Abstract
Importance
In clinical trials, transcatheter aortic valve replacement (TAVR) has been shown to improve symptoms and quality of life. As this technology moves into general clinical practice, it is critical to evaluate the health status outcomes among unselected patients treated with TAVR.
Design/Participants
Observational study of patients with severe aortic stenosis treated with TAVR in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (TVT) Registry.
Main Outcomes
Disease-specific health status was assessed at baseline and at 30 days (n=31,636) and 1 year after TAVR (n=7,014) with the Kansas City Cardiomyopathy Questionnaire-overall summary score (KCCQ-OS; range 0–100 points). We examined factors associated with health status at 1 year after TAVR using multivariable linear regression, with adjustment for baseline health status and accounting for clustering of patients within sites.
Results
Mean baseline KCCQ-OS was 42.3±23.7, indicating substantial health status impairment. Surviving patients had, on average, large improvements in health status at 30 days that persisted to 1 year, with a mean improvement in the KCCQ-OS of 27.6 points at 30 days and 31.9 points at 1 year. Worse baseline health status, older age, higher ejection fraction, lung disease, home oxygen, lower mean aortic valve gradient, prior stroke, diabetes, pacemaker, atrial fibrillation, slower gait speed, and non-femoral access were associated with worse health status at 1 year. Overall, 62.3% of patients had a favorable outcome at 1 year (alive with reasonable quality of life [KCCQ-OS ≥60] and no significant decline [≥10 points] from baseline) with the lowest rates seen among patients with severe lung disease (51.4%), on dialysis (47.7%), or with very poor baseline health status (49.2%).
Conclusion
In a national, contemporary clinical practice cohort of unselected patients, we found that improvement in health status following TAVR was similar to that seen in the pivotal clinical trials. While the health status results were favorable for the majority of patients, ~1 in 3 patients still had a poor outcome 1 year after TAVR. Continued efforts are needed to improve patient selection and procedural/post-procedural care in order to maximize health status outcomes of this evolving therapy.
Keywords: transcatheter aortic valve replacement, quality of life
Although the pivotal trials demonstrated both survival and quality of life (QOL) benefits of TAVR within select cohorts of patients and hospital centers,1–7 it is critical to understand how this novel technology performs as it moves beyond experienced centers and operators and outside of the strict inclusion and exclusion criteria of the clinical trials. In order to understand these outcomes more fully, the Society of Thoracic Surgeons (STS) and the American College of Cardiology (ACC) developed the STS/ACC Transcatheter Valve Therapy (TVT) Registry.8 In the TVT registry, unselected patients treated with TAVR between 2011 and 2013 experienced a 1-year survival rate of 76%,9 similar to that observed in the pivotal trials. In this elderly population with extensive comorbidity and impaired health status, however, it is unlikely that prolonged survival alone (without improved health status) would be viewed as a desirable outcome. The importance of improving and maintaining health status after TAVR in these patients has been recognized not only by patients and physicians but also by the Centers for Medicare and Medicaid Services, which requires the collection and monitoring of health status outcomes as part of the national registry. In order to better understand the full range of benefits of TAVR in clinical practice, we examined the short- and long-term health status outcomes of surviving patients after TAVR in the TVT registry.
METHODS
Study Sample and Protocol
Details of the design, structure, and data elements for the TVT Registry have been published previously.8,10 The registry was launched in 2011 as a joint initiative of the STS and ACC and now includes more than 450 clinical sites. Facilities are required to participate in the registry in order to obtain Medicare reimbursement; as such, TVT collects data on nearly all TAVR procedures performed outside of clinical trials in the US. Registry activities have been approved by a central institutional review board, and the Duke University School of Medicine institutional review board granted a waiver of informed consent for this study. Sites collect data on patient demographics, comorbidities, hemodynamics, functional status, patient-reported health status, and outcomes. The TVT Registry has been linked to Medicare administrative claims using direct patient identifiers by the Centers for Medicare & Medicaid Services in order to evaluate long-term patient outcomes, including hospitalizations and survival.9
Health Status Assessment and Poor Outcome Definition
The principal health status instrument for the TVT registry is the Kansas City Cardiomyopathy Questionnaire (KCCQ)—a patient-reported disease-specific health status survey developed to describe and monitor symptoms, functional status, and QOL in patients with heart failure11,12; it has also undergone psychometric testing in patients with severe aortic stenosis.13 The shortened, 12-item version of the KCCQ14 is collected as part of the TVT Registry at baseline and at 30 days and 1 year after TAVR. The KCCQ-12 assesses 4 domains related to valvular heart disease (physical limitation, symptom frequency, quality of life, social limitation), which are combined into an overall summary score (KCCQ-OS)—the primary outcome of this study. All domain scores and the KCCQ-OS range from 0 to 100 with higher scores indicating less symptom burden and better QOL. Linguistically and culturally validated translations of the KCCQ were provided to non-English speakers.
Congruent with prior studies, the KCCQ-OS was categorized as very poor (KCCQ-OS <25), poor (KCCQ-OS 25–49), fair (KCCQ-OS 50–74), and good QOL (KCCQ-OS ≥75).13,15 Changes in the KCCQ-OS of 5, 10, and 20 points correspond to small, moderate or large clinical improvements, respectively.16 In order to integrate QOL outcomes with survival, a favorable outcome at 1-year after TAVR was defined as survival with a reasonable QOL (KCCQ-OS score ≥60, roughly equivalent to New York Heart Association class I–II symptoms16,17) without any meaningful worsening (decrease of ≥ 10 points in the KCCQ-OS score from baseline to 1 year).18
Statistical Analysis
Baseline characteristics for the analytic cohort are presented as percentages for categorical variables and medians with interquartile ranges (IQR) for continuous variables. Patient mortality risk was estimated using STS Predicted Risk of Operative Mortality score, which includes 24 variables and has been validated for predicting in-hospital/30-day mortality following surgical aortic valve replacement.19 Changes in KCCQ scores from baseline were evaluated at 30-days and 1-year using paired t-tests. Mean KCCQ-OS scores at 1-year were compared among key subgroups using ANCOVA. These comparisons were adjusted for baseline KCCQ-OS scores except for the analysis that was stratified by baseline KCCQ-OS scores. Rates of favorable outcome at 1-year were estimated for each subgroup and compared using chi-square tests. We then examined factors associated with health status at 1 year after TAVR using multivariable linear regression with generalized estimating equations to account for clustering of patients within sites. Non-linearity (through use of linear splines) and two-way interactions were explored and retained in the model at a level of statistical significance of 0.05 or for clinical importance. As an exploratory analysis, we added 5-meter walk test results to the model to test this association with long-term health status. These results were categorized as ≥0.83 m/s (normal walker; reference group), 0.50 to <0.83 m/s (slow walker), and <0.50 m/s (slowest walker; also includes patients unable to walk).
Missing Data
Although KCCQ completion in TVT has been improving over time, missing data are an important consideration in any QOL analysis.10 The rate of missing KCCQ data was 18.3% at baseline, 30.6% at 30-days, and 55.7% at 1-year. To ensure that we examined a representative cohort of patients, we first limited our 1-year analyses to sites with ≥50% completion rates for the KCCQ. Second, we examined differences in baseline characteristics between surviving patients with and without 1-year KCCQ data. Third, to reduce the effect of selection bias, we used the inverse probability weighting framework to increase the weight of patients who were most like those with missing follow-up data.20 This was done by constructing a multivariable logistic regression model among patients eligible for 1-year follow-up to determine the probability of having missing follow-up KCCQ data. The model included all pre-specified patient-level factors and in-hospital major complications. We then weighted each of the patients in the analytic cohort by the inverse probability of the likelihood of having follow-up KCCQ data to better reflect the overall TAVR population. Both the analysis comparing the health status of patients in key sub-groups and the analysis examining predictors of health status outcomes report the results from this inverse propensity weighting.
The rates of missing data on patient-level factors and in-hospital major complications were all <2% except for 5-meter walk test, which was missing in 13%. Missing data were imputed to the most common category for categorical variables and to the median for continuous variables; 5-meter walk times were not imputed due to the degree of missingness. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, North Carolina), and statistical significance was defined as a 2-sided p-value of <0.05.
RESULTS
Study Sample
Between November 2011 and March 2016, 30,823 patients at 343 sites underwent TAVR and provided baseline QOL data as part of the TVT Registry. The 30-day cohort included 31,636 patients from 406 sites who survived 30-days and completed the KCCQ at baseline and follow-up (Supplemental Figure 1). There were 23,621 patients who underwent TAVR and were eligible for 1 year follow-up. After excluding patients from 179 sites with <50% KCCQ completion rates, the 1-year cohort included 7,014 patients from 169 sites who survived 1 year and completed the KCCQ at both baseline and follow-up. Patients who survived 1 year but were missing KCCQ data were more likely to have severe lung disease, higher STS mortality risk scores, and lower baseline KCCQ-OS scores, as compared with patients in the 1-year analytic cohort (Supplemental Table 1). Median age for the 1-year analytic cohort (the primary analysis) was 84 years (IQR 78–88), 49% were female, 32% had undergone prior bypass graft surgery, 36% had diabetes, and 11% were on home oxygen (Table 1). The median STS mortality risk score was 6.2% (IQR 4.2–9.4); mean aortic gradient was 43 mmHg (IQR 36–52); and 64% underwent TAVR via a femoral approach.
Table 1.
Baseline characteristics of analytic cohort
| 30-Day Cohort n=31,636 |
1-Year Cohort n=7,014 |
|
|---|---|---|
| Age (y) | 83 (77–87) | 84 (78–88) |
| Female | 15,288 (48.3) | 3,454 (49.2) |
| Caucasian race | 29,903 (95.0) | 6,655 (95.2) |
| Body surface area (m2) | 1.85 (1.68–2.02) | 1.84 (1.68–2.01) |
| Prior myocardial infarction | 7,677 (24.3) | 1,820 (26.0) |
| Prior coronary stenting | 11,072 (35.1) | 2.536 (36.2) |
| Prior bypass graft surgery | 9,026 (28.6) | 2,226 (31.8) |
| Peripheral artery disease | 9,637 (30.5) | 2,188 (31.2) |
| Atrial fibrillation/flutter | 12,828 (40.6) | 2,753 (39.3) |
| Permanent pacemaker | 5,081 (16.1) | 1,139 (16.2) |
| Ejection fraction (%) | 58 (47–63) | 58 (47–63) |
| Prior stroke | 3,787 (12.0) | 818 (11.7) |
| Diabetes mellitus | 11,790 (37.3) | 2,547 (36.3) |
| Treated with insulin | 4,290 (36.4) | 903 (35.5) |
| Severe lung disease | 4,232 (13.5) | 806 (11.5) |
| Home oxygen | 3,634 (11.5) | 757 (10.8) |
| Current smoker | 1,704 (5.4) | 344 (4.9) |
| Hemoglobin (g/dL) | 11.9 (10.6–13.1) | 12.0 (10.8–13.1) |
| Glomerular filtration rate (mL/min/1.73 m2) | 60.7 (44.8–76.7) | 61.6 (45.6–77.1) |
| Current dialysis | 1,115 (3.5) | 195 (2.8) |
| STS mortality risk score (%) | 6.3 (4.2–9.6) | 6.2 (4.2–9.4) |
| Aortic valve mean gradient (mmHg) | 43 (35–51) | 43 (36–52) |
| Moderate/severe aortic insufficiency | 6,270 (19.9) | 1,306 (18.7) |
| Moderate/severe mitral insufficiency | 9,238 (34.9) | 1,967 (33.3) |
| Femoral access site | 24,341 (77.0) | 4,506 (64.3) |
| Acuity of TAVR | ||
| Elective | 28,505 (90.1) | 6.353 (90.6) |
| Urgent | 2,189 (6.9) | 454 (6.5) |
| Shock/Inotropes/Assist Device | 846 (2.7) | 189 (2.7) |
| Emergency/Salvage/Cardiac Arrest | 96 (0.3) | 18 (0.3) |
| Baseline KCCQ-OS | 39.6 (24.0–59.4) | 41.7 (26.0–60.4) |
| 5-meter walk test (s) | 7.7 (6.0–10.0) | 7.7 (6.0–10.0) |
KCCQ-OS, Kansas City Cardiomyopathy Questionnaire-overall summary score; Data are expressed as n (%) or median (interquartile range)
Baseline Health Status
Among patients in the 30-day cohort, the mean baseline KCCQ-OS score was 42.3±23.7 (Table 2). Examining the subscales of the KCCQ, the QOL domain was the most impaired at 33.2±27.0, indicating that patients reported that their valve disease severely limited their enjoyment of life and that they would be dissatisfied if they had to spend the rest of their life feeling the same way. The social limitation domain, which describes how patients’ valve disease limits their participation in hobbies, household chores, and visiting others, was also severely impaired at 39.8±30.2. The physical limitations domain, which describes the degree to which patients are limited in bathing, walking, or hurrying, showed slightly less impairment at 43.2±27.7. The symptom frequency domain, which quantifies how often the patient had swelling, fatigue, shortness of breath, and orthopnea in the prior 2 weeks, was the least impaired at 51.8±26.2.
Table 2.
Mean KCCQ scores and unadjusted changes from baseline, on the basis of paired t-tests
| n | Mean ± SD (baseline) | Mean ± SD | Change from Baseline (95% CI) | P-Value | |
|---|---|---|---|---|---|
| 30 days after TAVR | |||||
| Overall Summary Score | 31,636 | 42.3 ± 23.7 | 69.9 ± 23.7 | 27.6 (27.3–27.9) | <0.001 |
| Physical Limitation | 27,788 | 43.2 ± 27.7 | 65.1 ± 28.8 | 21.4 (21.0–21.8) | <0.001 |
| Symptom Frequency | 31,611 | 51.8 ± 26.2 | 73.5 ± 23.4 | 21.6 (21.3–22.0) | <0.001 |
| Quality of Life | 31,437 | 33.2 ± 27.0 | 70.7 ± 27.9 | 37.5 (37.1–37.9) | <0.001 |
| Social Limitation | 26,838 | 39.8 ± 30.2 | 69.3 ± 30.5 | 29.0 (28.5–29.4) | <0.001 |
| 1 year after TAVR | |||||
| Overall Summary Score | 7,014 | 44.0 ± 23.3 | 75.9 ± 21.9 | 31.9 (31.3–32.6) | <0.001 |
| Physical Limitation | 5,980 | 44.8 ± 27.3 | 68.8 ± 28.4 | 23.2 (22.3–24.0) | <0.001 |
| Symptom Frequency | 7,009 | 53.9 ± 25.4 | 78.0 ± 21.9 | 24.0 (23.4–24.7) | <0.001 |
| Quality of Life | 6,973 | 34.7 ± 27.1 | 78.6 ± 24.8 | 43.9 (43.1–44.7) | <0.001 |
| Social Limitation | 5,965 | 41.3 ± 30.1 | 77.0 ± 27.7 | 35.0 (34.1–36.0) | <0.001 |
KCCQ, Kansas City Cardiomyopathy Questionnaire; TAVR, transcatheter aortic valve replacement
Short-term Health Status Outcomes
At 30-day follow-up, KCCQ-OS scores improved by an average of 27.6 points to 69.9±23.7 (Table 2). On average, each of the individual domains increased by >20 points from baseline to 30 days after TAVR, indicating large improvements. The largest improvement was observed in the QOL domain, where the mean improvement was 37.5 points, resulting in an average score of 70.7±27.9. The physical limitations domain was least impacted by TAVR, with a 21.4-point average improvement and a mean 30-day score of 65.1±28.8, although this still represents a large clinical change.
Long-term Health Status Outcomes
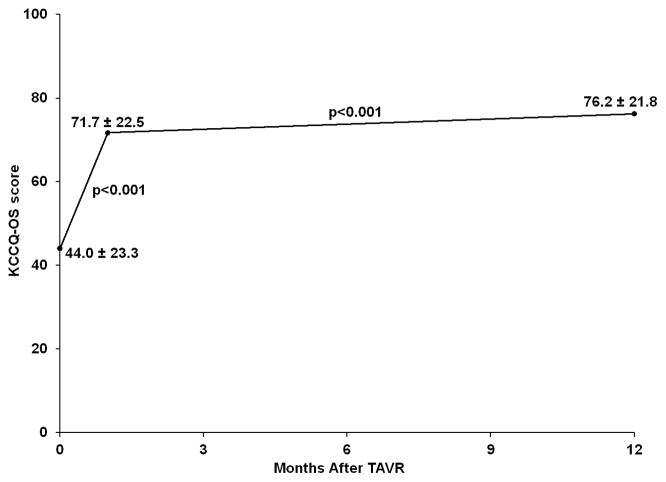
One year after TAVR, surviving patients continued to demonstrate substantial improvement in health status, with a mean KCCQ-OS score of 75.9±21.9 and a mean improvement from baseline of 31.9 points (Table 2). Similar to the 30-day results, the lowest domain score at 1 year was physical limitations with a mean score of 68.8±28.4, with the remaining domains ranging from 77.0 to 78.6. Integrating survival and QOL, 62.3% of patients had a favorable outcome at 1 year after TAVR. Among patients with a poor outcome, this was due to death in 19.4%, persistently poor QOL in 17.4%, and a decline in QOL in 4.9%. Among 6050 patients with health status assessment at all time points, KCCQ-OS scores increased significantly from baseline to 30 days and increased further from 30 days to 1 year (p<0.001 for both comparisons; Figure 1).
Figure 1.
Mean KCCQ-OS scores over time among 6050 patients with complete data
Subgroup Comparisons
There were notable differences in mean 1-year KCCQ-OS scores and the proportion of patients with a favorable outcome across subgroups of patients (Table 3). Older age, no prior CABG, chronic lung disease, low mean aortic gradient, and worse baseline health status were each associated with both worse health status after TAVR and lower rates of favorable outcome. Neither kidney function nor access site were associated with health status after TAVR (among survivors); however, patients with chronic renal impairment and patients who required transapical or transaortic access had lower rates of favorable outcomes, mainly due to increased rates of mortality among these patients. Patients with left ventricular systolic dysfunction had lower rates of favorable outcomes (presumably due to higher rates of death); however, if they survived, they actually had better long-term health status. There were no statistically significant differences in 1-year KCCQ-OS scores or rates of favorable outcomes according to sex. The lowest rates of favorable outcomes were among patients with severe lung disease (51.4%), requiring dialysis (47.7%), and with very poor baseline health status (49.2%).
Table 3.
Health status at 1-year after TAVR in key subgroups
| n | 1-Year Estimate (95% CI) | P-Value | Favorable Outcome1 | P-Value | |
|---|---|---|---|---|---|
| Sex | 0.492 | 0.911 | |||
| Male | 3560 | 74.5 (73.1–76.0) | 62.4% | ||
| Female | 3454 | 74.2 (72.8–75.6) | 62.3% | ||
| Age | 0.041 | <0.001 | |||
| <80 years | 2197 | 75.3 (73.8–76.7) | 63.6% | ||
| 80 to <90 years | 3750 | 74.3 (72.8–75.7) | 62.8% | ||
| ≥90 years | 1067 | 73.3 (71.6–75.0) | 58.2% | ||
| Prior CABG | 0.023 | <0.001 | |||
| Yes | 2226 | 75.3 (73.8–76.9) | 64.8% | ||
| No | 4785 | 74.0 (72.7–75.4) | 61.1% | ||
| Ejection fraction | <0.001 | 0.017 | |||
| <30% | 425 | 77.7 (75.3–80.0) | 57.9% | ||
| 30 to <45% | 1011 | 75.3 (73.5–77.1) | 60.9% | ||
| ≥45% | 5511 | 73.8 (72.4–75.1) | 63.0% | ||
| Mean aortic gradient | 0.003 | <0.001 | |||
| <40 mmHg | 2401 | 73.1 (71.5–74.7) | 59.4% | ||
| ≥40 mmHg | 4527 | 75.0 (73.6–76.3) | 63.9% | ||
| COPD | <0.001 | <0.001 | |||
| None/mild | 5282 | 75.3 (73.9–76.6) | 65.1% | ||
| Moderate | 891 | 73.3 (71.3–75.3) | 57.5% | ||
| Severe | 806 | 71.2 (69.2–73.1) | 51.4% | ||
| Renal function | 0.450 | <0.001 | |||
| Cr <2 mg/dL without dialysis | 6473 | 74.6 (73.3–75.9) | 63.5% | ||
| Cr ≥2 mg/dL without dialysis | 339 | 74.3 (71.6–77.0) | 53.6% | ||
| Dialysis | 195 | 72.5 (69.2–75.8) | 47.7% | ||
| Access site | 0.112 | <0.001 | |||
| Transfemoral | 4506 | 74.9 (73.6–76.2) | 65.1% | ||
| Transapical | 1821 | 73.5 (71.8–75.3) | 58.7% | ||
| Transaortic | 558 | 73.4 (71.0–75.7) | 54.1% | ||
| Other | 126 | 75.2 (71.0–79.4) | 62.3% | ||
| Baseline health status | <0.001 | <0.001 | |||
| 0 to <25 | 1633 | 66.2 (64.2–68.2) | 49.2% | ||
| 25 to <50 | 2634 | 72.8 (71.3–74.3) | 61.3% | ||
| 50 to <75 | 1900 | 78.0 (76.7–79.4) | 72.0% | ||
| 75 to 100 | 847 | 85.9 (84.4–87.4) | 73.8% |
Defined as alive, KCCQ-OS ≥60, and no worsening of KCCQ-OS from baseline (decline of <10 points)
Factors Associated with Health Status at 1 Year
In the multivariable model, patients who had better health status at baseline were more likely to have better health status at 1 year, with every 10-point increase in baseline KCCQ-OS score associated with a 2.5-point higher 1-year KCCQ-OS score (95% CI 2.2 to 2.8, p<0.001; Table 4). Older age was associated with worse 1-year health status, with every 5-year increase associated with a 1.0-point reduction in KCCQ-OS score (95% CI −1.4 to −0.7, p<0.001). Higher ejection fraction at baseline, severe lung disease, home oxygen, lower mean aortic valve gradient, prior stroke, diabetes, permanent pacemaker (prior to TAVR), atrial fibrillation, and non-femoral access were also associated with lower 1-year KCCQ-OS scores. In exploratory analysis among 6151 patients with 5-meter walk test data, both slow gait speed and slower gait speed (as defined previously) were associated with worse 1-year KCCQ-OS scores (mean difference −3.5 points [95% CI −5.0 to −2.1] and −5.7 points [95% CI −7.6 to −3.7], respectively).
Table 4.
Factors independently associated with 1-year KCCQ-OS scores after TAVR
| Estimate (95% CI) | P-value | |
|---|---|---|
| Baseline KCCQ-OS score (per 10 points) | +2.5 (+2.2 to +2.8) | <0.001 |
| Age (per 5 years) | −1.0 (−1.4 to −0.7) | <0.001 |
| Ejection fraction (per 5%) | −0.6 (−0.8 to −0.3) | <0.001 |
| Severe lung disease | −2.8 (−4.3 to −1.2) | <0.001 |
| Home oxygen | −5.3 (−7.3 to −3.2) | <0.001 |
| Mean aortic valve gradient (per 10 mmHg) | +0.9 (+0.5 to +1.3) | <0.001 |
| Prior stroke | −2.6 (−4.2 to −0.9) | 0.002 |
| Diabetes mellitus | −1.5 (−2.5 to −0.6) | 0.002 |
| Moderate/severe aortic insufficiency | +1.9 (+0.5 to +3.2) | 0.007 |
| Permanent pacemaker | −1.9 (−3.3 to −0.4) | 0.011 |
| Atrial fibrillation/flutter | −1.4 (−2.6 to −0.2) | 0.021 |
| Access site (non-femoral vs. femoral) | −1.2 (−2.4 to −0.1) | 0.038 |
KCCQ-OS, Kansas City Cardiomyopathy Questionnaire-overall summary score
Factors in the model that were not significantly associated with QOL (p>0.05): sex, race, body surface area, prior myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft surgery, peripheral artery disease, current smoker, hemoglobin, glomerular filtration rate, current dialysis, moderate/severe mitral insufficiency, moderate/severe tricuspid insufficiency, acuity of case
DISCUSSION
As the use of TAVR expands beyond the pivotal clinical trials—both to new centers and new patient populations—it is important to understand the short- and long-term outcomes of these procedures in unselected patients. While recent studies have demonstrated rates of survival and major complications that are generally consistent with those seen in the original clinical trials,9 the health status benefits of these procedures—particularly as assessed from the patient’s perspective—remain largely unexplored. Given the elderly population who are generally considered for TAVR, understanding the health status outcomes of these procedures is essential.
In this study, we used data from the TVT registry to explore the health status outcomes among unselected patients undergoing TAVR in current US practice. We found that most patients had substantial impairment in health status prior to TAVR and demonstrated considerable improvement after the procedure. In particular, by 30 days after TAVR, surviving patients demonstrated large improvements across all domains of the KCCQ, and there were further, albeit more modest, improvements from 30 days to 1 year. Worse baseline health status, older age, severe lung disease, home oxygen, higher baseline ejection fractions, lower mean aortic valve gradient, and slower gait speed were all associated with worse long-term health status. When we examined health status and survival as a composite endpoint, 38% of patients had a poor outcome at 1 year after TAVR, roughly half of which was due to death with the other half due to persistently poor or worse QOL. Patients with severe lung disease, end-stage renal disease, or very poor baseline health status had the highest rates of poor outcomes, with half being either dead or having persistently poor QOL at 1 year.
Comparison with Prior Studies
The QOL results in the TVT registry are generally similar to those observed in the clinical trials among patients at high and extreme surgical risk.2,3,6,7 Nonetheless, there are several subtle differences in outcomes between the pivotal trials and the TVT registry. For example, the changes in the KCCQ-OS at 30 days and 1 year in TVT of 27 and 32 points, respectively, are ~5–10 points greater than those seen in the PARTNER A and B and CoreValve clinical trials. Moreover, the 62% rate of favorable outcome at 1 year in TVT is modestly higher than seen in the pivotal trials, in which only 50–58% of patients were alive with good QOL at 1 year.7,21 The reasons for these differences are not entirely clear but may relate to differences between the patients treated in current practice as compared with the original trials. In our study, the median STS risk score was <7%—lower than both the PARTNER and CoreValve trials. While there are undoubtedly some patients treated in TVT who are even higher risk than those included in the pivotal trials (e.g., dialysis, very low ejection fraction, low mean gradient), the typical patient treated in current US practice appears to be somewhat lower risk than the trial participants, with fewer comorbidities that might hinder recovery of QOL after hospitalization. In addition, ongoing improvements in patient selection, procedural techniques, and post-procedural care are likely to have contributed to enhanced health status recovery post-TAVR.
In a recent analysis of a German TAVR registry, generic health status (EuroQol) improved in the majority of patients. However, the authors of this study noted that a sizable proportion of patients did not derive any meaningful benefit in terms of QOL. Importantly, a generic health status measure is not as sensitive to change as a disease-specific health measure,16 thereby limiting the ability to detect important changes in symptoms, function, and QOL. For example, in the CoreValve High Risk US Pivotal Trial, the 1-year changes in the KCCQ-OS had an effect size of ~1 versus ~0.2 for EuroQol-5D.7 Consequently, we believe that use of a generic heath status assessment will underestimate the extent of improvement in the TAVR population.
Clinical Implications
The current study findings are encouraging and suggest that the benefits of TAVR that have been previously demonstrated within carefully designed and conducted clinical trials can be extended to the commercial TAVR population. In the future, investigating the factors that underlie these outcomes could allow us to maximize the benefits of this approach. For example, using risk prediction models to estimate a patient’s pre-procedure likelihood of a successful outcome may allow providers to improve patient selection for TAVR.21 In addition, such models may be useful in guiding procedural and post-procedural care, by targeting patients at increased risk of a poor outcome for less invasive approaches, geriatric consultation, or more intensive rehabilitation and follow-up to help maximize recovery.
Limitations
Our study should be considered in light of the following potential limitations. First, the degree of missing health status data, both at baseline and follow-up, made the analyses challenging. We used a number of approaches to address this issue, including limiting our analyses to sites with higher rates of complete data and inverse propensity weighting. However, it is still possible that missing data biased our results. While longitudinal health status data are critical to the understanding of benefit of TAVR, collection of these data in an unselected cohort has been challenging,22 particularly when there is no specific reimbursement for data collection. Achieving more complete data will require continued efforts by registry leadership along with a belief by the sites that such data are important. As such, we hope that these analyses will highlight the importance of health status data for risk prediction and patient selection,23 assessing response to treatment, and identifying mechanisms to improve care.
Second, while we examined a number of covariates in our multivariable model some important factors, such as dementia and disability, were not included in the TVT data elements and thus could not be examined as potential predictors of long-term QOL. Third, the long-term QOL results are reported for surviving patients only. This is a key point given the high mortality rate among patients treated with TAVR, as it is possible that long-term health status benefits of TAVR in particular subgroups could have been missed due to differential attrition of the sickest patients in the comparator subgroup (e.g., renal dysfunction). To address this challenge we also reported a composite endpoint that integrates both survival and QOL; we believe that examining the results of both of these analyses is essential to fully understand the benefits of TAVR.
Conclusions
In this national cohort study of unselected US patients undergoing TAVR in clinical practice, we found that, on average, patient health status improved substantially following the procedure. The observed magnitude of health status improvement was similar to that seen in the pivotal clinical trials. Nonetheless, ~1 in 3 patients still had a poor outcome at 1 year after TAVR, half of which was due to death and half due to poor QOL. Therefore, continued efforts to optimize patient selection and refine procedural and post-procedural care to maximize health status recovery are needed to continue to improve the outcomes of these patients.
Supplementary Material
Acknowledgments
Zhuokai Li had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
FUNDING/SUPPORT: The STS/ACC TVT Registry™ is an initiative of the Society of Thoracic Surgeons and the American College of Cardiology. This research was supported by the American College of Cardiology’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the authors, and do not necessarily represent the official views of the NCDR or its associated professional societies identified at CVQuality.ACC.org/NCDR. The study sponsors were not involved in the design and conduct of the study; analysis and interpretation of the data; preparation of the manuscript; or decision to submit the manuscript for publication. Dr. Arnold is supported by a Career Development Grant Award (K23 HL116799) from the National Heart, Lung, and Blood Institute. Dr. Vora is supported by a T32 training grant (T32 HL069749) and L30 HL124592 from the NHLBI.
Footnotes
AUTHOR DISCLOSURES: Dr. Vemulapalli has received research support from the American College of Cardiology, Abbott Vascular, and AHRQ. Dr. Mack is a member of the Executive Committee of the PARTNER Trial of Edwards Lifesciences (uncompensated). Dr. Reynolds is a consultant to Medtronic and has received research grant support from Edwards Lifesciences and Medtronic. Dr. Cohen has received research grant support from Edwards Lifesciences, Medtronic, and Boston Scientific and consulting fees from Medtronic and Edwards Lifesciences.
References
- 1.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124(18):1964–1972. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 3.Osnabrugge RL, Arnold SV, Reynolds MR, et al. Health status after transcatheter aortic valve replacement in patients at extreme surgical risk: results from the CoreValve U.S. trial. JACC Cardiovasc Interv. 2015;8(2):315–323. doi: 10.1016/j.jcin.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 5.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds MR, Magnuson EA, Wang K, et al. Health-Related Quality of Life After Transcatheter or Surgical Aortic Valve Replacement in High-Risk Patients With Severe Aortic Stenosis: Results From the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A) J Am Coll Cardiol. 2012;60(6):548–558. doi: 10.1016/j.jacc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 7.Arnold SV, Reynolds MR, Wang K, et al. Health Status After Transcatheter or Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Increased Surgical Risk: Results From the CoreValve US Pivotal Trial. JACC Cardiovasc Interv. 2015;8(9):1207–1217. doi: 10.1016/j.jcin.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll JD, Edwards FH, Marinac-Dabic D, et al. The STS-ACC transcatheter valve therapy national registry: a new partnership and infrastructure for the introduction and surveillance of medical devices and therapies. J Am Coll Cardiol. 2013;62(11):1026–1034. doi: 10.1016/j.jacc.2013.03.060. [DOI] [PubMed] [Google Scholar]
- 9.Holmes DR, Jr, Brennan JM, Rumsfeld JS, et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313(10):1019–1028. doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]
- 10.Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310(19):2069–2077. doi: 10.1001/jama.2013.282043. [DOI] [PubMed] [Google Scholar]
- 11.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 12.Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7(2):235–242. doi: 10.1016/j.ejheart.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Arnold SV, Spertus JA, Lei Y, et al. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6(1):61–67. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 14.Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015 doi: 10.1161/CIRCOUTCOMES.115.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110(5):546–551. doi: 10.1161/01.CIR.0000136991.85540.A9. [DOI] [PubMed] [Google Scholar]
- 16.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Arnold SV, Spertus JA, Lei Y, et al. Use of the Kansas City Cardiomyopathy Questionnaire for Monitoring Health Status in Patients With Aortic Stenosis. Circulation. Heart failure. 2013;6(1):61–67. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 18.Arnold SV, Spertus JA, Lei Y, et al. How to Define a Poor Outcome After Transcatheter Aortic Valve Replacement: Conceptual Framework and Empirical Observations From the Placement of Aortic Transcatheter Valve (PARTNER) Trial. Circ Cardiovasc Qual Outcomes. 2013;6(5):591–597. doi: 10.1161/CIRCOUTCOMES.113.000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg. 2008;135(1):180–187. doi: 10.1016/j.jtcvs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 21.Arnold SV, Reynolds MR, Lei Y, et al. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129(25):2682–2690. doi: 10.1161/CIRCULATIONAHA.113.007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta BP, Grady KL, Fendler T, Jones PG, Spertus JA. Variation of Quality of Life Data Collection Across INTERMACS Sites. J Card Fail. 2015 doi: 10.1016/j.cardfail.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Arnold SV, Spertus JA, Vemulapalli S, et al. Association of Patient-Reported Health Status With Long-Term Mortality After Transcatheter Aortic Valve Replacement: Report From the STS/ACC TVT Registry. Circ Cardiovasc Interv. 2015;8(12) doi: 10.1161/CIRCINTERVENTIONS.115.002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.