Abstract
Inflammatory bowel diseases (IBD), including Crohn's disease and ulcerative colitis, are chronic relapsing disorders of the intestines. They cause severe problems, such as abdominal cramping, bloody diarrhea, and weight loss, in affected individuals. Unfortunately, there is no cure yet, and treatments only aim to alleviate symptoms. Current treatments include anti-inflammatory and immunosuppressive drugs that may cause severe side effects. This warrants the search for alternative treatment options, such as nutritional supplements, that do not cause side effects. Before their application in clinical studies, such compounds must be rigorously tested for effectiveness and security in animal models. A reliable experimental model is the dextran sulfate sodium (DSS) colitis model in mice, which reproduces many of the clinical signs of ulcerative colitis in humans. We recently applied this model to test the beneficial effects of a nutritional supplement containing vitamins C and E, L-arginine, and ω3-polyunsaturated fatty acids (PUFA). We analyzed various disease parameters and found that this supplement was able to ameliorate edema formation, tissue damage, leukocyte infiltration, oxidative stress, and the production of pro-inflammatory cytokines, leading to an overall improvement in the disease activity index. In this article, we explain in detail the correct application of nutritional supplements using the DSS colitis model in C57Bl/6 mice, as well as how disease parameters such as histology, oxidative stress, and inflammation are assessed. Analyzing the beneficial effects of different diet supplements may then eventually open new avenues for the development of alternative treatment strategies that alleviate IBD symptoms and/or that prolong the phases of remission without causing severe side effects.
Keywords: Medicine, Issue 119, inflammation, oxidative stress, DSS, adherens junction, vitamins, anti-oxidants, nutrition, intestinal epithelial permeability
Introduction
Colitis is an inflammatory condition of the colon that can cause diarrhea and abdominal pain. Colitis can be acute, in response to infection or to stress, or it can develop as a chronic disease, such as in ulcerative colitis (UC), which belongs to the group of inflammatory bowel diseases (IBD). Although the clinical signs of UC have been well described, the pathogenesis is still poorly understood1. It is accepted among experts that UC is a multifactorial disease, with genetic mutations and aberrant immune responses playing a major role2. However, environmental factors, such as style of living and nutrition, also contribute to disease development and progression3.
Unfortunately, IBD is not curable, but there are many treatment options that aim to alleviate clinical symptoms. Current treatments include anti-inflammatory drugs, such as sulfasalazine and corticosteroids; immunosuppressants, such as azathioprine; monoclonal antibodies that capture pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), or block adhesion molecules, such as integrins, to reduce excessive leukocyte recruitment; and inhibitors that target kinases that trigger pro-inflammatory pathways, such as Janus kinase (JAK)4. Not all patients respond to all treatments, so therapeutic strategies must be individualized5. Furthermore, most of these therapeutic drugs interfere with the metabolism and immune responses, often causing severe side effects. For this reason, alternative treatment options have been investigated.
Alternative treatments include probiotics and nutritional supplements, which have been applied in animal models and clinical trials with varying levels of success6,7. We recently found that the application of different single nutritional supplements, such as anti-oxidative vitamins or ω3-poly-unsaturated fatty acids (PUFAs), is inferior to the application of a combination of such supplements in alleviating colitis and cardiovascular disease symptoms8,9. These studies were performed in mice, so clinical studies must be performed in humans to determine whether these findings will also be applicable to humans. Before clinical studies are initiated, the effectiveness and safety of new treatment options must be evaluated in animal models.
For IBD, the dextran sulfate sodium (DSS) model has been widely used to study the mechanisms of disease development and the beneficial effects of drugs and nutritional supplements6,10. In most studies, only an acute disease over a time period of seven days is induced; nevertheless, the clinical signs observed in these animals closely resemble those observed in IBD patients (i.e., bloody diarrhea, weight loss, epithelial dysfunction, and immune cell infiltration)10. DSS induces erosions in the mucosa, resulting in barrier dysfunction and in increased intestinal epithelial permeability11. The exact mechanism remains unknown. However, a study suggests that DSS interacts with medium chain-length fatty acids to form nano-lipocomplexes that are able to enter epithelial cells and to induce inflammatory signaling pathways12. In this article, we describe in detail how colitis is induced and analyzed in mice, how nutritional supplements are applied by gavage to ensure a constant dosage in each animal, and how the effects of such supplements on various colitis symptoms are examined.
Protocol
All animal experiments have been approved by the Institutional Animal Care and Use Committee of Cinvestav.
1. Preparation of DSS Drinking Water and the Induction of Colitis
Prepare a 3.5% w/v dextran sulfate sodium (DSS) solution in autoclaved drinking water. DSS dissolves easily and it is not required to filter the final solution. NOTE: 7 days of DSS treatment induces severe colitis. If inducing mild colitis, 3 - 4 days of treatment are recommended. As long as the DSS drinking water stays clear, it is not necessary to change the water. However, if it turns turbid, it must be replaced.
Record the baseline weight of male mice. Ensure that they are 8 - 12 weeks of age and within a range of 21 - 25 g bodyweight. NOTE: The appropriate DSS concentration must be optimized in each laboratory. Results can greatly vary, depending upon the housing conditions. Concentrations between 2.5 - 5% are usually reported to induce strong colitis in C57Bl/6J WT mice10. DSS from different companies and different lots should be tested, as results may also vary with different sources of DSS.
Provide water ad libitum and replace it with fresh 3.5% DSS water if necessary.
2. Application of Nutritional Supplements by Gavage
Prepare a "feedable" solution of the desired nutritional supplements in an appropriate solvent. For this study, we used a mixture containing 200 mg/kg L-arginine, 83 mg/kg vitamin C, 46 mg/kg vitamin E, 77 mg/kg eicosapentaenoic acid (EPA), and 115 mg/kg docosahexaenoic acid (DHA) in a 1:1 solution of water and safflower oil.
Fill a 1-mL syringe connected to a gavage needle (15 G x 50 mm) with the nutritional supplement mixture. Wipe the outside of the gavage needle to remove any compound outside of the needle and to ensure proper dose application.
Restrain the mouse by grasping the base of the tail with one hand and by firmly gripping a skin fold at the back of the neck with the thumb and forefinger of the other hand. Place the tail between the third finger and the base of the thumb of the same hand that holds the neck.
While maintaining the mouse in an upright position, insert the needle into the left side of the animal's mouth and carefully follow the roof of the mouth to locate the esophagus. If no resistance is encountered, advance the needle towards the stomach. NOTE: Verify that the animal is breathing properly before proceeding.
Administer the mixture slowly, remove the tube by slowly pulling out the syringe, and release the mouse. Apply nutritional supplements once daily by gavage during the course of the colitis experiment.
3. Determination of the Disease Activity Index
NOTE: The disease activity index is the combination of the scores for weight loss, perianal bleeding, and stool consistency, which are obtained daily13.
Measure the bodyweight daily and assign a score of 0 to 4 according to the weight loss percentage (0: 0%, 1: 1 - 5%, 2: 5 - 10%, 3: 10 - 20%, and 4: > 20%). NOTE: Bodyweight loss is determined by calculating the difference in percent between the basal weight before the induction of DSS-colitis and the actual weight.
- To determine the level of perianal bleeding, collect a fresh stool sample and use the provided applicators to apply a thin smear on a slide from a guaiac fecal occult blood test kit. Use a fresh applicator for each sample.
- Close the cover on the front and open the window on the back of the slide. Apply two drops of the developer solution to the back at the sample location.
- Read the results after 30 s. Any trace of blue color is positive for occult blood. Assign a score of 0 to 4 (0: none, 0.5 - 2.5: positive guaiac fecal occult blood test (depending upon the signal strength), and 3 - 4: gross bleeding). NOTE: If blood in the stool is visible, the guaiac fecal occult blood test does not have to be performed, and a score of 3, 3.5, or 4 is assigned, depending upon the amount of visible blood.
Determine stool consistency by observing a fresh stool sample. Assign a score of 0 to 4 (0: normal/solid, 0.5 - 2.5: pasty stool, and 3 - 4: diarrhea).
4. Determining Intestinal Epithelial Permeability In Vivo by Evans Blue Assay
Anesthetize the animals by injecting them intraperitoneally with a mixture of ketamine (100 mg/kg of bodyweight) and xylazine (13 mg/kg of bodyweight) diluted in saline solution (0.9% NaCl), and assess the depth of anesthesia by monitoring the pinch withdrawal reflex.
Place the anesthetized animals in a supine position and perform a laparotomy to expose the intestines.
Locate the cecum and make a small incision in the proximal segment of the colon ascendens (ideally, immediately adjacent to the cecum).
Insert a feeding needle (G22) and secure it with a ligature using a common silk thread. Carefully flush abundant PBS through the tube to rinse out all feces from the colon. Instill Evans blue solution (1.5% w/v in PBS) into the colon until it reaches the anus.
Incubate the Evans blue for 15 min. Wash out the dye by flushing the tube with abundant PBS until the perianal washout is clear.
Euthanize the animals by cervical dislocation.
Excise the colon and rinse it again with abundant PBS. Rinse once with 1 mL of 6 mM N-acetylcysteine in PBS to eliminate any dye sticking to the colonic mucus.
Cut the colon longitudinally and rinse it once more with PBS and 1 mL of 6 mM N-acetylcysteine.
Record the weight and length. To extract the Evans blue dye, place the colon in 2 mL of N,N-dimethylformamide overnight at room temperature with gentle agitation. Measure the dye concentration spectrophotometrically at 610 nm.
5. Tissue Collection
Anesthetize the animals by injecting them intraperitoneally with a mixture of ketamine (100 mg/kg of bodyweight) and xylazine (13 mg/kg of bodyweight) diluted in saline solution (0.9% NaCl), and assess the depth of anesthesia by monitoring the pinch withdrawal reflex.
Euthanize the animals by cervical dislocation.
Place the animals in a supine position and perform a laparotomy to expose the abdominal cavity.
Using scissors, dissect the entire colon and record its length.
Flush the colon carefully several times with chilled PBS to remove the feces. Record the tissue weight and prepare for the following experiments, as described in steps 5.6 - 5.8.
For RNA isolation and myeloperoxidase (MPO) assays, cut off an appropriately-sized tissue sample (100 mg is usually sufficient), place it inside a tube, and snap-freeze it in liquid nitrogen. Store the tissues at -80 °C for future use.
For immunofluorescence staining and oxidative stress determination (see below), cut a small colon sample (0.5 - 1 cm) and submerse it in small aluminum cups filled with optimal cutting temperature (OCT) compound. Place the cups on dry ice and let them freeze slowly, in order to avoid bubble formation. Frozen tissue samples can be stored at -80 °C for future use.
For Swiss rolls14, open the colon longitudinally and carefully remove feces using forceps. Roll it up, with the mucosa facing inwards, using fine-tipped forceps or a thin, round wooden stick. Carefully place it inside an embedding cassette and continue with step 6.1. NOTE: DSS induces damage in the colon. However, damage distribution varies from the proximal to the distal colon, with most damage usually seen in the distal colon and rectum. Thus, we recommend analyzing the histology either in the distal colon or in Swiss roles of the entire colon.
6. Analysis of Colon Histology by Hematoxylin-eosin Staining
- Tissue Preparation
- For the histological analysis, submerge either the Swiss rolls or the small tissue pieces from certain colon regions of control and treated mice in 5 mL of 10% formaldehyde for 48 h at room temperature (RT) to achieve proper fixation. NOTE: All further steps in step 6 are carried out at RT, unless otherwise indicated.
- Wash them with tap water for 18 h.
- Dehydrate them with 15 mL of 70% ethanol for 1 h, 96% for 1 h, and absolute ethanol for 1 h. Repeat each step with fresh alcohol before proceeding to the next concentration.
- Continue the dehydration in a mixture of 7.5 mL of absolute ethanol and 7.5 mL of absolute xylene for 1 h. Repeat once before passing the samples to 15 mL of absolute xylene for 1 h. Repeat this step once with fresh xylene.
- Embedding Colon Tissue Samples in Paraffin
- Immerse the samples in 50 mL of liquid paraffin for 17 h. Repeat this step once but for only 3 h.
- Immerse the samples in cubes (plastic, square mold, size: 22 x 22 x 22 mm) in 810 mL of liquid paraffin.
- Allow the paraffin with the colon tissue samples to solidify for 24 h at RT.
- Cutting Tissue Cross-sections using a Microtome
- Cut colon samples using a microtome, according to the manufacturer's instructions, at a thickness of 5 µm.
- Collect the cuts in a water bath (1 L) containing 0.3% gelatin. This prevents the folding of tissue cross-sections. Recover the tissue sections and mount them on glass slides.
- Hematoxylin and Eosin Staining of Tissue Cross-sections
- Deparaffinize the samples for 18 h at 60 °C in an incubator. For this purpose, use a glass slide rack with a handle.
- After deparaffinization, immerse the slides in 70 mL of absolute xylene for 5 min. Repeat this step once with fresh xylene.
- Transfer the samples into 70 mL of absolute alcohol for 1 min. Repeat this step once with fresh alcohol.
- Incubate the colon tissue samples in 70 mL of ethanol 96% for 1 min. Repeat this step once with fresh alcohol.
- Wash the samples in tap water twice for 2 s.
- Incubate the tissues in Harris hematoxylin for 7 min.
- Wash the samples in tap water twice for 2 s.
- Incubate the samples in acidic alcohol (70 mL of ethanol 70% and 700 µL of 1 M hydrochloric acid) for 7 s.
- Wash the samples in tap water twice for 2 s.
- Incubate the tissue samples in 70 mL of lithium carbonate (1 g of lithium carbonate in 100 mL of distillated water) for 7 s.
- Wash the samples in tap water twice for 2 s.
- Incubate the samples in 70 mL of ethanol 80% for 1 min.
- Transfer the samples into 70 mL of Eosin-Y for 15 s.
- Incubate them in 70 mL of 96% ethanol for 1 min. Repeat this step once with fresh alcohol.
- Incubate the tissue samples in 70 mL of absolute ethanol (ethyl alcohol absolute anhydrous) for 1 min. Repeat this step once with fresh alcohol.
- Incubate the samples in 70 mL of a mixture of 35 mL of absolute alcohol and 35 mL of absolute xylene for 1 min.
- Transfer the samples into 70 mL of absolute xylene for 1 min. Repeat this step for 5 min in 70 mL of fresh absolute xylene.
- Add 80 µL of synthetic resin to the colon tissue samples, cover them with coverslips, and let them dry for 24 h at RT. Finally, analyze the samples using bright-field microscopy8.
7. Analyzing Junction Composition by Immunofluorescence
Prepare the tissue as described in step 5.7 and cut 8 µm-thick cross-sections using a cryostat.
Fix and permeabilize colon cross-sections with 96% ethanol at -20 °C for 20 min. NOTE: All subsequent incubations should be carried out at RT, unless otherwise stated, in a humid dark box to prevent drying and fluorochrome fading.
Air-dry samples, and then rinse them 3 times for 5 min each with 1x PBS
Incubate the samples with blocking solution (PBS containing 0.01% Tween and 2% BSA) for 2 h.
Remove the blocking solution and rinse it with PBS-0.01% Tween for 10 min.
During this time, dilute the primary antibodies to the desired concentrations in PBS-0.01% Tween.
Remove the washing solution, apply the antibody solution from the previous step, and incubate overnight at 4 °C in a humidified box.
Remove the antibody solution and wash 3 times with PBS-0.01% Tween for 5 min each and once with deionized water for 10 min, with very gentle agitation.
While washing, centrifuge fluorochromeconjugated, species-specific secondary antibodies at 14,000 x g for 20 min at 4 °C.
Dilute the secondary antibodies without precipitates as indicated by the provider in PBS-0.01% Tween, apply them, and incubate for 1 h at RT.
Repeat step 7.8 and remove the washing solution as completely as possible.
Pipette 4 µL of anti-fading agent over each sample on the slide.
Cover the samples with a coverslip.
Air dry for 1 h.
Seal slide with nail polish. Slides can be stored at -20 °C until further analysis.
Analyze on a confocal laser microscope8.
8. Determination of Oxidative Stress by Oxidation of Ethidium
After 7 days of DSS colitis, prepare tissue as described in step 5.7 and cut colon sections in a cryostat at a thickness of 5 µm. Mount cross-sections onto glass slides treated with poly-L-lysine solution and incubate the samples with 5 µM of dihydroethidium (DHE) in water at 37 °C for 30 min in the dark.
Wash the samples with PBS (pH 7.6) three times for 15 min each with gentle agitation at RT. Air dry the samples and add a drop of mounting medium to protect the fluorescence. Observe the fluorescence of oxidized ethidium on a laser-scanning confocal microscope (40X magnification, 568 nm wavelength).
9. Analysis of Leukocyte Recruitment by MPO Assay
After 7 days of DSS colitis, collect colon tissue and homogenize the samples (200 - 400 mg) with 1 mL of hexadecyltrimethylammonium (HTAB) buffer (0.5% HTAB in 50 mM phosphate buffer, pH 6.0) using a homogenizer on ice.
Rinse the tip of the homogenizer twice with 1 mL of HTAB buffer to include all tissue in the lysate and sonicate it five times at 40% amplitude for 10 s each. Freeze-thaw in liquid nitrogen three times.
Centrifuge at 14,000 x g for 15 min and collect the supernatant. Prepare 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) solution by mixing ABTS, 5 mL of sodium citrate (1 M in water), 0.1 mL of hydrogen peroxide, and 45 mL of bi-distilled water.
Mix 0.1 mL of each supernatant with 0.1 mL of ABTS solution in a 96-well flat-bottomed plate. After 10 min, measure the absorbance at 460 nm using a spectrophotometer.
10. Evaluating the Expression of Pro-inflammatory Cytokines by PCR
- Tissue Homogenization
- Homogenize 50 to 100 mg of colon tissue samples in 1 mL of an acid guanidinium thiocyanate-phenol-chloroform mixture using a power homogenizer.
- Incubate it at room temperature for 15 min.
- Phase Separation
- Add 0.2 mL of chloroform and shake vigorously for 30 s.
- Incubate at 4 °C for 30 min.
- Centrifuge at 12,000 x g for 60 min at 4 °C.
- Transfer the upper aqueous phase into a fresh tube (~500 µL).
- RNA Precipitation and Resuspension
- Add 0.5 mL of isopropyl alcohol and incubate the samples at 4 °C for 60 min.
- Centrifuge at 12,000 x g for 30 min at 4 °C.
- Remove the supernatant completely.
- Add 1 mL of ethanol 75% and vortex.
- Centrifuge at 12,000 x g for 30 min at 4 °C.
- Remove the supernatant.
- Allow the remaining ethanol to air-dry for 3-5 min. NOTE: It is important not to let the RNA pellet dry completely, as this will greatly decrease its solubility.
- Re-dissolve the pellet in 0.3 mL of diethylpyrocarbonate (DEPC)-treated water. Immediately transcribe the RNA samples into cDNA (more stable, see step 10.5) or store at -80 °C.
- RNA Quantification
- Dilute 1 µL of RNA with 99 µL of DEPC-treated water.
- Read the OD at 260 nm and 280 nm using a UV/VIS spectrophotometer to determine sample concentration and purity.
- cDNA Synthesis
- Mix RNA samples (1-5 µg) with 1 µL of Oligo(dT)12-18 (0.5 µg/µL) and DEPC-treated water (total volume of 12 µL) in RNase-free sterile tubes.
- Incubate at 70 °C for 10 min.
- Add 2 µL of 10x PCR Buffer, 1 µL of 50 mM MgCl2, 1 µL of 10 mM dNTP mix, and 2 µL of 0.1 M dithiothreitol (DTT).
- Incubate at 4 °C for 5 min.
- Add 1 µL of reverse transcriptase and incubate at 42 °C for 5 min.
- Incubate at 70 °C for 15 min.
- Incubate at 4 °C for 15 min. cDNA can be stored at -20 °C until further use.
- PCR
- Mix 2.5 µL of 10x PCR Buffer, 1.5 µL of 25 mM MgCl2, 0.5 µL of 10 mM dNTP mix, 0.25 µL of Taq DNA polymerase, 0.25 µL of each primer (10 µM) and cDNA (100 ng), and sterile water to a total volume of 25 µL.
- Run the samples on a 96-well thermal cycler. To prevent amplification of genomic DNA, forward and reverse primers should be located in different exons: IL1β-FW: GCAACTGTTCCTGAACTCAACT and IL1β-RE: TCTTTTGGGGTCCGTCAACT; IL6-FW: CCTTCCTACCCCAATTTCCAA and IL6-RE: AGATGAATTGGATGGTCTTGGTC; KC-FW: TGTCAGTGCCTGCAGACCAT and KC-RE: CCTGAGGGCAACACCTTCA; and Actin-FW: TATCCACCTTCCAGCAGATGT and Actin-RE: AGCTCAGTAACAGTCCGCCTA. Use the following PCR conditions: 95 °C for 5 min followed by 30 cycles at 95 °C for 15 s, 58 °C for 30 s, and 72 °C for 30 s, plus a final extension for 10 min at 72 °C.
Representative Results
Diet Supplements Can Protect from DSS Colitis
Anti-inflammatory and anti-oxidative diet supplements, such as vitamin C, vitamin E, the nitric oxide (NO)-source L-arginine, and ω3-polyunsaturated fatty acids (ω3-PUFAs), have been used with varying success in animal models to alleviate signs of inflammatory diseases3,6,15. Malnutrition, oxidative stress, and the production of pro-inflammatory cytokines can trigger both acute and chronic colitis3. In a previous study, we proved that a combination of micronutrients showed protective effects against DSS-induced colitis in vivo. Here, we provide detailed protocols on how these effects were measured8. First, we analyzed the effects of nutritional supplements on disease progression by determining the disease activity index (DAI), consisting of the three parameters of weight loss, stool consistency, and perianal bleeding. Representative results showed that DSS treatment led to a continuously-increasing DAI, as expected, reaching a maximum of 8.2 ± 0.46 on day seven (Figure 1A). Anti-inflammatory diet supplementation delayed the onset of colitis, but at later time points, when colitis was very strong, the protective effect was marginal and no longer statistically significant, suggesting that diet supplements are only effective when the colitis is not yet too strong. Importantly, dietary intervention could prevent the DSS-induced increase in intestinal epithelial permeability, which is an important feature of colitis (Figure 1B). DSS colitis led to reduced colon lengths, and this shortening was also ameliorated by the anti-inflammatory and anti-oxidative diet supplement (Figure 1C). Thus, clinical symptoms of colitis can indeed be alleviated by changes in diet composition.
Colon Tissue Damage and Disassembly of TJ and AJ during Colitis Is Attenuated by Anti-inflammatory and Anti-oxidative Diet Supplements
To analyze why colitis progression was less severe in animals receiving a diet supplement, tissue morphology was investigated after hematoxylin/eosin (H/E) staining of paraffin-embedded distal colon tissues. Control samples showed a normal morphology (Figure 2A, upper panel), whereas animals treated with 3.5% DSS showed typical signs of colitis (i.e., leukocyte infiltration, edema formation, crypt dysplasia, and ulcerations; Figure 2A, middle panel). Of note, parallel treatment with diet supplementation clearly alleviated colitis-induced morphological changes, with an overall morphology that was more comparable to the control group (Figure 2A, bottom panel). These observations were very similar to what has been reported for co-treatments with probiotic bacteria13 and clearly show that changes in diet can counteract the development of colitis.
We knew already that colitis-induced intestinal epithelial permeability could be counteracted by diet supplementation. Increased permeability is a hallmark of IBD, and it is in large part caused by the disassembly of the stabilizing adherens junctions (AJ) and tight junctions (TJ). Destabilization of epithelial cell contacts by the internalization of junctional proteins is induced by pro-inflammatory cytokines, which are abundantly produced during colitis16. We found, through the immunofluorescent staining of distal colon tissue, that the TJ molecule zonula occludens-1 (ZO-1) and the AJ molecule E-cadherin were internalized during DSS-induced colitis and that co-localization of these proteins was partly lost at crypt epithelial cell contacts (Figure 2B). Importantly, internalization of E-cadherin and ZO-1 was reduced when DSS-treated mice were co-treated with an anti-inflammatory and anti-oxidative diet supplement (Figure 2B). Overall, crypt and junction structures were more comparable to controls when the diet supplement was applied, suggesting that the observed protective effect was at least in part due to the preservation of tissue architecture.
Dietary Intervention Can Counteract Inflammatory Neutrophil Recruitment, Oxidative Stress, and the Production of Pro-inflammatory Cytokines
Another hallmark of IBD is uncontrolled neutrophil infiltration. Recruited neutrophils contribute to tissue damage by producing and releasing reactive oxygen species (ROS) and proteases17. Using an MPO activity assay, we found strong neutrophil recruitment in the colon in response to DSS, which was counteracted by diet supplementation (Figure 3A).
Oxidative stress is another important feature of colitis, caused by the release of ROS from recruited neutrophils. Thus, we analyzed the production of ROS in colon cross-sections. Figure 3B demonstrates that DSS treatment strongly increased oxidative stress. Of note, changes in diet completely prevented DSS-induced oxidative stress (Figure 3B), which is most likely a consequence of reduced neutrophil recruitment (Figure 3A).
Pro-inflammatory cytokines and chemokines are strongly produced by various cell types during colitis18. Such inflammatory mediators contribute to neutrophil recruitment and junction protein internalization-features that we already knew could be attenuated by changes in diet. Analysis of mRNA expression by RT-PCR revealed that DSS increased the expression of IL1β, IL-6, and keratinocyte-derived chemokine (KC), as expected (Figure 3C), and that anti-inflammatory diet supplementation diminished this DSS-induced increase (Figure 3C). These data imply that diet supplements can indeed serve to counteract clinical signs of IBD.
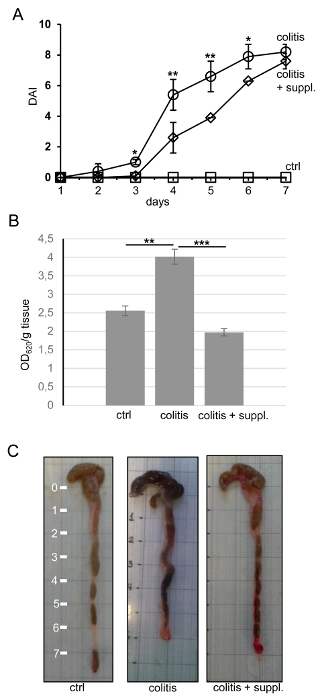 Figure 1. Nutritional Supplements Can Alleviate Disease Activity Index, Intestinal Epithelial Permeability, and Colon Shortening.(A) The disease activity index (DAI, values from 0 - 12) consists of the three parameters of weight loss, stool consistency, and perianal bleeding and was recorded daily in the experimental groups of control (ctrl), colitis, and colitis + nutritional supplement (colitis+suppl.). The control group did not show any signs of colitis, resulting in a DAI of 0 throughout the experimental period. n = 5 per group. (B) Intestinal epithelial permeability was measured by Evans blue leakage in the abovementioned experimental groups and was expressed as the absorption at 620 nm per g of tissue. n = 8 per group. All data are shown as the mean ± the standard deviation of the mean (SDM). *p< 0.05; **p< 0.01; ***p< 0.001. Statistics were calculated by one-way ANOVA. (C) Representative images of whole colons of the respective experimental groups after 7 days of treatment. The scale on the left is in cm. Please click here to view a larger version of this figure.
Figure 1. Nutritional Supplements Can Alleviate Disease Activity Index, Intestinal Epithelial Permeability, and Colon Shortening.(A) The disease activity index (DAI, values from 0 - 12) consists of the three parameters of weight loss, stool consistency, and perianal bleeding and was recorded daily in the experimental groups of control (ctrl), colitis, and colitis + nutritional supplement (colitis+suppl.). The control group did not show any signs of colitis, resulting in a DAI of 0 throughout the experimental period. n = 5 per group. (B) Intestinal epithelial permeability was measured by Evans blue leakage in the abovementioned experimental groups and was expressed as the absorption at 620 nm per g of tissue. n = 8 per group. All data are shown as the mean ± the standard deviation of the mean (SDM). *p< 0.05; **p< 0.01; ***p< 0.001. Statistics were calculated by one-way ANOVA. (C) Representative images of whole colons of the respective experimental groups after 7 days of treatment. The scale on the left is in cm. Please click here to view a larger version of this figure.
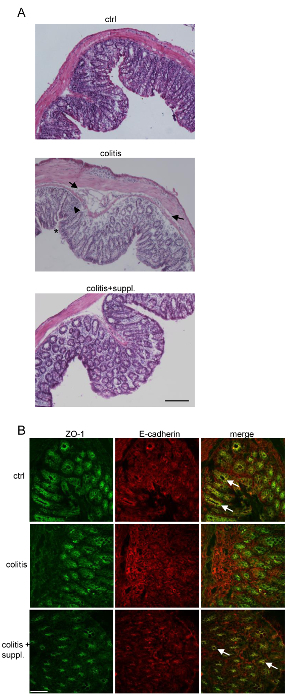 Figure 2. Tissue Morphology and Junction Architecture Are Better Preserved during Colitis when Applying Nutritional Supplements.(A) 5-µm paraffin cross-section of tissue biopsies from the distal colons of the respective experimental groups were stained with hematoxylin/eosin and analyzed for typical signs of colitis, such as edema formation (arrows), leukocyte infiltration (arrowheads), and ulceration (*). Overall tissue morphology of the colitis+suppl. group more resembles that of the control than the colitis group, proving an overall protective effect against DSS colitis. Images are representative of 3 independent tissue preparations per group. Images were taken using a 10X objective. Bar = 100 µm. (B) 8 μm colon cryo-sections of the respective groups were stained with antibodies against ZO-1 (green) and E-cadherin (red). Co-localization (yellow in the merged images; arrows) of E-cadherin and ZO-1 at crypt epithelial cell contacts, which is lost during colitis, is mostly preserved in the colitis + suppl. group. Images are representative of 3 independent tissue preparations. Bar = 50 µm. Please click here to view a larger version of this figure.
Figure 2. Tissue Morphology and Junction Architecture Are Better Preserved during Colitis when Applying Nutritional Supplements.(A) 5-µm paraffin cross-section of tissue biopsies from the distal colons of the respective experimental groups were stained with hematoxylin/eosin and analyzed for typical signs of colitis, such as edema formation (arrows), leukocyte infiltration (arrowheads), and ulceration (*). Overall tissue morphology of the colitis+suppl. group more resembles that of the control than the colitis group, proving an overall protective effect against DSS colitis. Images are representative of 3 independent tissue preparations per group. Images were taken using a 10X objective. Bar = 100 µm. (B) 8 μm colon cryo-sections of the respective groups were stained with antibodies against ZO-1 (green) and E-cadherin (red). Co-localization (yellow in the merged images; arrows) of E-cadherin and ZO-1 at crypt epithelial cell contacts, which is lost during colitis, is mostly preserved in the colitis + suppl. group. Images are representative of 3 independent tissue preparations. Bar = 50 µm. Please click here to view a larger version of this figure.
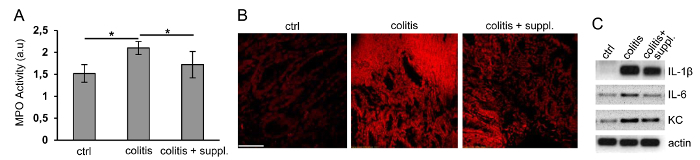 Figure 3. Neutrophil Recruitment, Oxidative Stress, and the Production of Pro-inflammatory Cytokines can be Reduced by Nutritional Supplements.(A) Myeloperoxidase (MPO) activity was measured to analyze the amount of leukocyte recruitment into colon tissues in the experimental groups: control (ctrl), colitis, and colitis + nutritional supplement (colitis+suppl.). n = 5 per group; *p< 0.05. Data are shown as the mean ± SDM. Statistics were calculated by one-way ANOVA. (B) The fluorescence of oxidized ethidium, depicted in red as the measure of oxidative stress in 8-µm cryo-sections of colon tissues, was recorded by laser confocal microscopy. Images are representative of 3 independent tissue preparations. Bar = 100 µm. (C) The production of the pro-inflammatory cytokines IL1β and IL-6 and the chemokine KC was determined by semi-quantitative RT-PCR in a 1.5% agarose gel (black and white were reverted for the sake of clarity). β-actin served as the housekeeping gene. Representative images of three independent cDNA preparations are shown. Please click here to view a larger version of this figure.
Figure 3. Neutrophil Recruitment, Oxidative Stress, and the Production of Pro-inflammatory Cytokines can be Reduced by Nutritional Supplements.(A) Myeloperoxidase (MPO) activity was measured to analyze the amount of leukocyte recruitment into colon tissues in the experimental groups: control (ctrl), colitis, and colitis + nutritional supplement (colitis+suppl.). n = 5 per group; *p< 0.05. Data are shown as the mean ± SDM. Statistics were calculated by one-way ANOVA. (B) The fluorescence of oxidized ethidium, depicted in red as the measure of oxidative stress in 8-µm cryo-sections of colon tissues, was recorded by laser confocal microscopy. Images are representative of 3 independent tissue preparations. Bar = 100 µm. (C) The production of the pro-inflammatory cytokines IL1β and IL-6 and the chemokine KC was determined by semi-quantitative RT-PCR in a 1.5% agarose gel (black and white were reverted for the sake of clarity). β-actin served as the housekeeping gene. Representative images of three independent cDNA preparations are shown. Please click here to view a larger version of this figure.
Discussion
In order to use nutritional supplements in clinical studies, the benefits and safety of such supplements must be carefully evaluated in vivo in animal studies. In the case of colitis, several appropriate animal models that resemble the clinical signs of IBD have been established, including chemical models using DSS, TNBS, or acetic acid; knock-out (KO) models such as IL10-KO; and immune-cell-mediated colitis using adoptive T-cell transfer19-21. The DSS model of colitis is a rapid and reliable method of inducing colitis that resembles many disease symptoms of human IBD and has been used in a plethora of studies investigating the molecular mechanisms of colitis22. Moreover, DSS colitis is reversible and thus also allows for the study of tissue healing after the colitogenic stimulus has been removed. The provided protocol describes in detail the DSS colitis model that has been previously optimized in our laboratory8.
A disadvantage is that the optimization of the DSS concentration must be done in every laboratory, since environmental factors greatly affect disease development and progression23. Additionally, DSS must be in a molecular weight range of 40 - 50 kDa to induce colitis. For the unexperienced researcher, it will be important to develop an eye for an unbiased assessment of the DAI parameters of stool consistency and perianal bleeding. If the assessment is performed by an unexperienced researcher, it is recommendable to have a colleague confirm the assessment, ideally in a blinded fashion.
To analyze the intestinal epithelial permeability for macromolecules, three major methods have been used throughout the literature: the Evans blue dye assay, as described here; the instillation of 4-kDa FITC-dextran in an intestinal loop; and the feeding of 4-kDa FITC-dextran13,24,25. In the latter assay, FITC-dextran is fed by gavage, and the amount of FITC dextran that crossed the epithelium and leaked into the blood stream is measured fluorometrically from serum samples. In this assay, the permeability of the entire gastrointestinal tract is measured, since the tracer passes all the way from the esophagus to the rectum and most of the tracer will pass before reaching the colon. In the loop model, permeability is measured in a confined intestinal region used for the loop (usually the small intestine)25. In contrast, the Evans blue assay has the advantage that only epithelial permeability in the colon is measured, since the tracer is directly instilled into the entire colon. Thus, using this assay, we can conclude that the colonic epithelium is protected by the diet supplement.
It is always important to record the colon weight and length after extraction and before using the tissues for other assays, because some data (e.g., leaked Evans blue in the permeability assay) are expressed per g of tissue. Furthermore, the weight-to-length ratio is an independent read-out of tissue damage26. In general, tissue morphology is analyzed using paraffin-embedded tissues stained with hematoxylin and eosin, as described here. Paraffin embedding has the advantage that the tissue morphology is much better conserved compared to other embedding methods. This allows for the unequivocal identification of different cell types within the mucosa (i.e., immune cells, epithelium, goblet cells, etc.) and the analysis of tissue alterations during colitis, such as edema formation, leukocyte infiltration, and epithelial apical erosions. On the other hand, paraffin has the disadvantage that immunofluorescent staining can only be performed after time-consuming epitope recovery. Therefore, we always prepare different frozen tissue biopsies embedded in OCT compound. Such tissue samples can be easily sectioned using a cryostat and show low levels of autofluorescence. We use ethanol for fixation by dehydration because neither does it interfere with actin filaments (as methanol does), nor does it trigger autofluorescence (as PFA does). Using these techniques, we found that an anti-inflammatory and anti-oxidative diet supplement can improve tissue morphology and junction architecture during colitis (compare Figure 2).
Excessive neutrophil recruitment is a critical event during colitis that is induced by the production of chemoattractants in response to inflammation17. Neutrophil recruitment is a physiological event because neutrophils contribute to the removal of the inflammatory cue and to subsequent wound healing. However, if this innate immune response is not properly controlled and terminated, neutrophils in the mucosa can contribute to tissue damage by releasing proteases and reactive oxygen species. Neutrophils contain MPO, which can be easily measured and quantified using a colorimetric assay, where the amount of MPO is directly proportional to the number of neutrophils. Figure 3A shows that neutrophil recruitment increases during colitis and that dietary intervention can prevent this excessive and uncontrolled immune response. In accordance with the abundant neutrophil presence in the mucosa, oxidative stress increases during colitis (Figure 3B).
Our diet supplementation clearly protects the mucosa from oxidative stress, which is most likely a consequence of reduced neutrophil recruitment. As mentioned, neutrophils are attracted by chemokines and the production of chemokines is induced by pro-inflammatory cytokines that are secreted by various cell types during colitis. Thus, we assumed that reduced neutrophil recruitment is a consequence of reduced cytokine and chemokine production. Figure 3C demonstrates that the pro-inflammatory cytokines IL-1β and IL-6, as well as the chemokine KC, are upregulated during DSS-induced colitis. The diet supplement reversed the levels of IL-6 back to control levels and ameliorated the increased production of IL-1β and KC. Of note, KC is the most important chemoattractant for neutrophils in mice.
Thus, it is tempting to speculate that the observed preservation of tissue morphology is due to reduced neutrophil infiltration, which is, in turn, a consequence of the reduced production of KC. Analyzing RNA production in DSS-treated tissues by RT-PCR is problematic when residual DSS is present in the isolated RNA. This is because DSS inhibits both reverse transcriptases and Taq-polymerases. If no amplification can be achieved, it will be necessary to further purify the RNA samples by LiCl-mediated precipitation, as described elsewhere27.
In summary, we describe in detail different methods for the analysis of tissue damage during DSS colitis and how this damage can be ameliorated by dietary supplements. Knowledge gained from such animal experiments can justify the establishment of patient cohorts to investigate the protective effects of the analyzed supplements in human IBD. Data obtained from clinical studies may then open new avenues to develop alternative treatment strategies for the management of IBD.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by grants from the Mexican Council for Science and Technology (Conacyt, 207268 and 233395 to Michael Schnoor). KFCO is a recipient of a Conacyt stipend (396260) to obtain an MSc degree.
References
- Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Neuman MG, Nanau RM. Inflammatory bowel disease: role of diet, microbiota, life style. Transl Res. 2011;160:29–44. doi: 10.1016/j.trsl.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Lowenberg M, D'Haens G. Next-Generation Therapeutics for IBD. Current Gastroenterol Rep. 2015;17:21. doi: 10.1007/s11894-015-0444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein CN. Does everyone with inflammatory bowel disease need to be treated with combination therapy. Curr Opin Gastroenterol. 2016. [DOI] [PubMed]
- Nanau RM, Neuman MG. Nutritional and probiotic supplementation in colitis models. Dig Dis Sci. 2012;57:2786–2810. doi: 10.1007/s10620-012-2284-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Nutrition and diet in inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29:216–221. doi: 10.1097/MOG.0b013e32835b9a40. [DOI] [PubMed] [Google Scholar]
- Vargas Robles H, et al. Experimental Colitis Is Attenuated by Cardioprotective Diet Supplementation That Reduces Oxidative Stress, Inflammation, and Mucosal Damage. Ox Med Cell Longev. 2016. p. 8473242. [DOI] [PMC free article] [PubMed]
- Vargas-Robles H, Rios A, Arellano-Mendoza M, Escalante BA, Schnoor M. Antioxidative diet supplementation reverses high-fat diet-induced increases of cardiovascular risk factors in mice. Ox Med Cell Longev. 2015;2015:467471. doi: 10.1155/2015/467471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014;104:25. doi: 10.1002/0471142735.im1525s104. Unit 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroui H, et al. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One. 2009;7:32084. doi: 10.1371/journal.pone.0032084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennigen R, et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:1140–1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- Park CM, Reid PE, Walker DC, MacPherson BR. A simple, practical 'swiss roll' method of preparing tissues for paraffin or methacrylate embedding. J Microsc. 1987;145:115–120. doi: 10.1111/j.1365-2818.1987.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Shen W, Gaskins HR, McIntosh MK. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J Nutr Biochem. 2013. [DOI] [PubMed]
- Bruewer M, et al. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Muc Immunol. 2012;5:354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- Yan Y, et al. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One. 2009;4:6073. doi: 10.1371/journal.pone.0006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JR, Viney JL. Overview of mouse models of inflammatory bowel disease and their use in drug discovery. Curr Prot Pharmacol, S.J. Enna. 2009. Chapter 5, Unit 5.57. [DOI] [PubMed]
- Ostanin DV, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:135–146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Kor J Physiol Pharmacol. 2014;18:279–288. doi: 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittem CG, Williams AD, Williams CS. Murine Colitis modeling using Dextran Sulfate Sodium (DSS) JoVE. 2010. [DOI] [PMC free article] [PubMed]
- Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Prot. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- Naydenov NG, et al. Nonmuscle Myosin IIA Regulates Intestinal Epithelial Barrier in vivo and Plays a Protective Role During Experimental Colitis. Sci Rep. 2016;6:24161. doi: 10.1038/srep24161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumagin R, Robin AZ, Nusrat A, Parkos CA. Transmigrated neutrophils in the intestinal lumen engage ICAM-1 to regulate the epithelial barrier and neutrophil recruitment. Muc Immunol. 2014;7:905–915. doi: 10.1038/mi.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukoetter MG, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viennois E, Chen F, Laroui H, Baker MT, Merlin D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res Notes. 2013;6:360. doi: 10.1186/1756-0500-6-360. [DOI] [PMC free article] [PubMed] [Google Scholar]


