Abstract
Aims
Traditionally, fasting and 2-hour post challenge plasma glucose have been used to diagnose diabetes. However, evidence indicates that clinically relevant pathophysiological information can be obtained by adding intermediate time-points to a standard oral glucose tolerance test (OGTT).
Methods
We studied a population-based sample of 3,666 Asian Indians without diabetes from the CARRS-Chennai Study, India. Participants underwent a three-point (fasting, 30-minute, and 2-hour) OGTT at baseline. Patterns of glycemic response during OGTT were identified using latent class mixed-effects models. After a median follow-up of two years, participants had a second OGTT. Logistic regression adjusted for diabetes risk factors was used to compare risk of incident diabetes among participants in different latent classes.
Results
We identified four latent classes with different glucose patterns (Classes 1–4). Glucose values for Classes 1, 2, and 4 ranked consistently at all three time-points, but at gradually higher levels. However, Class 3 represented a distinct pattern, characterized by high 30-minute (30minPG), normal fasting (FPG) and 2-hour (2hPG) plasma glucose, moderately high insulin sensitivity, and low acute insulin response. Approximately 22% of participants were categorized as Class 3, and had a 10-fold risk of diabetes compared to the group with the most favorable glucose response, despite 92.5% of Class 3 participants having normal glucose tolerance (NGT) at baseline.
Conclusions
Elevated 30minPG is associated with high risk of incident diabetes, even in individuals classified as NGT by a traditional OGTT. Assessing 30minPG may identify a subgroup of high-risk individuals who remained unidentified by traditional measures.
Keywords: Type 2 Diabetes, Asian Indian, Oral Glucose Tolerance Test, Diabetes Physiology
1. Introduction
Ever since the publication of the 1980 WHO Expert Committee recommendations, measurement of fasting plasma glucose (FPG) in combination with 2-hour post challenge glucose (2hPG) levels has been the cornerstone of hyperglycemia and diabetes diagnoses [1], thereby relying on two time points along the oral glucose tolerance test (OGTT) for hyperglycemic classification. In addition, in 2009 an international expert committee also recommended the use of the HbA1c assay as an additional diagnostic tool [2]. However, while HbA1c is a frequently used tool, cost, standardization, and certain clinical situations such as hemoglobinopathies and iron deficiency anemia make the accurate assessment of HbA1c difficult in many developing country settings [3]. Furthermore, type 2 diabetes develops through varying pathophysiological mechanisms [4], and while insulin resistance may be the primary defect in some individuals, dysfunction in insulin secretion has been seen as the earliest observed defect in others [5]. This diversity in pathophysiology is important for appropriate diagnosis, prevention, and treatment. However, it may be missed, not only when a summary measure of glycemia, such as HbA1c is used [6], but also when only two time points along the oral glucose tolerance test (OGTT) are assessed.
Recent epidemiological studies have indicated that intermediate measures along the OGTT, such as 30-minute plasma glucose (30minPG) or 1-hour plasma glucose (1hPG) concentrations may be stronger predictors of diabetes risk compared to traditional FPG or 2hPG values [7–11]. In addition, we recently showed in a pooled analysis of five cohorts with at least five time points during the OGTT, that there is a significant heterogeneity in glucose response curves, even if FPG and 2hPG levels are similar [12]. This heterogeneity was associated with distinct cardiometabolic risk profiles, but it is unknown whether long-term outcomes differ by different glucose response curve patterns. Furthermore, additional pathophysiological information regarding insulin secretion and insulin resistance can be obtained by adding intermediate measures to the OGTT [8]. Given that defects in early phase insulin secretion are unlikely to suppress hepatic glucose production and thereby cause increases in plasma glucose during early phases of the OGTT [4], assessing the early glycemic response (i.e. at 30 minutes) may have additional merits for identifying high risk individuals. This may be especially the case in populations such as Asian Indians, who are at high risk of diabetes and exhibit poor β-cell function, even at levels of mild dysglycemia [13]. We therefore propose to use a novel, data driven method, latent class mixed-effects models to investigate the heterogeneity in glycemic patterns that is observed during a three-point OGTT and compare the incidence of diabetes among individuals in each of the identified glycemic patterns in a population of Asian Indians living in urban India.
2. Materials and Methods
2.1 Study population
In brief, The Center for Cardiometabolic Risk Reduction in South Asia (CARRS) Surveillance Study is a multi-site, longitudinal study, representative of two urban cities in India (Chennai and Delhi) and one in Pakistan (Karachi). The CARRS-surveillance study has been approved by the Institutional Review Boards (IRBs) of Public Health Foundation of India, New Delhi, All India Institute of Medical Sciences, New Delhi, Madras Diabetes Research Foundation, Chennai, India, Aga Khan University, Karachi, Pakistan, and Emory University, Atlanta, USA. Baseline recruitment and data collection were done between 2010 and 2011[14], with first follow up between 2013 and 2014. In the present analysis, only data from the Chennai, India site was used, as this was the only site to collect fasting, 30minPG, and 2hPG samples. Chennai is a large, metropolitan city located in the South Indian state of Tamil Nadu with a population of approximately 4.65 million people [15]. Households were selected for participation using multi-stage random sampling technique in order to be representative of the city of Chennai [14]. A total of 6,920 individuals aged ≥ 20 were screened for participation, of which 6,906 (99%) provided questionnaire data and 876 (13%) reported a previous diabetes diagnosis. Fasting plasma glucose was obtained from 5,952 participants (86%). In those not reporting a previous diabetes diagnosis (6,113), 2hPG glucose was obtained from 4,051 participants (67%). For this study we limited our population to the 3,666 participants who were not previously diagnosed with diabetes, who did not have screen detected diabetes based on FPG or 2hPG at baseline, and who provided FPG samples or underwent a full OGTT.
2.2 Measurements
Height, weight, and waist circumference were obtained according to standard procedures. Total cholesterol (enzymatic colorimetric cholesterol oxidase peroxidase), high-density lipoprotein cholesterol (HDL; direct), and triglycerides (enzymatic methods) were measured using Roche/Boehringer-Mannheim Diagnostics respectively. Low density lipoprotein cholesterol was calculated using the Friedewald Formula [14], and serum insulin concentrations were measured using the electrochemiluminesence method (COBAS E 411; Roche Diagnostics). After at least an 8-hour overnight fast, a 75g OGTT was administered to participants without previously diagnosed diabetes and who were willing and able to participate in the glucose challenge. Plasma glucose levels were analyzed using the hexokinase/kinetic method [14] and were assessed in blood samples that were obtained from a peripheral vein just before glucose ingestion (time 0) and at 30 and 120 minutes post glucose challenge. Isolated impaired fasting glucose (iIFG) was defined by FPG of 6.1–6.9 mmol/l and 2hPG below 7.8 mmol/l. Isolated impaired glucose tolerance (iIGT) was defined as FPG < 6.1 mmol/l with 2hPG of 7.8–11.0 mmol/l. Combined IFG+IGT was defined as both FPG of 6.1–6.9 mmol/l and 2hPG of 7.8–11.0 mmol/l. Normal glucose tolerance was defined as both FPG < 6.1 mmol/l and 2hPG < 7.8 mmol/l [16]. At follow-up, incident type 2 diabetes was defined as FPG ≥ 7.0 mmol/l and/or 2hPG ≥ 11.1 mmol/l [16] or physician diagnosis.
BIGTT-SI0-30-120 and BIGTT-AIR0-30-120 were used to estimate the insulin sensitivity index (SI) and acute insulin response (AIR), respectively, as they incorporate glucose and insulin response at time 0, as well as 30 and 120 minutes post challenge, and have been shown to correlate highly with gold standard intravenous glucose tolerance tests [17].
2.3 Statistical analysis
Heterogeneity of glucose patterns during the OGTT was examined with latent class mixed-effects models. This method identifies the most heterogeneous trajectories (patterns) based on repeated glucose measurements (fasting, 30-min and 2-hour glucose) by letting slope coefficients vary between classes, i.e. glucose trajectories are identified for each class. The number of measurements per person (n=3) allowed us only to use a piecewise-linear specification for time, with a turning point at 30 minutes. The number of classes has to be pre-specified before fitting a model. Then the best model is selected based on measures of model fit and class sizes. We chose the optimal number of classes by minimizing the model’s Bayesian Information Criterion (BIC) while keeping the size of classes sufficiently large, a commonly employed modeling strategy. The method returns membership probabilities for each individual for each class, and the highest membership probability determines the class each participant is assigned to. More details of the latent class analysis were published previously [12]. We report and compare baseline characteristics and diabetes status at follow-up between the identified classes. Medians (Q1–Q3) and percentages are reported for continuous and categorical variables, respectively.
To investigate how different glucose response patterns during the OGTT were associated with future risk of diabetes, we used logistic regression with class membership at baseline as exposure and diabetes diagnosis (doctor diagnosed or screen-detected) at follow-up (binary) as outcome. Models were adjusted for follow-up duration, age, sex, smoking and waist circumference. In a large subgroup of participants with insulin measurements, we fitted models with further adjustment for estimates of insulin sensitivity. Statistical analyses were performed using the lcmm, lme4 and Epi packages in R (version 3.3.1).
3. Results
We identified four separate latent classes with different glucose patterns (Classes 1–4) comprised of 39%, 29%, 22% and 10% of participants respectively (Figure 1). Mean class membership probabilities were high, ranging from 0.77 to 0.89. Glucose levels for Classes 1, 2, and 4 ranked consistently at all three time points, with Class 1 characterized as having relatively low FPG, 30minPG, and 2hPG, values; Class 2 having moderate levels or FPG, 30minPG, and 2hrPG, and Class 4 having high glucose values at all three time points. Class 3 represented a distinct and unique pattern, with the second highest 30minPG (~10 mmol/L), but an FPG comparable to Class 2 and a 2hPG comparable to Class 1 (~5 mmol/L).
Fig 1.
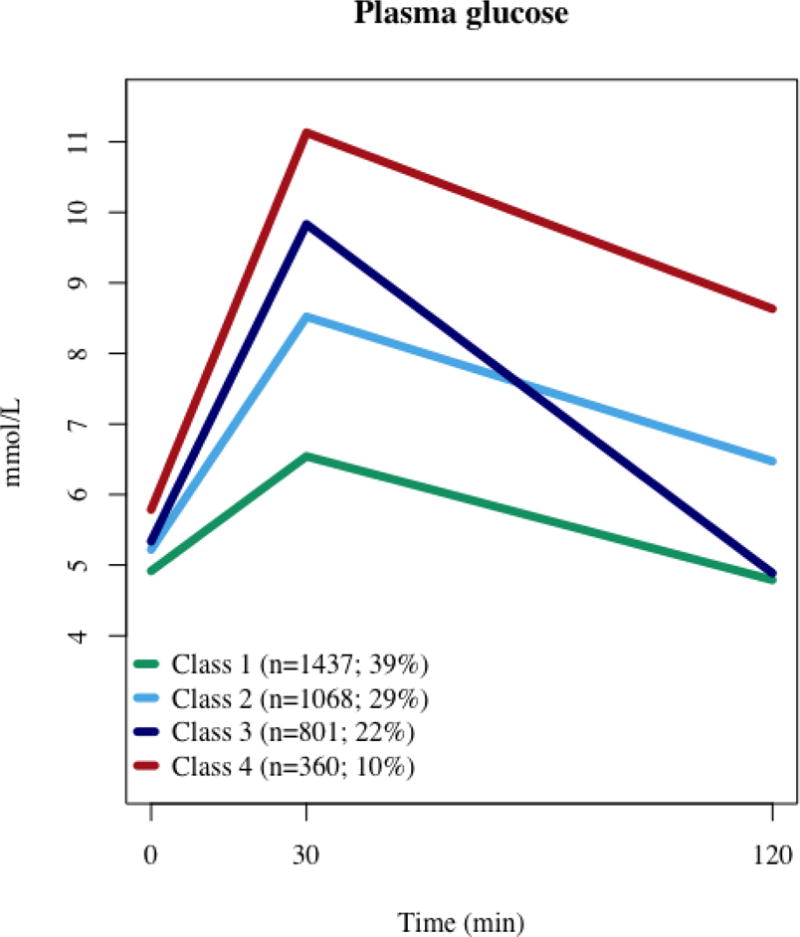
Heterogeneous plasma glucose patterns during the OGTT identified by latent class trajectory analysis
Table 1 describes the general characteristics of the study population as a whole and by glucose response class. In general, Class 1 had the most, and Class 4 had the least favorable cardio-metabolic risk profile. Classes 2 and 3 had similar cardio-metabolic risk profiles, aside from age and sex. Men were more likely to have the high 30minPG/normal 2hPG pattern and therefore more likely to be classified in Class 3. There were a high proportion of individuals with NGT in Class 1, and a high proportion of individuals with prediabetes (iIFG, iIGT, IFG+IGT) in Class 4. While at baseline there were no participants in Class 3 with iIGT, there were a fair number of participants with iIFG, as well as a high proportion of participants (92.5%) with NGT. Additionally, participants in Class 3 had a slightly higher median insulin sensitivity (+13%), but much lower acute insulin response than those in Class 2. Classes 3 and 4 had a similar median acute insulin response, which was much lower than in Classes 1 and 2. Actual insulin values were also different between latent classes. Classes 1, 2 and 3 had lower values at 2 hours than at 30 minutes, although they were still greater than in the fasting state. Contrarily, in Class 4, insulin levels further increased in the 30 minute to 2 hour period by ~20%. Similarly to the glucose pattern seen in Class 3, those in Class 4 exhibited the largest relative decline in insulin levels between 30 minutes and 2 hours.
Table 1.
Baseline characteristics of cardiometabolic risk factors and follow-up status by latent class
| N | Overall 3666 |
Class 1 1437 (39%) |
Class 2 1068 (29%) |
Class 3 801 (22%) |
Class 4 360 (10%) |
|---|---|---|---|---|---|
| Age (year) | 37 (30–45) | 35 (28–42) | 39 (31–47) | 37 (30–45) | 44 (37–52) |
| Sex (male %) | 41.8 | 36.3 | 41.8 | 49.3 | 47.5 |
| BMI (kg/m2) | 25.2 (22.1–28.4) | 23.8 (20.8–27.0) | 26.1 (23.1–29.0) | 25.8 (22.8–28.5) | 27.1 (24.2–30.4) |
| Height (cm) | 156 (150–163) | 155 (150–162) | 156 (150–163) | 157 (151–165) | 157 (150–163) |
| Waist circumference (cm) | 83 (75–91) | 79 (71–87) | 85 (78–93) | 85 (78–92) | 89 (82–96) |
| TC (mmol/L) | 4.6 (4.0–5.3) | 4.4 (3.9–5.1) | 4.7 (4.2–5.3) | 4.7 (4.1–5.4) | 4.9 (4.4–5.5) |
| LDL (mmol/L) | 2.9 (2.4–3.4) | 2.7 (2.3–3.3) | 3.0 (2.5–3.5) | 3.0 (2.5–3.5) | 3.1 (2.6–3.5) |
| HDL (mmol/L) | 1.1 (0.9–1.2) | 1.1 (0.9–1.2) | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) |
| Triglycerides (mmol/L) | 1.3 (0.9–1.8) | 1.1 (0.8–1.5) | 1.3 (1.0–1.8) | 1.4 (1.0–1.9) | 1.6 (1.2–2.2) |
| Smoking (%) | 17.6 | 16.6 | 16.4 | 19.1 | 21.4 |
| Glucose0 (mmol/L) | 5.1 (4.8–5.5) | 4.9 (4.6–5.2) | 5.2 (4.9–5.5) | 5.3 (5.0–5.7) | 5.7 (5.4–6.2) |
| Glucose30 (mmol/L) | 8.2 (6.9–9.5) | 6.6 (5.8–7.2) | 8.5 (8.0–9.0) | 9.7 (9.1–10.4) | 10.9 (10.3–11.8) |
| Glucose120 (mmol/L) | 5.5 (4.7–6.4) | 4.8 (4.2–5.4) | 6.4 (5.8–7.0) | 4.9 (4.3–5.6) | 8.5 (7.7–9.6) |
| Insulin0 (pmol/L) | 47 (32–68) | 40 (29–59) | 51 (36–71) | 47 (32–69) | 60 (47–83) |
| Insulin30 (pmol/L) | 325 (224–506) | 298 (204–455) | 345 (240–549) | 340 (235–542) | 358 (235–523) |
| Insulin120 (pmol/L) | 240 (164–398) | 191 (147–292) | 291 (183–479) | 233 (167–373) | 435 (293–675) |
| BIGTT-SI0-30-120 | 8.1 (5.0-11.0) | 10.4 (7.8-12.9) | 6.8 (4.4-9.4) | 7.7 (5.0-10.1) | 4.2 (2.5-5.8) |
| BIGTT-AIR0-30-120 | 1974 (1489–2695) | 2156 (1712–2863) | 2081 (1594–2860) | 1556 (1207–2218) | 1573 (1197–2211) |
| Glucose tolerance status (%) | |||||
| NGT | 86.7 | 98.8 | 88.1 | 92.5 | 21.4 |
| iIFG | 4.1 | 1.0 | 4.2 | 7.5 | 8.1 |
| iIGT | 6.6 | 0.1 | 7.1 | 0.0 | 45.8 |
| IFG + IGT | 2.6 | 0.0 | 0.6 | 0.0 | 24.7 |
| Follow-up time (y) | 1.9 (1.3–2.2) | 1.9 (1.4–2.2) | 1.8 (1.3–2.2) | 1.9 (1.3–2.2) | 1.8 (1.3–2.3) |
| Missing follow-up (%) | 29.9 | 30.5 | 32.1 | 28.5 | 23.9 |
| DM at follow-up (%)* | 5.3 | 0.6 | 4.0 | 6.6 | 23.4 |
Among those who participated at follow-up
After a median follow-up of 1.9 years, 70% of participants (N=2,571) attended a follow-up clinical examination. Class 4, with the least favorable glucose pattern, had the least loss to follow-up (24% vs. ~29–32% in other classes). Among those who had a follow-up visit, 137 participants (5.3%) had newly diagnosed diabetes (self-reported (n=11) or screen detected (n=126) at the follow-up examination). The incidence varied greatly by latent class (Table 2). While only a few participants developed diabetes in Class 1 (N=6; 0.6%), in Class 4, one in four participants did so. Participants in Class 4 had by far the highest risk of diabetes compared to all other classes, regardless of model adjustment. However, despite Class 3 having more participants with NGT than Class 2 at baseline, there was a higher risk of incident diabetes in Class 3 compared to Class 2 in all adjusted models. Additional adjustment for insulin sensitivity did not explain differences between classes. Between Classes 2, 3 and 4, Class 3 had by far the highest proportion of participants with NGT at baseline among those who developed diabetes (78.9% compared to 34.5% in Class 2 and 6.25% in Class 4), although absolute numbers were relatively low (30 out of 38 in Class 3).
Table 2.
Association between glucose patterns during an OGTT (latent class membership) and diabetes incidence (odds ratios are presented with 95% CIs having Classes 1, 2 and 3 as reference categories)
| N | Class 1 999 (39%) |
Class 2 725 (28%) |
Class 3 573 (22%) |
Class 4 274 (11%) |
|---|---|---|---|---|
| Model 1 Adjustment: follow-up time |
Ref. | 6.9 (3.0–18.6) | 12.1 (5.5–32.2) | 53.7 (24.8–140.7) |
| 0.14 (0.05–0.33) | Ref. | 1.8 (1.1–2.9) | 7.8 (4.9–12.7) | |
| 0.08 (0.03–0.18) | 0.6 (0.3–0.9) | Ref. | 4.4 (2.9–6.9) | |
|
| ||||
| Model 2 Adjustment: Model 1 + age, sex |
Ref. | 6.6 (2.9–17.7) | 11.9 (5.4–31.5) | 48.5 (22.1–128.3) |
| 0.15 (0.06–0.35) | Ref. | 1.8 (1.1–3.0) | 7.4 (4.6–12.1) | |
| 0.08 (0.03–0.19) | 0.6 (0.3–0.9) | Ref. | 4.1 (2.6–6.4) | |
|
| ||||
| Model 3 Adjustment: Model 2 + waist ircumference, smoking |
Ref. | 5.5 (2.4–14.8) | 9.7 (4.3–25.8) | 35.5 (16.0–94.8) |
| 0.18 (0.07–0.42) | Ref. | 1.8 (1.1–3.0) | 6.5 (4.0–10.8) | |
| 0.05 (0.01–0.15) | 0.5 (0.3–0.9) | Ref. | 3.5 (2.2–5.7) | |
4. Discussion
Using a novel latent class analysis approach to identify heterogeneity in response to an oral glucose load, our analyses revealed four distinct patterns of glycemic response. While glucose levels for Classes 1, 2, and 4 ranked consistently at all three time-points, a distinct pattern was noted in Class 3, and was characterized by high 30minPG, but relatively low FPG and 2hPG levels. Participants in Class 3 were also characterized by moderate insulin sensitivity and low acute insulin response. However, compared to Classes 1 or 2, there was a higher risk of incident diabetes in Class 3, despite more than 90% of its participants being classified as NGT at baseline.
The results of our work are in accordance with a recent study noting an independent association between elevated 30minPG and incident diabetes in Asian Indians with impaired glucose tolerance at baseline [18]. Similarly to our work, results of this study also indicated that participants with higher levels of 30minPG were more likely to have increased prevalence of IFG and decreased β-cell function compared to those with lower 30minPG levels [18], thereby suggesting that elevated 30minPG may be a surrogate marker of abnormal insulin release. Several studies have also indicated that intermediate time points, particularly 1hPG are stronger predictors of incident diabetes risk compared to traditionally used measures [7,9–11,19]. A recent study assessing the longitudinal association of an elevated 1hPG with incident diabetes over 25 years in a non-diabetic cohort found that 1hPG had greater sensitivity and specificity compared to 2hPG in terms of predicting cumulative incident diabetes [20]. In aggregate, these results support the notion that the restricted OGTT, yielding only FPG and 2hPG glucose measures may fail to identify a subset of individuals at high risk. Diagnostic criteria are often a compromise between the need for pathophysiological precision on the one hand and low costs and practical convenience on the other. Our study has shown that clinically relevant pathophysiological information can be obtained by adding a 30-minute glucose measurement to a standard OGTT. Whether this finding can or should be translated to a different approach to diabetes screening and diagnosis, depends on a wider set of public health considerations.
The lack of 1hPG measures is a limitation of the present study. However, previous work has demonstrated that while 30minPG levels do not have as strong an association with incident diabetes as 1hPG, they have a stronger association than fasting or 2-hour measures [7]. Given that early phase glucose increases during an OGTT may be reflective of defects in early phase insulin secretion [4], the inclusion of 30minPG in risk assessments may have additional benefits in populations where poor insulin secretion might be the primary defect in diabetes development. Strengths of our study include the utilization of a large, population-based sample. In addition, the longitudinal nature of the study allowed for the assessment of incident diabetes at follow-up. Despite the short median follow-up time (2 years), this allowed us to extend our previous findings on the heterogeneity of glucose response curves [12], however further research is necessary to combine the detailed assessment of glucose response curves with even longer follow-up time to be able to examine long-term outcomes (e.g. incidence of diabetes, cardiovascular events).
In conclusion, while traditionally, individuals with pre-diabetes (IFG or IGT) are those considered to be at high risk for diabetes development [21], the results of our study noted that in an urban Indian population a large proportion of those who develop incident diabetes are normoglycemic at baseline, but can be identified as high risk by high 30minPG values. Therefore, while the optimal time point for identifying future diabetes risk remains unknown, clinically relevant pathophysiological data can be gathered via the addition of intermediate measures during the OGTT such as 30minPG. The inclusion of these measures may be especially useful in high risk populations such as Asian Indians who may have poorer insulin response compared to other ethnic groups, and thereby are at greater diabetes risk even at glucose levels considered normal by fasting or 2-hour measures.
Highlights.
Latent class analysis identifies distinct OGTT glucose patterns among Asian Indians.
A high 30-min glucose peak was associated with increased risk of diabetes.
High proportion of those with a elevated 30-min glucose and T2DM at follow-up were NGT at baseline.
Internal time points during the OGTT add clinically useful information to traditional measures.
Acknowledgments
A.H., K.M.V.N. and D.R.W. are supported by the Danish Diabetes Academy. The Danish Diabetes Academy is funded by the Novo Nordisk Foundation. The CARRS study is funded in whole or in part by the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), Department of Health and Human Services, under Contract No. HHSN268200900026C, and the United Health Group, Minneapolis, MN, USA.
Role of the Funding Source: The funding sources had no involvement in the collection, analysis, and interpretation of the data, the writing of the report, or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Parts of this study were presented at the Global Partnerships to Advance NCD Research within the Sustainable Development Goal Agenda conference, August 8–9, 2016, Atlanta, GA, USA.
Conflict of interest statement
The authors disclose no conflict of interest relevant to this manuscript.
References
- 1.World Health Organization. Technical Report Series. Geneva: World Health Org.; 1980. Expert Committee on Diabetes Mellitus; p. 646. [PubMed] [Google Scholar]
- 2.International Expert Committee. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unnikrishnan R, Mohan V. Challenges in estimation of glycated hemoglobin in India. Diabetes Technol Ther. 2013;15:897–9. doi: 10.1089/dia.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–9. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- 5.Faerch K, Hulman A, Solomon TP. Heterogeneity of Pre-diabetes and Type 2 Diabetes: Implications for Prediction, Prevention and Treatment Responsiveness. Curr Diabetes Rev. 2016;12:30–41. doi: 10.2174/1573399811666150416122903. [DOI] [PubMed] [Google Scholar]
- 6.Faerch K, Johansen NB, Witte DR, Lauritzen T, Jørgensen ME, Vistisen D. Relationship between insulin resistance and β-cell dysfunction in subphenotypes of prediabetes and type 2 diabetes. J Clin Endocrinol Metab. 2015;100:707–16. doi: 10.1210/jc.2014-2853. [DOI] [PubMed] [Google Scholar]
- 7.Alyass A, Almgren P, Akerlund M, Dushoff J, Isomaa B, Nilsson P, et al. Modelling of OGTT curve identifies 1 h plasma glucose level as a strong predictor of incident type 2 diabetes: results from two prospective cohorts. Diabetologia. 2014;58:87–97. doi: 10.1007/s00125-014-3390-x. [DOI] [PubMed] [Google Scholar]
- 8.Jagannathan R, Sevick MA, Li H, Fink D, Dankner R, Chetrit A, et al. Elevated 1-hour plasma glucose levels are associated with dysglycemia, impaired beta-cell function, and insulin sensitivity: a pilot study from a real world health care setting. Endocrine. 2016;52:172–5. doi: 10.1007/s12020-015-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care. 2007;30:1544–8. doi: 10.2337/dc06-1331. [DOI] [PubMed] [Google Scholar]
- 10.Priya MM, Amutha A, Pramodkumar TA, Ranjani H, Jebarani S, Gokulakrishnan K, et al. β-Cell Function and Insulin Sensitivity in Normal Glucose-Tolerant Subjects Stratified by 1-Hour Plasma Glucose Values. Diabetes Technol Ther. 2015;18:29–33. doi: 10.1089/dia.2015.0065. [DOI] [PubMed] [Google Scholar]
- 11.Priya M, Anjana RM, Chiwanga FS, Gokulakrishnan K, Deepa M, Mohan V. 1-hour venous plasma glucose and incident prediabetes and diabetes in Asian indians. Diabetes Technol Ther. 2013;15:497–502. doi: 10.1089/dia.2013.0025. [DOI] [PubMed] [Google Scholar]
- 12.Hulman A, Simmons RK, Vistisen D, Tabak AG, Dekker JM, Alssema M, et al. Heterogeneity in glucose response curves during an oral glucose tolerance test and associated cardiometabolic risk. Endocrine. doi: 10.1007/s12020-016-1126-z. [DOI] [PubMed] [Google Scholar]
- 13.Staimez LR, Weber MB, Ranjani H, Ali MK, Echouffo-Tcheugui JB, Phillips LS, et al. Evidence of Reduced β-Cell Function in Asian Indians With Mild Dysglycemia. Diabetes Care. 2013;36:2772–8. doi: 10.2337/dc12-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair M, Ali MK, Ajay VS, Shivashankar R, Mohan V, Pradeepa R, et al. CARRS Surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health. 2012;12:701. doi: 10.1186/1471-2458-12-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Directorate of Census Operations Tamil Nadu. District Census Handbook Chennai. 2011 Available from: http://www.censusindia.gov.in/2011census/dchb/3302_PART_B_DCHB_CHENNAI.pdf. Accessed 14 Jul 2016.
- 16.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. Geneva: World Health Org.; 2006. [Google Scholar]
- 17.Hansen T, Drivsholm T, Urhammer SA, Palacios RT, Vølund A, Borch-Johnsen K, et al. The BIGTT Test A novel test for simultaneous measurement of pancreatic β-cell function, insulin sensitivity, and glucose tolerance. Diabetes Care. 2007;30:257–62. doi: 10.2337/dc06-1240. [DOI] [PubMed] [Google Scholar]
- 18.Chamukuttan S, Ram J, Nanditha A, Shetty AS, Sevick MA, Bergman M, et al. Baseline level of 30-min plasma glucose is an independent predictor of incident diabetes among Asian Indians: analysis of two diabetes prevention programmes. Diabetes Metab Res Rev. 2016 doi: 10.1002/dmrr.2799. [DOI] [PubMed] [Google Scholar]
- 19.Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes. Diabetes Care. 2009;32:281–6. doi: 10.2337/dc08-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergman M, Chetrit A, Roth J, Jagannathan R, Sevick M, Dankner R. One-hour post-load plasma glucose level during the OGTT predicts dysglycemia: Observations from the 25 year follow-up of the Israel Study of Glucose Intolerance, Obesity and Hypertension. Diabetes Res Clin Pract. 2016;120:221–8. doi: 10.1016/j.diabres.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]


