Abstract
Background
The aim of the current study was to explore the anti-arthritic effect of pinitol via assessing its effect on various inflammatory mediators and its possible mechanism of action.
Material/Method
We assessed the anti-arthritic effect of pinitol in a formaldehyde- and CFA-induced arthritic model in Wistar Swiss albino strain rats divided into 6 groups. The rats received different doses of pinitol and indomethacin for 28 days. The arthritic index and body weight were determined at regular intervals, together with hepatic, hematological, and antioxidant parameters. The expression of proinflammatory cytokines (e.g., IL-6, TNF-α, and IL-1β) and inflammatory mediators (e.g., COX-2 and VEGF) were also estimated with histopathological evaluation of the joint tissue of rats. A docking study of pinitol with PTPN22 was also carried out.
Results
The CFA-induced model rats developed redness and nodules in the tail and front paws, and the arthritic control (AC) group rats showed similar symptoms, which were decreased by pinitol administration. The body weight of AC group rats was decreased, while pinitol-treated rats showed considerably increased body weight. Hematological, hepatic, and antioxidant parameters were altered by pinitol in a dose-dependent manner. Pinitol significantly decreased the elevated concentration of proinflammatory cytokines and inflammatory mediators, with improvement in histopathological condition. The docking study suggested that pinitol efficiently interacted with PTPN22 via Arg59, Tyr60, Leu106, and Lys138 by creating close interatomic hydrogen bonds and hydrophobic contacts.
Conclusions
Pinitol showed anti-arthritic effects via reduction of proinflammatory cytokines and inflammatory mediators via inhibition of PTPN22.
MeSH Keywords: Anti-Inflammatory Agents, Molecular Docking Simulation, Plant Development
Background
Rheumatoid arthritis (RA) is a systematic, chronic, immune-derived inflammatory disease, which affects about 1% of young people in developed countries. It damages the peripheral joint, ligaments, and tendons, which leads to joint deformity and destruction, as well as physical disability [1]. The pathological conditions include damage of gristle, joint swelling, and destruction of cartilage and subchondral bone. RA conditions worsen with increased sedentary lifestyle with less physical activity. Other reasons for the increased prevalence of arthritis are poor diet, lifestyles changes, chemical toxicity, and pollution [2]. Various other factors played a critical role in the pathophysiology of developing the painful and crippling syndrome of arthritis [3].
Proinflammatory cytokines such as IL-6, IL-1β, and TNF-α play a significant role in the pathophysiology of RA, and these proinflammatory cytokines have been targeted for the development of new RA drugs [4]. Proinflammatory cytokines induce inflammation via several mechanisms, which further induce the angiogenesis, leucocyte linkage, and tissue degradation in RA disease. Another mechanism for development of RA is generation of free radicals (FR), especially reactive oxygen species (ROS)/reactive nitrogen species (RNS), which induce cartilage breakdown via either degradative effect on matrix or activation of MMPs [5].
Non-steroidal anti-inflammatory drugs (NSAIDs) are the drugs of first choice, but NSAIDs having various serious adverse effects (e.g., ulceration, reversal of platelet activation, gastric erosion, gastrointestinal hemorrhage, and liver toxicity), which further increase the risk of hemorrhage. Various clinical studies have evaluated several drugs as anti-arthritic agents but at present there is still no highly effective anti-arthritis drug with less adverse effects. Piascledine (a mixture of soybean oil and avocado) is an herbal formulation used as the anti-arthritis drug to relieve inflammatory arthritic symptoms [6,7]. Various other small molecules have also been examined to discover new molecules for use in RA therapy.
The utilization of indigenous plants as medicine has a long history and may be advantageous for use in anti-inflammatory and antioxidant therapy. We searched for a novel plant-based drug endowed with anti-inflammatory and antioxidant action with fewer adverse effects for use in RA therapy [8]. Thus, in the current study, we evaluated the protective effect of pinitol against chemical-induced acute and chronic inflammation.
Material and Methods
Animals
Wistar Swiss albino rats (130–180 g; male; age 4–5 months) were utilized in this experimental study. The rats were obtained from the institutional animal house and were kept in individual polypropylene cages in the standard experimental condition (temperature 25±2°C; relative humidity 60–80% and 12/12 h dark/light cycle). The rats had ad libitum access to a standard pellet diet and water. The whole animal study was approved from the Institutional Animal Ethics Committee.
Experimental study
Formaldehyde-induced arthritis
Formaldehyde was used for the induction of acute arthritis. Rats were divided into 6 groups with 6 animals in each group:
Group I: Normal control (NC)
Group II: NC+pinitol (20 mg/kg)
Group III: Arthritic control (AC)
Group IV: AC+ pinitol (5 mg/kg)
Group V: AC+ pinitol (10 mg/kg)
Group VI: AC+ pinitol (20 mg/kg)
Group VII: AC+ indomethacin (10 mg/kg).
We used a screw-gauge micrometer for determination of baseline ankle diameter.
We injected 0.1 ml of formaldehyde (2% v/v) into the left hind paw of all rats except for those in the NC and NC+pinitol (20 mg/kg) groups on day 1 and 3 [9]. Inflammation was estimated by measuring the joint diameter at different time intervals using the screw-gauge micrometer [10].
Complete Freund’s adjuvant (CFA) induced chronic inflammation (arthritis)
The animals were divided into 6 groups and each group contains 6 rats:
Group I: Normal control (NC)
Group II: NC+pinitol (20 mg/kg)
Group III: Arthritic control (AC)
Group IV: AC+ pinitol (5 mg/kg)
Group V: AC+ pinitol (10 mg/kg)
Group VI: AC+ pinitol (20 mg/kg)
Group VII: AC+ indomethacin (10 mg/kg).
CFA containing Mycobacterium tuberculosis (10 mg per 1 mL sterile paraffin oil) was used for the induction of arthritis on day 0. All groups received the pre-determined treatment 1 day before the induction of CFA and continuing for 4 weeks (28 days) [11]. Inflammation was estimated by measuring joint diameter at different time points using the screw-gauge micrometer [12].
Biochemical parameters
For the estimation of biochemical parameters, tissue was removed from excised angle joints of rats in all groups, stored at −80°C, and weighed. A tissue homogenate (10%) was prepared using phosphate buffer (0.1 M, pH=7.4, ice-cold) and further used for the assessment of endogenous glutathione and malonyldialdehyde concentration. Another part of the homogenate was centrifuged at 10 000 rpm and the resulting supernatant was used for the estimation of protein concentration and nitrite level (NO) in the serum.
Biochemical parameters
Biochemical parameters – alkaline phosphatase (ALP), aspartate transaminase (AST) and alanine transaminase (ALT) – were determined using a previously published method [13±–15], and blood profile – white blood cell (WBC), erythrocyte sedimentation rate (ESR), hemoglobin (Hb), and red blood cells (RBC) – was assessed.
Antioxidant parameters
On day 28, rats in all groups were killed with excess of diethyl ether and the cartilage was isolated from the rats for the estimation the antioxidant parameters – glutathione (GSH) malondialdehyde (MDA), glutathione peroxidase (GPx), and superoxide dismutase (SOD) – using a previously published method with minor modifications [13–15].
Arthritic index in adjuvant induced rats
A visual scoring system was used to estimate the degree of arthritis in the rats, with the following categories: score 0, no change; score 1, enlargement and erythema; and score 2, coarse inflammation and erythema of the limb, inability to move the limb, and coarse abnormality. The maximum possible score was recorded 16, and scoring was performed at regular intervals [2, 11].
Inflammatory mediators
We assessed proinflammatory cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α), and inflammatory mediator NF-kB using the instructions provided by the manufacturer.
Docking interaction
Docking calculations were carried out using DockingServer (Bikadi, Hazai, 2009). Gasteiger partial charges were added to the ligand atoms. Non-polar hydrogen atoms were merged, and rotatable bonds were defined. Docking calculations were carried out on the crystal structure of human tyrosine phosphatase PTPN22 protein model using pinitol as the ligand. Essential hydrogen atoms, Kollmann united atom type charges, and solvation parameters were added with the aid of AutoDock tools (Morris, Goodsell et al., 1998). Affinity (grid) maps of 60×60×60 Å grid points and 0.375 Å spacing were generated using the Autogrid program (Morris, Goodsell et al., 1998). AutoDock parameter set- and distance-dependent dielectric functions were used in the calculation of the van der Waals and the electrostatic terms, respectively. Docking simulations were performed using the Lamarckian genetic algorithm (LGA) and the Solis and Wets local search method (Solis and Wets, 1981). Initial position, orientation, and torsions of the ligand molecules were set randomly. All rotatable torsions were released during docking. Each docking experiment was derived from 10 different runs that were set to terminate after a maximum of 250 000 energy evaluations. The population size was set to 150. During the search, a translational step of 0.2 Å, and quaternion and torsion steps of 5 were applied.
Statistical analysis
Analysis of variance (ANOVA) was used for the determination of data and all data are presented as mean ±SEM via using the Graph Pad Prism Version 7.0. P<0.05, P<0.01, and P<0.001 were considered as significant.
Results
Effect of pinitol on formaldehyde-induced inflammation
The acute anti-arthritic effect of pinitol was compared with formaldehyde-induced acute inflammation. The assessment made on the 10th days and at regular interval for evaluating the thickness of the paw of rats in all groups. Formaldehyde-induced inflammation group rats showed increased paw edema, which was significantly (P<0.001) decreased by pinitol in a dose-dependent manner (Figure 1).
Figure 1.
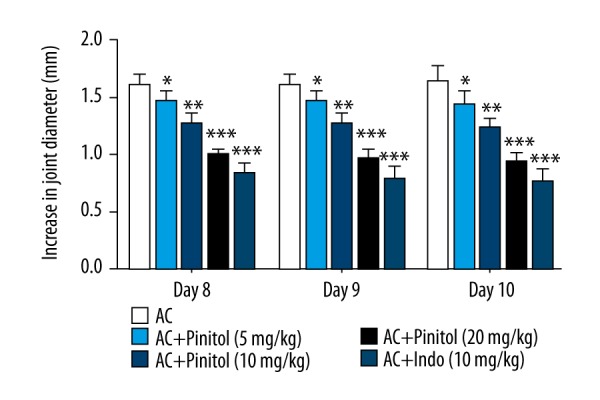
Effect of pinitol against the formaldehyde induced acute inflammation. The data are expressed in mean ±SEM (n=number of animals in each group=6). The comparisons were made by ANOVA followed by Dunnett’s test. * P<0.05 is considered as significant. ** P<0.01 is considered as very significant. *** P<0.001 is considered as extremely significant.
Effect of pinitol on CFA-induced arthritis
The rats showed swelling (redness) after the injection of CFA and this redness turned into swelling on day 7 (first stage of swelling). Subsequently, this redness persisted for 14 days, further leading to the development of swelling and redness into the front paw. The swelling reached a maximum at the third week (25 days). CFA-induced AC group rats showed similar results as the normal control rats. In contrast, rats treated with pinitol and indomethacin showed decreased redness and swelling in all paws at end of the study (Figure 2).
Figure 2.
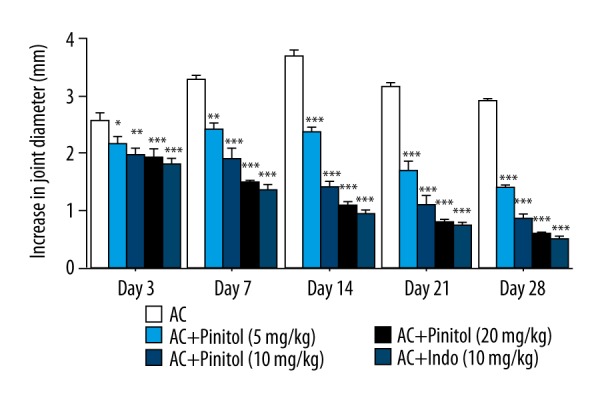
Effect of pinitol against the complete fruend’s adjuvant induced chronic inflammation. The data are expressed in mean ± SEM (n=number of animals in each group=6). The comparisons were made by ANOVA followed by Dunnett’s test. * P<0.05 is considered as significant. ** P<0.01 is considered as very significant. *** P<0.001 is considered as extremely significant.
The body weight significantly increased in all groups except for the AC control group. At the regular time intervals, all groups showed increased body weight in a dose-dependent manner. Pinitol (20 mg/kg) group rats showed the greatest increase in body weight as compared to other groups. the AC control group rats showed decreased body weight compared to their initial body weight (Figure 3).
Figure 3.
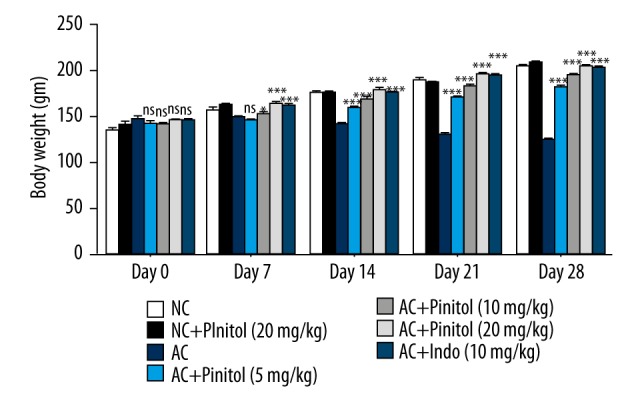
Effect of pinitol on the body weight of different group of rats in complete fruend’s adjuvant induced chronic inflammation. The data are expressed in mean ±SEM (n=number of animals in each group=6). The comparisons were made by ANOVA followed by Dunnett’s test. * P<0.05 is considered as significant. ** P<0.01 is considered as very significant. *** P<0.001 is considered as extremely significant.
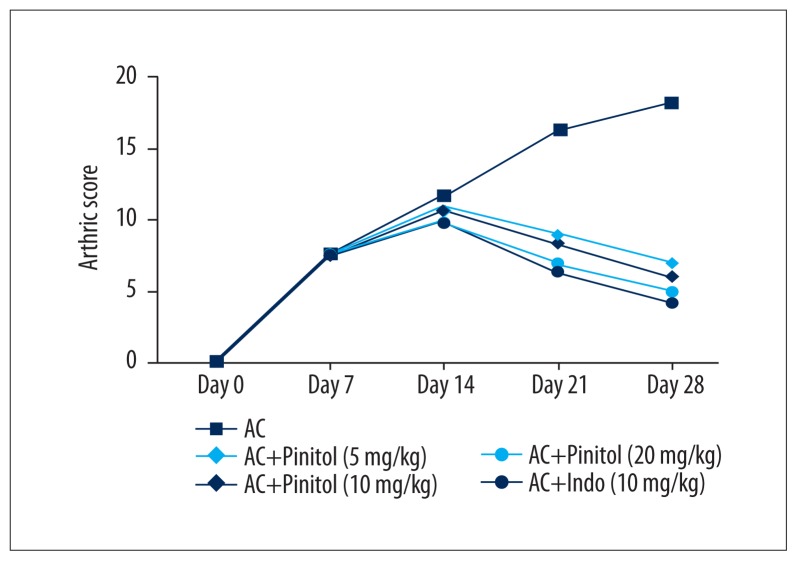
Another sign of arthritis is arthritic index, which was estimated on the basis of visual scoring. AC control group rats showed elevated arthritic score, which were increased at the end of the study. Pinitol-treated rats and indomethacin-treated rats had lower arthritic scores compared to the AC control group rats (Figure 4).
Figure 4.
Effect of pinitol on the arthritic score of complete fruend’s adjuvant induced chronic inflammation rats.
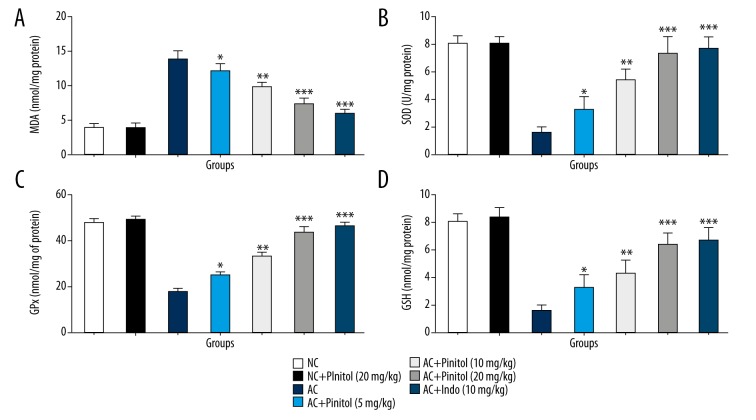
Effect of pinitol on antioxidant parameters
AC control group rats showed increased levels of MDA and reduced levels of GPx, SOD, and GSH as compared to NC rats, suggesting alteration in the level of endogenous antioxidant parameter due to increased generation of free radicals, which play a significant role in the expansion of arthritis. Pre-treatment with pinitol causes modulation of in vivo antioxidant status, which confirmed the antioxidant effect of pinitol. The same result was observed in the indomethacin group rats (Figure 5).
Figure 5.
Effect of pinitol on the antioxidants parameter of different group of rats in complete fruend’s adjuvant induced chronic inflammatory rats. The antioxidant parameters were determined in term of (A) MDA, (B) SOD, (C) GPx and (D) GSH. The data are expressed in mean ±SEM (n=number of animals in each group=6). The comparisons were made by ANOVA followed by Dunnett’s test. * P<0.05 is considered as significant. ** P<0.01 is considered as very significant. *** P<0.001 is considered as extremely significant.
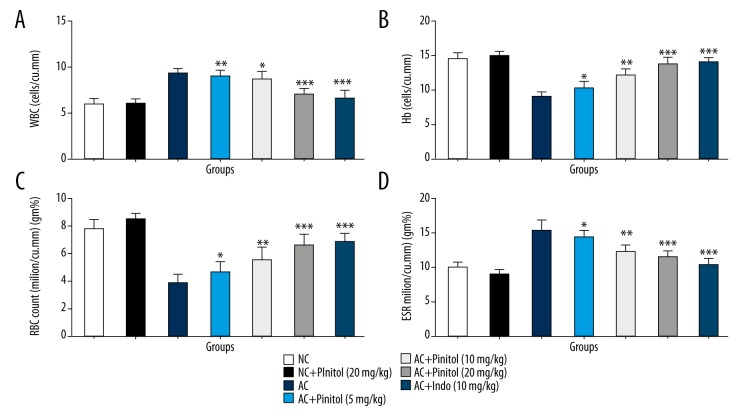
Effect of pinitol on biochemical parameters
In arthritis, the WBC and ESR levels were elevated and RBC and Hb levels were decreased due to injury. A similar pattern was observed in the AC control group rats as compared to the NC group rats, suggesting hematological abnormality. Pinitol dose-dependently altered the hematological abnormality, showing an anti-arthritic effect (Figure 6).
Figure 6.
Effect of pinitol on the haematological parameter of different group of rats in complete fruend’s adjuvant induced chronic inflammatory rats. The antioxidant parameters were determined in term of (A) WBC, (B) Hb, (C) RBC and (D) ESR. The data are expressed in mean ±SEM (n=number of animals in each group=6). The comparisons were made by ANOVA followed by Dunnett’s test. * P<0.05 is considered as significant. ** P<0.01 is considered as very significant. *** P<0.001 is considered as extremely significant.
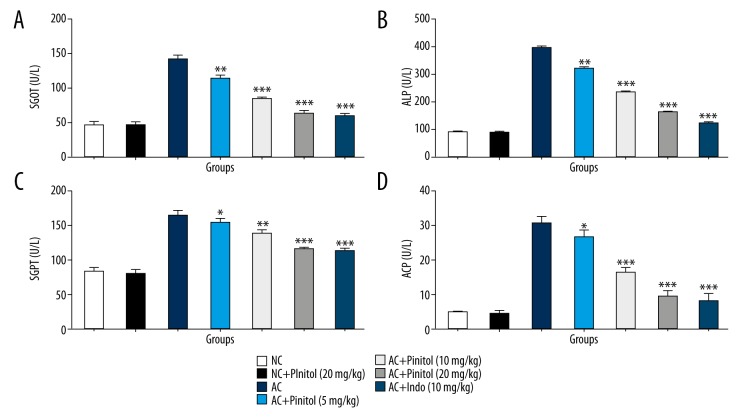
Due to arthritis, the hepatic parameters SGOT, SGPT, ALP, and ALT were altered due to the leakage of the inflammatory mediator into the serum. In the CFA-induced rat model, the AC rats showed altered levels of hepatic parameters, which was significantly (P<0.001) restored by pinitol treatment in a dose-dependent manner (Figure 7).
Figure 7.
Effect of pinitol on the hepatic parameter of different group of rats in complete fruend’s adjuvant induced chronic inflammatory rats. The antioxidant parameters were determined in term of (A) SGOT, (B) ALP, (C) SGPT and (D) ACP. The data are expressed in mean ±SEM (n=number of animals in each group=6). The comparisons were made by ANOVA followed by Dunnett’s test. * P<0.05 is considered as significant. ** P<0.01 is considered as very significant. *** P<0.001 is considered as extremely significant.
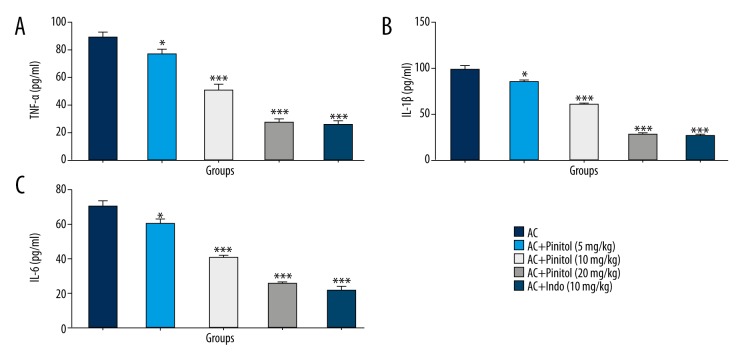
Effect of pinitol on inflammatory mediators
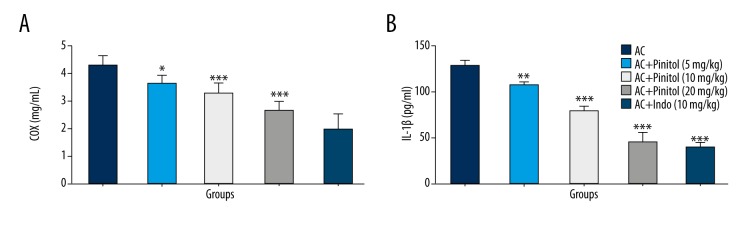
Various evidence shows that inflammation is crucial in the spread of arthritis within the body. It is a painful disease, and causes severe pain as the disease progresses. Proinflammatory cytokines play a critical role in the spread of arthritis. In arthritis, the level of proinflammatory cytokines is increased in the serum and synovial fluid. The AC control group rats showed increased level of proinflammatory cytokines, which was significantly (P<0.001) altered by pinitol treatment in a dose-dependent manner (Figure 8). The same was observed in COX-2 and VEGF levels. The levels of COX-2 and VEGF were increased in the AC group, but pinitol and indomethacin down-regulated COX-2 and VEGF levels (Figure 9).
Figure 8.
Effect of pinitol on the pro-inflammatory cytokines of different group of rats in complete fruend’s adjuvant induced chronic inflammatory rats. The antioxidant parameters were determined in term of (A) TNF-α, (B) IL-1β and (C) IL-6. The data are expressed in mean ±SEM (n=number of animals in each group=6). The comparisons were made by ANOVA followed by Dunnett’s test. * P<0.05 is considered as significant. ** P<0.01 is considered as very significant. *** P<0.001 is considered as extremely significant.
Figure 9.
Effect of pinitol on the inflammatory mediator of different group of rats in complete fruend’s adjuvant induced chronic inflammatory rats. The antioxidant parameters were determined in term of (A) COX and (B) VEGF. The data are expressed in mean ±SEM (n=number of animals in each group=6). The comparisons were made by ANOVA followed by Dunnett’s test. * P<0.05 is considered as significant. ** P<0.01 is considered as very significant. *** P<0.001 is considered as extremely significant.
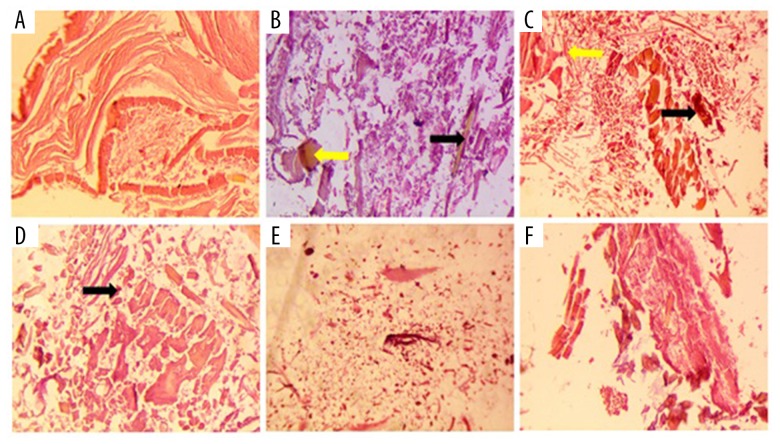
Effect of pinitol on histopathology
In the histopathological study, the NC group rats had normal articular surface tissue, whereas the AC control group rats had hyperplasia in the articular section with degradation of connective tissue. The pinitol treatment caused reduction of this defect of the tissue, showing its anti-arthritic effect (Figure 10).
Figure 10.
Histopathological effect of pinitol on adjuvant induced arthritic rats. (A) NC – no changes; (B) CFA control: displayed the bone erosion along with deformity, mononuclear cell infiltration and existence of leukocytes infiltration (red arrow) and synovitis (blue arrow); (C) AC+Pintiol (5 mg/kg) suggest the existence of leukocytes infiltration (black arrow); (D) AC+Pintiol (10 mg/kg) same Pinitol (5 mg/kg) was observed; (E) AC+Pinitol (20 mg/kg) and (F) AC+Indo (10 mg/kg): showed the recovered histopathological profile.
Docking study
The pinitol docking study was carried out with 3D crystal structure of human tyrosine phosphatase PTPN22. Pinitol showed excellent interaction with the PTPN22 receptor with estimated free energy of binding of −3.32 kcal/mol with estimated inhibition constant of Ki 3.6 6 mM (Table 1, Figure 11). Table 1 and Figure 12 also show the formation of numerous interactions of pinitol with amino acid residues, such as Arg59, Tyr60, Leu106, and Lys138 via hydrogen bonding and hydrophobic contacts.
Table 1.
Docking interaction and scoring of pinitol with PTPN22.
| Est. free energy of binding | Est. inhibition constant, Ki | vdW + Hbond + desolv energy | Electrostatic energy | Total intermolecular energy |
|---|---|---|---|---|
| −3.32kcal/mol | 3.66 mM | −3.78 kcal/mol | −0.17 kcal/mol | −3.94 kcal/mol |
Figure 11.
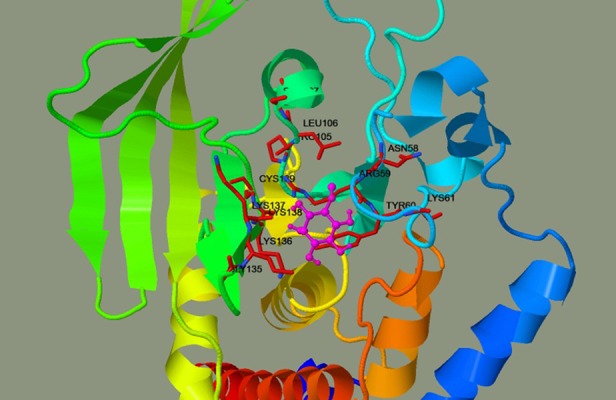
Docking interaction of pinitol with PTPN22. The pinitol was shown in magenta and interacting residue shown in maroon.
Figure 12.
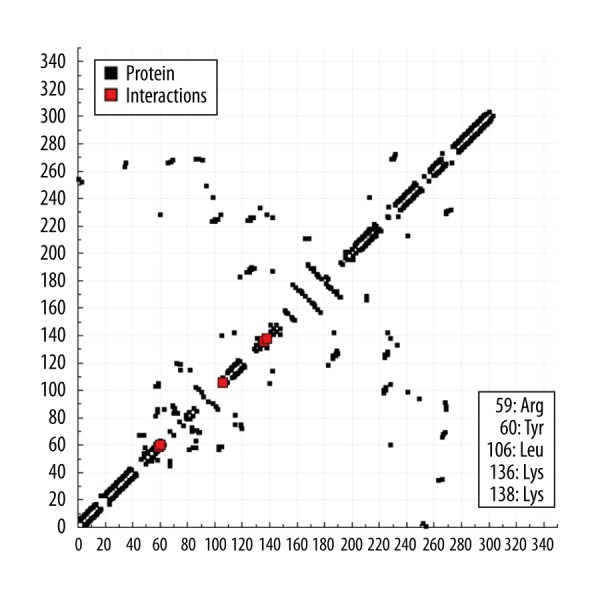
The hydrogen bond plot of pinitol with PTPN22.
Discussion
The CFA-induced arthritis model has been extensively used to evaluate and explain the action of recently developed anti-arthritis drug. Despite the established anti-inflammatory potential of pinitol, we assessed its anti-inflammatory effect against chronic induced inflammation. We used the CFA-induced model due to its immunological chronic inflammation, which has similar symptoms as human arthritis. CFA up-regulates the monocytes/macrophages and lymphocyte migration into the synovial cavity of damaged areas. It also developed swelling and redness in the injected foot during the 24-h periods with irritation [16]. During CFA-induced arthritis, it starts the release of inflammatory mediators, which play an important part in tissue demolition, fibrosis, and vascular deformity over time. Various researchers suggested that inflammatory mediators play an important role in initiation of the immune response. CFA induces the inflammation in 2 ways: firstly, it develop the swelling into the hind paw; and secondly, causes development of swelling into the front paw and expand the nodules in the tail and ears [17]. In the present study, we found that AC rats developed nodules in the tail and ears, which was significantly down-regulated by pinitol in a dose-dependent manner. Pinitol reduces humoral immune reaction due to its ability to reduce acute inflammation via decreasing vascular permeability and down-regulating other inflammatory mediators such as COX-2. Pinitol also reduces the secondary inflammation lesion, which is the consequence of delayed hypersensitivity reaction. The results also suggest that pinitol had an important effect on CFA-induced arthritis symptoms, suggesting that it might be effective in RA therapy [18].
Several researchers suggested that body weight is another indicator for estimating the potential effect of anti-arthritis drugs. AC rats showed decreased body weight at end of the experimental study, which was significantly (P<0.001) increased in the pinitol pre-treated rats. The AC rats showed decreased body weight due to altered metabolic activity and increased severity of arthritis [8,11]. These effects were dose-dependently altered by pinitol, which was further supported by the anti-arthritic effect of pinitol.
Anemia is the most common problem, which is occluded during arthritis. Anemia occurs due to changes in the bone marrow during inflammatory arthritis, together with gastrointestinal blood loss, which affects the incorporation of RBC through iron and decreases RBC content [19,20]. During arthritis, Hb and RBC levels are reduced, which is linked to anemia because of deformability of erythrocytes. WBCs are considered a significant parameter of the immune system, which is linked with the initiation of inflammation and other inflammatory-related infectious diseases. In arthritis, WBCs are elevated due to amplification of the IL-1β level. We have also observed increase in the level of ESR due to initiation of production of endogenous proteins (e.g., fibrinogen and α/β globulin), which also contributes to the progression of disease [21,22]. CFA-induced arthritic rats showed decreased levels of Hb due to destruction of premature RBCs and slow response of bone marrow erythropoietin. In arthritis, the Hb and RBC levels declined and boosted the ESR and WBC levels. A similar pattern was found in the CFA-induced arthritic control group rats. On the other hand, pre-treatment with pinitol dose-dependently altered the hematological profile, suggesting its anti-anemic and anti-arthritic effects.
RNS/ROS/FR forms in the body as a result of numerous exogenous and endogenous chemicals and reagents. These generated radicals react with the other molecules, destroying DNA, protein, cartilage, and the lipid membrane [6,23]. Endogenous antioxidants (e.g., GPx, SOD, CAT, MDA, and GSH) establish equilibrium between free and toxicant radicals. During the arthritic condition, secretion of inflammatory mediators is initiated, which initiates the production of ROS/free radicals in the inflamed area and causes alteration of the content of endogenous antioxidants. Many researchers suggest that during the CFA-induced arthritic condition, macrophages and granulocytes start the deposition of synovial fluid in the inflamed area and generate H2O2 and O2 radicals. Lipid peroxidation (LPO) is a common indicator of lipid damage, and malonaldehyde (MDA) (an indicator of LPO) is elevated during arthritis and is considered to be a significant marker of inflammatory disease. SOD and GSH are first-line antioxidant defence markers involved in the endogenous defence system [24,25]. SOD converts O2 radical to H2O2, which protects cells from injury due to the toxic effect of O2 anions. Other antioxidant enzymes such as GPx decreased the presence of GSH to form water and oxidized glutathione. Pinitol causes dose-dependent alteration of the antioxidant enzymes and has anti-arthritic effects.
Several studies suggested that proinflammatory cytokines play a significant role in the pathogenesis of chronic inflammation (arthritis). Pleiotropic proinflammatory cytokines such as TNF-α play a significant role in the expansion of inflammation (acute and chronic), linked with lymphocytes and neutrophils in endothelial cells via damaging tissue [16,26]. TNF-α, commonly present in the synovial fluid and sera of arthritic patients, was considered as a laboratory and clinical marker for the estimation of RA disease. Other proinflammatory cytokines such as IL-6 and IL1β are involved in the expansion of inflammatory reaction. All these proinflammatory cytokines were involved in the production of macrophage chemotactic protein-1 (MCP-1). IL-6 starts generating via monocytes, T cells and fibroblasts, which stimulate and initiate the induction of MCP-1 and osteoclast discrimination [18,27]. Other factors (IL-1β and TNF-α activated via nuclear factor and osteoclasts) are responsible for the obliteration and reabsorption of bones.
Conclusions
All the proinflammatory cytokines were involved in elevating the clinical signs of arthritis and development of arthritis symptoms, joint swelling, and decrease in body weight. AC rats showed up-regulated levels of proinflammatory cytokines, which was found to be significantly (P<0.001) altered by pinitol in a dose-dependent manner. Moreover, the docking study suggests human tyrosine phosphatase PTPN22 as a possible target for pinitol as an anti-arthritic agent.
Footnotes
Source of support: Departmental sources
References
- 1.Kumar V, Bhatt PC, Rahman M, et al. Melastoma malabathricum linn attenuates complete freund’s adjuvant-induced chronic inflammation in wistar rats via inflammation response. BMC Complement Altern Med. 2016;16:510. doi: 10.1186/s12906-016-1470-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Kumar V, Alabbasi FA, Verma A, et al. Umbelliferone β-D-galactopyranoside exerts an anti-inflammatory effect by attenuating COX-1 and COX-2. Toxicol Res (Camb) 2015;4:1072–84. [Google Scholar]
- 3.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: Roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;423:7–16. [PubMed] [Google Scholar]
- 4.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–42. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 5.Vasanthi P, Nalini G, Rajasekhar G. Status of oxidative stress in rheumatoid arthritis. Int J Rheum Dis. 2009;12:29–33. doi: 10.1111/j.1756-185X.2009.01375.x. [DOI] [PubMed] [Google Scholar]
- 6.Wruck CJ, Fragoulis A, Gurzynski A, et al. Role of oxidative stress in rheumatoid arthritis: Insights from the Nrf2-knockout mice. Ann Rheum Dis. 2011;70:844–50. doi: 10.1136/ard.2010.132720. [DOI] [PubMed] [Google Scholar]
- 7.Tak PP, Zvaifler NJ, Firestein GS, Greene DR. Rheumatoid arthritis and p53: How oxidative stress, might alter the course of inflammatory diseases. Immunol Today. 2000;21:78–82. doi: 10.1016/s0167-5699(99)01552-2. [DOI] [PubMed] [Google Scholar]
- 8.Kumar V, Verma A, Ahmed D, et al. Fostered antiarthritic upshot of moringa oleifera lam. Stem bark extract in diversely induced arthritis in wistar rats with plausible mechanism. International Journal of Pharmaceutical Sciences and Research. 2013;4:3894–901. [Google Scholar]
- 9.Kumar V, Anwar F, Verma, et al. Therapeutic effect of umbelliferon-α-D-glucopyranosyl-(2(I)→1(II))-α-D-glucopyranoside on adjuvant-induced arthritic rats. J Food Sci Technol. 2014;52:3402–11. doi: 10.1007/s13197-014-1403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaithwas G, Majumdar DK. Effect of L. usitatissimum (flaxseed/linseed) fixed oil against distinct phases of inflammation. ISRN Inflamm. 2013;2013:1–4. doi: 10.1155/2013/735158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar V, Al-Abbasi FA, Ahmed D, et al. Paederia foetida linn. Inhibits adjuvant induced arthritis by suppression of PGE2 and COX-2 expression via nuclear factor-κB. Food Funct. 2015;6:1652–66. doi: 10.1039/c5fo00178a. [DOI] [PubMed] [Google Scholar]
- 12.Anand R, Kaithwas G. Anti-inflammatory potential of alpha-linolenic acid mediated through selective COX Inhibition: Computational and experimental data. Inflammation. 2014;37:1297–306. doi: 10.1007/s10753-014-9857-6. [DOI] [PubMed] [Google Scholar]
- 13.Chauhan S, Devi U, Kumar VR, et al. Dual inhibition of arachidonic acid pathway by mulberry leaf extract. Inflammopharmacology. 2015;23:65–70. doi: 10.1007/s10787-014-0223-y. [DOI] [PubMed] [Google Scholar]
- 16.Alvarado-Vazquez PA, Morado-Urbina CE, Castañeda-Corral G, et al. Intra-articular administration of an antibody against CSF-1 receptor reduces pain-related behaviors and inflammation in CFA-induced knee arthritis. Neurosci Lett. 2015;584:39–44. doi: 10.1016/j.neulet.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 17.Billiau A, Matthys P. Collagen-induced arthritis and related animal models: How much of their pathogenesis is auto-immune, how much is auto-inflammatory? Cytokine Growth Factor Rev. 2011;22:339–44. doi: 10.1016/j.cytogfr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Coradini K, Friedrich RB, Fonseca FN, et al. A novel approach to arthritis treatment based on resveratrol and curcumin co-encapsulated in lipid-core nanocapsules: In vivo studies. Eur J Pharm Sci. 2015;78:163–70. doi: 10.1016/j.ejps.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Patil CR, Rambhade AD, Jadhav RB, et al. Modulation of arthritis in rats by toxicodendron pubescens and its homeopathic dilutions. Homeopathy. 2011;100:131–37. doi: 10.1016/j.homp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Loredo-Pérez AA, Montalvo-Blanco CE, Hernández-González LI, et al. High-fat diet exacerbates pain-like behaviors and periarticular bone loss in mice with CFA-induced knee arthritis. Obesity. 2016;24:1106–15. doi: 10.1002/oby.21485. [DOI] [PubMed] [Google Scholar]
- 21.Koo ST, Lee CH, Choi H, et al. The effects of pressure on arthritic knees in a rat model of CFA-induced arthritis. Pain Physician. 2013;16:95–102. [PubMed] [Google Scholar]
- 22.Ozawa J, Kurose T, Kawamata S, Yamaoka K. Morphological changes in hind limb muscles elicited by adjuvant-induced arthritis of the rat knee. Scand J Med Sci Sports. 2010;20(1):e72–79. doi: 10.1111/j.1600-0838.2009.00900.x. [DOI] [PubMed] [Google Scholar]
- 23.El-Sayed RM, Moustafa YM, El-Azab MF. Evening primrose oil and celecoxib inhibited pathological angiogenesis, inflammation, and oxidative stress in adjuvant-induced arthritis: novel role of angiopoietin-1. Inflammopharmacology. 2014;22(5):305–17. doi: 10.1007/s10787-014-0200-5. [DOI] [PubMed] [Google Scholar]
- 24.Dwivedi S, Saquib Q, Al-Khedhairy A, et al. Characterization of coal fly ash nanoparticles and induced oxidative DNA damage in human peripheral blood mononuclear cells. Sci Total Environ. 2012;437:331–38. doi: 10.1016/j.scitotenv.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Mena S, Ortega A, Estrela JM. Oxidative stress in environmental-induced carcinogenesis. Mutation Mutat Res. 2009;674(1–2):36–44. doi: 10.1016/j.mrgentox.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Parvathy SS, Masocha W. Gait analysis of C57BL/6 mice with complete freund’s adjuvant-induced arthritis using the Catwalk system. BMC Musculoskelet Disord. 2013;14:14. doi: 10.1186/1471-2474-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croci T, Zarini E. Effect of the cannabinoid CB1 receptor antagonist rimonabant on nociceptive responses and adjuvant-induced arthritis in obese and lean rats. Br J Pharmacol. 2007;150:559–66. doi: 10.1038/sj.bjp.0707138. [DOI] [PMC free article] [PubMed] [Google Scholar]