Abstract
The present methodology teaches the investigator how to measure and use the LAV as a surrogate of chronic elevations in Left Ventricular diastolic pressure through echocardiography, as well as to obtain measurements of the Aorta and PA diameter in mice.
Mice older than 10 d of age can be analyzed using the present technique. The technique is composed of 3 main steps: set-up, image acquisition, and image analysis. The set-up step consists of getting the mouse anesthetized with 1% isoflurane, shaving it, and taping it in a supine position to a heated EKG board where the image acquisition will take place. The image acquisition step consists of learning to identify the cardiac structures and obtaining all the required images with its correspondent probe and axis in order to be able to calculate volumes and diameters. The image analysis step consists of measuring the previously acquired images with the aid of computer software.
Advantages of the proposed technique include a fast (15 min) procedure that would allow the researcher to evaluate interventions in a non-invasive, non-terminal approach and therefore follow the same mouse over time; each mouse can be used as its own control. This fact plus having the same operator perform all the acquisition and analysis for the entire experiment minimizes the limitation of operator-dependency. The present methodology is useful for mouse researchers in cardiovascular and pulmonary medicine.
Keywords: Medicine, Issue 120, Echocardiography, Murine, Left Atrium, Pulmonary Artery, Diastolic Dysfunction, Heart Failure, Aging, Pulmonary Hypertension, Non-Invasive
Introduction
The present methodology teaches the investigator how to measure and use the LAV, Aorta diameter and PA diameter with 2D echocardiography in mice under isoflurane. Heart Failure with preserved Ejection Fraction (HFpEF) in elderly people affects up to 10% in those 80 years and older1 and results in significant morbidity and mortality. Significant mortality also occur from Pulmonary Hypertension (PH), an insidious disease process presenting with similar symptoms as HFpEF, in which elevated pulmonary artery pressures lead to exertional dyspnea, progressive right heart failure, and often death.2 The increasing prevalence of both HFpEF and PH signify the need for developing a method that allows for accurate evaluation and monitoring of interventions in murine models in a non-invasive, non-terminal approach.
Aging leads to a deterioration of diastolic function via alterations in ventricular-arterial stiffening, vascular dysfunction, inflammation3, impaired calcium regulation4, decreased β-adrenergic responsiveness, and physical deconditioning producing slowed active relaxation and increased passive stiffness. Over time this leads to increased LV filling pressure and compensatory enlargement of the LA.5
Though other etiologies such as valvular dysfunction (mitral regurgitation or stenosis) and infiltrative processes cause elevations in pressure and volume in the LA1, the European Society of Cardiology supports the addition of LA size as a noninvasive reflection of LV function.6
Correlations between LA volume and invasive measures of diastolic function have been studied in mice. LA volume correlated with differences in function determined invasively within age groups for both 14- and 31-months-old mice. In the 14-months-old mice, LA volume correlated with three standard invasive measures of diastolic function −dP/dtmin (r2 = .5, p <0.05), Tau (time constant of relaxation, (r2 = .6, p <0.05), and left ventricular end diastolic pressure (r2 = .25, p <0.05). For the 31 months-old mice, the correlations between LA volume and -dP/ dtmin (r2 = .92, p <0.05) and LVEDP (r2 = .61, p <0.05) were apparent though the relationship with Tau was less clear. Therefore, LA volume increased with diastolic impairment not only across groups but within age groups.7
Studies of cardiac function in murine models using catheter techniques to evaluate cardiac performance, although rigorous and reliable, are limited due to their incompatibility with repeated assessments.8 Alternatives to invasive measures such as MRI and 3D echocardiography may also be more accurate than 2D echocardiographic techniques, but they are more expensive; 2D echocardiography is considered adequate for LA volume evaluation. 9,10
Assessment of the LA volume and PA diameter with echocardiography allows the discrimination between models that produce primary increases in pulmonary artery resistance resulting in an increase in PA diameter with no change in LA pressure or LAV from those where the Pulmonary Artery and Left Atrium both enlarge as a result of elevated filling pressures on the left side of the heart. This approach was taken by Scalia et al. who showed that in people, the echocardiographic Pulmonary Artery to Left Atrial Ratio (ePLAR) is a parameter to accurately differentiate between patients with pre-pulmonary capillary hypertension and post-capillaryhypertension.11
Protocol
All animals were cared for in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals at Baylor College of Medicine.
1. Set-up
Turn on the echocardiographic system. Start the software by selecting "Cardiac Measurements" from the drop down menu displayed and click "Initialize".
To begin a new study click on the "New" button on the upper part of the screen and enter the demographic information of the mouse in study. For example mouse ID, sex, date of birth as well as the name of the operator doing the study.
Turn on the temperature-controlled EKG board by clicking the "TEMP ON/OFF" button. Set it to a temperature of 39 °C using the up and down arrows.
To start setting up the anesthesia chamber, first make sure the oxygen and isoflurane levels are appropriate in each of the delivery systems.
Place the mouse in the anesthesia chamber and induce with isoflurane and oxygen until recumbent and then transfer it to face mask. Confirm that the mouse is properly anesthetized by pinching its paw and checking for lack of movement. Maintain the isoflurane at the lowest possible level that avoids a positive paw withdrawal response.
Place the mouse in a supine position on the temperature-controlled EKG board and tape each paw to the correspondent transducer element on the board. Optionally, use transducer cream to improve the signal.
Apply artificial tears ophthalmic lubricant ointment on the mouse's eyes to prevent dryness.
Once the mouse is taped to the EKG board, observe an EKG tracing in the lower part of the monitor. Monitor the heart rate throughout the whole study to ensure best results. A heart rate of 400 beats/min is ideal. Heart rates below this range may suggest isoflurane effects.
Make sure the mouse keeps receiving oxygen and isoflurane by face mask.
Use an electric razor to shave the body fur from the mouse's anterior thorax and upper abdominal area. Use depilatory cream if necessary to further ensure fur removal.
Put a generous amount of transducer gel in the chest of the mouse.
2. Image Acquisition
- Left Atrium measurement
- Locate the handle beneath the EKG board. Use the handle to adjust the angle of the movable EKG board in which the mouse was placed. Begin by adjusting the EKG board to a 30 - 45° angle along the X axis and 0° angle along the Y axis. Authors refer to it as "supine" position. Use this position for all of the Left Atrium measurements acquisition.
- Place the 25 MHz scan head in the echocardiographic movable arm clamp in a long axis view. Make sure to select the Cardiac Measurement Filter and 2D B-Mode in the echocardiographic system.
- Notice that the echocardiographic set has X and Y axis adjustment torques that can be moved clockwise or counterclockwise. Rotate them to get a long axis view of the heart at the level of the aortic outflow tract and acquire a 5 s movie recording. Acquire the scan by pressing the "Cine Store" button on the keyboard of the echocardiographic system. Use this to calculate the Superior-Inferior measurement of the LA.
- After the movie acquisition, switch the echocardiographic view to M-Mode by pressing the "M-Mode" button on the keyboard of the echocardiographic system.
- Observe a sagittal view and a cursor. Place the cursor so that it encompasses the whole LA at the level of the aortic outflow tract. Acquire 3 images of this new view. Acquire the scan by pressing the "Frame Store" button on the keyboard of the echocardiographic system. Use this to calculate the Anterior-Posterior measurement of the LA.
- Return to B-Mode by pressing the "B-Mode" button on the keyboard of the echocardiographic system.
- Rotate the movable arm clamp of the set 90° clockwise. This will set the scan head in a short axis view.
- Using the Y axis torque find the intraarticular septum. Make sure to visualize the wall of the atrium. Acquire a 5 s movie recording. Acquire the scan by pressing the "Cine Store" button on the keyboard of the echocardiographic system. Use this to calculate the Medio-Lateral measurement of the LA.
 Figure 1: "Supine" Position. Representation of a mouse in the "Supine" Position. Approximately 30 - 45° angle along the X axis and 0° angle along the Y axis. This position is used to acquire all of the Left Atrium measurements. Please click here to view a larger version of this figure.
Figure 1: "Supine" Position. Representation of a mouse in the "Supine" Position. Approximately 30 - 45° angle along the X axis and 0° angle along the Y axis. This position is used to acquire all of the Left Atrium measurements. Please click here to view a larger version of this figure.
- Great Vessels Measurement
- Continue by exchanging the 25 MHz scan head for the 30 MHz scan head. Remove the 25 MHz scan head from the movable arm clamp and place the 30 MHz scan head instead. Make sure to select the Abdominal Measurements Filter and 2D B-Mode in the echocardiographic system.
- Locate the handle beneath the EKG board. Use the handle to adjust the angle of the movable EKG board in which the mouse lies. Begin by adjusting the EKG board to a 5° angle along the X axis and 60° angle along the Y axis. Use this podition for the acquisition of the Aorta and Pulmonary Artery measurements. Refer to this as "standing-up" position.
- Using the X and Y axis adjustment torques in the echocardiographic set, get a long axis view of the heart at the level of the aortic outflow tract and acquire a 5 s movie recording. Acquire the scan by pressing the "Cine Store" button on the keyboard of the echocardiographic system. Use this to calculate the Aortic Diameter.
- After the movie acquisition, rotate the movable arm clamp of the set 90° clockwise. This will set the scan head in a short axis view.
- Using the Y axis torque locate the Pulmonary Artery at the level of its bifurcation and acquire a 5 s movie recording. Acquire the scan by pressing the "Cine Store" button on the keyboard of the echocardiographic system. Use this to calculate the Pulmonary Artery Diameter. NOTE: The researcher may use Doppler imaging as a tool to ensure correct identification of the Pulmonary Artery. If desired, once completing step 2.2.5 press the "PW" button on the keyboard of the echocardiographic system to obtain Doppler imaging. If characteristic pulmonary wave tracings are seen, the researcher can be reassured that the correct location was identified.
 Figure 2: "Standing-up" Position. Representation of a mouse in the "Standing-up" Position. Approximately 5° angle along the X axis and a 60° angle along the Y axis. This position is used to acquire the Aorta and Pulmonary Artery diameters. Please click here to view a larger version of this figure.
Figure 2: "Standing-up" Position. Representation of a mouse in the "Standing-up" Position. Approximately 5° angle along the X axis and a 60° angle along the Y axis. This position is used to acquire the Aorta and Pulmonary Artery diameters. Please click here to view a larger version of this figure.
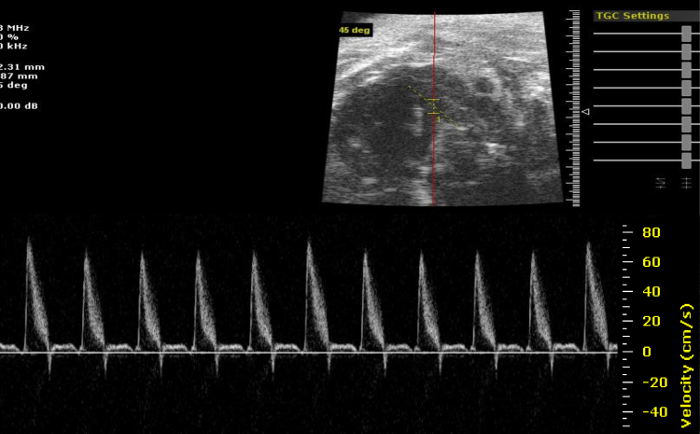 Figure 3: Pulmonary Artery Doppler. Characteristic Pulmonary Artery Doppler imaging. The researcher may use Doppler imaging as a tool to ensure correct identification of the Pulmonary Artery. Please click here to view a larger version of this figure.
Figure 3: Pulmonary Artery Doppler. Characteristic Pulmonary Artery Doppler imaging. The researcher may use Doppler imaging as a tool to ensure correct identification of the Pulmonary Artery. Please click here to view a larger version of this figure.
3. Post Procedure Animal Recovery
To safely remove the mouse from the EKG board, remove the gel from the chest of mouse and remove the tape from the extremities.
Turn off the anesthesia and oxygen delivery systems. Return the mouse to its cage and do not leave the mouse unattended until it has regained sufficient consciousness to maintain sternal recumbency.
4. Image Analysis
- Image Transferring
- Use a USB flash drive to transfer the images collected in the echocardiographic system into a computer. Insert the USB flash drive into a USB port in the echocardiographic system.
- Select the study or studies to transfer by clicking on the study name(s).
- Click on the "Copy To" button located on the upper part of the screen. A new window showing files and directories will appear. Select the directory corresponding to the USB flash drive just inserted and click "OK".
- Remove the USB flash drive from the echocardiographic system and insert it into a USB port in the computer to be used for analysis.
- Launch the echocardiographic software. Click on the "Copy From" button located on the upper part of the screen. Observe a new window showing files and directories. Select the studies to be copied from the directory corresponding to the USB flash drive just inserted and click "OK".
- After copying the files, observe them in the study list. To begin analyzing a study, double click on its name and select the name of the operator analyzing.
- Left Atrium Analysis NOTE: For all the following atrial measurements use the EKG as a guide to select the P-wave as the point where the researcher wants to measure the LA. This corresponds to the pre-atrial contraction time when the LA is at its largest capacity.
- Select the movie clip corresponding to the Superior-Inferior dimension of the Left Atrium (Step 2.1.3). Determine the pre-atrial contraction time using the EKG as a guide. This way the cavity is measured at its largest capacity.
- From the tool bar on the top of the screen, click on "Tools" and then "Measurements". A new window will appear. Click on the "Linear distance Measurement Tool" icon (it corresponds to the first icon on the left upper corner of the window). Measure the cavity at its largest diameter.
- Select the image corresponding to the Anterior-Posterior dimension of the Left Atrium (Step 2.1.5). Determine the pre-atrial contraction time using the EKG as a guide. This way the cavity is measured at its largest capacity. To measure it repeat step 4.2.2.
- Select the image corresponding to the Medio-Lateral dimension of the Left Atrium (Step 2.1.8). Determine the pre-atrial contraction time using the EKG as a guide. This way the cavity is measured at its largest capacity. Measure the cavity from the interatrial septum to the opposing wall in a diagonal fashion. To measure it repeat step 4.2.2. NOTE: Repeat steps 4.2.1 thru 4.2.4 at least 3 times and acquire the average values to minimize error.
- Great Vessels Analysis NOTE: For all the following great vessels measurements use the EKG as a guide to select a time after the QRS complex as the point where the researcher wants to measure. This corresponds to the point where the great vessels are at their largest capacity.
- Select the movie clip corresponding to the Aorta (Step 2.2.3). Determine a point after the QRS using the EKG. Measure the diameter at a 90° angle using the vessel wall as a reference. To measure it repeat step 4.2.2
- Select the movie clip corresponding to the Pulmonary Artery (Step 2.2.5). Determine a point after the QRS using the EKG. Measure the diameter at a 90° angle using the vessel wall as a reference. Select a plane above the pulmonary artery bifurcation. To measure it repeat step 4.2.2.
Representative Results
Assessment of the LA volume and PA diameter with echocardiography allows the discrimination between models where increased pulmonary artery resistance results in increased PA diameter with no change in LAV from those where the Pulmonary Artery and Left Atrial enlargement are both a result of elevated filling pressures on the left side of the heart. Images displaying the differences in the two pathophysiologies are shown in Figure 4. Images of the LA and PA are from young, hypoxia induced pulmonary hypertension and age related LV diastolic dysfunction. The Pulmonary Hypertension models develop an increase in PA diameter without change in Left Atrium volume over a 4 weeks period. In comparison, the old animals develop an increase in both their Pulmonary Artery diameter and Left Atrium volume suggesting that backward propagation of pressure drive the increase in PA diameter in this group.
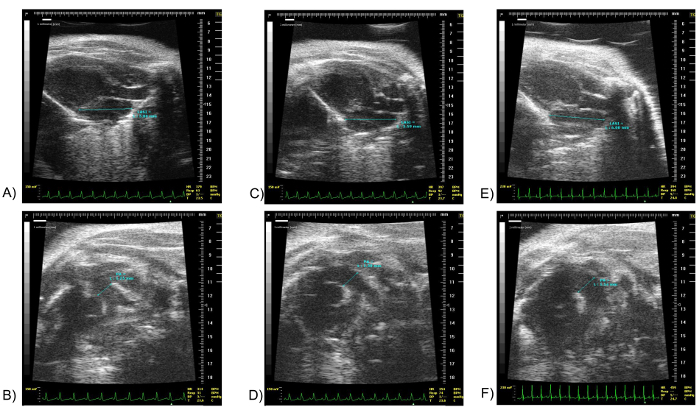 Figure 4: LA Superior-Inferior and PA Diameter on a Normal Mouse, a Pulmonary Hypertension Model Mouse and an Old Mouse with Age-associated LV Diastolic Dysfunction. Images (A) and (B) show the LA Superior-Inferior and PA diameter respectively of a 15 weeks old normal mouse. Images (C) and (D) show the LA Superior-Inferior and PA diameter respectively of a 15 weeks old Pulmonary Hypertension model mouse. Pulmonary hypertension was induced by exposure to 10% hypoxia for four weeks and the PA systolic pressure was increased 75% by right heart catheter studies. Please note the difference in PA diameter. Images (E) and (F) show the LA Superior-Inferior and PA diameter respectively of a 21 months old mouse with elevated LV end diastolic pressure. The filling pressure was increased almost 300% in the old mice. Please note that both the LA and PA diameter are larger compared to the 15 weeks old mice. Scale units in millimeters. The marker "Δ" denotes the point in the EKG at which the image shown was obtained. LA SI: Left Atrium Superior-Inferior, PA: Pulmonary Artery. Please click here to view a larger version of this figure.
Figure 4: LA Superior-Inferior and PA Diameter on a Normal Mouse, a Pulmonary Hypertension Model Mouse and an Old Mouse with Age-associated LV Diastolic Dysfunction. Images (A) and (B) show the LA Superior-Inferior and PA diameter respectively of a 15 weeks old normal mouse. Images (C) and (D) show the LA Superior-Inferior and PA diameter respectively of a 15 weeks old Pulmonary Hypertension model mouse. Pulmonary hypertension was induced by exposure to 10% hypoxia for four weeks and the PA systolic pressure was increased 75% by right heart catheter studies. Please note the difference in PA diameter. Images (E) and (F) show the LA Superior-Inferior and PA diameter respectively of a 21 months old mouse with elevated LV end diastolic pressure. The filling pressure was increased almost 300% in the old mice. Please note that both the LA and PA diameter are larger compared to the 15 weeks old mice. Scale units in millimeters. The marker "Δ" denotes the point in the EKG at which the image shown was obtained. LA SI: Left Atrium Superior-Inferior, PA: Pulmonary Artery. Please click here to view a larger version of this figure.
To ensure accuracy in the results it is necessary that measurements are taken at their appropriate timing in the cardiac cycle. When measuring the LAV, the researcher wants to measure the atrium at its largest capacity for all three measures, this means before atrial contraction. If the LA appendage is seen in any mice in the Superior-Inferior view, the authors recommend to ignore it when doing the measurement. When measuring the great vessels the researcher wants to measure them at their largest capacity, meaning after the QRS or just after the ventricles have pumped blood into the vessels. Figures 5 through 9 show images corresponding to the same animal, measured at different time points on the EKG to illustrate the differences between a correct and incorrect measurement.
The researcher can now calculate the LAV using the formula of the prolate ellipse9,10 as in the following example:
(4 * π * Superior-Inferior Dimension * Anterior-Posterior Dimension * Medio-Lateral Dimension) / (3*2*2*2)
In Figure 5, a "Correct" measurement is that obtained using the EKG as a guide to ensure the researcher is measuring at the largest capacity of the cavity. In this case the atria is at its largest capacity when measured just at the P-wave. (A,C). An "Incorrect" measurement is that obtained at any point other than the P-wave. It tends to give smaller dimensions as the left atrium contracts or fills. (B,D). In Figure 6, a "Correct" measurement is that obtained using the EKG as a guide to ensure the researcher is measuring at the largest capacity of the cavity. In this case the atria is at its largest capacity when measured just at the P-wave (LA(1)). An "Incorrect" measurement is that obtained at any point other than the P-wave. It tends to give smaller dimensions as the left atrium contracts or fills. (LA(2)). In Figure 7, a "Correct" measurement is that obtained using the EKG as a guide to ensure the researcher is measuring at the largest capacity of the cavity. The atria is at its largest capacity when measured just at the P-wave. (A,C). An "Incorrect" measurement is that obtained at any point other than the P-wave. (B,D). *The authors think the cine is extremely helpful for determining the limits of the LA. The researcher should be able to see the LA move throughout the cardiac cycle. In Figure 8, a "Correct" measurement is that obtained using the EKG as a guide to ensure the researcher is measuring at the largest capacity of the vessel. In this case the aorta is at its largest capacity just after the QRS complex. (A,C). An "Incorrect" measurement is that obtained at any point other than that just after the QRS complex. It tends to give smaller dimensions as the aorta contracts or fills. (B,D). In Figure 9, a "Correct" measurement is that obtained using the EKG as a guide to ensure the researcher is measuring at the largest capacity of the vessel. In this case the pulmonary artery is at its largest capacity just after the QRS complex. (A,C). An "Incorrect" measurement is that obtained at any point other than just after the QRS complex. It tends to give smaller dimensions as the pulmonary artery contracts or fills. (B,D).
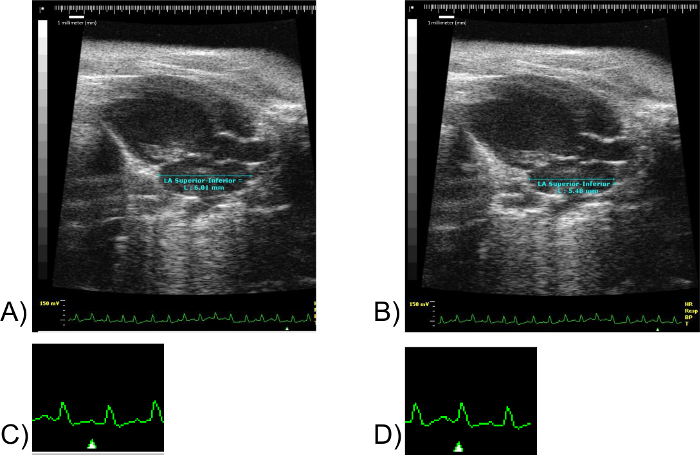 Figure 5: LA Superior-Inferior Measurements. Images (A) and (B) correspond to the same mouse specimen. Scale units in millimeters. Image (C) corresponds to the lower right fragment of EKG of Image (A). Not to scale. Image (D) corresponds to the lower right fragment of the EKG of Image (B). Not to scale. Note the marker "Δ" in images (C) and (D). The marker "Δ" denotes the point in the EKG at which the image shown was obtained. Please click here to view a larger version of this figure.
Figure 5: LA Superior-Inferior Measurements. Images (A) and (B) correspond to the same mouse specimen. Scale units in millimeters. Image (C) corresponds to the lower right fragment of EKG of Image (A). Not to scale. Image (D) corresponds to the lower right fragment of the EKG of Image (B). Not to scale. Note the marker "Δ" in images (C) and (D). The marker "Δ" denotes the point in the EKG at which the image shown was obtained. Please click here to view a larger version of this figure.
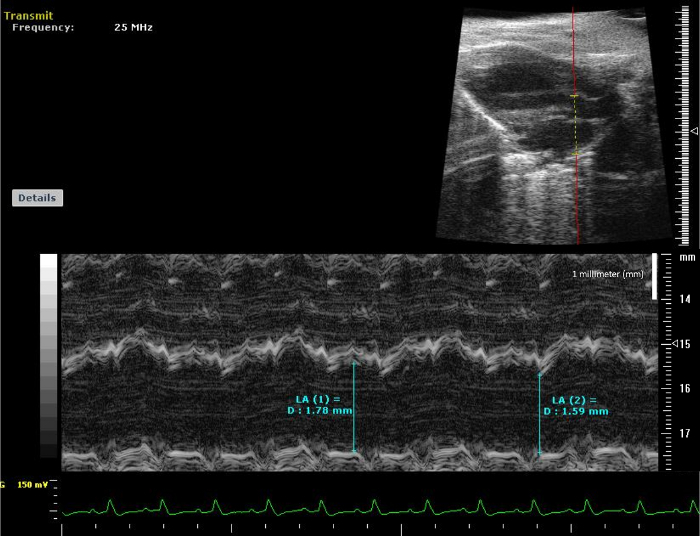 Figure 6: LA Anterior-Posterior Measurements. M-Mode view of Left Atrium. The image corresponds to an M-Mode view of Left Atrium in order to obtain the Anterior-Posterior Measurement. Please click here to view a larger version of this figure.
Figure 6: LA Anterior-Posterior Measurements. M-Mode view of Left Atrium. The image corresponds to an M-Mode view of Left Atrium in order to obtain the Anterior-Posterior Measurement. Please click here to view a larger version of this figure.
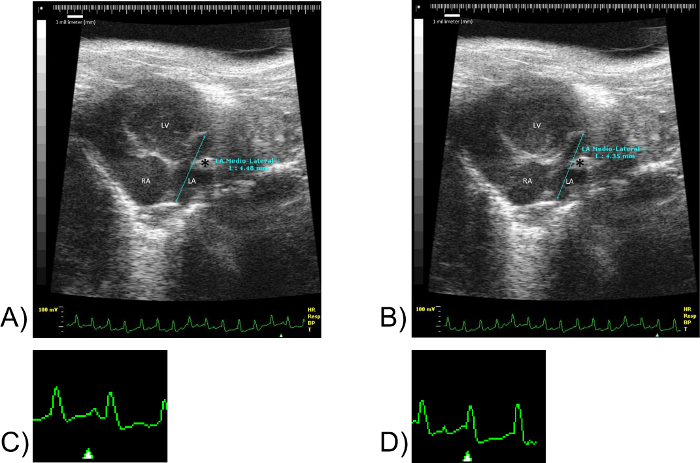 Figure 7: LA Medio-Lateral Measurements. Images(A) and (B) correspond to the same mouse specimen. Scale units in millimeters. (C) corresponds to a fragment of EKG of (A). Not to scale. (D) corresponds to a fragment of the EKG of (B). Not to scale. Note the marker "Δ" in images (C) and (D). The marker "Δ" denotes the point in the EKG at which the image shown was obtained. LV: Left ventricle, RA: Right atrium, LA: Left atrium. Please click here to view a larger version of this figure.
Figure 7: LA Medio-Lateral Measurements. Images(A) and (B) correspond to the same mouse specimen. Scale units in millimeters. (C) corresponds to a fragment of EKG of (A). Not to scale. (D) corresponds to a fragment of the EKG of (B). Not to scale. Note the marker "Δ" in images (C) and (D). The marker "Δ" denotes the point in the EKG at which the image shown was obtained. LV: Left ventricle, RA: Right atrium, LA: Left atrium. Please click here to view a larger version of this figure.
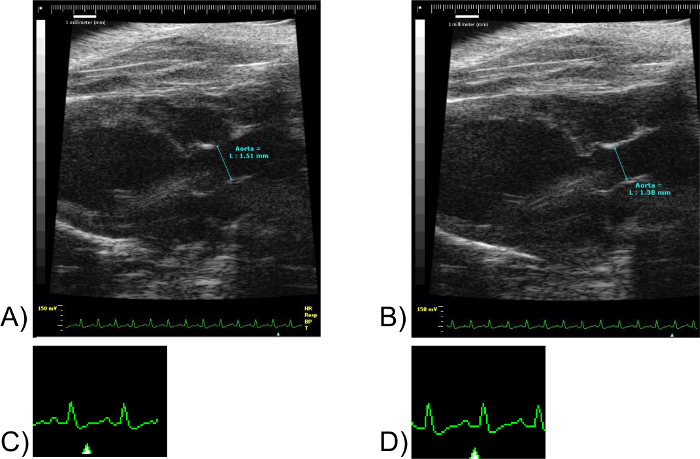 Figure 8: Aorta Measurements. Images(A) and (B) correspond to the same mouse specimen. Scale units in millimeters. (C) corresponds to a fragment of EKG of (A). Not to scale. (D) corresponds to a fragment of the EKG of (B). Not to scale. Note the marker "Δ" in images (C) and (D). The marker "Δ" denotes the point in the EKG at which the image shown was obtained. Please click here to view a larger version of this figure.
Figure 8: Aorta Measurements. Images(A) and (B) correspond to the same mouse specimen. Scale units in millimeters. (C) corresponds to a fragment of EKG of (A). Not to scale. (D) corresponds to a fragment of the EKG of (B). Not to scale. Note the marker "Δ" in images (C) and (D). The marker "Δ" denotes the point in the EKG at which the image shown was obtained. Please click here to view a larger version of this figure.
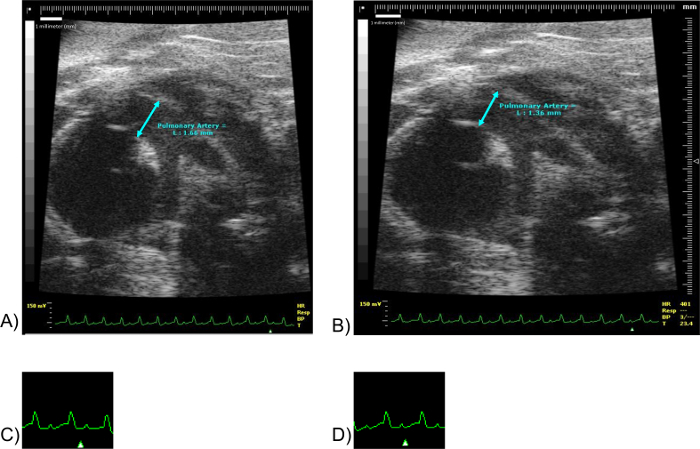 Figure 9: Pulmonary Artery Measurements. Images (A) and (B) correspond to the same mouse specimen. Scale units in millimeters. (C) corresponds to a fragment of EKG of (A). Not to scale. (D) corresponds to a fragment of the EKG of (B). Not to scale. Note the marker "Δ" in images (C) and (D). The marker "Δ" denotes the point in the EKG at which the image shown was obtained. Please click here to view a larger version of this figure.
Figure 9: Pulmonary Artery Measurements. Images (A) and (B) correspond to the same mouse specimen. Scale units in millimeters. (C) corresponds to a fragment of EKG of (A). Not to scale. (D) corresponds to a fragment of the EKG of (B). Not to scale. Note the marker "Δ" in images (C) and (D). The marker "Δ" denotes the point in the EKG at which the image shown was obtained. Please click here to view a larger version of this figure.
To demonstrate the reproducibility and consistency of this technique, the authors offer two examples derived from their own work. The first one presents a group of 19 months old mice that underwent baseline echocardiography and subsequent echocardiography one week later. The authors collected the measurements for Left Atrium and Great Vessels as previously described and obtained the following results. The left side graph shows each individual mouse's Left Atrium Volume calculated using the prolate ellipse formula9,10. The right side graph shows each individual mouse's Pulmonary Artery Diameter. The data showed variation of only a few percent.
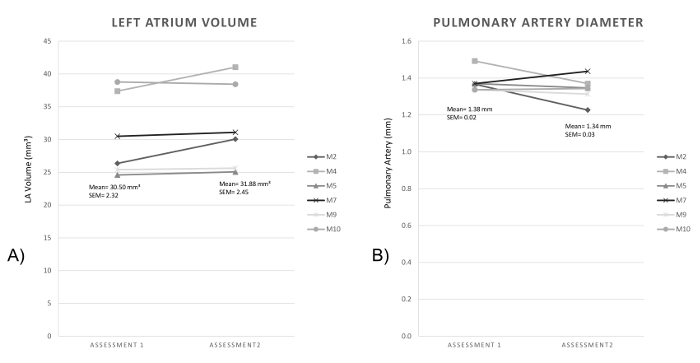 Figure 10: Left Atrium Volume and Pulmonary Artery Diameter on two subsequent assessments. (A) shows the Left Atrium Volume in mm³ of six 19 months old mice on 2 subsequent assessments. (B) shows the Pulmonary Artery diameter in mm of the same 19 months old mice group. Please click here to view a larger version of this figure.
Figure 10: Left Atrium Volume and Pulmonary Artery Diameter on two subsequent assessments. (A) shows the Left Atrium Volume in mm³ of six 19 months old mice on 2 subsequent assessments. (B) shows the Pulmonary Artery diameter in mm of the same 19 months old mice group. Please click here to view a larger version of this figure.
As a second example, the authors present two images of the same 21 months old mouse acquired on the same day by two different researchers. The first two panels correspond to the images acquired by Researcher #1 and the second two panels correspond to the images acquired by Researcher #2. Please note that the difference in "Time Gain Compensation" does not affect the measurements. This example demonstrate the great importance of selecting the right point in QRS while measuring the cavity size, as previously described.
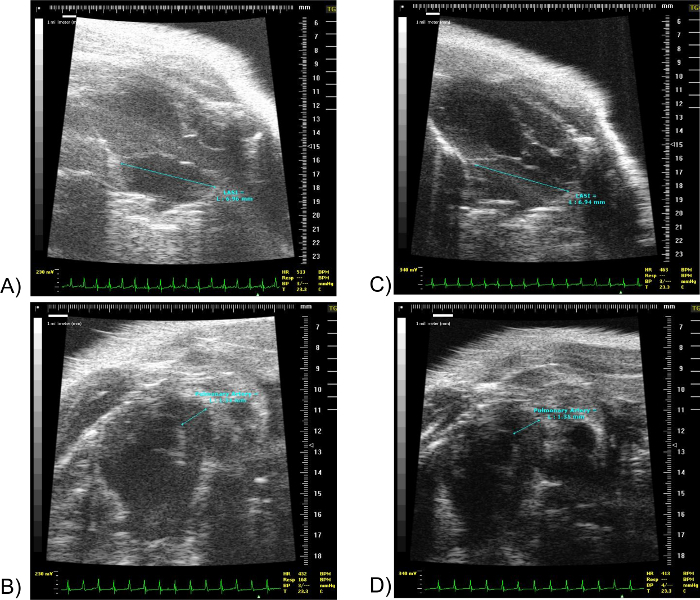 Figure 11: LA Superior-Inferior and PA diameter of the same 19 months old mouse acquired by two different researchers. (A) shows the Left Atrium Superior-Inferior diameter. (B) shows the PA diameter of the same mouse. Both images were acquired by the same researcher on the same day. (C) shows the Left Atrium Superior-Inferior diameter. (D) shows the PA diameter of the same mouse. Both images were acquired by a second researcher on the same day. Scale units in millimeters. The marker "Δ" denotes the point in the EKG at which the image shown was obtained. LA SI: Left Atrium Superior-Inferior. Please click here to view a larger version of this figure.
Figure 11: LA Superior-Inferior and PA diameter of the same 19 months old mouse acquired by two different researchers. (A) shows the Left Atrium Superior-Inferior diameter. (B) shows the PA diameter of the same mouse. Both images were acquired by the same researcher on the same day. (C) shows the Left Atrium Superior-Inferior diameter. (D) shows the PA diameter of the same mouse. Both images were acquired by a second researcher on the same day. Scale units in millimeters. The marker "Δ" denotes the point in the EKG at which the image shown was obtained. LA SI: Left Atrium Superior-Inferior. Please click here to view a larger version of this figure.
Discussion
There are three critical steps to successfully measure the LAV, Aorta and PA diameters. During the Set-up it is important to completely remove the fur on the chest; failure to do so will result in interference with image quality. During the Image Acquisition steps it is important to obtain each image with their corresponding probe and filter as the frames per second vary from probe to probe: that is 25 MHz and Cardiac Filter for the assessment of the LAV and 30 MHz and Abdominal Filter for assessment of the Aorta and PA diameters. The researcher may be limited sometimes by poor image acquisition if the mouse has a great amount of fat or if a rib makes it difficult to get a good window. During the Image Analysis steps it is important to measure the cavities at their maximum capacities as described in the Representative Results section. Use the EKG as guidance.
Echocardiography is known to be operator dependent. This technique requires familiarization with the cardiac anatomy and echocardiographic windows for best image acquisition as well as to reduce the time of the study. Familiarization becomes relevant to prevent complications derived from the prolonged use of isoflurane such as slowing of the heart rate and hemodynamic compromise. To prevent this, close monitoring of the animal must be ensured by maintaining the heart rate above 400 beats/min throughout the study.
This technique offers many advantages compared to other available techniques to evaluate cardiac function in mice. Compared to invasive catheterization it is a non-invasive, non-terminal technique that allows serial measurements; compared to MRI, it is cheaper and faster8. This new technique for measuring LAV and PA diameter through 2D echocardiography opens doors to monitor interventions and further research in cardiopulmonary sciences.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the Huffington Center on Aging at Baylor College of Medicine, Geriatrics, CVS DeBakey Heart Center at Houston Methodist Hospital and BCM, NIH RO-1 HL 13870 to ML Entman, as well as a Career Development Award # IK2BX002410 from the United States Department of Veterans Affairs (LMP), Biomedical Laboratory Research and Development Program.
We are immensely grateful to Mark Entman M.D. for sharing his wisdom and supporting this work.
References
- Upadhya B, Taffet GE, Ping C, Kitzman DW. Heart failure with preserved ejection fraction in the elderly: scope of the problem. Journal of Molecular and Cellular Cardiology. 2015;83:73–87. doi: 10.1016/j.yjmcc.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclaughlin VV, Archer SL, et al. ACCF / AHA Expert Consensus Document Expert Consensus Document ACCF / AHA 2009 Expert Consensus Document on Pulmonary Hypertension A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association. Circulation. 2009. pp. 2250–2294. [DOI] [PubMed]
- Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, Entman ML. Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. Journal of Molecular and Cellular Cardiology. 2011;50(1):248–256. doi: 10.1016/j.yjmcc.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate CA, Taffet GE, Hudson EK, Blaylock SL, McBride RP, Michael LH. Enhanced calcium uptake of cardiac sarcoplasmic reticulum in exercise-trained old rats. Am J Physiol Heart Circ Physiol. 1990;258(2):H431–H435. doi: 10.1152/ajpheart.1990.258.2.H431. [DOI] [PubMed] [Google Scholar]
- Kitzman DW, Daniel KR. Diastolic Heart Failure in the Elderly. Heart Failure Clinics. 2007;3(4):437–453. doi: 10.1016/j.hfc.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Sanderson JE, Rusconi C, et al. How to diagnose diastolic heart failure a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007. pp. 2539–2550. [DOI] [PubMed]
- Medrano G, Hermosillo-Rodriguez J, et al. Left Atrial Volume and Pulmonary Artery Diameter Are Noninvasive Measures of Age-Related Diastolic Dysfunction in Mice. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2015. pp. 1–10. [DOI] [PMC free article] [PubMed]
- Franco F, Thomas GD, et al. Magnetic Resonance Imaging and Invasive Evaluation of Development of Heart Failure in Transgenic Mice With Myocardial Expression of Tumor Necrosis Factor- Circulation. 1999;99:448–454. doi: 10.1161/01.cir.99.3.448. [DOI] [PubMed] [Google Scholar]
- Jenkins C, Bricknell K, Marwick TH. Use of real-time three-dimensional echocardiography to measure left atrial volume: Comparison with other echocardiographic techniques. Journal of the American Society of Echocardiography. 2005;18(9):991–997. doi: 10.1016/j.echo.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Ujino K, Barnes ME, et al. Two-dimensional echocardiographic methods for assessment of left atrial volume. The American journal of cardiology. 2006;98(9):1185–1188. doi: 10.1016/j.amjcard.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Scalia GM, Scalia IG, et al. ePLAR - The echocardiographic Pulmonary to Left Atrial Ratio - A novel non-invasive parameter to differentiate pre-capillary and post-capillary pulmonary hypertension. International Journal of Cardiology. 2016;212:379–386. doi: 10.1016/j.ijcard.2016.03.035. [DOI] [PubMed] [Google Scholar]


