Abstract
Background
The presence of spinal cord (SC) magnetic resonance imaging (MRI) lesions can be expected to affect the mobility of people with relapsing–remitting MS (pwRRMS), but reports are ambiguous.
Objective
The objective of this paper is to determine whether the presence of SC MRI lesions in early diagnosed pwRRMS could be considered a predictor of long-term disability.
Methods
pwRRMS with an SC MRI performed within two years from the onset of symptoms and followed up for at least seven years were included. Patients were grouped into: (a) pwRRMs with at least one SC T2 MRI lesion, and (b) pwRRMs without SC T2 MRI lesions. The primary end point was to evaluate the effects of independent factors on reaching an Expanded Disability Status Score (EDSS) of 4.0.
Results
A total of 239 pwRRMS matched the required criteria: 116 in the group with SC lesions and 123 in the group without SC lesions. At baseline, there were no statistical differences between the two groups. The presence of SC lesions (Exp(b) 4.4, CI 2.1–9.0, p < 0.001) and higher basal EDSS (Exp(b) 3.3, CI 2.3–4.8, p < 0.001) proved to be the best predictors of reaching EDSS 4.0.
Conclusion
The presence of T2 SC MRI lesions detected early from MS onset of RRMS predicts a worse prognosis.
Keywords: Multiple sclerosis, all spinal cord, MRI
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) characterized by demyelinating lesions. These lesions can be detected by magnetic resonance imaging (MRI) T2-weighted images, and they are localized either in the brain and the spinal cord (SC).1 Focal abnormalities in the SC are present in most people with MS (pwMS), affecting the cervical region more frequently than the thoracic and lumbar regions.2 The importance of SC lesions involving clinically eloquent pathways (pyramidal tracts, spinothalamic tracts and posterior columns) lies in their presumed greater potential to result in clinical symptoms in MS than brain lesions.3 Several clinical, demographic, and additional features have been described with the risk of long-term disability;4 however, there has long been debate as to whether performing an SC magnetic resonance imaging (MRI) scan in the early phase of the spectrum of demyelinating diseases, including clinically isolated syndrome (CIS) and relapsing–remitting MS (RRMS), is a useful diagnostic tool.5–7 An increasing demand for MRI scanning in pwMS is a matter of question in clinical practice, and the new recommendations from an experts’ committee on the assessment of the course of MS recommend annual brain MRI scans for all patients with RRMS.8 The question whether there are specific indications for SC imaging in CIS and early diagnosed RRMS is still open; however, these recommendations come with a significant cost to health services.6
In the current study, we studied a large cohort of people with RRMS (pwRRMS) to determine whether the presence of lesions in SC T2 MRI at the diagnosis of pwRRMS could predict a worse prognosis in terms of disability.
Methods
A monocenter, retrospective review of prospectively collected data was performed.
All pwMS admitted to an MS center were prospectively entered into a computerized database since January 1995. Clinical and MRI data were collected in-house in Excel files until 2000, and then iMed© software (iMed, Merck Serono SA; Geneva) was released and used for the collection of pwRRMS data. Clinic visits usually occurred every six months, and unscheduled visits occurred if a pwRRMS had an exacerbation. iMed© software was installed in our clinic in 2001. In order to store patient demographic and disease-related information regarding the previous period 1995–2000, a senior trained neurologist (F.P.) entered into the iMed software those data that were previously collected in the medical records sheets. Afterwards S.L.F., a trained neurologist, double-checked whether the information reported was correct.
Inclusion criteria were: i) clinically definite RRMS according to Poser and McDonald 2001 criteria;9,10 ii) disease onset between January 1995 and December 2007 (to enable a disease duration of more than seven years at the last follow-up visit); and iii) an SC MRI scan performed within two years from the disease onset. Other possible diagnoses involving SC (as neuromyelitis optica or other causes of myelitis) were excluded.
The RR course was defined as a history of relapses and remissions without progressive deterioration at the beginning of the disease.11
At the baseline assessment, the following demographic and clinical characteristics were systematically collected: sex, age at disease onset and age at the last follow-up, disease duration, time to diagnosis, number of relapses in the first two and seven years from the onset, first disease-modifying treatment, switch to another therapy, and disability level. Disability was assessed by the Expanded Disability Status Scale (EDSS)12 in scheduled visits every six months. EDSS was assessed far (at least 30 days) from any relapse event, so as to consider a stable disability level. Secondary progression was defined as the progressive disability with or without superimposed relapses for at least six months.
MRI techniques
Brain and SC MRI sequences were acquired with a 1.0 and 1.5 Tesla system. Scanning sessions included T1-weighted, T2-weighted images and a fluid-attenuated inversion recovery (FLAIR) sequence. Moreover, the T1-weighted images were acquired before and 10 minutes after an intravenous injection of gadolinium-diethylenetriamine-penta-acetic acid (0.1 mmol/kg) (Gd-DTPA). For SC MRI, sagittal fast spin-echo dual-echo T2-weighted and sagittal T1-weighted spin-echo images were obtained. FLAIR imaging was not acquired in the SC because it has been recognized not to be sensitive to detect SC lesions. Brain and SC MRI scans were performed every year. The number of lesions on T2-weighted MRI were recorded.
Standard protocol approvals, registrations, and patient consent
The study protocol was approved by the local ethics committee and was conducted in accordance with the ethical principles of the Declaration of Helsinki and with the appropriate national regulations. Patients provided written informed consent.
Statistical analysis
The Shapiro-Wilk test has been used to verify the normal distribution of the continuous variables. Descriptive statistics were calculated in the total MS sample and in the two groups based on the presence of spinal lesions. Descriptive characteristics and clinical measures were compared between the two groups with the non-parametric Mann-Whitney U test and chi-square test (for discrete variables).
The effect of the presence of SC impairment (at least one SC lesion on T2-sequence) on the time to reach EDSS 4.0 was studied with the Kaplan-Meier survival curves. A univariate analysis was run to assess the impact of the dichotomized SC MRI criteria (presence or not of SC T2 lesions) on the progression to EDSS 4.0. Then, to evaluate the effect of other independent variables (including the number of SC lesions) that might have influenced the risk of reaching EDSS 4.0, a multiple Cox regression analysis was run. Progression to an EDSS score of 4.0 was used as the dependent variable, while the other variables (age, sex, disease duration, basal EDSS, number of basal brain T2 lesions, number of basal SC MRI T2 lesions, the presence or not of SC T2 lesions, treatment (disease-modifying drugs, that is interferons and glatiramer acetate, or immunosuppressant therapy) were used as independent variables. In order to avoid issues in interpreting the results of multivariate analysis due to the inclusion of both the presence of SC lesions and number of SC lesions, we re-ran the same analysis another two times: once with both the above variables and once with only the variable “number of SC lesions.” The probability of the F value for variables entry into the model was set at 0.05, whereas the probability of the F value for removal of variables was set at 0.10. All statistical tests were applied with a two-tailed analysis and 0.05 as the level of significance. The statistical software SPSS version 20 was used for all analyses (IBM SPSS Statistics 20, IBM©, Armonk, NY, USA).
Results
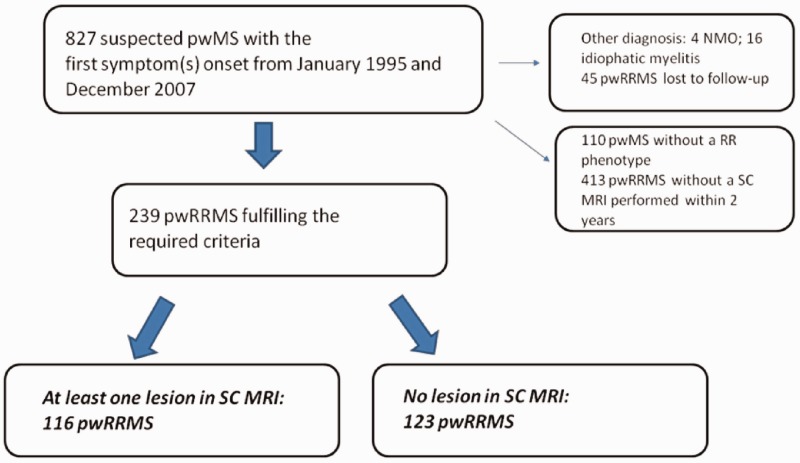
From January 1995 to December 2007, 827 individuals with suspected MS (489 women, 338 men) and 239 pwRRMS (162 women and 77 men) fulfilled the criteria to be included in this study. A total of 116 pwRRMS had at least one lesion on SC MRI (group with SC lesions), while for the other 123 pwRRMS the SC MRI did not reveal any spinal lesions (group without SC lesions) (see Figure 1). Table 1 shows the demographic, clinical and MRI characteristics for the total MS sample and for the two groups.
Figure 1.
Patients' selection flow chart.
pwMS: people with multiple sclerosis; SC: spinal cord; MRI: magnetic resonance imaging; NMO: neuromyelitis optica; pwRRMS: people with relapsing–remitting multiple sclerosis.
Table 1.
Demographic and clinical characteristics of the total study sample and subgroups based on the presence of SC lesions in T2 MRI.
| Total sample |
Subgroups based on T2 SC MRI |
|||
|---|---|---|---|---|
|
(n = 239) |
SC lesions | No SC lesions |
p value |
|
| (n = 116) | (n = 123) | |||
| Age at last follow-up (years, mean ± SD) | 41.6 ± 9.4 | 41.1 ± 9.2 | 42.0 ± 9.7 | nsa |
| Age at time of inclusion (years, mean ± SD) | 31.9 ± 10.8 | 31.5 ± 10.6 | 32.3 ± 11.2 | nsa |
| Gender, | ||||
| F/M (n) | 162/77 | 78/38 | 84/39 | nsb |
| Disease duration (months, mean ± SD) | 104.2 ± 28.7 | 99.3 ± 31.4 | 108.8 ± 25.1 | 0.026a |
| Lag time diagnosis (months, mean ± SD) | 24 ± 19.5 | 25.5 ± 21.1 | 22.5 ± 17.8 | nsa |
| Lag time therapy (months, mean ± SD) | 26.5 ± 20.9 | 27.6 ± 22.2 | 25.5 ± 19.7 | nsa |
| Initial symptom | ||||
| Spinal | 84 | 72 | 12 | <0.001b |
| Other symptoms | 155 | 44 | 111 | <0.001b |
| Number of relapses in the first two years (mean ± SD) | 2.9 ± 1.4 | 2.7 ± 1.3 | 2.9 ± 1.5 | ns |
| Number of relapses in the first seven years (mean ± SD) | 5.4 ± 2.5 | 4.3 ± 2.1 | 5.7 ± 2.8 | ns |
| Basal EDSS score (mean ± SD) | 1.5 ± 0.9 | 1.7 ± 0.9 | 1.4 ± 0.8 | <0.05b |
| Reach EDSS score of 4.0, n (%) | 51 (21.3) | 41 (35.3) | 10 (8.1) | <0.001b |
| Reach switching therapy, n (%) | 44 (18.4) | 34 (29.3) | 10 (8.1) | <0.001b |
| Number of brain T2 lesions, (mean ± SD) | 16.9 ± 14.7 | 20.8 ± 18.0 | 13.3 ± 9.7 | ns |
| Number of spine T2 lesions, (mean ± SD) | 1.0 ± 0.9 | 1.5 ± 0.8 | / | / |
: t test; b: chi-square Pearson test; SC: spinal cord; MRI: magnetic resonance imaging; EDSS: Expanded Disability Status Scale; F: female; M: male; SD: standard deviation.
There were no differences between the groups in gender distribution of age either at the beginning of the disease or at last follow-up. Time to diagnosis (lag-time onset-diagnosis), time to start the first therapy, number of lesions on T2 brain MRI scan, number of relapses one year before enrollment and disease duration were similar in both groups. The initial symptoms were SC syndrome in 84 patients (35.1%), polyregional syndrome in 70 patients (29.3%), optic neuritis in 47 patients (19.7%) and brainstem syndrome in 38 patients (15.9%). Out of 116 in the group with SC lesions, 34 (29.3%) switched from a first-line therapy to a second-line therapy, while out of 123 in the group without SC lesions, 10 (8.1%) switched to a second-line therapy (p < 0.001). Significant differences were found between the two groups for disease duration, with longer duration in the group without SC lesions; and baseline EDSS, with higher scores for the group with SC lesions. Forty-one pwRRMS with SC lesions (35.3%) reached EDSS > 4.0; only 10 (8.1%) pwRRMS without SC lesions reached an EDSS of 4.0 (χ2 25.6, p < 0.001). One pwRRMS in the group with SC lesions and no pwRRMS in the group without SC lesions had an EDSS value of 4.0 at baseline evaluation. The mean number of SC lesions in pwRRMS in the group with SC lesions was 1.5. At seven-year follow-up the mean value was 2.1. In the pwRRMS without SC lesions 41 pwRRMS experienced SC lesions during the follow-up period.
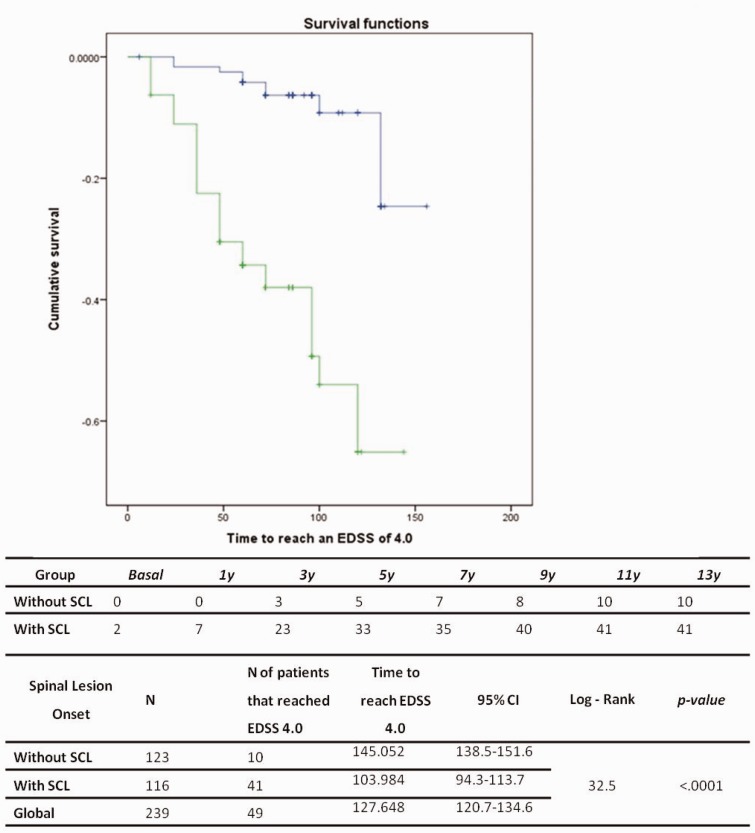
The Kaplan-Meier survival analysis showed that pwRRMS with at least one lesion on SC MRI reached a score of 4.0 in the EDSS sooner (mean time 145 months in the group without SC lesions versus 103.9 months in the group with SC lesions, log rank 32.5, p < 0.001) (Figure 2).
Figure 2.
Kaplan-Meier suvival analysis of the time to reach EDSS 4.0 according the presence of spinal cord MRI lesion. EDSS: Expanded Disability Status Scale; SCL: spinal cord lesion; CI: confidence interval.
In order to first evaluate the relationship between the presence of SC T2 lesions and the hazard of reaching EDSS 4.0, we ran a Cox univariate regression analysis. The presence of SC lesions showed an Exp(b) value of 6.0, confidence interval (CI) 2.9–12.2, p < 0.001. Next, the multiple Cox-regression analysis was run. The assumption of proportional hazard was assessed by log-minus-log plots that yield parallel survival curves. The independent covariates that were retained in the model were the base EDSS and the presence of SC lesions with an Exp(b) value of 3.3, CI 2.3–4.8, p < 0.001 for basal EDSS, and an Exp(b) value of 4.4, CI 2.1–9.0, p < 0.001 for the presence of SC lesions. The same analysis was run without including the number of SC lesions (only the presence of SC lesion variable). We found that the basal EDSS and the presence of SC lesions were retained in the model with an Exp(b) value of 3.3, CI 2.3–4.8, p < 0.001 for the basal EDSS and Exp(b) 4.4, CI 2.1–9.0, p < 0.05 for the presence of SC lesions. Finally, the same analysis was run including only the variable “number of SC lesions.” We found that the basal EDSS and the number of SC lesions were retained in the model with the following Exp(b): 1.4, CI 1.1–1.8, p < 0.05 for the number of SC lesions, and 3.6, CI 2.5–5.2 for the basal EDSS.
Discussion
The aim of this study was to investigate retrospectively whether the presence of SC MRI lesions at the beginning from MS onset influenced the disability accrual in long-term follow-up. We found that the presence of SC lesions detected early at the time of diagnosis (within two years from disease onset) and EDSS level at our baseline assessment were significantly correlated to higher level of disability at long-term follow-up.
Abnormal SC MRIs were found in up to 83% of patients with early diagnosis of MS; focal SC lesion was the most common pattern found.13–16 In a small population, Cohen et al.17 found that upper cervical SC atrophy most strongly correlates with physical disability in MS.
It has long been debated whether there are specific indications for performing SC MRI in pwRRMS with a CIS and/or at the early stages of RRMS; but there has been no consensus so far.
Barkhof 7 argued that because SC imaging may be of value in satisfying the McDonald criteria and because it may have some prognostic value in relation to disability accrual, it is better to perform a routine SC MRI along with the brain sequences at the time of the first imaging procedure.
However, MRI scanning is expensive, and given the economic issues that many radiological departments are facing, placing significant demands on the MRI services could be problematic.6 Moreover, since spinal lesions are believed to be mostly symptomatic,13 MRIs of the SC are not routinely performed or recommended on a regular basis in the absence of spinal symptoms.18 However, asymptomatic spinal lesions do occur13,15,19 and are often independent of brain lesions.13 A recent study enrolling 103 pwRRMS showed that asymptomatic spinal lesions occur in one-quarter of clinically stable patients with RRMS, even if their presence did not increase the risk of disability progression.20 Sombekke et al.21 observed that CIS patients with asymptomatic spinal lesions have a significantly greater probability of converting to clinically defined MS as compared to those patients without asymptomatic spinal lesions.
Hence, we believe that the information provided by an early SC MRI scan can help the physician to better address the best management of pwRRMS, and early biomarkers of prognosis and therapy responsiveness are strongly warranted in order to delay or halt progression of the disease.22
The landscape of treatment available for RRMS has dramatically changed in recent years with the introduction of a plethora of new therapeutic agents.23 Many of the drugs in development are treatments adopted from oncology and rheumatology such as monoclonal antibodies, and their goal is to induce activity-free disease in pwRRMS.24 It is still a matter of challenge whether an early aggressive treatment could be the approach to pursue.25 The challenge is now to understand at early stages of the disease which drug may work better in a specific group of pwRRMS. For these reasons analyzing which are the strong predictors factors of reaching disability could help physicians to pave the way to a personalized therapy.
Our study has enrolled a large cohort of pwRRMS at early stages of the disease who were followed-up for a long observational period. Coret et al.26 found that the presence of MRI SC lesions (retrospectively detected within three years from onset) predicted a worse prognosis in a cohort of 25 pwRRMS who were followed up for 10 years.
All pwRRMS were carefully assessed for the inclusion criteria, and the follow-up allowed confirmation of RRMS diagnosis and discarding other causes of myelitis. In conclusion, we found that performing early SC MRI (within the first two years from onset) could play a central role in establishing the prognosis of RRMS. This could also be used for monitoring disease activity in clinical settings, and for application in future MS clinical trials.
Limitations of the study
This study has several limitations. The retrospective design suffers from recall bias (number of relapse, exact date of disease onset, time elapsed between onset and diagnosis, etc.), but the relatively narrow entry window from onset to database inclusion of four years has limited the bias due to recall of information. The retrospective nature of the study limited the interpretation of data in terms of a direct risk analysis. PwRRMS received different treatments, hampering us from understanding the role of a specific therapy on reaching disability milestones. We are aware that using only T2 sequences would have hindered the identification of lesions in the brain and in the SC as well; however, we have to underline that proton density and/or short tau inversion recovery (STIR) sequences were more recently adopted in our real-world setting of routine scans. We did not considered oligoclonal bands for the missing data recorded, even if it has been demonstrated that their presence can influence conversion to clinically defined MS and disability accumulation.
Acknowledgments
Author contributions are as follows: Dr D’Amico, Dr Leone and Dr Lo Fermo participated in the design, analysis, interpretation, writing of the manuscript for important intellectual content. Dr Patti participated in the analysis, interpretation, writing, critical review for important intellectual content and in the final approval of the manuscript. Dr Zappia participated in the interpretation and critical review of the manuscript, contributed important intellectual content, and gave final approval of the manuscript.
Conflicts of interest.
Dr Zappia has served on scientific advisory boards and received honoraria from UCB-Union Chimique Belge and Lundbeck, and has received scientific grants from the Italian Medicines Agency (AIFA) and Novartis.
Dr Patti has served on scientific advisory boards for Teva, Biogen-Idec, Bayer-Schering and Novartis, and has received honoraria as a speaker for Teva, Biogen, Merck Serono, Bayer-Schering, Genzyme/Sanofi and Novartis.
Dr D’Amico and Dr Leone have received funding for travel by Teva, Biogen, Merck Serono, Bayer-Schering, Genzyme/Sanofi and Novartis.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med 2000; 343: 938–952. [DOI] [PubMed] [Google Scholar]
- 2.Honig LS, Sheremata WA. Magnetic resonance imaging of spinal cord lesions in multiple sclerosis. J Neurol Neurosurg Psychiatry 1989; 52: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Riordan JI, Losseff NA, Phatouros C, et al. Asymptomatic spinal cord lesions in clinically isolated optic nerve, brain stem, and spinal cord syndrome suggestive of demyelination. J Neurol Neurosurg Psychiatry 1998; 64: 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Confavreux C, Vukusic S. Natural history of multiple sclerosis: A unifying concept. Brain 2006; 129: 606–616. [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson M. Spinal cord MRI should always be performed in clinically isolated syndrome patients: Commentary. Mult Scler 2014; 20: 1690–1691. [DOI] [PubMed] [Google Scholar]
- 6.Rovira A, Tintoré M. Spinal cord MRI should always be performed in clinically isolated syndrome patients: No. Mult Scler 2014; 20: 1686–1687. [DOI] [PubMed] [Google Scholar]
- 7.Barkhof F. Spinal cord MRI should always be performed in clinically isolated syndrome patients: Yes. Mult Scler 2014; 20: 1688–1689. [DOI] [PubMed] [Google Scholar]
- 8.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis guidelines for research protocols. Ann Neurol 1983; 13: 227–231. [DOI] [PubMed] [Google Scholar]
- 10.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50: 121–127. [DOI] [PubMed] [Google Scholar]
- 11.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996; 46: 907–911. [DOI] [PubMed] [Google Scholar]
- 12.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 13.Bot JC, Barkhof F, Polman CH, et al. Spinal cord abnormalities in recently diagnosed MS patients: Added value of spinal MRI examination. Neurology 2004; 62: 226–233. [DOI] [PubMed] [Google Scholar]
- 14.Hittmair K, Mallek R, Prayer D, et al. Spinal cord lesions in patients with multiple sclerosis: Comparison of MR pulse sequences. AJNR Am J Neuroradiol 1996; 17: 1555–1565. [PMC free article] [PubMed] [Google Scholar]
- 15.Lycklama à Nijeholt GJ, Castelijns JA, Weerts J, et al. Sagittal MR of multiple sclerosis in the spinal cord: Fast versus conventional spin-echo imaging. AJNR Am J Neuroradiol 1998; 19: 355–360. [PMC free article] [PubMed] [Google Scholar]
- 16.Lycklama à Nijeholt GJ, Uitdehaag BM, Bergers E, et al. Spinal cord magnetic resonance imaging in suspected multiple sclerosis. Eur Radiol 2000; 10: 368–376. [DOI] [PubMed] [Google Scholar]
- 17.Cohen AB, Neema M, Arora A, et al. The relationships among MRI-defined spinal cord involvement, brain involvement, and disability in multiple sclerosis. J Neuroimaging 2012; 22: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filippi M, Rocca MA, De Stefano N, et al. Magnetic resonance techniques in multiple sclerosis: The present and the future. Arch Neurol 2011; 68: 1514–1520. [DOI] [PubMed] [Google Scholar]
- 19.Okuda DT, Mowry EM, Cree BA, et al. Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology 2011; 76: 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zecca C, Disanto G, Sormani MP, et al. Relevance of asymptomatic spinal MRI lesions in patients with multiple sclerosis. Mult Scler. Epub ahead of print 12 October 2015. [DOI] [PubMed]
- 21.Sombekke MH, Wattjes MP, Balk LJ, et al. Spinal cord lesions in patients with clinically isolated syndrome: A powerful tool in diagnosis and prognosis. Neurology 2013; 80: 69–75. [DOI] [PubMed] [Google Scholar]
- 22.Katsavos S, Anagnostouli M. Biomarkers in multiple sclerosis: An up-to-date overview. Mult Scler Int 2013; 2013: 340508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartung HP, Kieseier BC. The new therapeutic landscape in multiple sclerosis: Exciting times and new perspectives. Curr Opin Neurol 2014; 27: 243–245. [DOI] [PubMed] [Google Scholar]
- 24.Bevan CJ, Cree BA. Disease activity free status: A new end point for a new era in multiple sclerosis clinical research? JAMA Neurol 2014; 71: 269–270. [DOI] [PubMed] [Google Scholar]
- 25.Edan G, Le Page E. Induction therapy for patients with multiple sclerosis: Why? When? How? CNS Drugs 2013; 27: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coret F, Bosca I, Landete L, et al. Early diffuse demyelinating lesion in the cervical spinal cord predicts a worse prognosis in relapsing–remitting multiple sclerosis. Mult Scler 2010; 16: 935–941. [DOI] [PubMed] [Google Scholar]