Abstract
Objectives
To identify the variability of short- and long-term survival outcomes among closed Phase III randomized controlled trials with small sample sizes comparing SBRT (stereotactic body radiation therapy) and surgical resection in operable clinical Stage I non-small cell lung cancer (NSCLC) patients.
Patients and Methods
Clinical Stage I NSCLC patients who underwent surgery at our institution meeting the inclusion/exclusion criteria for STARS (Randomized Study to Compare CyberKnife to Surgical Resection in Stage I Non-small Cell Lung Cancer), ROSEL (Trial of Either Surgery or Stereotactic Radiotherapy for Early Stage (IA) Lung Cancer), or both were identified. Bootstrapping analysis provided 10,000 iterations to depict 30-day mortality and three-year overall survival (OS) in cohorts of 16 patients (to simulate the STARS surgical arm), 27 patients (to simulate the pooled surgical arms of STARS and ROSEL), and 515 (to simulate the goal accrual for the surgical arm of STARS).
Results
From 2000 to 2012, 749/873 (86%) of clinical Stage I NSCLC patients who underwent resection were eligible for STARS only, ROSEL only, or both studies. When patients eligible for STARS only were repeatedly sampled with a cohort size of 16, the 3-year OS rates ranged from 27–100%, and 30-day mortality varied from 0–25%. When patients eligible for ROSEL or for both STARS and ROSEL underwent bootstrapping with n=27, the 3-year OS ranged from 46–100%, while 30-day mortality varied from 0–15%. Finally, when patients eligible for STARS were repeatedly sampled in groups of 515, 3-year OS narrowed to 70–85%, with 30-day mortality varying from 0–4%.
Conclusion
Short- and long-term survival outcomes from trials with small sample sizes are extremely variable and unreliable for extrapolation.
Keywords: lung cancer, surgery, stereotactic body radiation therapy, clinical trials
1. Introduction
For clinical Stage I non-small cell lung cancer (NSCLC) patients, lobectomy with mediastinal lymph node sampling has been the standard of care, with 5-year survival rates exceeding 80%. [1,2] Resection allows for local control and nodal sampling allows the possibility of upstaging (occurring in 15–35% of patients). [3–5] Increased use of video-assisted thoracoscopic surgery (VATS) have decreased rates of postoperative complications substantially [6,7]. For inoperable or high risk patients, stereotactic body radiation therapy (SBRT) has emerged as an alternative to surgery [8,9].
To examine SBRT’s role in treating operable Stage I NSCLC patients, two Phase 3 randomized controlled trials were created: the STARS trial in the United States (Randomized Study to Compare CyberKnife to Surgical Resection in Stage I Non-small Cell Lung Cancer) and the ROSEL Trial in the Netherlands (Trial of Either Surgery or Stereotactic Radiotherapy for Early Stage (IA) Lung Cancer). To compare overall survival (OS) outcomes, STARS required 1030 randomized patients, while ROSEL called for 960 patients. [10,11] However, both trials failed to accrue and closed, with STARS randomizing 36 patients and ROSEL enrolling 22 patients.
Chang and colleagues recently published a post-hoc analysis combining enrolled patients from STARS and ROSEL including 27 surgical patients. [12] We hypothesize that this pooled analysis remains underpowered with unstable and inconclusive results. By using our institutional data from clinical Stage I NSCLC surgical patients, we evaluated whether creating similarly sized simulations of a) the pooled analysis and b) the target accrual goal would identify variable ranges of 30-day mortality and 3-year OS.
2. Patients and Methods
2.1 Patients
This study received approval by our Institutional Review Board (IRB) at Washington University in St. Louis. We used our prospectively maintained clinical Stage I NSCLC database to identify surgical patients that would have been eligible for STARS and/or ROSEL. Inclusion and exclusion criteria for these trials are listed in Table 1.
Table 1.
| Trial name | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| STARS |
|
|
| ROSEL |
|
|
2.2 Statistical Analysis
Univariate analysis was performed to compare patient and tumor characteristics. Chi-square and Fisher’s exact tests were applied to categorical variables as appropriate based on group size. Independent sample t-tests were applied to normally distributed continuous variables, and reported as mean and standard deviation. P values < 0.05 were considered statistically significant. Follow-up time was calculated from the date of surgery to date of death or last known follow-up. Kaplan-Meier analysis and the log-rank test was applied to determine differences in three-year OS among patient groups. All statistical analyses were performed using SAS, Version 9.3, 2011, Cary, NC.
2.3 Bootstrapping Methods
A bootstrap analysis was used to analyze the potential range of 30-day mortality and 3-year OS values. This was done by resampling our data set (with replacement to give equal probabilities of selection with each iteration) 10,000 times. Repeated simulations of 30-day mortality rates and 3-year OS of the pooled STARS and ROSEL surgical arm (n=27) and the STARS surgical arm enrollment goal (n=515) were performed. Mean values and interquartile ranges (IQR) are reported for each of the distributions generated. Bootstrapping analysis was performed in R, Version 3.1.0, R Core Team (2012), R Foundation for Statistical Computing, Vienna, Austria. [13] Normality of the three-year survival distributions was confirmed using quantile-quantile (QQ) plots.
3. Results
3.1 Patient Characteristics
From 2000 to 2012, 214/873 (24.5%) of our operative clinical Stage I patients were hypothetically eligible for both STARS and ROSEL, 508/873 (58.0%) for STARS only, and 27/873 (3.1%) for ROSEL only (Table 2). Therefore, 749/873 (85.8%) of clinical Stage I NSCLC patients at our institution would have been eligible for one or both of these trials. The most common reason for patient ineligibility from both trials was failing the pulmonary function criteria of STARS (FEV1 >40%), while also having a clinical T1b or T2 tumor (a ROSEL exclusion).
Table 2.
Clinical characteristics of clinical stage I NSCLC patients that received surgical resection from 2000–2012, that were eligible for either STARS only, or both STARS and ROSEL.
| Patient Characteristics | Patients eligible for STARS only (n = 508) | Patients eligible for STARS and ROSEL (n = 214) | P Value |
|---|---|---|---|
|
| |||
| Age | 66.64 (± 10.10) | 65.81 (± 10.07) | 0.39 |
|
| |||
| ACE (Adult Comorbidity Evaluation) Score | |||
| 0 | 59 (7.7%) | 26 (16.9%) | 0.95 |
| 1 | 199 (35.2%) | 77 (41.4%) | |
| 2 | 129 (30.2%) | 55 (20.3%) | |
| 3 | 75 (17.0%) | 32 (12.3%) | |
|
| |||
| Gender | |||
| Male | 241 (47.4%) | 94 (43.9%) | 0.39 |
| Female | 267 (52.6%) | 120 (56.1%) | |
|
| |||
| Race | |||
| Caucasian | 435 (85.6%) | 186 (86.9%) | 0.58 |
| Other | 73 (14.4%) | 27 (12.6%) | |
|
| |||
| Prior Malignancy | |||
| No | 314 (61.8%) | 154 (72.0%) | 0.01 |
| Yes | 193 (38.0%) | 59 (27.6%) | |
|
| |||
| FEV1 % Predicted | 81.79 (± 0.19) | 80.53 (± 0.20) | 0.40 |
| DLCO % Predicted | 74.79 (± 0.23) | 74.90 (± 0.19) | 0.43 |
|
| |||
| Smoking History | |||
| Current | 174 (34.3%) | 76 (35.5%) | 0.92 |
| Never | 54 (10.6%) | 21 (9.8%) | |
| Past | 280 (55.1%) | 117 (54.7%) | |
|
| |||
| Number of Comorbidities | |||
| 0 | 154 (30.3%) | 70 (32.7%) | 0.94 |
| 1 | 184 (36.2%) | 74 (34.6%) | |
| 2 | 98 (19.3%) | 40 (18.7%) | |
| ≥3 | 72 (14.2%) | 30 (14.0%) | |
|
| |||
| Tumor Location | |||
| Left lower lobe | 70 (13.8%) | 33 (15.4%) | 0.71 |
| Left upper lobe | 150 (29.5%) | 65 (30.4%) | |
| Right lower lobe | 86 (16.9%) | 30 (14.0%) | |
| Right upper lobe | 172 (33.9%) | 77 (36.0%) | |
| Right middle | 30 (5.9%) | 9 (4.2%) | |
|
| |||
| Parenchymal Location | |||
| Central | 182 (35.8%) | 0 (0.00%) | <0.01 |
| Peripheral | 326 (64.2%) | 214 (100%) | |
|
| |||
| Clinical T stage | |||
| T1a | 132 (26.0%) | 214 (100%) | <0.01 |
| T1b | 257 (50.6%) | 0 (0.00%) | |
| T2 | 119 (23.4%) | 0 (0.00%) | |
|
| |||
| Pathology | |||
| Adenocarcinoma | 310 (61.0%) | 144 (67.3%) | 0.05 |
| Squamous | 143 (28.2%) | 42 (19.6%) | |
| Other | 55 (10.8%) | 28 (13.1%) | |
|
| |||
| Preoperative Lesion Size (cm) | 2.55 (± 0.79) | 1.56 (± 0.37) | <0.01 |
|
| |||
| Resection type | |||
| Bilobectomy or pneumonectomy | 29 (5.7%) | 1 (0.5%) | <0.01 |
| Lobectomy | 400 (78.7%) | 163 (76.2%) | |
| Segmentectomy | 31 (6.1%) | 9 (4.2%) | |
| Wedge | 48 (9.5%) | 41 (19.2%) | |
|
| |||
| Surgical approach | |||
| Thoracotomy | 303 (59.7%) | 126 (58.9%) | 0.70 |
| VATS | 203 (40.0%) | 86 (40.2%) | |
| Sternotomy | 2 (0.4%) | 2 (0.9%) | |
3.2 Short- and Long-Term Outcomes in Combined Surgical Arm Simulation
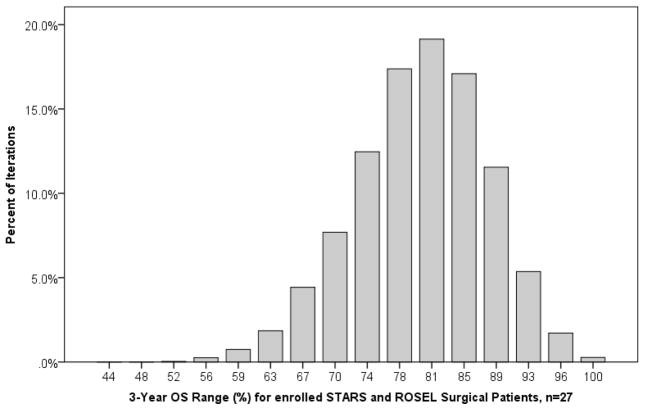
Patients eligible for either STARS and/or ROSEL (n=749) were sampled 10,000 times in groups of 27, to simulate the combined surgical arms of STARS and ROSEL. Here, 3-year OS rates ranged from 46% to 100%, with a mean value of 79%, IQR 73–84% (Figure 1). In groups of 27, repeated samples from this population demonstrated a 30-day mortality rate that varied from 0–15% (mean 1.6%, IQR 0–4%).
Figure 1.
Results of 3-year OS estimates when 749 clinical Stage I NSCLC patients eligible for STARS and/or ROSEL were sampled 10,000 times (with replacement) in groups of 27, to simulate the actual combined surgical enrollment arms of STARS and ROSEL.
3.3 Short- and Long-Term Outcomes in Surgical Arm Target Accrual Simulation
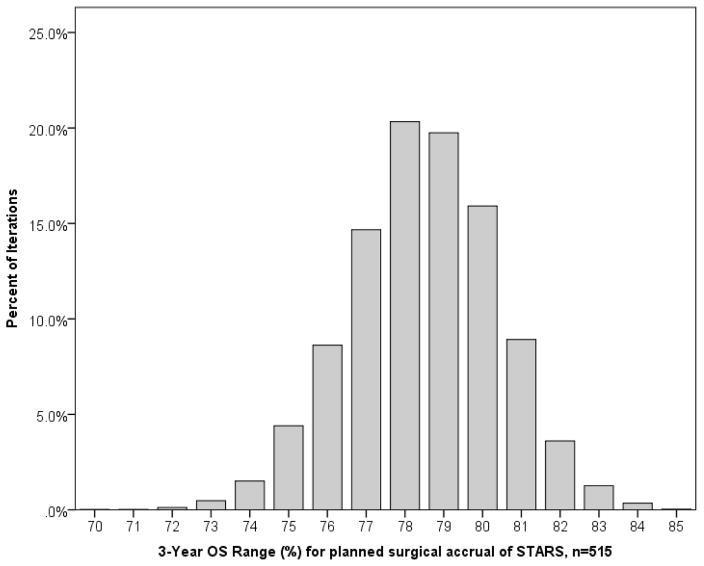
Patients eligible for STARS or both STARS and ROSEL (n=722) were sampled 10,000 times in groups of 515, to simulate the actual accrual target for the STARS surgical arm. Here, the 3-year OS range became more narrow with values of 70% to 85%, and a mean value of 78.5%, IQR 77–80% (Figure 2). Similarly, the range of 30-day mortality rates also narrowed, varying from 0–4%, with a mean of 1.4%, IQR 1–2%.
Figure 2.
Results of 3-year OS estimates when 722 clinical Stage I NSCLC patients that would have been eligible for STARS were sampled 10,000 times (with replacement) in groups of 515, to simulate the anticipated surgical enrollment arm of STARS.
3.4 Comparative Short- and Long-Term Survival by Treatment Arm
To evaluate for potential survival differences among patients in the 3 potential trial eligibility groups, a Kaplan-Meier analysis was performed. There was no difference among 3-year OS rates between resected patients eligible for STARS only (78.5%, 95% CI 74.6–81.8%), ROSEL only (81.5%, 95% CI 61.1–91.8%), or both STARS and ROSEL (84.6%, 95% CI 79.0–88.8%), p=0.15. There was no difference in our 30-day mortality between the three patient eligibility groups: 6/508 (1.2%) for STARS, 1/27 (3.7%) for ROSEL, and 5/214 (2.3%) for STARS and ROSEL, p=0.19.
4.0 Discussion
Our analysis demonstrates that when repeated simulations are performed using the number of surgical patients actually accrued to STARS and ROSEL, the variability of 30-day mortality and 3-year OS is so large that conclusions from these type of analyses should be considered exploratory. In the combined surgical arm simulation (n=27), possible values for 3-year OS of the 10,000 iterations ranged from 46% or 100%, depending on the specific group of patients randomly selected from our database. The mean 3-year OS of the 10,000 iterations did approach our population's actual survival rates, representing the most common survival tendency, while there was a marked reduction of the variability in bootstrap simulations of 3-year OS rates using the STARS target accrual sample size (n=515), as would be expected.
The pooled analysis of STARS and ROSEL by Chang and colleagues has recently received a high level of correspondence. [14–17] A major theme of these concerned commentaries questions the conclusion that “SBRT might lead to better OS compared to surgery for operable Stage I NSCLC” despite very few follow-up events in the study populations: 6 deaths in the combined surgical arm and 1 death in the SBRT arm. [12] By comparison, ROSEL was powered for 960 participating patients, with 400 anticipated events (deaths) for analysis. [11] While we did not perform this analysis on SBRT patients (typically an inoperable or extremely high risk population at our institution, and not eligible for STARS/ROSEL), the small SBRT sample size in the pooled analysis (n=31) would lead to similar ranges of outcome variability.
The finding of a 95% 3-year OS for SBRT patients from the pooled STARS and ROSEL analysis is possibly a non-representative result. In an institutional review of over 80 operable patients receiving SBRT, the 5-year OS rates for Stage IA tumors was 72% and for Stage IB tumors 62%. [18] A recent propensity matched analysis between Stage I NSCLC patients (biopsy-proven) receiving either VATS lobectomy or SBRT demonstrated a significant improvement in 3-year OS for surgical patients (86.1% versus 60.2% for SBRT, p<0.0001), 3-year cause-specific survival (90.4% versus 71.5%, p<0.0001), and 3-year recurrence-free survival (77.0% versus 43.3%, p<0.0001). [19] Another review of operable SBRT patients from the Netherlands (from a ROSEL site) found a 3-year OS rate of 84.7%. [20] A recent propensity-matched analysis between SBRT and surgical patients found a 5-year OS of 53% versus 80% respectively, but did not reach significance (p=0.089). [21]
There are limitations to this study. While our analysis included patients eligible for STARS and/or ROSEL, this does not represent a cohort of patients that were entered into the randomization process. Hence, we cannot adjust for patients that would have declined participation in a trial. However, in STARS and ROSEL, only 58 patients were recruited over an enrollment period of 5.5 years at 38 centers, and begs the question how representative this group is. [14, 17] Of the eligible patients screened for STARS, more than half were not enrolled secondary to treatment preference (with 82% of patients opting for surgery). [12] Additionally, STARS and ROSEL (as well as our early surgical years) have a higher proportion of thoracotomy cases, while VATS has increasingly become the standard surgical approach, with improved in-hospital mortality. [22, 23]
Without reinforcing the exploratory nature of the findings of a pooled analysis, many may misinterpret the results. Few patients (and physicians) feel equipoise between these therapies, and the use of non-traditional randomization schemes to account for patient choice may be helpful.
Conclusions
Given the variability in 3-year OS and 30-day mortality in small sample sizes, the findings of equivalence between SBRT and surgery in a pooled analysis of STARS and ROSEL should be considered exploratory, and not definitive.
Highlights.
Trials that close with poor accrual in Stage I NSCLC have variable survival rates
Simulating trial accrual goals has survival results similar to clinical experience
Conclusions for SBRT in operable Stage I NSCLC cannot be made from such trials
Acknowledgments
Pamela Samson was funded by NIH Cardiothoracic T32 HL0777 and Varun Puri was funded through NIH K07CA178120 and K12CA167540-02 during the creation and analysis of this project, and during the writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SEER Fact Sheets: Lung and Bronchus Cancer. Surveillance, Epidemiology, and End Results Program. 2014 Oct; http://seer.cancer.gov/statfacts/html/lungb.html.
- 2.Whitson B, Groth S, Duval S, et al. Surgery for Early-Stage Non-Small Cell Lung Cancer: A Systematic Review of Video-Assisted Thoracoscopic Surgery Versus Thoracotomy Approaches to Lobectomy. Ann Thorac Surg. 2008;86:2008–18. doi: 10.1016/j.athoracsur.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140(2):377–86. doi: 10.1016/j.jtcvs.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 4.Licht PB, Jorgensen OD, Ladegaard L, et al. A National Study of Nodal Upstaging after Thoracoscopic Versus Open Lobectomy for Clinical Stage I Lung Cancer. Ann Thorac Surg. 2013;96:943–50. doi: 10.1016/j.athoracsur.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg. 2013;96(4):1171–7. doi: 10.1016/j.athoracsur.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 6.Laursen LO, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg. 2015 doi: 10.1093/ejcts/ezv205.. [DOI] [PubMed] [Google Scholar]
- 7.Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;83(6):1965–70. doi: 10.1016/j.athoracsur.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 8.Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, et al. Stereotactic body radiation therapy for early-stage non-small cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75(3):677–82. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 9.Taremi M, Hope A, Dahele M, Pearson S, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys. 2012;82(2):967–73. doi: 10.1016/j.ijrobp.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 10. [Accessed August 2015];Randomized Study to Compare CyberKnife to Surgical Resection in Stage I Non-small Cell Lung Cancer (STARS) https://clinicaltrials.gov/archive/NCT00840749/2009_02_09.
- 11. [Accessed August 2015];Trial of Either Surgery or Stereotactic Radiotherapy for Early Stage (IA) Lung Cancer (ROSEL) https://clinicaltrials.gov/ct2/show/NCT00687986.
- 12.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small cell lung cancer: a pooled analysis of two randomized trials. Lancet Oncol. 2015;16:630–37. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canty AJ. Resampling Methods in R: The Boot Package. R News. 2002;2(3):2–7. [Google Scholar]
- 14.Cao C, D’Amico T, Demmy T, Dunning J, et al. Surgery versus SABR for resectable non-small cell lung cancer (Correspondence) Lancet Oncol. 2015;16(8):e370. doi: 10.1016/S1470-2045(15)00036-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Tian J, Wang C. Surgery versus SABR for resectable non-small cell lung cancer (Correspondence) Lancet Oncol. 2015;16(8):e371. doi: 10.1016/S1470-2045(15)00063-7. [DOI] [PubMed] [Google Scholar]
- 16.Hamaji M, Groth S, Sugarbaker D, Burt B. Surgery versus SABR for resectable non-small cell lung cancer (Correspondence) Lancet Oncol. 2015;16(8):e372. doi: 10.1016/S1470-2045(15)00091-1. [DOI] [PubMed] [Google Scholar]
- 17.Meyers BF, Puri V, Broderick SR, Samson P, et al. Lobectomy versus stereotactic body radiotherapy for stage I non-small cell lung cancer: Post hoc analysis dressed up as level-1 evidence. J Thorac Cardiovasc Surg. 2015 doi: 10.1016/j.jtcvs.2015.06.086.. S0022–5223(15)01237-4. [DOI] [PubMed] [Google Scholar]
- 18.Onishi H, Shirato H, Nagata Y, Hiraoka M, et al. Stereotactic Body Radiotherapy (SBRT) for Operable Stage I Non-small Cell Lung Cancer: Can SBRT Be Comparable to Surgery? Int J Radiation Oncol Biol Phys. 2011;81(5):1352–1358. doi: 10.1016/j.ijrobp.2009.07.1751. [DOI] [PubMed] [Google Scholar]
- 19.Hamaji M, Chen F, Matsuo Y, Kawaguchi A, et al. Video-Assisted Thoracoscopic Lobectomy Versus Stereotactic Radiotherapy for Stage I Lung Cancer. Ann Thorac Surg. 2015;99:1122–9. doi: 10.1016/j.athoracsur.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Lagerwaard FJ, Verstegen NE, Haasbeek C, Slotman BJ, et al. Outcomes of Stereotactic Ablative Radiotherapy in Patients with Potentially Operable Stage I Non-Small Cell Lung Cancer. Int J of Radiation Biol Phys. 2012;83(1):348–353. doi: 10.1016/j.ijrobp.2011.06.2003. [DOI] [PubMed] [Google Scholar]
- 21.Mokhles S, Verstegen N, Maat A, et al. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: Results from propensity score analysis. Lung Cancer. 2015;87:283–289. doi: 10.1016/j.lungcan.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Ceppa DP, Kosinski AS, Berry MF, Tong BC, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg. 2012;256:487–93. doi: 10.1097/SLA.0b013e318265819c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cattaneo SM, Park BJ, Wilton AS, Seshan VE, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85:231–6. doi: 10.1016/j.athoracsur.2007.07.080. [DOI] [PubMed] [Google Scholar]