NK cells, which have a number of intracellular adapter alterations that impact FcγRIIIa signaling.30 These results also show for the first time that cytokine-induced memory-like NK cells have enhanced IFN-γ responses to FcγRIIIa triggering, as well as enhanced antibody-dependent cellular cytotoxicity in vitro, suggesting that memory-like NK cells will be effective responders to antibody-opsonized targets. Thus, therapeutic monoclonal antibodies or other approaches to activate via FcγRIIIa may represent a strategy to further enhance the anti-leukemic targeting and triggering of cytokine-induced memory-like NK cells. While compelling, these in vitro results will require further study using appropriate in vivo models in order to elucidate memory-like NK cell response patterns in an adoptive immunotherapy setting.
In most conventional NK cell adoptive transfer studies,31–33 effective targeting of donor NK cells to patient AML blasts requires a self-KIR licensed NK cell to have a KIR-ligand (HLA-C1, C2, or Bw4) mismatch with the patient AML. This restricts the size of the “leukemia-reactive” NK cell pool of a donor, potentially limiting AML elimination.34 Here, we demonstrate that IL-12/15/18 pre-activation results in an expanded pool of leukemia-reactive NK cells via rescue of unlicensed (no self-KIR) NK cells. This provides a new rational for applying memory-like differentiation to allogeneic NK cell adoptive immunotherapy for leukemia patients, and suggests that consideration of the licensing status of donor NK cells will be less impactful for leukemia responses. Recently, allogeneic memory-like NK cells were shown to substantially reduce AML burden in vivo. This was evident in both murine xenograft models with K562 leukemia, as well as in a first-in-human phase 1 clinical trial of adoptively-transferred HLA-haploidentical memory-like NK cells into patients with relapsed or refractory AML.19 This study investigated the other aspect of KIR/KIR-ligand interaction: how inhibitory KIR present on donor NK cells interact with KIR-ligand expressing (matched) or non-expressing (mismatched) AML. The results showed that memory-like NK cells triggered with AML were not subject to the same KIR/KIR-ligand based inhibition as conventional NK cells.19 Collectively, these two studies demonstrate that adoptive NK cell immunotherapy strategies utilizing cytokine-induced memory-like NK cells are able to circumvent the typical KIR/KIR-ligand considerations that impact selection of NK cell donors via several mechanisms: enhancement of unlicensed NK cell anti-AML responses and the ability to ignore inhibitory KIR signals when responding to AML. These data suggest that a larger pool of individuals may thus be utilized when selecting donors for memory-like NK cell AML therapy, compared to conventional NK cell therapy.
Supplementary Material
Highlights.
Cytokine-induced memory-like NK cell differentiation enhances NK cell responses.
Memory-like differentiation augments licensed and unlicensed NK cell function.
Unlicensed NK cell anti-tumor responses are restored via memory-like differentiation.
FcγRIIIa stimulates enhanced IFN-γ production and killing by memory-like NK cells.
Mechanisms of memory-like functionality are distinct from HCMV-adapted NK cells.
Acknowledgments
We thank Drs. Megan Cooper, Rizwan Romee, Anthony French, and Daniel Link for insightful discussion. This work was supported by grants from the Howard Hughes Medical Institute (Medical Fellow Award, J.A.W.), Translational TL1 Program UL1 TR000448 (J.A.W.), NIH/NCI F32 CA200253 (M.M.B-E.), Siteman Cancer Center Team Science and Siteman Investment Program Awards (T.A.F.), and Gabrielle’s Angel Foundation for Cancer Research (T.A.F.), Leukemia SPORE developmental research grant (T.A.F.). The Siteman Cancer Center Flow Cytometry and Immunomonitoring Laboratory Core resources were utilized for this study, supported by NCI Cancer Center Support Grant P30CA91842.
Footnotes
Financial Disclosure Statement: The authors declare no competing financial interests.
Authorship
J.A.W, T.A.F conceived of the project, designed experiments, and wrote the manuscript with input from all other authors; J.A.W., M.M.B-E., M.R., J.W.L, B.A.J. T.S., S. A-L. performed experiments; J.A.W., T.A.F. analyzed data. All authors reviewed data and approved of the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Long EO, Sik Kim H, Liu D, Peterson ME, Rajagopalan S. Controlling Natural Killer Cell Responses: Integration of Signals for Activation and Inhibition. Annu Rev Immunol. 2013;31(1):227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 5.Cooley S, Xiao F, Pitt M, et al. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110(2):578–586. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anfossi N, André P, Guia S, et al. Human NK Cell Education by Inhibitory Receptors for MHC Class I. Immunity. 2006;25(2):331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Sunwoo JB, Yang L, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A. 2008;105(8):3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105(11):4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J, Heller G, Chewning J, Kim S, Yokoyama WM, Hsu KC. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179(9):5977–5989. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 10.Orr MT, Murphy WJ, Lanier LL. “Unlicensed” natural killer cells dominate the response to cytomegalovirus infection. Nat Immunol. 2010;11(4):321–327. doi: 10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fauriat C, Ivarsson Ma, Ljunggren H-G, Malmberg K-J, Michaëlsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 2010;115(6):1166–1174. doi: 10.1182/blood-2009-09-245746.. [DOI] [PubMed] [Google Scholar]
- 12.Juelke K, Killig M, Thiel A, Dong J, Romagnani C. Education of hyporesponsive NK cells by cytokines. Eur J Immunol. 2009;39(9):2548–2555. doi: 10.1002/eji.200939307. [DOI] [PubMed] [Google Scholar]
- 13.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34(6):251–258. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Leary JG, Goodarzi M, Drayton DL, Yu H, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7(5):507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 15.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106(6):1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romee R, Schneider SE, Leong JW, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209(13):2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8(357):357. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrien-Elliott MM, Wagner JA, Fehniger TA. Human Cytokine-Induced Memory-Like Natural Killer Cells. J Innate Immun. 2015;7(6):563–571. doi: 10.1159/000382019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooley S, Weisdorf DJ, Guethlein La, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411–2419. doi: 10.1182/blood-2010-05-283051.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113(3):726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venstrom JM, Pittari G, Gooley Ta, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367(9):805–816. doi: 10.1056/NEJMoa1200503.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosario M, Liu B, Kong L, et al. The IL-15-based ALT-803 complex enhances Fc RIIIa-triggered NK cell responses and in vivo clearance of B cell lymphomas. Clin Cancer Res. 2016;22(3):596–608. doi: 10.1158/1078-0432.CCR-15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du J, Lopez-Verges S, Pitcher B, et al. CALGB 150905 (Alliance): rituximab broadens the antilymphoma response by activating unlicensed NK cells. Cancer Immunol Res. 2014;2(9):878–889. doi: 10.1158/2326-6066.CIR-13-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang T, Scott JM, Hwang I, Kim S. Cutting Edge: Antibody-Dependent Memory-like NK Cells Distinguished by FcRγ Deficiency. J Immunol. 2013;190(4):1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlums H, Cichocki F, Tesi B, et al. Cytomegalovirus Infection Drives Adaptive Epigenetic Diversification of NK Cells with Altered Signaling and Effector Function. Immunity. 2015;42(3):443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J, Zhang T, Hwang I, et al. Epigenetic Modification and Antibody-Dependent Expansion of Memory-like NK Cells in Human Cytomegalovirus-Infected Individuals. Immunity. 2015;42(3):431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rölle A, Brodin P. Immune Adaptation to Environmental Influence: The Case of NK Cells and HCMV. Trends Immunol. 2016;37(3):233–243. doi: 10.1016/j.it.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 32.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28(6):955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 34.Ruggeri L, Aversa F, Martelli MF, Velardi A. Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol Rev. 2006;214(1):202–218. doi: 10.1111/j.1600-065X.2006.00455.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.