Abstract
Discoveries in tumor immunology and subsequent clinical advances in cancer immunotherapy have revealed that the immune system is not oblivious to tumor progression, but heavily interacts with developing neoplasia and malignancy. A major factor preventing immune destruction is the establishment of a highly immunosuppressive tumor microenvironment (TME), which provides architecture to the tumor, supports indirect means of immunosuppression such as the recruitment of tolerogenic cells like regulatory T cells and MDSCs, and represents a zone of metabolically dearth conditions. T-cell activation and consequent effector function are cellular states characterized by extreme metabolic demands, and activation in the context of insufficient metabolic substrates results in anergy or regulatory differentiation. Thus, T cells must endure both immunosuppression (coinhibitory molecule ligation, regulatory T cells, and suppressive cytokines) but also a sort of metabolic suppression in the tumor microenvironment. Here I will review the general features of the tumor microenvironment, identify the metabolic demands of activated effector T cells, discuss the known metabolic checkpoints associated with intratumoral T cells, and propose strategies for generating superior antitumor T cells, whether in vitro for adoptive cell therapy or through in vivo reinvigoration of the existing immune response.
Checkpoint blockade and adoptive cell therapies for cancer treatment
The immune system has the ability to remove specifically-targeted cells with extreme precision, a feature that is very desirable for cancer therapy, as most traditional therapies fail to remove ‘every last cell’. Although it was long thought that cancer represented a disease state that was ‘too’ self, and thus progressed outside of immune control, it is now clear that clinically apparent cancer is likely the product of a failure of surveillance and early elimination of neoplasia by the immune system.(1)
For many years, researchers and clinicians sought to reinvigorate or supplement immunity to cancer, using vaccines or cytokine therapy, understanding and utilizing what was known of immune regulation and evasion at the time. Understanding the negative regulation of the immune response opened the door for blockade of these coinhibitory checkpoints, the first of which was the blockade of the B7 ligand CTLA-4. A coinhibitory molecule acting as an antagonist to CD28 signaling, CTLA-4 represents a molecule that, when blocked, enhances T-cell priming (2). Its blockade via the monoclonal antibody ipilimumab results in an unleashing of the immune response, resulting in durable antitumor responses in melanoma (3). Once researchers revealed that T-cell inhibition likely occurs locally in the tissues, as well as at the level of priming, it became possible to more directly enhance tumor-infiltrating T cells.
PD-1, a coinhibitory checkpoint receptor upregulated upon activation and ligated in the tissues, now represents a key target in cancer immunotherapy (4). The PD-1 axis is a coinhibitory interaction that occurs between T cells and tumors, or those other cells that the tumor has conscripted (4). Tumor cells sense immune activation, in part via IFNγ and other microenvironmental factors, and upregulate PD-L1 as a means to inhibit immune destruction (5,6). Blockade of PD-1 signaling through monoclonal antibodies like nivolumab and pembrolizumab has resulted in long-term durable responses in many patients, with less immune-related toxicities than ipilimumab treatment (7). However, it is important to note that, like their predecessors, this next wave of immunotherapies has similar problems: although a significant proportion of patients respond well to checkpoint blockade, the majority of patients will have little or no response (4). Combination therapies (8), optimal sequencing, and stratification based on various biomarkers have edged this proportion higher, but there are few predictors of those that will have an immunotherapeutic response (9), and even fewer alternatives for those patients that do not respond.
However, as our understanding of how the T-cell response is negatively regulated through coinhibitory receptors opened the door for checkpoint blockade, new studies of additional mechanisms by which T cells are regulated through additional, potentially non-immunologic mechanisms may shed new light on the extension of immunotherapies to additional patients.
We now appreciate that T cells and tumor cells do not interact in isolation, as antigen presenter and antigen detector. In fact a very distinct constellation of cellular players characterize what is commonly known as the tumor microenvironment (10). Suppressive regulatory T cells, myeloid-derived suppressor cells, and tumor-associated macrophages can generate an additional zone of immunoregulation that blankets the additional inhibitory ligations that T cells endure, often driven by the cancer cells themselves (11). Although these cells are currently being targeted through various modalities and utilized as biomarkers of response, behind the veil of these cells lies a distinct metabolic landscape, heavily impacted by the deregulated bioenergetics of the tumor and further exacerbated by altered angiogenesis and stromal deregulation (12).
Cancer cells divide continuously and unrestrainedly, fueling their proliferation through metabolic deregulation (13). Nucleotides and membranes must be generated and proteins must be duplicated, creating a major energetic demand on the cancer cell (13). Early biochemical work showed that tumor cells utilize glucose fermentation (‘Warburg effect’), generating lactic acid rather than oxidizing glucose in the mitochondria, to generate biomass in the mitochondria required for cell growth (14). Tumor cells are, thus, hungry, and in the spatially-restricted tumor microenvironment, act as a sink for nutrients and oxygen. Indeed, metabolic profiling has confirmed that tumors contain low concentrations of glucose, glutamine, and oxygen, while having high amounts of the byproducts of tumor cell metabolism, including lactic acid, glutamate, and ketone bodies (15). Importantly, although deregulated metabolism is an ‘emerging hallmark’ of cancer and certainly a common phenotype of tumor cells, the degree of this deregulation can be quite heterogeneous, from patient to patient and even within individuals (13,16). Indeed, as cancers develop, individual cancer cells can adopt distinct metabolic patterns that can be associated or even causative in altered differentiation. Although more differentiated tumor cells tend to rely on glycolysis, cancer stem cells are heavily dependent on mitochondrial function for the maintenance of a stemness phenotype (13). At the individual level of cancer stem cells, numerous studies have shown great heterogeneity in cancer cells with stem properties, which can prioritize specific glycolytic or oxidative pathways as their primary metabolic phenotype, expertly reviewed elsewhere (17). Thus, the metabolic status of an individual's tumor microenvironment can be quite distinct, induced by the very nature of the tumor cell itself. Indeed, oncogenic mutations can promote distinct metabolic profiles in the tumor cell. For example, Myc can promote glycolysis and glutamine metabolism in tumor cells (18,19), and Ras can promote glucose uptake and associated hexosamine and nucleotide synthesis (20,21). Thus, tumor cell metabolic deregulation can be highly variable, dependent on many host- and tumor-derived factors. The changes induced by this metabolic deregulation are not trivial, and any other cells with high metabolic demands will effectively function at a loss unless bolstered to outcompete cancer cells for nutrients.
Race cars and semi-trucks: fueling the effector and memory phase
Naïve T cells, quiescent for a lifetime, have barely detectable metabolic activity, likely as a means to preserve clonality throughout ontogeny (22). However, upon activation T cells grow considerably for about 24 hours and then rapidly divide, performing cell cycles measured as quickly as 2 h (23,24). During this effector phase, T cells engage aerobic glycolysis, fermenting glucose much like cancer cells do, in order to preserve mitochondria for biosynthesis and limit oxidative damage (25). They also become very dependent on glutamine, engaging glutaminolysis pathways almost immediately upon T-cell receptor (TCR)–elicited Myc transcription (26). This ‘high octane’ metabolism is not simply important for generating ATP, reducing intermediates, and nucleotides (through the pentose phosphate pathway) to support proliferation, but actively contributes to the effector program of T cells through translational control of effector cytokines. Specifically, GAPDH, like many glycolytic enzymes, can ‘moonlight’ as an RNA-binding protein during non-glycolytic periods (27). Glucose uptake and processing is also coupled to other effector T-cell responses, like calcium flux, and maintaining lineage stability (28,29). Indeed glucose metabolism also can play roles in modulating epigenetic stability at the level of histones and DNA methylation (29). Thus, glucose (and its availability) can directly contribute to effector T-cell function, as well as provide energy to support their robust proliferation.
Mitochondria, too, play a vital role in T-cell activation. Although the fate of the majority of glucose lies outside of the mitochondria, activated T cells still utilize mitochondrial pathways for oxidative phosphorylation as well as many biosynthetic pathways (30-32). Importantly, mitochondria are also important for the final steps in the oxidation of other fuel sources, like amino acids and lipids, representing ‘diesel engines’ that can burn ‘less refined’ fuel. They are also critically important for calcium buffering, and generating reactive-oxygen species (33,34). Mitochondria are dynamically regulated: quantitatively within the cell through mitochondrial biogenesis (35,36), qualitatively through the development of ‘fissed’ or ‘fused’ mitochondrial networks (37), and spatially through cytoskeletal tethering into and out of the immunologic synapse (38,39). As T cells progress to their memory phase, they further upregulate mitochondrial biogenesis to build spare respiratory capacity, a measurement of oxidative reserve used in stress situations (40). Memory T cells also engage in mitochondrial fusion, forming networks of mitochondria to effect higher oxidative phosphorylation function (37). In this way, memory cells are bioenergetically primed, able to respond with vigor to reencounter with an antigen, but also able to re-enter the quiescent state to persist, potentially never seeing cognate antigen again. Thus mitochondrial pathways are associated in T cells with longevity and persistence, as well as metabolic plasticity, effectively being able to oxidize a multitude of other fuel sources outside of glucose.
Metabolic defects in tumor-infiltrating T cells
The persistent nature of a tumor, a source of long-term antigen and inflammatory signals, has been likened to that of chronic viral infection, producing phenotypically similar exhausted T cells, which still perform but at limited efficacy (41). Thus, studies both in cancer and experimental models of chronic viral infection have revealed much about the nature of T cells in this type of condition. Although in vitro studies have revealed much about the signaling of some of these T-cell subsets, it has been difficult to model the microenvironments associated with T-cell exhaustion with sufficient accuracy. Work identifying transcriptional signatures associated with exhausted T cells using the lymphocytic choriomeningitis virus system indeed revealed no ‘smoking gun’ for the T-cell exhaustion phenotype (41). Rather, exhaustion, both in cancer and chronic infection, represents a number of non-redundant deficiencies that seem to synergize to induce T-cell dysfunction. Indeed, exhausted T cells have a distinct metabolic transcriptional signature that is induced early, and in a distinct manner, from the effector responses of acute infections (42). However, many of these changes are not transcriptionally induced, but rather are limited by post-translational or microenvironmental changes.
The metabolic defects of the tumor microenvironment are important to understand, as rapid proliferation, cytokine production, and cytolytic activity, important cellular functions for T cells, each have high energetic demands (43). Sustaining that effector response thus requires overcoming several metabolic barriers: sufficient metabolic substrates (glucose, glutamine, fatty acids, oxygen), cellular machinery to utilize these substrates (glycolytic enzymes, mitochondria, reducing intermediates), and nutrient sensors to mobilize this cellular machinery (mTOR, AMPK, Myc, ERRα) (Fig. 1). Studies exploring the interface between these metabolic pathways and immunity have garnered much interest, both at the basic level as well as in various disease states, and this new field of ‘immunometabolism’ has revealed that bioenergetics act as a sort of primordial mechanism for immunoregulation.
Figure 1. Metabolic barriers to antitumor immunity.
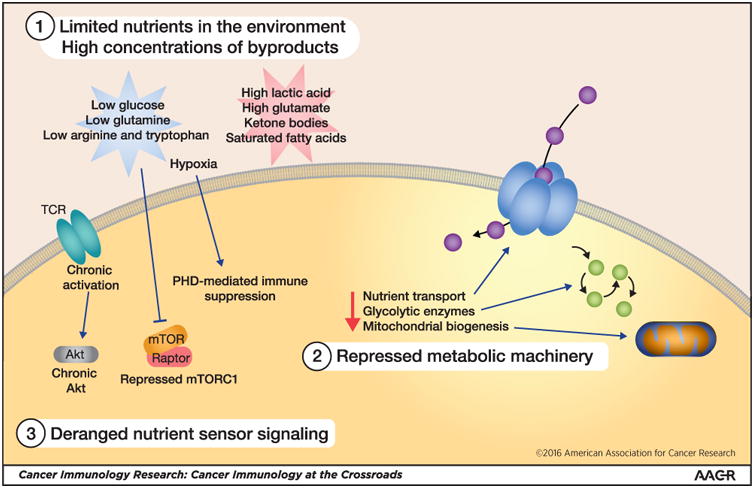
(1) Altered tumor cell metabolism creates a microenvironment with low concentrations of primary metabolic substrates for T cells, including glucose, glutamine, and oxygen. Metabolites produced by tumor cells like lactic acid are in high concentrations and can be immunosuppressive. (2) In addition, signals in the tumor microenvironment can repress the glycolytic machinery, as well as pathways involved in mitochondrial biogenesis. (3) Finally, nutrient sensing machinery can also be deregulated, resulting in a failure to mobilize these important pathways to promote sustained T-cell responses in the tumor microenvironment.
Studies of T-cell tolerance brought to light the impact of metabolites and nutrient sensing in T-cell function; this has been extensively reviewed elsewhere (44). Kinases like mTOR limit nutrients through nutrient sensing and other pathways during T-cell stimulation, which not only impacts immediate effector function, but can have long-term effects on the differentiation and future function of these cells (45). Although many of these studies were conducted in vitro, applying that rationale to the tumor microenvironment has revealed that in this physiologic environment in which nutrients are limiting, T cells fail by similar mechanisms. Glucose, a primary fuel for effector T cells, is limiting in the tumor microenvironment, consumed by tumor cells themselves (28,46). This not only limits the immediate effector function of T cells, but also limits their ability to take up glucose as well. Immunotherapies like PD-1 blockade may have a bystander effect of limiting tumor glucose consumption, thus tipping the balance in favor of antitumor immunity (46). Importantly, glucose metabolism also plays a critical role in promoting calcium signaling, which is critical for TCR-induced NFAT activation and subsequent effector function (28). Thus, the competition for glucose remains a major limiting factor for T-cell function in the tumor, but other metabolic substrates are limiting in the tumor microenvironment. Glutamine, arginine, tryptophan, and oxygen are all present at lowered concentrations in the tumor microenvironment, driven, at least in part, by tumor cell metabolism and competition. This limits T-cell metabolism and consequent effector function (19,47-49). Improving T-cell metabolism for therapy need not be a heroic undertaking, however. Arginine supplementation of therapeutic T cells has been shown to improve their metabolism and can heighten antitumor responses in a model of adoptive cell therapy (50). This approach of directly ‘feeding’ the T cells to promote their antitumor function may be of value in the future. It may also be attractive to utilize strategies to remodel the metabolism of the microenvironment itself, rather than providing T cells nutrients directly. These strategies could include, for example, identification and inhibition of a tumor specific pathway that promotes the consumption of a T-cell primary fuel. Inhibiting oncogenic pathways that promote glycolysis might also lower tumor glucose consumption, providing fuel for T cells. Alternatively, utilizing inhibitors that are more broadly active but affect tumor cells disproportionately may be an attractive strategy. This may involve targeting metabolic drugs to tumors with antibodies, or, more simply, utilizing pharmacologic inhibitors which may be more actively taken up by tumor cells. Thus, it may be possible to lower a metabolic barrier through carefully designed strategies to inhibit tumor cell metabolism, creating an environment more permissive to T-cell function and consequent response to immunotherapy.
However, T cells are not only limited by the availability of nutrients in the tumor microenvironment. Rather, the metabolic machinery can also be repressed in tumor-infiltrating T cells. As mentioned above, T cells in the tumor microenvironment can repress the machinery required to process glucose, including glucose transporters and glycolytic enzymes (28,46,51). Our group has found that mitochondria, which require an active process termed ‘mitochondrial biogenesis’ for their replication during cell division, are also limiting in tumor-infiltrating T cells (52). Tumor-infiltrating T cells have poor mitochondrial morphology, repressed mitochondrial function, and lower total mitochondrial mass (52). This process occurred independently of PD-1 blockade, suggesting that the mitochondrial repression occurs in parallel to PD-1-induced coinhibition. Similar loss of oxidative metabolism is observed in exhausted T cells from chronic viral infection, including repression of mitochondrial biogenesis programs (42). Reprogramming of T cells to enforce mitochondrial biogenesis resulted in superior antitumor and antiviral T-cell function (42,52). Thus, T cells lack the machinery to process available nutrients, another major barrier to effective antitumor immunity.
The nutrient-sensing machinery itself can be deranged in tumor-infiltrating T cells. Mitochondrial biogenesis is repressed through chronic Akt activation, which represses the activity of the PPARγ coactivator 1 (PGC1α) pathway, a transcriptional coactivator supporting this biogenesis pathway (53). In addition, several groups have observed repression of the mTORC1 pathway in exhausted T cells, which has major metabolic effects on tumor-infiltrating T cells, including the inhibition of glycolysis, mitochondrial activity, and translation, as well as the induction of autophagy (42,52). The oxygen sensing machinery plays a major role in immunosuppression in the tumor microenvironment (54). Whereas hypoxia can have both activating and suppressive functions in T cells (55), mitigating tumor hypoxia through respiratory supplemental oxygenation can improve immunotherapeutic responses in mice (48). Thus, T cells in tumor microenvironments must overcome a number of metabolic barriers to be able to fuel their antitumor function.
Retrofitting T cells in immunotherapy: built for speed or the long haul
Since the identification of tumor-infiltrating lymphocytes, researchers and clinicians have been trying to harness the power of T cells for therapy. In hematologic malignancies, mismatched hematopoetic cell transplants have long utilized the broad reactivity of T cells against alloantigens to target host tumors (graft-versus-leukemia), and the power of this response can be observed in the toxic and potentially lethal side effects of graft-sversus-host disease (56). Specificity, in the form of chimeric antigen receptors or TCR-engineered T cells, has allowed the harnessing of this ‘serial-killer’ response to generate durable immunity to several hematologic malignancies, targeting cell-surface antigens like CD19. These re-engineered T cells have the capacity to kill tumor cells extraordinarily efficiently, clearing so much tumor that cytokine release must be mitigated therapeutically. However, this approach may not be directly applicable for immunotherapy of solid tumors. Responses to CAR- and TCR-engineered T cells in solid tumors have been underwhelming, and in many cases accompanied by significant off-target toxicities (57).
Effector T cells can sense antigen upon presentation, and receive many other signals in the form of costimulation/coinhibition and cytokines, but T cells are merely integrating signals: they know nothing about the persistence, scope, or nature of an antigen. Thus, during an effector phase, the hard-wired response is to proliferate rapidly and kill quickly, with the ‘hope’ that this is sufficient to eliminate infection. They do this at the expense of their continued longevity, favoring glycolysis over mitochondrial metabolism. Metabolic choices thus may determine the long-term potential of these cells, a supposition supported experimentally in models of TIL therapy (58,59). This ‘shock-and-awe’ response is likely sufficient to clear most acute infections, but in the case of a solid tumor, which smolders with continuing cell division, it may simply be not enough. Efforts to reinvigorate or redirect T cells to cancer have been focused on creating the ‘best’ effector cells that will infiltrate the tumor and lyse cancer cells with reckless abandon. However, these effector cells (whether endogenously re-invigorated or adoptively transferred) are not engineered for the long haul; thus clinical responses are infrequent or incomplete. In the solid-tumor microenvironment, nutrients are insufficient to support this type of response; glucose is limiting, acidosis makes regeneration of NAD+ by LDH more difficult, and hypoxia prevents any residual mitochondrial activity (55). Clues from the CAR T-cell studies and the study of intratumoral T cells have taught us that memory-like T cells are superior antitumor T cells. Memory T cells are imbued with higher mitochondrial activity, more fused mitochondrial networks, and the ability to utilize additional substrates to fuel their secondary responses, all of which are desirable in long-lived T cells and in a nutrient-poor condition (30,37,40). Thus, promoting mitochondrial biogenesis to support memory states and decreasing hypoxia to allow for mitochondrial function are key factors in generating better long-lived T cells. Indeed, reprogramming T cells to favor mitochondrial biogenesis through enforced expression of PGC1α resulted in enhanced antitumor response, even with small numbers of cells (52). In other words, making a cell ‘metabolically’ resemble a memory cell promotes memory-like states. This type of manipulation may be critical for the generation of long-term durable immune responses like those needed for the eradication of solid tumors.
Concluding remarks
Recent clinical successes have brought cancer immunotherapy into the mainstream, providing hope to patients with advanced cancers and revealing new pathways by which we might bolster antitumor immunity to benefit a larger proportion of patients. Those successes were enabled through the improved understanding of how immunity was regulated. It is clear that deregulated metabolism, a common feature of cancer cells, can also act as a negative regulator of immune cell function through the generation of an environment with a dearth of nutrients, coupled to the exploitation of the basic wiring of T-cell signaling. Modulating those intratumoral energetics, whether through reprogramming of T cells, selective targeting of tumor cell metabolism, or other undiscovered means, has the potential to further transform how the immune system is harnessed for cancer treatment.
Acknowledgments
The author is supported by the University of Pittsburgh Cancer Institute (start-up funds and CCSG P30CA047904-28), the Sidney Kimmel Foundation for Cancer Research (SKF-015-039), and a Stand Up To Cancer – AACR Innovative Research Grant (SU2C-AACR-IRG-04-16).
Footnotes
Conflict of Interest Statement: The author has no conflicts of interest to disclose.
References
- 1.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Advances in immunology. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 2.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5271–9. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer cell. 2015;27:450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nature medicine. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 6.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nature medicine. 2003;9:562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 7.Mahoney KM, Freeman GJ, McDermott DF. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clinical therapeutics. 2015;37:764–82. doi: 10.1016/j.clinthera.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. The Lancet Oncology. 2016 doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shien K, Papadimitrakopoulou VA, Wistuba II. Predictive biomarkers of response to PD-1/PD-L1 immune checkpoint inhibitors in non-small cell lung cancer. Lung cancer (Amsterdam, Netherlands) 2016;99:79–87. doi: 10.1016/j.lungcan.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Current opinion in immunology. 2016;39:1–6. doi: 10.1016/j.coi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Current opinion in immunology. 2013;25:268–76. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Ackerman D, Simon MC. Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends in cell biology. 2014;24:472–8. doi: 10.1016/j.tcb.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science (New York, NY) 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer research. 2006;66:8927–30. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 15.Justus CR, Sanderlin EJ, Yang LV. Molecular Connections between Cancer Cell Metabolism and the Tumor Microenvironment. International journal of molecular sciences. 2015;16:11055–86. doi: 10.3390/ijms160511055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warmoes MO, Locasale JW. Heterogeneity of glycolysis in cancers and therapeutic opportunities. Biochemical pharmacology. 2014;92:12–21. doi: 10.1016/j.bcp.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. Br J Cancer. 2016;114:1305–12. doi: 10.1038/bjc.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. The Journal of biological chemistry. 2000;275:21797–800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 19.Shroff EH, Eberlin LS, Dang VM, Gouw AM, Gabay M, Adam SJ, et al. MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:6539–44. doi: 10.1073/pnas.1507228112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiaradonna F, Sacco E, Manzoni R, Giorgio M, Vanoni M, Alberghina L. Ras-dependent carbon metabolism and transformation in mouse fibroblasts. Oncogene. 2006;25:5391–404. doi: 10.1038/sj.onc.1209528. [DOI] [PubMed] [Google Scholar]
- 21.Ying H, Kimmelman Alec C, Lyssiotis Costas A, Hua S, Chu Gerald C, Fletcher-Sananikone E, et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell. 2012;149:656–70. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. Journal of immunology (Baltimore, Md : 1950) 2001;167:6869–76. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 23.Yoon H, Kim TS, Braciale TJ. The Cell Cycle Time of CD8(+) T Cells Responding In Vivo Is Controlled by the Type of Antigenic Stimulus. PloS one. 2010;5:e15423. doi: 10.1371/journal.pone.0015423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinjyo I, Qin J, Tan SY, Wellard CJ, Mrass P, Ritchie W, et al. Real-time tracking of cell cycle progression during CD8(+) effector and memory T-cell differentiation. Nature Communications. 2015;6:6301. doi: 10.1038/ncomms7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimeloe S, Burgener AV, Graehlert J, Hess C. T cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology. 2016 doi: 10.1111/imm.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–82. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O'Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–51. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–28. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nature immunology. 2013;14:1064–72. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- 30.van der Windt GJ, O'Sullivan D, Everts B, Huang SC, Buck MD, Curtis JD, et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14336–41. doi: 10.1073/pnas.1221740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–77. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 32.Ron-Harel N, Sharpe AH, Haigis MC. Mitochondrial metabolism in T cell activation and senescence: a mini-review. Gerontology. 2015;61:131–8. doi: 10.1159/000362502. [DOI] [PubMed] [Google Scholar]
- 33.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nature reviews Molecular cell biology. 2012;13:566–78. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 34.Wenner CE. Targeting mitochondria as a therapeutic target in cancer. Journal of cellular physiology. 2012;227:450–6. doi: 10.1002/jcp.22788. [DOI] [PubMed] [Google Scholar]
- 35.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2011;1813:1269–78. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. The American Journal of Clinical Nutrition. 2011;93:884S–90S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buck MD, O'Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwindling C, Quintana A, Krause E, Hoth M. Mitochondria positioning controls local calcium influx in T cells. Journal of immunology (Baltimore, Md : 1950) 2010;184:184–90. doi: 10.4049/jimmunol.0902872. [DOI] [PubMed] [Google Scholar]
- 39.Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, et al. T cell activation requires mitochondrial translocation to the immunological synapse. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14418–23. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nature reviews Immunology. 2015;15:486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, et al. Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion. Immunity. 2016;45:358–73. doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delgoffe GM, Powell JD. Feeding an army: The metabolism of T cells in activation, anergy, and exhaustion. Molecular immunology. 2015 doi: 10.1016/j.molimm.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nature reviews Immunology. 2014;14:435–46. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. Journal of immunology (Baltimore, Md : 1950) 2009;183:6095–101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–41. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. The Journal of clinical investigation. 2013;123:3678–84. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatfield SM, Kjaergaard J, Lukashev D, Schreiber TH, Belikoff B, Abbott R, et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Science translational medicine. 2015;7:277ra30. doi: 10.1126/scitranslmed.aaa1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cham CM, Gajewski TF. Metabolic mechanisms of tumor resistance to T cell effector function. Immunologic research. 2005;31:107–18. doi: 10.1385/IR:31:2:107. [DOI] [PubMed] [Google Scholar]
- 50.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell. 2016;167:829–42.e13. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao L, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nature immunology. 2015 doi: 10.1038/ni.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, et al. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity. 2016;45:374–88. doi: 10.1016/j.immuni.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spiegelman BM. Transcriptional control of energy homeostasis through the PGC1 coactivators. Novartis Foundation symposium. 2007;286:3–6. discusssion 6-12, 162-3, 96-203. [PubMed] [Google Scholar]
- 54.Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, et al. Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche. Cell. 2016;166:1117–31.e14. doi: 10.1016/j.cell.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Ertl HC. Starved and Asphyxiated: How Can CD8(+) T Cells within a Tumor Microenvironment Prevent Tumor Progression. Frontiers in immunology. 2016;7:32. doi: 10.3389/fimmu.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garber HR, Mirza A, Mittendorf EA, Alatrash G. Adoptive T-cell therapy for Leukemia. Molecular and cellular therapies. 2014;2:25. doi: 10.1186/2052-8426-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beatty GL, O'Hara M. Chimeric antigen receptor-modified T cells for the treatment of solid tumors: Defining the challenges and next steps. Pharmacology & therapeutics. 2016 doi: 10.1016/j.pharmthera.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sukumar M, Liu J, Mehta GU, Patel SJ, Roychoudhuri R, Crompton JG, et al. Mitochondrial Membrane Potential Identifies Cells with Enhanced Stemness for Cellular Therapy. Cell metabolism. 2016;23:63–76. doi: 10.1016/j.cmet.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. The Journal of clinical investigation. 2013;123:4479–88. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]


