Abstract
Despite decades of investigation, the neuronal and molecular bases of motivational states remain mysterious. We have recently developed a novel, reductionist, and scalable system for in-depth investigation of motivation using the mating drive of male Drosophila melanogaster (Drosophila), the methods for which we detail here. The behavioral paradigm centers on the finding that male mating drive decreases alongside fertility over the course of repeated copulations and recovers over ~3 d. In this system, the powerful neurogenetic tools available in the fly converge with the genetic accessibility and putative wiring diagram available for sexual behavior. This convergence allows rapid isolation and interrogation of small neuronal populations with specific motivational functions. Here we detail the design and execution of the satiety assay that is used to measure and alter courtship motivation in the male fly. Using this assay, we also demonstrate that low male mating drive can be overcome by stimulating dopaminergic neurons. The satiety assay is simple, affordable, and robust to influences of genetic background. We expect the satiety assay to generate many new insights into the neurobiology of motivational states.
Keywords: Neuroscience, Issue 120, Motivation, Mating drive, Drosophila, Dopamine, Courtship, Behavior
Introduction
Work in Drosophila has provided deep and pioneering insight into many biological phenomena, including the nature of the gene1, principles of embryonic development2, circadian rhythms3, and the development and wiring of the nervous system4,5,6. Motivation remains far less well understood than these phenomena, perhaps because of the limitations on the systems that have been studied thus far. Motivation in the fly is primarily studied in the context of hunger, which presents many challenges due to their vanishingly small food intake per feeding bout and exoskeleton which precludes overt signs of fat deposition. Consequently, there is a need to expand the systems used to study motivation in the fly.
We describe a behavioral framework for the study of mating drive in Drosophila. This system takes advantage of the neurogenetic tools in the fly as well as the accessibility7,8,9,10,11,12 and the putative connectome of its sexually dimorphic circuitry8,13. In addition, much of the innate14,15,16,17,18,19,20,21 and learned22,23,24 sensory-motor circuitry controlling courtship has been worked out in detail, providing a rare opportunity to locate the exact circuit node upon which motivation impinges. We recently reported that, in the fly, as in humans, dopamine levels are central to mating drive25,26,27. We have gained genetic access to the relevant dopamine-producing and receiving neurons in the fly, facilitating detailed molecular- and circuit-level analyses of this conserved phenomenon using the assays we describe here25.
We add to the behavioral assays in Zhang et al.25 a new flat behavioral arena that allows video scoring, which we call a 2-dimensional (2-D) satiety assay, an important improvement over previous methods. Consequently, the new assay is more scalable and quantifiable, and therefore more suitable for genetic screens of genes and neurons involved in motivation. We use this new assay, together with courtship assays and neurogenetic manipulations, to demonstrate how to measure and alter mating drive in the fly.
Protocol
NOTE: This protocol describes the preparation (Sections 1 - 3), execution (Section 4), and analysis (Section 4) of 2-D satiety assays. Then, using dopaminergic stimulation as an example, Section 5 shows how to combine thermogenetic stimulation with 2-D satiety assays to induce hypersexuality. Section 6 describes 3 ways to verify the results of 2-D satiety assays. Finally, Section 7 shows how to measure the recovery of mating drive in male flies.
1. Fabricating 8- and 32-chamber Behavioral Arenas
NOTE: Each behavioral arena consists of several layers of laser-cut plastic sheets held together by hex screws and thumb nuts at each of the four corners (Figure 1A).
- Fabricate the layers of the arena. Fabricate each layer using a laser cutter to cut sheets of acrylic plastic to the appropriate shape (see Figure 1B, 1C for products). NOTE: Many research institutions have laser cutters in their machine shops or maker spaces. If the institution has access to a laser cutter, continue with the following steps. If not, then follow the instructions in Step 1.2 instead.
- For each layer, open the design pattern in the laser cutter's software. NOTE: The designs can be found in appropriately named PDF files (e.g., 8-Chamber Arena - Layer 2 - Doors.pdf) in Supplemental Material 1. This file also contains annotations for the type of plastic to be used (e.g., 1/8 inch (i.e. 3.175 mm) clear acrylic). The exact thickness of each layer is not critical. The main requirement is to allow 0.12 inches (3 mm) or more of free vertical space in the chambers.
- Adjust the laser settings (power, focus, etc.) for the type and thickness of plastic to be used. Place plastic in the laser cutter bed and run the laser cutter. NOTE: These settings will vary widely based on the laser cutter model and the strength of its laser. Find appropriate settings based on past experience or by making test cuts on scrap plastic. The deep hexagonal engravings are included in the design to recess the hex screw heads. This feature allows for one-handed tightening of the thumb nuts and allows the arenas to rest flat on the bench (without protruding screw heads). Nevertheless, making deep engravings is often hard to achieve, and time-consuming, using a laser cutter. This step is optional and can be skipped without affecting the behavioral results.
- When cutting the door frame (Layer 2, Figure 1A), also use appropriate laser settings for engraving the chamber numbers included in the design (as in Figure 1B, 1C).
Place an order with an online laser cutting fabricator. Upload all the PDF design files for the 8- and/or 32-chamber arena and specify the material type (e.g., 1/8 inch (i.e. 3.175 mm) clear acrylic) based on the annotations in the files. The results will appear as in Figures 1B (8-chamber arena) and 1C (32-chamber arena).
Assemble arenas using four hex screws and four thumb nuts to align and hold together the plastic layers (Figure 1A). The arenas will appear as in Figure 1D, 1E (8-chamber) and Figure 1F, 1G (32-chamber). NOTE: The food wall (Layer 4 in Figure 1A) is used for 2-D satiety assays only and is omitted in 32-chamber arenas, which are used in courtship assays.
2. Food Preparation for 2-D Satiety Assay
NOTE: Because a 2-D satiety assay spans 4.5 h, fly food is used in the arena to provide nutrition and water. This protocol uses, but is not restricted to, the conventional cornmeal-agar fly food.
Partially assemble the 2-D satiety arena (8-chamber) with Layers 3 - 6 (see Figure 1A for layer assignments) and tighten it with thumb nuts and hex screws.
Place fresh fly food into a clean, microwave-safe bottle. Add just enough water to cover the bottom surface of the bottle (Figure 2A).
Microwave food on high for 30 - 45 s. Check progress and stir every 15 s until melted (Figure 2B). CAUTION! Fly food easily boils over.
Use a blunted 1,000-µL pipette tip (Figure 2C) to slowly transfer ~1 mL melted food into each chamber (Figure 2D).
Allow food to resolidify at 4 °C for ~10 min (Figure 2E) before completely assembling the arena (Figure 2F).
Use the completed arena for experiments (see Section 4) or store at 4 °C.
Store the leftover food at 4 °C and reuse.
3. Preparing Virgin Females for Behavioral Assays
NOTE: 2-D satiety assays use large numbers of virgin female flies (~120 - 160 for a full behavioral arena) that are difficult to collect using standard methods. This section describes an alternative approach using the hs-hid transgene on the Y chromosome28, which has been used successfully in courtship assays25,29. The stock used to generate white-eyed virgin females has the genotype w1118(X)/hs-hid(Y);+/+;+/+ (Bloomington stock number 24638).
Flip flies into a new bottle once 20 - 50% of the pupae have eclosed (see Figure 3A for example) and there are enough males (5 - 10) to propagate the stock.
Heat shock the original bottle in a 37 °C hot water bath for 1 h to sufficiently activate hs-hid to kill males (Figure 3B). After the heat shock, only females will eclose from these bottles and so will remain virgin until the assays. NOTE: Make sure all sides of the bottle are evenly heated (see Figure 3A for water line). Extend this time for up to 2 h if 1 h is not sufficient to euthanize all males.
Isolate collected females for at least 3 d before experiments to ensure that they are old enough to be sexually receptive25,29. NOTE: Y-chromosome-less males occasionally arise from this stock. These males have white eyes and are unaffected by the heat shock because they do not contain the hs-hid transgene. They cannot produce sperm and therefore cannot decrease receptivity in females30,31.
4. Performing and Scoring 2-D Satiety Assays
NOTE: It is important to control the age of the male flies as mating drive changes with age. This protocol uses males that are 3 - 4 d old25.
Set the incubator to the desired temperature (e.g., 23 °C for standard, RT experiments) and humidity (>30%). For incubators without humidity control, leaving a cup of water in the incubator is sufficient.
Place a 2-D satiety assay arena in the incubator for ~30 min to equilibrate the temperature and to prevent condensation in the chambers during the course of the experiment. The rotating doors should be in the open position.
Use a fly aspirator32 to aspirate 15 - 20 virgin females into each chamber of the arena (Figure 4). NOTE: It is important that the females and males are not anesthetized on the same day as the assay. In our experience, doing so may impact female receptivity and male mating behavior.
Aspirate 1 male into each chamber.
Place the loaded arena in the incubator under a standard consumer camcorder (Figure 5) and film for 4.5 h.
Score videos by noting when each male fly starts and ends each mating (i.e. copulation). This step may be time-consuming at first. With practice, one can score the videos at 5x speed or higher. NOTE: Since flies mate for ~20 min at ~23 °C (shorter duration at higher temperatures)29, one can skim through the first 15 min of each mating.
After logging all mating times, sum the number of matings for each fly (see representative Results).
Use the provided code to generate an ethogram. See Supplemental Material 2 for the code, instructions, and example data.
Use CO2 or cold temperature to anesthetize the flies before removing them.
5. Using Thermogenetic Manipulation to Reverse Satiety in 2-D Satiety Assays
NOTE: This protocol tests whether thermogenetic stimulation of a defined neuronal population can overcome satiety. The experimental flies express the heat-sensitive cation channel TrpA1 in defined populations of neurons (UAS-TrpA1). The steps below apply to the stimulation of dopaminergic neurons (TH-Gal4), which have been shown to promote mating drive25. These steps can be used in conjunction with other neuronal drivers to discover other populations that promote mating drive.
To thermogenetically induce mating behaviors in satiated flies, increase the temperature in the incubator (e.g., from 23 °C to 28.5 °C) after completing a full 4.5 h 2-D satiety experiment.
After the incubator reaches the desired temperature, continue heat stimulation and score the matings (i.e. copulations) of the males for 1 h (see Representative Results). NOTE: The temperature and length of stimulation may vary depending on the genotype so it is necessary to troubleshoot the optimal conditions that prevent overstimulation of neurons while still producing a robust phenotype.
6. Using Courtship and Locomotion to Verify Satiety
NOTE: This section describes 3 optional methods that can be used to verify the results of 2-D satiety assays. They are not required for every assay.
- Use courtship assays to verify satiety.
- Aspirate one male and one virgin female into each of the 32 chambers in a courtship arena.
- Film the flies for ~20 min using a standard consumer camcorder.
- Score the courtship index (CI) to quantify courtship25; this is the fraction of time spent performing courtship behavior (singing, following, etc.) and mating (i.e. copulation) over a 5 min window. This window starts from the first instance of courtship behavior. If a male fly does not start courtship in the first 15 min, its CI is 0. Naïve WT flies should show a CI greater than 0.9, whereas a fully satiated fly should have a CI below 0.2. Other courtship measures may also be considered23,33.
- Scoring courtship in 2-D satiety assays
- Score the amount of time that a male fly spends courting, over a time period of a 2-D satiety assay (e.g., the first h). Courtship is defined as the male performing any step of the ritual (singing, following, etc.). Unlike in Section 6.1.3, this step does not include time the male spends mating.
- Divide the courtship time above by the total time the male was not mating (i.e. not copulating) in the same time period. For example, if a male fly courts for 15 min and mates for 20 min over the first h, the ratio is 15 min / (60 min - 20 min) = 0.375. The ratio is shown as "Fraction of nonmating time in courtship" in Representative Results.
- Using locomotion assay to evaluate exhaustion
- Partially assemble the 32-chamber arena with all parts but the contrast sheet.
- Aspirate one male fly (no female flies) into each of the 32 chambers in a courtship arena.
- Film the flies for ~10 min using a standard consumer camcorder.
- Track the flies with the MTrack2 plugin in ImageJ (http://valelab.ucsf.edu/~nstuurman/ijplugins/MTrack2.html).
7. Recovering Mating Drive after 2-D Satiety Assay
Perform a 2-D satiety assay as described in Section 4, but aspirate the male flies out at the end of the assay.
Isolate the male flies at 23 °C for the desired number of days.
Perform a 2-D satiety assay with the recovered males and score their matings (i.e. copulations) for just 1 h(see Representative Results).
Representative Results
To characterize Drosophila mating drive, 3-day-old, WT Canton-S males were tested in a 2-D satiety assay. Over the course of the assay (4.5 h), males mate an average of 4.8 ±0.3 (mean ±standard error of mean, SEM) times. Matings initiate mostly in the first 2 h (78%) (Figure 6A, 6B) and become less frequent as the assay progresses (Figure 6A, 6B). This decrease is not due to the lack of mating partners (74% females remain unmated throughout the assay) or physical fatigue (Figure 6F). Rather, this effect is likely explained by a decrease in the male's courtship during the assay, from 42.6 ±8.0% (mean ±SEM) (1st h) to 2.0 ±0.7% (mean ±SEM) (last hour) of nonmating time (Figure 6C). This decline in courtship is also seen when the males are tested in courtship assays with new females (Figure 6D, 6E), and recovers after the males have been isolated from females for 3 d (Figure 6G, 6H). These results show that internally maintained mating drive in male flies can be satiated in a 2-D satiety assay and can recover over time.
The 2-D satiety assay can also be easily combined with Drosophila neural manipulations. We recently reported that thermogenetic stimulation of dopaminergic neurons reverses mating drive in satiated male flies25. Using 2-D satiety assays, we find that dopaminergic stimulation (TH>TrpA1, 28.5 °C) at the end of the satiety assay increased both courtship (Figure 7A, 7C, red) and copulation (Figure 7A, 7B, red). The reversal effects are not observed with parental control genotypes (Figure 7A - 7C, black and gray). All of these results are consistent with the recent study25 and suggest that the 2-D satiety assay, along with the auxiliary assays (courtship and locomotion), can be used to dissect molecular and neuronal components of mating drive.
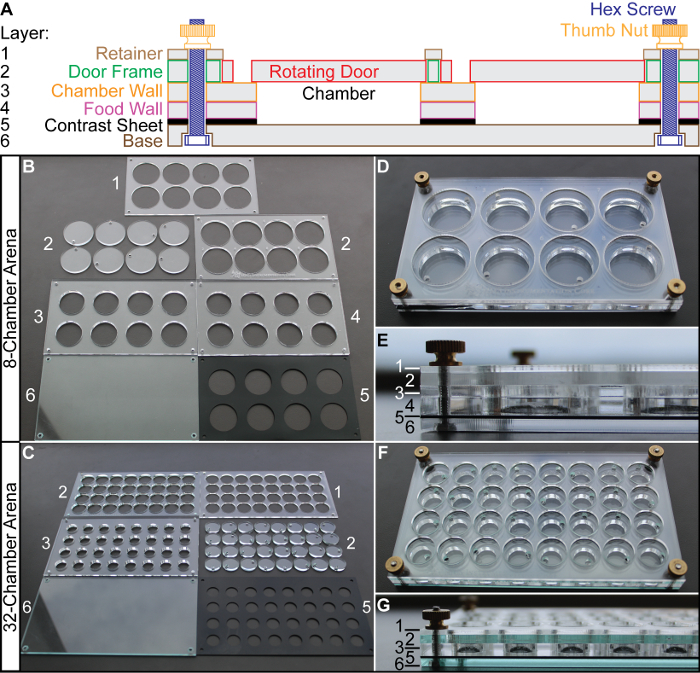 Figure 1. Designing and Assembling Behavioral Arenas. An 8-chamber arena (used for 2-D satiety assays) consists of 6 layers held together by thumb nuts and hex screws (A). A 32-chamber arena (used for courtship assays) is manufactured similarly, but without the food wall (layer 4). The arena parts are cut out with a laser cutter and the layers are numbered as (B) for 8-chamber and (C) for 32-chamber. The assembled 8-chamber arenas are shown in (D) (front view) and (E) (side view with numbered layers). The assembled 32-chamber arenas assembled in a similar fashion (F, G), but Layer 4 (food wall) is omitted (G) since no fly food is in the arena for courtship assays. Please click here to view a larger version of this figure.
Figure 1. Designing and Assembling Behavioral Arenas. An 8-chamber arena (used for 2-D satiety assays) consists of 6 layers held together by thumb nuts and hex screws (A). A 32-chamber arena (used for courtship assays) is manufactured similarly, but without the food wall (layer 4). The arena parts are cut out with a laser cutter and the layers are numbered as (B) for 8-chamber and (C) for 32-chamber. The assembled 8-chamber arenas are shown in (D) (front view) and (E) (side view with numbered layers). The assembled 32-chamber arenas assembled in a similar fashion (F, G), but Layer 4 (food wall) is omitted (G) since no fly food is in the arena for courtship assays. Please click here to view a larger version of this figure.
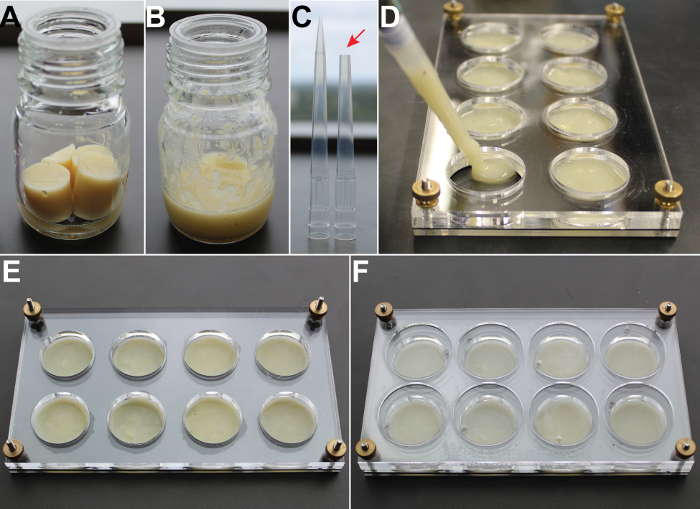 Figure 2. Preparing 2-D Satiety Assays with Food. Standard Drosophila food is placed in a microwave-safe jar with water (A). The food is microwaved until melted (B). A blunted pipette tip (C, arrow) is used to transfer food to chambers of a partially assembled behavioral arena (D). The food is re-solidified at 4 °C (E) before the behavioral arena is completely assembled (F). Please click here to view a larger version of this figure.
Figure 2. Preparing 2-D Satiety Assays with Food. Standard Drosophila food is placed in a microwave-safe jar with water (A). The food is microwaved until melted (B). A blunted pipette tip (C, arrow) is used to transfer food to chambers of a partially assembled behavioral arena (D). The food is re-solidified at 4 °C (E) before the behavioral arena is completely assembled (F). Please click here to view a larger version of this figure.
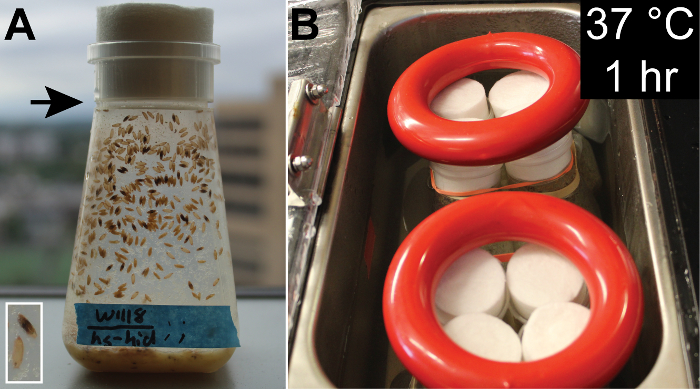 Figure 3: Generating Virgin Females from the w1118(X)/hs-hid(Y) Stock. Fly bottles should be heat shocked when 50 - 80% of the pupae are uneclosed as indicated by their opaqueness (A). The inset image shows samples of uneclosed (top) and eclosed (bottom) pupae (A). Bottles are weighed down and submerged in 37 °C hot water bath for 1 h with water level just above the bottom of the bottle stoppers to ensure even heating (B). See the black arrow in (A) for waterline. Please click here to view a larger version of this figure.
Figure 3: Generating Virgin Females from the w1118(X)/hs-hid(Y) Stock. Fly bottles should be heat shocked when 50 - 80% of the pupae are uneclosed as indicated by their opaqueness (A). The inset image shows samples of uneclosed (top) and eclosed (bottom) pupae (A). Bottles are weighed down and submerged in 37 °C hot water bath for 1 h with water level just above the bottom of the bottle stoppers to ensure even heating (B). See the black arrow in (A) for waterline. Please click here to view a larger version of this figure.
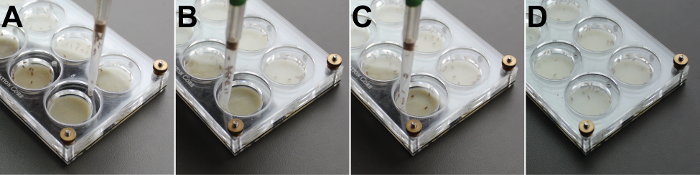 Figure 4: Loading Flies into a Behavioral Arena. Gently aspirate and hold 15 - 20 females in the aspirator (A). The pipette tip is used to open the rotating door (B), and the flies gently released into the chamber (C). Use the aspirator to close the rotating door (D). Please click here to view a larger version of this figure.
Figure 4: Loading Flies into a Behavioral Arena. Gently aspirate and hold 15 - 20 females in the aspirator (A). The pipette tip is used to open the rotating door (B), and the flies gently released into the chamber (C). Use the aspirator to close the rotating door (D). Please click here to view a larger version of this figure.
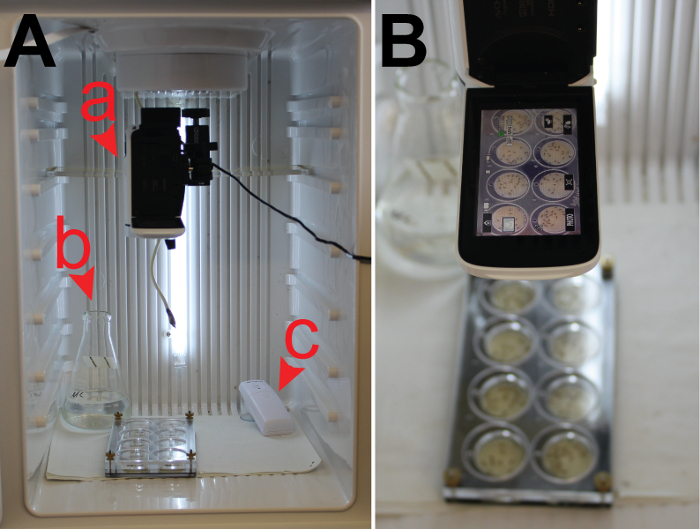 Figure 5: Performing and Recording a 2-D Satiety Assay. (A) A standard consumer camcorder (a) is used to record the assay in an incubator set to the desired temperature. The incubator contains a flask of water (b) and a humidity detector (c) to ensure the appropriate humidity level (>30%). The prepared 2-D satiety assay is filmed under the camcorder (B). Please click here to view a larger version of this figure.
Figure 5: Performing and Recording a 2-D Satiety Assay. (A) A standard consumer camcorder (a) is used to record the assay in an incubator set to the desired temperature. The incubator contains a flask of water (b) and a humidity detector (c) to ensure the appropriate humidity level (>30%). The prepared 2-D satiety assay is filmed under the camcorder (B). Please click here to view a larger version of this figure.
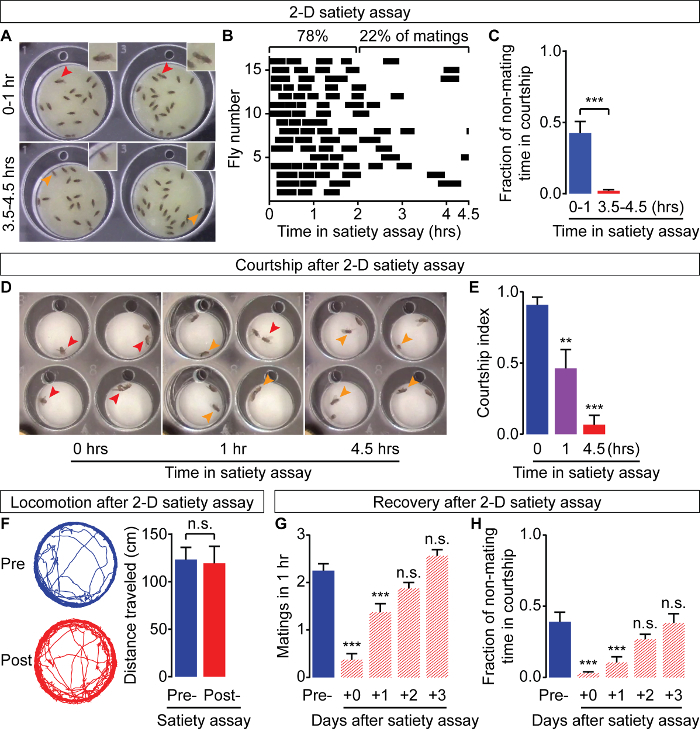 Figure 6: Satiation and Recovery of Fy Mating Drive. In a 2-D satiety assay, male flies mate frequently during the first 2 hours of the assay (A, B) but decrease their courtship (C) and matings (B) as the assay progresses. The progressive decrease in courtship behavior is maintained when males are transferred to a courtship assay with new females after completing a 2-D satiety assay of 0 h, or 1 h, or 4.5 h (D, E). Red arrows point to males exhibiting courtship and mating behaviors, while orange arrows point to non-mating, non-courting flies (A, D). The decline in sexual behaviors is not a result of physical exhaustion, as males show equivalent levels of locomotor activity before and after the 2-D satiety assay (F). Males gradually recover their mating (G) and courtship (H) levels over 3 d of isolation from females. In this figure, ***p <0.001, **p <0.01, n.s. not significant for t-test (C, F) and one-way ANOVA with Tukey post-test (E, G, H). N = 15 - 16 for each condition for all experiments. Error bars represent standard error of the mean (SEM). Please click here to view a larger version of this figure.
Figure 6: Satiation and Recovery of Fy Mating Drive. In a 2-D satiety assay, male flies mate frequently during the first 2 hours of the assay (A, B) but decrease their courtship (C) and matings (B) as the assay progresses. The progressive decrease in courtship behavior is maintained when males are transferred to a courtship assay with new females after completing a 2-D satiety assay of 0 h, or 1 h, or 4.5 h (D, E). Red arrows point to males exhibiting courtship and mating behaviors, while orange arrows point to non-mating, non-courting flies (A, D). The decline in sexual behaviors is not a result of physical exhaustion, as males show equivalent levels of locomotor activity before and after the 2-D satiety assay (F). Males gradually recover their mating (G) and courtship (H) levels over 3 d of isolation from females. In this figure, ***p <0.001, **p <0.01, n.s. not significant for t-test (C, F) and one-way ANOVA with Tukey post-test (E, G, H). N = 15 - 16 for each condition for all experiments. Error bars represent standard error of the mean (SEM). Please click here to view a larger version of this figure.
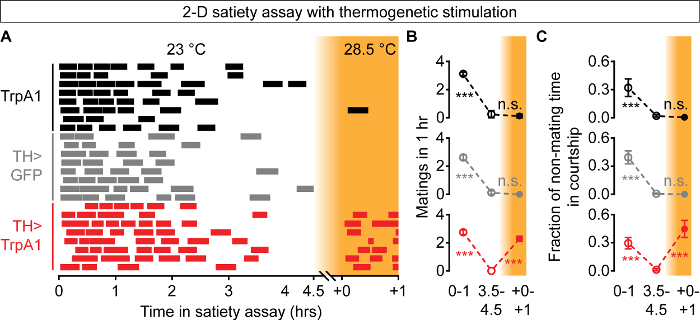 Figure 7: Thermogenetic Satiety Reversal in Male Flies. As in the standard 2-D satiety assay, males show a decrease in matings over the course of the experiment, but thermogenetic stimulation of dopaminergic neurons (TH>TrpA1, red), but not in parental-control genotypes (black and gray), reinstates mating drive in satiated males (A). No matings are scored when the X-axis is broken in (A). This reversal of mating drive can be quantified using either mating numbers (B) or courtship (C). X-axis numbers in (B) and (C) refer to time in (A). Orange background color indicates thermogenetic stimulation (A - C). In this figure, ***p <0.001, n.s. not significant for interactions between genotype and temperature in two-way ANOVA with Bonferroni post-test (B, C). N = 8 for each genotype (B, C). Error bars represent SEM. Please click here to view a larger version of this figure.
Figure 7: Thermogenetic Satiety Reversal in Male Flies. As in the standard 2-D satiety assay, males show a decrease in matings over the course of the experiment, but thermogenetic stimulation of dopaminergic neurons (TH>TrpA1, red), but not in parental-control genotypes (black and gray), reinstates mating drive in satiated males (A). No matings are scored when the X-axis is broken in (A). This reversal of mating drive can be quantified using either mating numbers (B) or courtship (C). X-axis numbers in (B) and (C) refer to time in (A). Orange background color indicates thermogenetic stimulation (A - C). In this figure, ***p <0.001, n.s. not significant for interactions between genotype and temperature in two-way ANOVA with Bonferroni post-test (B, C). N = 8 for each genotype (B, C). Error bars represent SEM. Please click here to view a larger version of this figure.
Supplemental Material 1. Please click here to download this file.
Supplemental Material 2. Please click here to download this file.
Discussion
Motivational states can be satiated, maintained, and recovered34. We present a 2-D satiety assay that quickly and robustly measures all of these aspects of mating drive in the fly. This assay opens up the possibility of using advanced fly genetic manipulations to study the molecular and circuit components of a motivated behavior.
The satiety assay relies on the male's ability to successfully court and copulate, and to terminate copulations at the appropriate time. Though hyposexual flies court less, low-courtship flies are not necessarily hyposexual; they may, for example, have trouble recognizing or tracking the females35. For this reason, the satiety assay is best suited for testing hypersexuality, a phenotype that cannot be reliably observed in a standard courtship assay because naïve wild-type males show courtship indices approaching 1. Hypersexuality in the satiety assay should be considered relative to the age of the male-in our experience, older males tend to mate slightly more frequently than the 3-day-old males used here.
We used thermogenetic stimulation of dopaminergic neurons to exemplify the neurogenetic manipulations that can be used in this system to investigate the components underlying motivation. In addition, researchers can also use thermogenetic stimulation throughout a 2-D satiety assay and look for hypersexual males that are slower to reach satiety. Of course, satiety assays can also be combined with neuronal silencing tools36,37, optogenetics38, genetic mutations39,40,41, RNAi knockdown28,42,43,44, etc. These resources make possible a thorough interrogation of motivation in the fly that is currently unachievable in other systems.
The satiety assay is affordable and scalable. Each arena can be manufactured for ~10 U.S. dollars' worth of materials (plus laser cutting costs, if any) and occupies less space than a paperback book. Scoring the videos is also a relatively simple task. A trained experimenter can score a 4.5 h video with 8 male flies in ~1.5 h. For more high-throughput screening, one can score only the last 2 h, when normal flies show very low levels of mating drive. Alternatively, one can spot check the assays every 30 min, as this will capture the majority of the ~20 min-long matings.
We hope this system will be widely adapted and will contribute to the emergence of Drosophila as a powerful system for unlocking the secrets of motivation.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors thank Mike Crickmore, Dragana Rogulja, and Michelle Frank for comments on the manuscript. Pavel Gorelik provided technical support for manufacturing the behavioral arenas. This work was conducted in Mike Crickmore's lab and is also supported by the Whitehall Foundation (Principal Investigator: Dragana Rogulja). S.X.Z. is a Stuart H.Q. and Victoria Quan Fellow at Harvard Medical School.
References
- Sturtevant AH, Bridges CB, Morgan TH. The spatial relations of genes. Proceedings of the National Academy of Sciences of the United States of America. 1919;5(5):168–173. doi: 10.1073/pnas.5.5.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Ortega JA, Hartenstein V. The Embryonic Development of Drosophila melanogaster. Berlin Heidelberg: Berlin, Heidelberg: Springer; 1985. [Google Scholar]
- Hall JC. Systems Approaches to Biological Rhythms in Drosophila. Methods in Enzymology. 2005;393:61–185. doi: 10.1016/S0076-6879(05)93004-8. [DOI] [PubMed] [Google Scholar]
- Luo L. Rho GTPases in neuronal morphogenesis. Nature reviews. Neuroscience. 2000;1(3):173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- Schmucker D, Clemens JC, et al. Drosophila Dscam Is an Axon Guidance Receptor Exhibiting Extraordinary Molecular Diversity. Cell. 2000;101(6):671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell. 1993;75(5):827–830. doi: 10.1016/0092-8674(93)90525-u. [DOI] [PubMed] [Google Scholar]
- Stockinger P, Kvitsiani D, et al. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121(5):795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Yu JY, Kanai MI, Demir E, Jefferis GSXE, Dickson BJ. Cellular Organization of the Neural Circuit that Drives Drosophila Courtship Behavior. Current biology. 2010;20(18):1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Zhou C, Pan Y, Robinett CC, Meissner GW, Baker BS. Central Brain Neurons Expressing doublesex Regulate Female Receptivity in Drosophila. Neuron. 2014;83(1):149–163. doi: 10.1016/j.neuron.2014.05.038. [DOI] [PubMed] [Google Scholar]
- Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nature neuroscience. 2010;13(4):458–466. doi: 10.1038/nn.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436(7049):395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- Kimura KI, Ote M, Tazawa T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438(7065):229–233. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GSXE. Sexual dimorphism in the fly brain. Current biology. 2010;20(18):1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowney EJ, Iguchi S, Bussell JJ, Scheer E, Ruta V. Multimodal Chemosensory Circuits Controlling Male Courtship in Drosophila. Neuron. 2015;87(5):1036–1049. doi: 10.1016/j.neuron.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallman BR, Kim H, Scott K. Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. eLife. 2015;4:e11188. doi: 10.7554/eLife.11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69(3):509–522. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Zhou C, Franconville R, Vaughan AG, Robinett CC, Jayaraman V, Baker BS. Central neural circuitry mediating courtship song perception in male Drosophila. eLife. 2015;4:e08477. doi: 10.7554/eLife.08477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron. 2011;69(3):498–508. doi: 10.1016/j.neuron.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Kohatsu S, Yamamoto D. Visually induced initiation of Drosophila innate courtship-like following pursuit is mediated by central excitatory state. Nature Communications. 2015;6:6457. doi: 10.1038/ncomms7457. [DOI] [PubMed] [Google Scholar]
- Fan P, Manoli DS, et al. Genetic and neural mechanisms that inhibit Drosophila from mating with other species. Cell. 2013;154(1):89–102. doi: 10.1016/j.cell.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446(7135):542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Ejima A, Smith BPC, et al. Generalization of Courtship Learning in Drosophila Is Mediated by cis-Vaccenyl Acetate. Current Biology. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleman K, Vrontou E, Krüttner S, Yu JY, Kurtovic-Kozaric A, Dickson BJ. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature. 2012;489(7414):145–149. doi: 10.1038/nature11345. [DOI] [PubMed] [Google Scholar]
- Pan Y, Baker BS. Genetic Identification and Separation of Innate and Experience-Dependent Courtship Behaviors in Drosophila. Cell. 2014;156(1-2):236–248. doi: 10.1016/j.cell.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Rogulja D, Crickmore MA. Dopaminergic Circuitry Underlying Mating Drive. Neuron. 2016;91(1):168–181. doi: 10.1016/j.neuron.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Bowers MB, Van Woert M, Davis L. Sexual behavior during L-dopa treatment for Parkinsonism. The American journal of psychiatry. 1971;127(12):1691–1693. doi: 10.1176/ajp.127.12.1691. [DOI] [PubMed] [Google Scholar]
- Sacks OW. Awakenings. New York: Vintage Books; 1999. [Google Scholar]
- Dietzl G, Chen D, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448(7150):151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Crickmore MA, Vosshall LB. Opposing dopaminergic and GABAergic neurons control the duration and persistence of copulation in Drosophila. Cell. 2013;155(4):881–893. doi: 10.1016/j.cell.2013.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Chen S, Busser S, Liu H, Honegger T, Kubli E. Gradual Release of Sperm Bound Sex-Peptide Controls Female Postmating Behavior in Drosophila. Current biology. 2005;15(3):207–213. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451(7174):33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- Pellegrino M, Nakagawa T, Vosshall LB. Single Sensillum Recordings in the Insects Drosophila melanogaster and Anopheles gambiae. Journal of Visualized Experiments. 2010;36(36):1–5. doi: 10.3791/1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R, Cook A. The Attractiveness to males of female Drosophila melanogaster: effects of mating, age and diet. Animal behaviour. 1975;23:521–526. doi: 10.1016/0003-3472(75)90129-3. [DOI] [PubMed] [Google Scholar]
- Toates FM. Motivational Systems (Problems in the Behavioural Sciences) 1986.
- Hall JC. The mating of a fly. Science. 1994;264(5166):1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- Simpson JH. Mapping and manipulating neural circuits in the fly brain. Advances in genetics. 2009;65(9):79–143. doi: 10.1016/S0065-2660(09)65003-3. [DOI] [PubMed] [Google Scholar]
- Venken KJT, Simpson JH, Bellen HJ. Genetic Manipulation of Genes and Cells in the Nervous System of the Fruit. Neuron. 2011;72(2):202–230. doi: 10.1016/j.neuron.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, et al. Independent optical excitation of distinct neural populations. Nature methods. 2014;11(3):338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen HJ, Levis RW, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167(2):761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC, Stern D, et al. The Berkeley Drosophila Genome Project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153(1):135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Cook KR, et al. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nature genetics. 2004;36(3):288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Kaufman TC, Gelbart WM. Research resources for Drosophila: the expanding universe. Nature reviews. Genetics. 2005;6(3):179–193. doi: 10.1038/nrg1554. [DOI] [PubMed] [Google Scholar]
- Ni JQ, Liu LP, et al. A Drosophila resource of transgenic RNAi lines for neurogenetics. Genetics. 2009;182(4):1089–1100. doi: 10.1534/genetics.109.103630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni JQ, Zhou R, et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nature. 2011;8(5):405–407. doi: 10.1038/nmeth.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]


