Abstract
Background
Folate, vitamin B12 and homocysteine concentrations during pregnancy are important factors for early development and may persistently influence kidney function in the offspring. We examined the associations of folate, vitamin B12, and homocysteine concentrations during pregnancy with kidney outcomes in school-aged children.
Study design
Population-based prospective cohort study from fetal life onwards.
Settings & participants
This study was performed among 4,226 pregnant women and their children.
Predictors
Folate, vitamin B12 and homocysteine blood concentrations measured in early pregnancy (median gestational age 13.2 weeks (25th to 75th percentiles 12.2, 14.8) and at birth (cord blood).
Outcomes & measurements
At the median age of 6.0 years (25th to 75th percentiles 5.9, 6.3) we measured combined kidney volume with ultrasound, estimated glomerular filtration rate based on creatinine (eGFRcreat) and cystatin C (eGFRcystC) concentrations and microalbuminuria.
Results
We observed that higher maternal folate concentrations were associated with larger childhood combined kidney volume, whereas higher maternal vitamin B12 concentrations were associated with higher childhood eGFRcystC (p-values <0.05). These associations were independent of homocysteine concentrations. Higher maternal homocysteine concentrations were associated with smaller combined kidney volume and lower childhood eGFRcystC (p-values <0.05). The association of maternal homocysteine concentrations with childhood eGFRcystC was largely explained by combined kidney volume. Higher cord blood homocysteine concentrations were associated with larger combined kidney volume and lower eGFRcystC (p-values <0.05). Folate, vitamin B12 or homocysteine concentrations were not associated microalbuminuria.
Limitations
Observational study, so causality cannot be established.
Conclusion
Our findings suggest that folate, vitamin B12 and homocysteine concentrations during fetal life are associated with offspring kidney development. However, the effect sizes are small. Further studies are needed to replicate these findings and assess the causality and consequences for kidney health in later life.
Keywords: methyl donors, folate, homocysteine, vitamin B12, kidney function, kidney development, pediatrics, epidemiology
Introduction
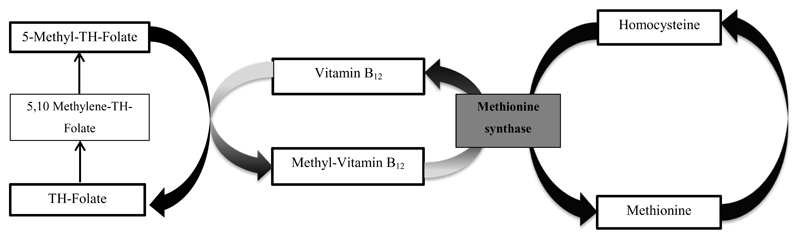
Adverse fetal nutritional exposures may have persistent consequences for kidney health in later life. Specifically, suboptimal nutritional exposures during fetal life may affect nephrogenesis, leading to a reduced number of nephrons and smaller kidneys, which subsequently leads to glomerular hyperfiltration and sclerosis.1, 2 The importance of early life nutrition for later kidney outcomes is illustrated by studies showing both associations of severe maternal undernutrition, preterm birth, and low birth weight with the risk of kidney disease in adulthood.3, 4,5 Not much is known about specific maternal nutritional exposures that persistently affect offspring kidney development.4, 6 Folate is an essential B-vitamin, important for cell growth and replication and is together with vitamin B12, an important methyl donor in many reactions, including the production of thymidine for DNA synthesis, polyamine synthesis and biosynthesis of methionine from homocysteine.7–9 Folate and vitamin B12 contribute to lowering homocysteine concentrations (Figure 1).10 Animal studies indicate that elevated homocysteine concentrations may lead to glomerular damage, and folic acid supplementation lowers creatinine concentration and urinary albumin excretion induced by hyperhomocysteinemia.11 Also, studies in adults have shown associations of elevated homocysteine concentrations with an accelerated decline in kidney function.12 Based on these findings, we hypothesized that lower folate and vitamin B12 concentrations and higher homocysteine concentrations during fetal life may affect nephrogenesis and lead to smaller kidneys with a lower kidney function.
Figure 1. Folate, vitamin B12 and homocysteine metabolism.
Schematic representation of homocysteine metabolism. Folate and vitamin B12 work closely together on homocysteine metabolism, handing off methyl groups to each other. TH-Folate is converted to 5,10-Methylene-TH-Folate which is further reduced to 5- Methyl-TH-Folate. With the demethylation of 5-Methyl-TH-Folate, the methyl group is donated into the methionine cycle. Vitamin B12 is involved directly in the transfer of the methyl group to homocysteine, through methionine synthase. Methionine synthase is a vitamin B12- dependent enzyme that catalyzes the formation of methionine from homocysteine The methionine cycle begins with homocysteine that accepts the methyl group from the folate pool through 5- Methyl-TH-Folate. Abbreviations: TH- Folate, Tetrahydro- folate.
Therefore, we examined, in a population-based prospective cohort study among 4,226 mothers and their children the associations of folate, vitamin B12 and homocysteine concentrations during first trimester of pregnancy and at birth with kidney outcomes in school-aged children.
Methods
Subjects
This study was embedded in the Generation R Study, an ongoing population-based prospective cohort study from fetal life onward in Rotterdam, the Netherlands.13 The study was conducted according to the guidelines of the Helsinki Declaration and approved by the Medical Ethics Committee of Erasmus University Medical Center, Rotterdam (MEC-2007-413). Written informed consent was obtained from parents. At the age of 6 years, all participating children and their mothers were invited to participate in detailed measurements. Of 8,879 mother prenatally included in the study, 6,128 had measurements on folate, vitamin B12 or homocysteine concentrations. Of the 6,057 singleton live-born children from mothers with nutritional data available, 70% of them attended the follow-up visit at the age of 6 years. Children with successful information on at least one of the kidney measurements were included in the study (N = 4,226) (Fig S1).
Maternal and fetal folate, vitamin B12 and homocysteine concentrations
In early pregnancy (median gestational age 13.2 weeks (25th to 75th percentile 12.2, 14.8)) venous samples were drawn and stored at room temperature before being transported to the regional laboratory for processing. Cord blood samples were taken immediately after delivery (40.1 weeks of gestation, 25th to 75th percentiles 39.3–41.0 weeks).13 To analyze folate, vitamin B12 and homocysteine concentrations, ethylenediaminetetraacetic acid plasma samples (folate, homocysteine) and serum samples (vitamin B12) were picked and transported to the Department of Clinical Chemistry at the Erasmus University Medical Centre, Rotterdam. After thawing, folate, homocysteine and vitamin B12 concentrations were analyzed using an immunoelectrochemoluminescence assay on the Architect System. These methods are described in detail elsewhere.14 Folate concentrations were defined as: deficient when < 7nmol/l or normal when ≥ 7 nmol/l.15
Folic acid supplement intake
Information on folic acid supplement use (0.4–0.5 mg) and the initiation of supplementation was obtained by questionnaires at the enrolment of the study. We categorized folic acid supplement use into three groups: 1) periconceptional use; 2) start when pregnancy was known; 3) no use during pregnancy. Detailed information on folic acid supplement intake is described elsewhere.16
Childhood kidney outcomes
As previously described, children’s kidney outcomes were assessed at a median age of 6.0 years (25th to 75th percentiles 5.9, 6.3) in a dedicated research center in the Sophia Children’s Hospital in Rotterdam.17 Kidney volume was measured with ultrasound, using an ATL-Philips HDI 5000 instrument (Seattle, WA, USA), equipped with a 2.0-5.0MHz curved array transducer. We calculated kidney volume using the equation for a prolate ellipsoid.18 Combined kidney volume was calculated by summing right and left kidney volume. Non-fasting blood samples were drawn by antecubital venipuncture. Creatinine concentrations were measured with enzymatic methods and cystatin C concentrations with a particle enhanced immunoturbidimetric assay (using Cobas 8000 analyzers, Roche, Almere, the Netherlands). Estimated glomerular filtration rate (eGFR) was calculated according to the revised Schwartz 2009 formula: eGFRcreat = 36.5 * (height (cm) / serum creatinine (μmol/l)),19 and Zappitelli’s formula based on cystatin C concentrations: eGFRcystC = 75.94 / [CysC1.17].20 Urine creatinine (μmol/l) and urine albumin (μg/l) concentrations were determined with a Beckman Coulter AU analyzer, creatinine concentrations were measured with the Jaffe reaction. Microalbuminuria was defined as an albumin-creatinine ratio between 2.5 and 25 mg/mmol for boys and between 3.5 and 25 mg/mmol for girls.21 Detailed information on kidney measures is described elsewhere.17
Covariates
We obtained information on maternal age, ethnicity, educational level, vitamins supplementation, smoking and alcohol usage during pregnancy using questionnaires.13 We assessed maternal energy intake at enrollment using a validated semi-quantitative food frequency questionnaire.22 Ethnicity and educational level were defined according to the classification of Statistics Netherlands.23 Maternal pre-pregnancy height and weight were self-reported and pre-pregnancy body mass index (BMI) was calculated (kg/m2). We measured maternal blood pressure in early and late pregnancy by using the Omron 907 automated digital oscillometric spygmanometer.24 Information on child’s sex, birthweight and gestational age was available from medical records and hospital registries. We obtained information on breastfeeding from postnatal questionnaires.17 At the age of 6 years, child height and weight were determined and BMI (kg/m2), and body surface area (BSA) (m2) were calculated.25
Statistical analysis
First, we performed a non-response analysis by comparing subject characteristics between children with and without follow-up kidney measurements by using T-tests, Chi-square tests and Mann-Whitney tests. Second, we used multivariable linear and logistic regression models to assess the associations of maternal first trimester and fetal cord blood folate, vitamin B12 and homocysteine concentrations with combined kidney volume, eGFRcreat and eGFRcystC, and risk of microalbuminuria in school-aged children. Folate, vitamin B12 and homocysteine concentrations were analysed continuously per standard deviation (SD)-increase to enable comparison between effect estimates. The regression models were first adjusted for child’s sex, and age at kidney measurements (basic models), and subsequently also for maternal age, education, body mass index, blood pressure, vitamins supplementation, smoking, alcohol use, energy intake during pregnancy, and for child’s birth weight, gestational age at birth, breastfeeding, and body surface area at the age of 6 years (confounder model). These covariates were included in the regression models based on previous literature or a change of >10% in effect estimates. To explore if the observed associations of folate and vitamin B12 with kidney outcomes were independent of homocysteine concentrations, we additionally adjusted the confounder model for homocysteine concentrations (homocysteine model). In a separate model we additionally adjusted the kidney function measures for kidney volume (kidney size model). Third, we used the same models to assess the associations between maternal folic acid supplement intake and childhood kidney outcomes. These analyses, were performed among 3,291 mothers who had information on folic acid supplements. Whether the associations of folate or vitamin B12 or homocysteine and kidney outcomes differed by sex or birthweight we analyzed the interaction terms. Since the interaction terms were not significant we did not stratify our analyses. To prevent bias associated with missing data, we used multiple imputations (n=5) only for covariates with missing values on the basis of the correlation of missing variables with other participant characteristics, according to the Markov Chain Monte Carlo method.26 Detailed information on multiple information procedure is given in Supplementary Materials. Subjects characteristics before and after imputation and the percentages of missing values are shown in Table S1. Statistical analyses were performed using the Statistical Package of Social Sciences version 21.0 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp).
Results
Subject characteristics
Table 1 shows the characteristics of the study population stratified on folate concentrations. The values are based on the original data. Table S2 shows folate, vitamin B12 and homocysteine concentrations per supplement group of folic acid use. The correlation coefficients of the investigated variables are given in Table S3. Maternal and cord blood folate, vitamin B12 and homocysteine concentrations correlation coefficients ranged from r= 0.34 to 0.46. Maternal folate and vitamin B12 concentrations were weakly negatively correlated with child height and weight, whereas homocysteine concentrations were weakly positively correlated with child height and weight (Table S3). Results from the non-response analyses are given in Table S4. Mothers whose children had kidney follow-up measurements had higher folate and vitamin B12, and lower homocysteine concentrations compared to mothers whose children did not have kidney follow-up measurements.
Table 1. Subject characteristics according to folate levels (N = 4,149).
| Folate deficient (< 7nmol/l) N = 277 | Normal folate (≥7nmol/l) N = 3,872 | |
|---|---|---|
| Maternal characteristics | ||
| Maternal age (y) | 27.5 (5.6) | 30.6 (4.8) |
| Pre-pregnancy body mass index(kg/m2) | 23.7 (21.0, 27.0) | 22.6 (20.8, 25.2) |
| Missing, n (%) | 60 (22) | 629 (16) |
| Gestational age at intake (wk) | 14.2 (12.6, 16.1) | 13.2 (12.1, 14.6) |
| Early pregnancy systolic blood pressure (mmHg) | 114 (12) | 116 (12) |
| Missing, n (%) | 3 (1) | 21 (0.5) |
| Early pregnancy diastolic blood pressure (mmHg) | 67 (10) | 68 (10) |
| Missing, n (%) | 3 (1) | 21 (0.5) |
| Late pregnancy systolic blood pressure (mmHg) | 119 (12) | 119 (12) |
| Missing, n (%) | 14 (5.1) | 120 (3.1) |
| Late pregnancy diastolic blood pressure (mmHg) | 69 (10) | 69 (9) |
| Missing, n (%) | 14 (5.1) | 120 (3.1) |
| Education level, n (%) | ||
| - No higher education | 204 (73.6) | 1,824 (47.1) |
| - Higher education | 44 (15.9) | 1,867 (48.2) |
| - Missing, n (%) | 29 (10.5) | 181 (4.7) |
| Ethnicity, n (%) | ||
| - European | 99 (35.7) | 2,555 (66.0) |
| - Non-European | 167 (60.3) | 1,264 (32.6) |
| - Missing, n (%) | 11 (4) | 53 (1.4) |
| Smoking during pregnancy, n (%) | ||
| - Never & until pregnancy was known | 154 (55.6) | 2,940 (75.9) |
| - Continued | 90 (32.5) | 545 (14.1) |
| - Missing, n (%) | 33 (11.9) | 387 (10) |
| Alcohol during pregnancy, n (%) | ||
| - Never & until pregnancy was known | 171 (61.7) | 1,939 (50.1) |
| - Continued | 59 (21.3) | 1,515 (39.1) |
| - Missing, n (%) | 47 (17) | 418 (10.8) |
| Folic acid supplements use, n (%) | ||
| - No | 166 (59.9) | 515 (13.3) |
| - Start 1st to 10 weeks | 23 (8.3) | 1,022 (26.4) |
| - Start periconceptional | 15 (5.4) | 1,492 (38.5) |
| - Missing, n (%) | 73 (26.4) | 843 (21.8) |
| Maternal calories intake (kcal) | 2,010 (599) | 2,050 (549) |
| Missing, n (%) | 76 (27.4) | 700 (18.1) |
| Vitamin supplements use, n (%) | ||
| - No | 211 (76.2) | 2,193 (56.6) |
| - Yes | 17 (6.1) | 1,114 (28.8) |
| - Missing, n (%) | 49 (17.7) | 565 (14.6) |
| Folate plasma concentrations (nmol/l) | 6.1 (5.4, 6.6) | 17.9 (11.6, 25.4) |
| Vitamin B12 serum concentrations (pmol/l) | 154.5 (115.8, 200.8) | 173.0 (131.0, 232.0) |
| Missing, n (%) | 7 (2.5) | 235 (6.1) |
| Homocysteine plasma concentrations (μmol/l) | 8.4 (7.1, 10.0) | 6.8 (6.0, 7.8) |
| Missing, n (%) | 7 (2.5) | 62 (1.6) |
| Infant characteristics | ||
| Girls, n (%) | 135 (48.7) | 1,941 (50.1) |
| Gestational age at birth (wk) | 40.1 (39.3, 40.9) | 40.1 (39.3, 41.0) |
| Birth weight (g) | 3,309 (596) | 3,447 (548) |
| Missing, n (%) | - | 4 (0.1) |
| Breastfeeding, n (%) | ||
| - No | 20 (7.2) | 231 (6.0) |
| - Yes | 178 (64.3) | 2,940 (75.9) |
| - Missing, n (%) | 79 (28.5) | 701 (18.1) |
| Cord blood folate concentrations (nmol/l) | 16.6 (13.2, 21.2) | 21.1 (16.5, 27.3) |
| Cord blood vitamin B12 concentrations (pmol/l) | 267.5 (210.3, 379.8) | 302.0 (220.0, 422.0) |
| Cord blood homocysteine concentrations (μmol/l) | 9.8 (8.4, 12.3) | 9.3 (7.4, 10.6) |
| Child characteristics at 6y visit | ||
| Age (y) | 6.1 (5.9, 6.5) | 6.0 (5.9, 6.2) |
| Height (cm) | 120.0 (6.9) | 119.3 (5.9) |
| Missing, n (%) | - | 6 (0.2) |
| Weight (kg) | 23.0 (20.8, 27.5) | 22.4 (20.4, 25.0) |
| Missing, n (%) | - | 6 (0.2) |
| Body mass index (kg/m2) | 16.1 (15.3, 18.1) | 15.8 (15.0, 16.9) |
| Missing, n (%) | - | 6 (0.2) |
| Body surface area (m2) | 0.9 (0.1) | 0.9 (0.1) |
| Missing, n (%) | - | 6 (0.2) |
| Combined kidney volume (cm3) | 122.1 (28.6) | 120.0 (23.2) |
| Creatinine (μmol/l) | 38.2 (5.2) | 37.3 (5.6) |
| Cystatin C (mg/l) | 783.1 (74.8) | 784.4 (81.8) |
| eGFRcreat ( ml/min/1.73m2) | 117.1 (14.8) | 119.2 (16.3) |
| eGFRcystC ( ml/min/1.73m2) | 102.4 (13.5) | 102.5 (14.7) |
| Microalbuminuria, n (%) | 24 (8.7) | 279 (7.2) |
Values are frequency counts and percentages for categorical variables, means (SD) for continuous variables with a normal distribution, or medians (25th to 75th percentiles) for continuous variables with a skewed distribution. Values are based on the original data. Folate deficiency was defined as a folate concentration < 7 nmol/l. Abbreviations: GFRcreat estimated glomerular filtration rate calculated based on creatinine blood levels: eGFRcystC estimated glomerular filtration rate calculated based on cystatin C blood levels.
Maternal and fetal folate, vitamin B12 and homocysteine concentrations and childhood kidney outcomes
Table 2 shows that a 1-SD higher maternal folate concentration was associated with a 1.16 cm3 (95% confidence interval ((CI) 0.47, 1.85) larger childhood combined kidney volume. No other associations were observed of maternal folate concentrations with other kidney outcomes. A 1-SD higher maternal vitamin B12 concentration was associated with 1.00 ml/min/1.73m2 (95% CI 0.43, 1.57) higher childhood eGFRcystC. The effects estimates were similar when we adjusted for maternal homocysteine concentrations (homocysteine models) and childhood combined kidney volume (kidney size model). Similarly, a 1-SD higher maternal homocysteine concentration was associated with a -1.44 cm3 (95% CI -2.09, -0.79) smaller combined kidney volume and a -0.57 ml/min/1.73m2 (95% CI -1.13, -0.02) lower childhood eGFRcystC. The association of maternal homocysteine concentrations with childhood eGFRcystC was largely explained by combined kidney volume. None of the exposures were associated with the risk of microalbuminuria. The results from basic models are given in Table S5.
Table 2. Associations of maternal folate, vitamin B12 and homocysteine concentrations during pregnancy with kidney outcomes at the age of 6 years (N = 4,226).
| Difference in outcome measure (95% Confidence Interval) | ||||
|---|---|---|---|---|
| First trimester maternal concentrations | Combined kidney volume (cm3) | eGFRcreat (ml/min/1.73m2) | eGFRcystC (ml/min/1.73m2) | Microalbuminuria (odds ratio) |
| Folate | ||||
| Confounder Model |
1.16 (0.47, 1.85)** N = 3,818 |
0.01 (-0.65, 0.67) N = 2,792 |
-0.03 (-0.63, 0.58) N = 2,792 |
0.97 (0.85, 1.10) N = 4,011 |
| Homocysteine Model |
1.02 (0.32, 1.72)** N = 3,757 |
-0.06 (-0.74, 0.61) N = 2,741 |
-0.11 (-0.73, 0.51) N = 2,741 |
0.96 (0.84, 1.10) N = 3,944 |
| Kidney size Model | -0.24 (-0.90, 0.42) N = 2,601 |
0.01 (-0.59, 0.61) N = 2,601 |
0.95 (0.83, 1.09) N = 3,686 |
|
| Vitamin B12 | ||||
| Confounder Model | 0.47 (-0.17, 1.11) N = 3,666 |
0.20 (-0.43, 0.83) N = 2,663 |
1.00 (0.43, 1.57)** N = 2,663 |
1.06 (0.95, 1.19) N = 3,849 |
| Homocysteine Model | 0.20 (-0.45, 0.85) N = 3,563 |
0.22 (-0.42, 0.85) N = 2,579 |
0.95 (0.36, 1.53)** N = 2,579 |
1.07 (0.96, 1.20) N = 3,737 |
| Kidney size Model | 0.09 (-0.53, 0.71) N = 2,481 |
0.96 (0.39, 1.52)** N = 2,482 |
1.08 (0.96, 1.21) N = 3,538 |
|
| Homocysteine | ||||
| Confounder Model |
-1.44 (-2.09, -0.79)** N = 3,779 |
-0.55 (-1.15, 0.05) N = 2,755 |
-0.57 (-1.13, -0.02)* N = 2,755 |
1.08 (0.98, 1.20) N = 3,969 |
| Kidney size Model | -0.35 (-0.97, 0.28) N=2,566 |
-0.52 (-1.10, 0.06) N = 2,556 |
1.10 (0.99, 1.23) N = 3,649 |
|
Values are linear and logistic regression coefficients (95% confidence interval). Confounder model is adjusted for maternal characteristics (age, body mass index before pregnancy, blood pressure in early pregnancy, ethnicity, education, vitamins supplementation, smoking, alcohol consumption , energy intake during pregnancy), and child characteristics (birthweight, gestational age, sex, breastfeeding, age and body surface area at 6 year visit). Homocysteine model is confounder model additionally adjusted for homocysteine concentrations during pregnancy. Kidney size model is confounder model additionally adjusted for child combined kidney volume. *p < 0.05, **p<0.01. Maternal folate, vitamin B12 and homocysteine concentrations were analyzed per 1 standard deviation in folate, vitamin B12 and homocysteine. Abbreviations: eGFRcreat, estimated glomerular filtration rate based on creatinine concentrations; eGFRcystC, estimated glomerular filtration rate based on cystatin C concentrations.
Table 3 shows that a 1-SD higher fetal cord blood homocysteine concentrations was associated with a 1.27 cm3 (95% CI 0.46, 2.08) larger childhood combined kidney volume and a -1.02 ml/min/1.73m2 (95% CI -1.76, -0.28) lower eGFRcystC,. The effect estimates on eGFRcystC remained similar after additional adjustment for kidney size. Higher cord blood homocysteine concentrations was associated with lower eGFRcreat after additional adjustment for kidney size -0.91 ml/min/1.73m2 (95% CI -1.71, -0.12). No other associations were observed of cord blood folate and vitamin B12 concentrations with kidney outcomes. The results from basic models are given in Table S6.
Table 3. Associations of cord blood folate, vitamin B12 and homocysteine concentrations with kidney outcomes at the age of 6 years (N = 2,674).
| Difference in outcome measure (95% Confidence Interval) | ||||
|---|---|---|---|---|
| Cord blood concentrations | Combined kidney volume (cm3) | eGFRcreat (ml/min/1.73m2) | eGFRcystC (ml/min/1.73m2) | Microalbuminuria (odds ratio) |
| Folate | ||||
| Confounder Model | 0.36 (-0.46, 1.18) N = 2,384 |
0.41 (-0.36, 1.17) N = 1,753 |
0.51 (-0.19, 1.22) N = 1,753 |
0.97 (0.83, 1.13) N = 2,517 |
| Homocysteine Model | 0.74 (-0.12, 1.59) N = 2,308 |
0.30 (-0.50, 1.10) N = 1,702 |
0.31 (-0.43, 1.06) N = 1,702 |
1.00 (0.85, 1.17) N = 2,439 |
| Kidney size Model | 0.37 (-0.39, 1.14) N = 1,625 |
0.48(-0.24, 1.20) N = 1,625 |
0.97 (0.82, 1.14) N = 2,306 |
|
| Vitamin B12 | ||||
| Confounder Model | -0.47 (-1.27, 0.34) N = 2,413 |
-0.52 (-1.28, 0.25) N = 1,776 |
0.35 (-0.36, 1.05) N = 1,776 |
0.99 (0.84, 1.15) N = 2,548 |
| Homocysteine Model | -0.05 (-0.90, 0.80) N = 2,271 |
-0.76 (-1.56, 0.05) N = 1,674 |
0.17 (-0.58, 0.92) N = 1,674 |
1.00 (0.85, 1.17) N = 2,403 |
| Kidney size Model | -0.48 (-1.25, 0.29) N = 1,645 |
0.42 (-0.30, 1.14) N = 1,645 |
1.00 (0.85, 1.18) N = 2,335 |
|
| Homocysteine | ||||
| Confounder Model |
1.27 (0.46, 2.08)** N = 2,311 |
-0.74 (-1.53, 0.06) N = 1,705 |
-1.02 (-1.76, -0.28)** N = 1,705 |
1.09 (0.95, 1.26) N = 2,443 |
| Kidney size Model |
-0.91 (-1.71, -0.12)* N = 1,579 |
-1.14 (-1.89, -0.39)** N = 1,579 |
1.09 (0.93, 1.27) N = 2,236 |
|
Values are linear and logistic regression coefficients (95% confidence interval). Confounder model is adjusted for maternal characteristics (age, body mass index before pregnancy, blood pressure in late pregnancy, ethnicity, education, vitamins supplementation, smoking and alcohol consumption, energy intake during pregnancy), and child characteristics (birthweight, gestational age, sex, breastfeeding, age and body surface area at the age of 6year visit). Homocysteine model is confounder model additionally adjusted for homocysteine concentrations at birth. Kidney size model is confounder model additionally adjusted for child combined kidney volume. *p < 0.05, **p<0.01. Cord blood folate, vitamin B12 and homocysteine concentrations were analyzed per 1 standard deviation in folate, vitamin B12 and homocysteine. Abbreviations: eGFRcreat, estimated glomerular filtration rate based on creatinine concentrations; eGFRcystC, estimated glomerular filtration rate based on cystatin C concentrations.
Table 4 shows that there was no association between folic acid supplement intake and kidney outcomes.
Table 4. Associations of maternal folic acid supplements intake during pregnancy with kidney measurements at the age of 6 years (N = 3,291).
| Difference in outcome measure (95% Confidence Interval) | ||||
|---|---|---|---|---|
| Folic Acid Supplement Use | Combined kidney volume (cm3) n = 3,025 | eGFRcreat (ml/min/1.73m2) n= 2,211 | eGFRcystC (ml/min/1.73m2) n= 2,215 | Microalbuminuria (odds ratio) n = 3,179 |
| Basic Model | ||||
| No (n = 696) | -1.63 (-3.78, 0.53) | 0.18 (-1.58, 1.94) | 0.50 (-1.14, 2.14) | 0.77 (0.54, 1.10) |
| Started when pregnancy was known (n = 1,065) | -0.89 (-2.74, 0.97) | -0.64 (-1.94, 0.66) | 0.86 (-0.56, 2.28) | 0.71 (0.52, 0.97) |
| Started periconceptional (n = 1,530) | Reference | Reference | Reference | Reference |
| Confounder Model | ||||
| No (n = 696) | -2.08 (-4.32, 0.15) | 1.47 (-0.61, 3.55) | 1.14 (-0.79, 3.08) | 0.82 (0.54, 1.25) |
| Started when pregnancy was known (n = 1,065) | -1.49 (-3.16, 0.18) | -0.27 (-1.83, 1.30) | 1.17 (-0.29, 2.63) | 0.75 (0.54, 1.03) |
| Started periconceptional (n = 1,530) | Reference | Reference | Reference | Reference |
Values are linear and logistic regression coefficients (95% confidence interval). Basic model is adjusted for child’s sex and age at 6 year visit. Confounder model is additionally adjusted for maternal characteristics (age, body mass index before pregnancy, blood pressure in early pregnancy, ethnicity, education, vitamin supplements, smoking, alcohol consumption, energy intake during pregnancy), and child characteristics (birthweight, gestational age, breastfeeding, age and body surface area at the age of 6y). * p < 0.05. Abbreviations: eGFRcreat, estimated glomerular filtration rate based on creatinine concentrations; eGFRcystC, estimated glomerular filtration rate based on cystatin C concentrations.
Discussion
Results from this population-based prospective cohort study suggest that maternal higher folate and lower homocysteine concentrations are associated with larger childhood combined kidney volume, whereas maternal higher vitamin B12 and lower homocysteine concentrations are associated with higher childhood eGFRcystC. Lower cord blood homocysteine concentrations were associated with smaller childhood combined kidney volume and higher eGFR.
Interpretation of main findings
Various lines of investigation suggest that an adverse fetal nutrition may have persistent consequences for kidney health in later life. As nephrogenesis continues until 36 weeks of gestation and largely ceases thereafter, adverse fetal nutritional exposures may have persistent impact on kidney function in later life.1, 2 For this study, we specifically hypothesized that low folate and vitamin B12 concentrations and higher homocysteine concentrations during fetal life may affect nephrogenesis and lead to persistently smaller kidneys with a lower kidney function.
Not many studies have explored the effect of maternal folate concentrations or folic acid supplements during pregnancy on childhood kidney measures. Results from a trial follow-up study in Rural Bangladesh among 3,267 mother and their 4 to 5 year old children indicated no effect of early maternal multiple micronutrient supplementation or food supplementation with iron and folate on offspring’s kidney volume.27 A study exploring the effect of maternal folic acid supplements during pregnancy has suggested that folic acid supplements alone are a risk factor for congenital anomalies of kidney and urinary tract.28 In our study only few children (n=6) had evidence of congenital kidney abnormalities. We did not observe any association of maternal folic acid supplements or cord blood folate concentrations with childhood kidney volume. However, our results suggest that higher maternal folate concentrations in early pregnancy are associated with a larger childhood combined kidney volume, independent of homocysteine concentrations. Specifically, folate concentrations in early pregnancy may affect child kidney volume. Folate concentrations are more directly related to the biological processes in the body than self-reported folic acid intake. Per se, they are a more precise measure of actual folate status, allowing for detection of potential subtle effects. This can explain why maternal folate concentrations were associated with childhood kidney volume, whereas folic acid supplementation was not. Furthermore, a continuous measure of folate concentration has statistically more power to detect differences compared to the categorical approach of folic acid supplementation. A study among young adults suggested that folic acid supplementation in subjects with low dietary content of folic acid was associated with decreased creatinine concentrations and subsequently higher eGFR.29 Another study among 6 to 8 year old children in rural Nepal suggested that maternal folic acid supplementation reduced the risk of microalbuminuria.30 Folate is important for homocysteine metabolism. Animal studies have shown that folic acid supplementation attenuates glomerular damage induced by hyperhomocysteinemia.11 In our study, we did not observe any association of maternal or cord blood folate concentrations or folic acid supplementations with eGFR or microalbuminuria in 6 year old children. The differences in results may be explained by the different study populations. Vitamin B12 leads together with folate to lower homocysteine concentrations. We observed that higher vitamin B12 concentrations during fetal life were associated with a higher eGFRcystC. To the best of our knowledge, no previous studies have explored the relationship of vitamin B12 with kidney function measures in children. Epidemiological studies in renal failure patients suggested that parenteral vitamin B12 lowers homocysteine concentrations independent of serum vitamin B12 concentrations.31 Previous studies have shown that after adjustment for homocysteine concentrations, higher vitamin B12 concentrations were associated with an increased risk of albuminuria. In individuals with high baseline homocysteine concentrations, increased vitamin B12 was associated with reduced kidney function.32 Specifically, among hyperhomocysteinaemic patients vitamin-B supplementation modulated cystatin C concentrations.33 In our study the observed associations of vitamin B12 with eGFR were independent of homocysteine concentrations. We did not observe any association of vitamin B12 concentrations with childhood microalbuminuria. Further research is needed to observe the effect of early life folate and vitamin B12 with kidney outcomes in later life.
Our results suggest that higher maternal homocysteine concentrations were associated with smaller combined kidney volume and lower eGFR. Nephrogenesis starts in early pregnancy, therefore high homocysteine concentrations since early pregnancy may influence on nephron formation. As we have previously reported, kidney size is positively correlated with eGFR.34 We observed that an increased kidney volume explained the associations of maternal homocysteine concentrations with kidney function. Higher cord blood homocysteine concentrations were associated with larger combined kidney volume and lower eGFR. Our findings suggest time specific effects of homocysteine concentrations on kidney volume. It may be that a constant exposure to high homocysteine concentration induces kidney hypertrophy in originally smaller kidneys. To the best of our knowledge, there are no other studies in children to compare our findings. In line with our findings, in adults elevated homocysteine concentrations are associated with an accelerated decline in eGFR.12, 35 Our findings are also supported by experimental studies in rats suggesting that elevated homocysteine concentrations can be an important pathogenic factor in glomerular damage.11 Results from a study among 340 adults aged 50-75 years, suggested that increased homocysteine concentrations influence on the development of microalbuminuria.36 In our study, we did not observe any association of homocysteine concentrations with the risk of microalbuminuria. It may be that the effects of impaired kidney growth on microalbuminuria may not be detectable during childhood, and become evident later in life. Altogether, these findings suggest time specific effects of homocysteine concentrations on kidney development. Previous studies using data from the same cohort have observed that low folate and high homocysteine but not vitamin B12 concentrations were associated with fetal growth restriction.14 However, a previous study did not observe any association of maternal folate, vitamin B12 or homocysteine concentrations with blood pressure at the age of 6 years.16
Although we observed small effect sizes, our results may be important from a population-based perspective. In the Netherlands, the food supplies are not fortified with folic acid, but women are advised to use folic acid supplements (400 μg/day) prior to and up to the 10-12th week of pregnancy. Although the results presented in this study do not provide a basis to make causal statements, they support population-strategies to increase folate and vitamin B12 concentrations and subsequently lower homocysteine concentrations in pregnant women.
Potential mechanisms
From our observational study, it is not possible to establish the causality for the observed associations. However, some biological mechanisms may link maternal folate, vitamin B12 and homocysteine concentrations with childhood kidney outcomes. Both observational and experimental studies relate low folate and high homocysteine concentrations with endothelial dysfunction and altered vascular development.12, 37–39 High homocysteine concentrations impair endothelial vasodilatation by inhibiting the generation of endothelial mediators and promoting adhesion between neutrophil and endothelial cells.40 Homocysteine may also decrease levels of adenosine in plasma and interstitial tissue, and induce proliferation and apoptosis of glomerular mesangial cells, which leads directly to renal vascular injury.41 Suboptimal vascular development and endothelial dysfunction could lead to hypertension in childhood which could be a predictor to chronic kidney diseases in later life.2, 4 Also, maternal diet programs the embryonic kidney, altering cell turnover and gene expression at a time when nephrons and glomeruli have yet to form.42 A beneficial effect of methyl donors, folate and vitamin B12 concentrations on childhood kidney outcomes support the hypothesis of epigenetic changes that program later kidney health.
Methodological considerations
To the best of our knowledge, this is the largest prospective population-based cohort study examining the associations of folate, vitamin B12 and homocysteine concentrations during fetal life with childhood kidney outcomes. We measured folate, vitamin B12 and homocysteine concentrations during early pregnancy and at birth, assessing critical periods of kidney development. Cord blood folate and vitamin B12 concentrations were higher compared to maternal concentrations. This is also reported previously in literature.43 According to Dutch recommendations, we assume that after the 10-12th gestational week women did not take folic acid supplements. We obtained information on maternal folic acid supplementation using questionnaires. Mothers who did not take folic acid supplements in pregnancy had lower folate and higher homocysteine concentrations compared to mothers that started folic acid supplements periconceptional. Furthermore, these mothers had lower folate and vitamin B12 and higher homocysteine concentrations as compared to normal ranges of these concentrations during pregnancy.44 Of the total group of singleton live-born children 70% had available information on kidney measurements. Selection bias in follow-up studies mainly arises from loss to follow-up than from non-response at baseline. Mothers of children without follow-up kidney measurements had lower folate and higher homocysteine concentrations, were younger, more with a non-European origin, smoked more frequently and used less alcohol during pregnancy, compared to mothers of children with available kidney measurements. Loss to follow-up would lead to selection bias if the associations of maternal first trimester micronutrient concentrations with childhood kidney outcomes would be different between those included and those not included in the final analyses. Because this is difficult to determine, selection bias cannot be excluded.
We performed detailed measurements on kidney outcomes. Kidney size was used as a measure of kidney development. Ultrasonography is a reliable method to measure kidney volume.18 Kidney size is correlated with the number of glomeruli and can be used in epidemiologic studies as a measure of kidney development.1 BSA is a well-known predictor of kidney volume. Replacement of BSA with BMI did not change the results. Similar effect estimates to combined kidney volume were observed when we explored Kidney volume/ BSA as an additional outcome.45 To estimate GFR, we used the Schwartz formula based on creatinine concentrations and height, and also Zappitelli’s formula based on cystatin C concentrations.19, 20 In our analyses we observed small differences in effect estimates between eGFRcreat and eGFRcystC, with slightly stronger effect estimates for eGFRcystC. Random urine sample was used to evaluate the presence of microalbuminuria.46 Our results apply to a relatively healthy sample of pregnant women and children. It may be that our findings could underestimate the true effect measures. Therefore, the generalizability of our results should be interpreted with caution in other populations. Finally, although we adjusted for a large number of potential maternal and childhood confounders, residual confounding, like child nutritional status, can still be present.
In conclusion, results from our prospective study, suggest that folate, vitamin B12 and homocysteine concentrations during fetal life affect offspring kidney measures. The effect sizes presented in the study are quite small, and the results could reflect confounding. Our findings should be considered as hypothesis generating and need further replication. Further follow-up studies are needed to examine the long term consequences for the risk of kidney diseases in later life.
Supplementary Material
Acknowledgments
The Generation R Study is conducted by the Erasmus University Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of participating mothers, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
Financial support
The general design of the Generation R Study was made possible by financial support from Erasmus Medical Center, Rotterdam; Erasmus University, Rotterdam; the Dutch Ministry of Health, Welfare and Sport; and the Netherlands Organization for Health Research and Development (ZonMw). K.Miliku has been financially supported through Erasmus Mundus Western Balkans (ERAWEB), a project funded by the European Commission. OH Franco works in ErasmusAGE, a research center funded by Nestlé Nutrition (Nestec Ltd.), Metagenics Inc. and AXA. V.W.V. Jaddoe received an additional grant from the Netherlands Organization for Health Research and Development (VIDI 016.136.361) and an European Research Council Consolidator Grant (ERC-2014-CoG-648916). The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript.
Abbreviations
- CI
confidence interval
- eGFRcreat
estimated glomerular filtration rate based on creatinine concentrations
- eGFRcystC
estimated glomerular filtration rate based on cystatin C concentrations
Footnotes
Financial Disclosure: None.
Author contributions
Research idea and study design: KM, VWVJ; data acquisition: AH, OHF, VWVJ; data analysis/interpretation: KM, OHF, AH, EAPS and VWVJ; statistical analysis: KM, AM; supervision or mentorship: VWVJ. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. KM and VWVJ take responsibility that this study have been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1.Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21(6):898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 2.Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis. 1994;23(2):171–175. [PubMed] [Google Scholar]
- 3.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJP. Low birth weights contribute to the high rates of early-onset chronic renal failure in the southeastern United States. Archives of Internal Medicine. 2000;160(10):1472–1476. doi: 10.1001/archinte.160.10.1472. [DOI] [PubMed] [Google Scholar]
- 4.Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes-a global concern. Nat Rev Nephrol. 2015;11(3):135–149. doi: 10.1038/nrneph.2014.251. [DOI] [PubMed] [Google Scholar]
- 5.Painter RC, Roseboom TJ, van Montfrans GA, et al. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J Am Soc Nephrol. 2005;16(1):189–194. doi: 10.1681/ASN.2004060474. [DOI] [PubMed] [Google Scholar]
- 6.Langley-Evans SC, Langley-Evans AJ, Marchand MC. Nutritional programming of blood pressure and renal morphology. Arch Physiol Biochem. 2003;111(1):8–16. doi: 10.1076/apab.111.1.8.15136. [DOI] [PubMed] [Google Scholar]
- 7.Glier MB, Green TJ, Devlin AM. Methyl nutrients, DNA methylation, and cardiovascular disease. Mol Nutr Food Res. 2014;58(1):172–182. doi: 10.1002/mnfr.201200636. [DOI] [PubMed] [Google Scholar]
- 8.Berti C, Decsi T, Dykes F, et al. Critical issues in setting micronutrient recommendations for pregnant women: an insight. Matern Child Nutr. 2010;6(Suppl 2):5–22. doi: 10.1111/j.1740-8709.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashworth CJ, Antipatis C. Micronutrient programming of development throughout gestation. Reproduction. 2001;122(4):527–535. [PubMed] [Google Scholar]
- 10.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340(19):1449–1454. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 11.Cao L, Lou X, Zou Z, et al. Folic acid attenuates hyperhomocysteinemia-induced glomerular damage in rats. Microvasc Res. 2013;89:146–152. doi: 10.1016/j.mvr.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Levi A, Cohen E, Levi M, Goldberg E, Garty M, Krause I. Elevated serum homocysteine is a predictor of accelerated decline in renal function and chronic kidney disease: A historical prospective study. Eur J Intern Med. 2014;25(10):951–955. doi: 10.1016/j.ejim.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Jaddoe VWV, van Duijn CM, Franco OH, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27(9):739–756. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- 14.Bergen NE, Jaddoe VW, Timmermans S, et al. Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: the Generation R Study. Bjog. 2012;119(6):739–751. doi: 10.1111/j.1471-0528.2012.03321.x. [DOI] [PubMed] [Google Scholar]
- 15.Simpson JL, Bailey LB, Pietrzik K, Shane B, Holzgreve W. Micronutrients and women of reproductive potential: required dietary intake and consequences of dietary deficiency or excess. Part I--Folate, Vitamin B12, Vitamin B6. J Matern Fetal Neonatal Med. 2010;23(12):1323–1343. doi: 10.3109/14767051003678234. [DOI] [PubMed] [Google Scholar]
- 16.van den Hil LCL, Taal HR, de Jonge LL, et al. Maternal first-trimester dietary intake and childhood blood pressure: the Generation R Study. British Journal of Nutrition. 2013;110(8):1454–1464. doi: 10.1017/S0007114513000676. [DOI] [PubMed] [Google Scholar]
- 17.Miliku K, Voortman T, Bakker H, Hofman A, Franco OH, Jaddoe VW. Infant Breastfeeding and Kidney Function in School-Aged Children. Am J Kidney Dis. 2015;66(3):421–428. doi: 10.1053/j.ajkd.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Geelhoed JJ, Taal HR, Steegers EA, et al. Kidney growth curves in healthy children from the third trimester of pregnancy until the age of two years. The Generation R Study. Pediatr Nephrol. 2010;25(2):289–298. doi: 10.1007/s00467-009-1335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zappitelli M, Parvex P, Joseph L, et al. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006;48(2):221–230. doi: 10.1053/j.ajkd.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 21.Donaghue KC, Chiarelli F, Trotta D, et al. ISPAD Clinical Practice Consensus Guidelines 2006-2007. Microvascular and macrovascular complications. Pediatr Diabetes. 2007;8(3):163–170. doi: 10.1111/j.1399-5448.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 22.Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, et al. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr. 1998;52(8):588–596. doi: 10.1038/sj.ejcn.1600611. [DOI] [PubMed] [Google Scholar]
- 23.Statistics Netherlands. Immigrants in the Netherlands 2004 (Allochtonen in Nederland 2004) Den Haag/Heerlen: Statistics Netherlands (Centraal Bureau voor de Statistiek); 2004. [Google Scholar]
- 24.El Assaad MA, Topouchian JA, Darne BM, Asmar RG. Validation of the Omron HEM-907 device for blood pressure measurement. Blood Press Monit. 2002;7(4):237–241. doi: 10.1097/00126097-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–311. discussion 312-303. [PubMed] [Google Scholar]
- 26.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkesworth S, Wagatsuma Y, Kahn AI, et al. Combined food and micronutrient supplements during pregnancy have limited impact on child blood pressure and kidney function in rural Bangladesh. J Nutr. 2013;143(5):728–734. doi: 10.3945/jn.112.168518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groen In 't Woud S, Renkema KY, Schreuder MF, et al. Maternal risk factors involved in specific congenital anomalies of the kidney and urinary tract: A case-control study. Birth Defects Res A Clin Mol Teratol. 2016;106(7):596–603. doi: 10.1002/bdra.23500. [DOI] [PubMed] [Google Scholar]
- 29.Mierzecki A, Makarewicz-Wujec M, Kloda K, Kozlowska-Wojciechowska M, Pienkowski P, Naruszewicz M. Influence of folic acid supplementation on coagulation, inflammatory, lipid and kidney function parameters in subjects with low and moderate content of folic acid in the diet. Kardiol Pol. 2014 doi: 10.5603/KP.a2014.0209. [DOI] [PubMed] [Google Scholar]
- 30.Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP, Jr, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J Nutr. 2009;139(8):1575–1581. doi: 10.3945/jn.109.106666. [DOI] [PubMed] [Google Scholar]
- 31.Elian KM, Hoffer LJ. Hydroxocobalamin reduces hyperhomocysteinemia in end-stage renal disease. Metabolism. 2002;51(7):881–886. doi: 10.1053/meta.2002.32800. [DOI] [PubMed] [Google Scholar]
- 32.McMahon GM, Hwang SJ, Tanner RM, et al. The association between vitamin B12, albuminuria and reduced kidney function: an observational cohort study. BMC Nephrol. 2015;16:7. doi: 10.1186/1471-2369-16-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tobin KA, Holven KB, Retterstol K, et al. Cystatin C levels in plasma and peripheral blood mononuclear cells among hyperhomocysteinaemic subjects: effect of treatment with B-vitamins. Br J Nutr. 2009;102(12):1783–1789. doi: 10.1017/S0007114509991048. [DOI] [PubMed] [Google Scholar]
- 34.Ladefoged J, Pedersen F. Relationship between roentgenological size of the kidney and the kidney function. J Urol. 1968;99(3):239–240. doi: 10.1016/S0022-5347(17)62684-3. [DOI] [PubMed] [Google Scholar]
- 35.Francis ME, Eggers PW, Hostetter TH, Briggs JP. Association between serum homocysteine and markers of impaired kidney function in adults in the United States. Kidney Int. 2004;66(1):303–312. doi: 10.1111/j.1523-1755.2004.00732.x. [DOI] [PubMed] [Google Scholar]
- 36.Jager A, Kostense PJ, Nijpels G, et al. Serum homocysteine levels are associated with the development of (micro)albuminuria: the Hoorn study. Arterioscler Thromb Vasc Biol. 2001;21(1):74–81. doi: 10.1161/01.atv.21.1.74. [DOI] [PubMed] [Google Scholar]
- 37.Birn H. The kidney in vitamin B12 and folate homeostasis: characterization of receptors for tubular uptake of vitamins and carrier proteins. Am J Physiol Renal Physiol. 2006;291(1):F22–36. doi: 10.1152/ajprenal.00385.2005. [DOI] [PubMed] [Google Scholar]
- 38.Oosterbaan AM, Steegers EA, Ursem NT. The effects of homocysteine and folic acid on angiogenesis and VEGF expression during chicken vascular development. Microvasc Res. 2012;83(2):98–104. doi: 10.1016/j.mvr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Martin H, Lindblad B, Norman M. Endothelial function in newborn infants is related to folate levels and birth weight. Pediatrics. 2007;119(6):1152–1158. doi: 10.1542/peds.2006-2706. [DOI] [PubMed] [Google Scholar]
- 40.Haynes WG. Hyperhomocysteinemia, vascular function and atherosclerosis: effects of vitamins. Cardiovasc Drugs Ther. 2002;16(5):391–399. doi: 10.1023/a:1022130217463. [DOI] [PubMed] [Google Scholar]
- 41.Chen YF, Li PL, Zou AP. Effect of hyperhomocysteinemia on plasma or tissue adenosine levels and renal function. Circulation. 2002;106(10):1275–1281. doi: 10.1161/01.cir.0000027586.64231.1b. [DOI] [PubMed] [Google Scholar]
- 42.Welham SJ, Riley PR, Wade A, Hubank M, Woolf AS. Maternal diet programs embryonic kidney gene expression. Physiol Genomics. 2005;22(1):48–56. doi: 10.1152/physiolgenomics.00167.2004. [DOI] [PubMed] [Google Scholar]
- 43.Ziffer H, Baker H, Pasher I, Sobotka H. A comparison of maternal and foetal folic acid and vitamin B12 at parturition. Br Med J. 1958;1(5077):978–979. doi: 10.1136/bmj.1.5077.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009;114(6):1326–1331. doi: 10.1097/AOG.0b013e3181c2bde8. [DOI] [PubMed] [Google Scholar]
- 45.Scholbach T, Weitzel D. Body-surface-area related renal volume: a common normal range from birth to adulthood. Scientifica (Cairo) 2012;2012:949164. doi: 10.6064/2012/949164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55(1):24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.