Abstract
The intestinal epithelium has important transport and barrier functions that play key roles in normal physiological functions of the body while providing a barrier to foreign particles. Impaired epithelial transport (ion, nutrient, or drugs) has been associated with many diseases and can have consequences that extend beyond the normal physiological functions of the transporters, such as by influencing epithelial integrity and the gut microbiome. Understanding the function and regulation of transport proteins is critical for the development of improved therapeutic interventions. The biggest challenge in the study of epithelial transport is developing a suitable model system that recapitulates important features of the native intestinal epithelial cells. Several in vitro cell culture models, such as Caco-2, T-84, and HT-29-Cl.19A cells are typically used in epithelial transport research. These cell lines represent a reductionist approach to modeling the epithelium and have been used in many mechanistic studies, including their examination of epithelial-microbial interactions. However, cell monolayers do not accurately reflect cell-cell interactions and the in vivo microenvironment. Cells grown in 3D have shown to be promising models for drug permeability studies. We show that Caco-2 cells in 3D can be used to study epithelial transporters. It is also important that studies in Caco-2 cells are complemented with other models to rule out cell specific effects and to take into account the complexity of the native intestine. Several methods have been previously used to assess the functionality of transporters, such as everted sac and uptake in isolated epithelial cells or in isolated plasma membrane vesicles. Taking into consideration the challenges in the field with respect to models and the measurement of transport function, we demonstrate here a protocol to grow Caco-2 cells in 3D and describe the use of an Ussing chamber as an effective approach to measure serotonin transport, such as in intact polarized intestinal epithelia.
Keywords: Cellular Biology, Issue 121, transport function, stripping mucosa, Ussing Chamber, 3D Caco-2, staining of 3D cells, serotonin transporter, SERT
Introduction
The intestinal epithelium is equipped with various transport proteins (channels, ATPases, co-transporters and exchangers) that perform numerous functions ranging from the absorption of nutrients, electrolytes, and drugs to the secretion of fluid and ions in the lumen. Transport proteins generate electrochemical gradients that permit the movement of ions or molecules in a vectorial manner. This is achieved by the asymmetric distributions of the transport systems in the apical and basolateral membranes of polarized epithelial cells. In addition, tight junctions, which tether adjacent epithelial cells, play an important role in this process by serving as a barrier to the intramembrane diffusion of components between the apical and basolateral membrane domains. Appropriate model systems mimicking these characteristic features of the native intestine (i.e. polarity, differentiation, and tight junction integrity) are critical for the study of the functionality of epithelial transport systems.
With respect to models, the typical cell lines used currently in intestinal epithelial transport research are Caco-2, a model of fully differentiated, absorptive small intestinal epithelial cells; and T84 cells or HT-29 subclones, models of crypt-derived large intestinal epithelial cells1. Conventionally, these cell lines are grown as monolayers in plastic surfaces or in coated transwell inserts. Trans-well cell culture inserts to some extent resemble the in vivo environment by allowing polarized cells to feed basolaterally. However, a limitation in conventional 2D culture systems is that the cells are forced to adapt to an artificial, flat, and rigid surface. Thus, the physiological complexity of the native epithelia is not accurately reflected in a 2D system. This limitation has been overcome by methods to grow cells in 3D in a specific microenvironment, such as gelatinous protein mixture, containing a variety of extracellular matrix components2,3. Nevertheless, the in vitro cultures cannot simulate the complexity of the intestinal epithelium, which has multiple cell types and region-specific architecture in the intestine. Thus, the studies using cell culture models require further validation in the native intestine. Using the in vitro 3D cell culture and mouse intestinal mucosa, we describe here simple methods to study the regulation of the intestinal serotonin transporter (SLC6A4, SERT).
The SERT transporter regulates the extracellular availability of an important hormone and neurotransmitter, 5-hydroxytryptamine (5-HT), by rapidly transporting it through a Na+/Cl--dependent process. SERT is a known target of anti-depressants and has recently emerged as a novel therapeutic target of GI disorders, such as diarrhea and intestinal inflammation. Methods to investigate 5-HT uptake in intestinal epithelial cells have been previously described. For example, Caco-2 cells grown on plastic supports or permeable inserts have been shown to exhibit fluoxetine-sensitive 3H-5-HT uptake at both apical and basolateral domains4. The measurement of SERT function in isolated Brush Border Membrane Vesicles (BBMVs) prepared from human organ donor small intestines has also been described by us5. 3H-5-HT uptake in human BBVMs was shown to be fluoxetine-sensitive and Na+/Cl--dependent, and it exhibited saturation kinetics with a Km of 300 nM5. Utilizing similar methods, we also previously measured SERT function as 3H-5-HT uptake in mouse intestinal BBMVs6. However, the preparation of pure plasma membrane vesicles requires a large amount of tissue mucosa. Other methods, such as the radiographic visualization of 3H-5-HT uptake sites, have also been shown previously in guinea pig and rat small intestines7.
The Ussing chamber provides a more physiological system to measure the transport of ions, nutrients, and drugs across various epithelial tissues. The main advantage of the Ussing chamber technique is that it enables the precise measurement of the electrical and transport parameters of intact, polarized intestinal epithelium. Further, to minimize the influence of the intrinsic neuromuscular system, seromuscular stripping of intestinal mucosa can be performed to investigate the regulation of transporters in the epithelium8.
We demonstrate that Caco-2 cells grown in 3D on gelatinous protein form a hollow lumen expressing distinct apical and basolateral markers. These cells show higher expression of SERT than 2D Caco-2 cells. Methods to grow 3D cells and to perform immunostaining or RNA and protein extraction are described. In addition, we describe methods to study SERT function and regulation by TGF-β1, a pleiotropic cytokine, in small intestinal mucosa by utilizing an Ussing chamber technique.
Protocol
1. Study of Intestinal Transporters in a 3D Culture System of Caco-2: Growing 3D Caco-2 Cell Culture on a Gelatinous Protein Mixture
Thaw growth factor reduced gelatinous protein mixture on ice for 8 h (or O/N) at 4 °C. Once thawed, make 1 mL or 500 µL aliquots for use or store at -20 °C for later use.
On the day of culture, precool the culture plates (for RNA and protein extraction) or chambered slides (for immunostaining) on ice. Add 30 µL of gelatinous protein mixture to each well of an eight-well chambered-glass slide (or 120 µL per well of a 6-well plate) and spread evenly. Take care not to generate air bubbles during the process.
Place the slides/plates inside a cell culture incubator at 37 °C for 15 - 30 min and allow the gelatinous protein mixture to solidify.
While the gelatinous protein mixture is solidifying, trypsinize a confluent flask of Caco-2 cells.
Count the number of cells and spin them at 500 x g in a tabletop centrifuge for 5 min. Re-suspend the cell pellet in 3D Caco-2 Medium as needed.
Seed the glass chamber slides at 4,000 cells per well (for plates, 20,000 per well). Allow the cells to grow in a 5% CO2 humidified incubator at 37 °C. Change medium every 3 - 4 d.
2. Immunofluorescence Staining for Epithelial Transporters in 3D Caco-2 Cells: Day 1
Aspirate the medium and fix the cells with 400 µL of 2% paraformaldehyde (PFA) (in PBS with the pH adjusted to 8.5 with NaOH) for 30 min at RT. NOTE: PFA solution should not be stored for more than 1 week at 4 °C, and freshly made solution can be used. CAUTION: PFA is highly toxic and a potential carcinogen. Always handle it with caution in a chemical hood and using personal protective equipment.
Rinse the wells twice with 1x PBS and store the slides at 4 °C in PBS (400 µL/well). Once fixed, store them at 4 °C for up to one week.
Warm the chamber slides to RT (this will harden the gelatinous protein mixture bed and make it easy to aspirate during the washing steps).
Permeabilize the cells with 0.5% Triton in PBS for no longer than 15 min.
Prepare PBS-glycine buffer (130 mM NaCl, 7 mM Na2HPO4, 3.5 mM NaH2PO4, and 100 mM Glycine). Rinse 3x with 1x PBS-glycine for 10 min each at RT.
Prepare immunofluorescence buffer (IF): 130 mM NaCl, 7 mM Na2HPO4, 3.5 mM NaH2PO4, 100 mM Glycine, 7.7 mM NaN3, 0.1% BSA, 0.2% TritonX-100, and 0.05% Tween-20.
Wash 3x in IF buffer for 10 min each at RT. Block in 5% Normal Goat Serum (NGS) in IF buffer. Incubate with primary antibody diluted in IF + 1% goat serum, 200 µL/well, for 1 - 2 h at RT. Wash with IF buffer 3x for 10 min each.
Incubate with secondary antibody (1:200) diluted in IF + 1% NGS, 200 µL/well, for 1 h at RT.
Wash with IF buffer three times for 10 min each. Keep the slides in the dark from this step on. Lift the plastic chambers from the glass slides by placing the chamber slide in a black holder and sliding a white lifter through the holder until its edge contacts the edge of the wells. Gently pull up the chambers (the holder and lifter should come with the culture slides).
Mount with slow anti-fade medium and allow it to dry for 10 min at RT.
Once dried completely, seal the slides with nail polish and image them with confocal microscopy. Store the slides at 4 °C for two weeks or at -20 °C for several months.
3. RNA and Protein Extraction from 3D Caco-2 Cells
Treat the cells cultured on gelatinous protein mixture for 12 - 14 d with a test agent, such as TGF- β1 (10 ng/mL, 1 h).
After treatment, wash the cells with 1x PBS. Keep the cells on ice for 30 min to liquefy the gelatinous protein mixture.
Wash the wells with chilled PBS and collect the cells in individual centrifuge tubes. Collect the cells using low-speed centrifugation at 500 x g and 4 °C for 10 min. Extract the RNA using standard procedures and use real time RT-PCR.
For the protein extraction, lyse the cells using 1x cell lysis buffer supplemented with 1x protease cocktail inhibitor. After adding cell lysis buffer to the pellet, lyse the cells by sonication and remove the cell debris by centrifugation at 7,500 x g and 4 °C for 10 min.
4. Measurement of 5-HT Uptake in Mouse Ileum Utilizing an Ussing Chamber: Intestinal Preparation and Seromuscular Stripping
Following euthanasia, dissect the mice and remove the small intestines (after the stomach and before the cecum). Divide the small intestine into three parts. Discard the middle third, use the proximal third as jejunum, and use the distal third as ileum. Avoid pulling the intestine from its mesenteric attachment to prevent damage to the epithelium. Keep the tissue section cold by covering it with ice-cold Krebs-Ringer bicarbonate (KBR) buffer.
Open the intestine longitudinally using scissors. Incubate freshly excised intestinal sections in ice-cold, gassing KBR containing 1 µM indomethacin for 10 min before the seromuscular stripping.
Pin the intestinal section (~1 cm in length) mucosal-side down to a plate containing 7% agarose or cured silicone elastomer (0.5 cm thick).
Strip the seromuscular layers under a dissection stereomicroscope with bottom illumination. Cut the seromuscular layer using a feather scalpel blade. Use fine forceps to obtain the edge of the layer along the longitudinal axis of the intestine. NOTE: During the stripping procedure, the bottom lighting of the stereomicroscope helps to identify and avoid areas with Peyer's patches.
After the completion of the seromuscular stripping, mount the mucosa carefully on the pins of an Ussing half-chamber. During the whole process, take care to hold the stripped mucosal tissue by the edges to avoid tearing it.
5. Equilibration and Tissue Treatment
Prepare Kreb's solution: 115 mM NaCl, 25 mM NaHCO3, 2.4 mM K2HPO4, 1.2 mM CaCl2, 1.2 mM MgCl2, and 0.4 mM KH2PO4 at pH 7.4, gassed with 95% O2/5% CO2 at 37 °C. Add 10 mM glucose to the serosal bath to serve as an energy substrate and 10 mM mannitol to the mucosal bath to maintain the osmotic balance. NOTE: Kreb's solution is supplemented with L-ascorbic acid (100 µM) and pargyline (100 µM, monoamine oxidase inhibitor) to prevent intracellular 5-HT degradation.
Insert the slider into the chamber to expose both the apical (luminal) side and the basolateral (serosal) side of the tissue to Kreb's solution.
After an equilibration period of 10 min, pretreat the ileal tissue with fluoxetine (10 µM) for 30 min on the apical side for Jm-s (mucosal-to-serosal flux).
Treat the tissue with TGF-β1 (10 ng/mL) for 1 h on basolateral side and then incubate it with 3[H]-5-HT (20 nM) for 30 min on the apical side.
6. Quantification of Mucosal to Serosal Flux (Jm-s) and 3[H]-5-HT Accumulation in the Tissue
Collect 0.75 mL aliquots from the serosal reservoir (total of 5 mL) to measure the radioactivity in a liquid scintillation counter and to calculate mucosal-to-serosal (Jm-s) flux rates; fluoxetine-sensitive flux represents SERT-mediated 3[H]-5-HT uptake.
To avoid hydrostatic pressure differences across the mucosa during a flux period, take samples from the serosal side and replace them with identical volumes of bath medium. NOTE: The flux calculation corrects for the dilution of the end sample (4.25 mL / 5 mL = 0.85).
For the quantification of 3[H]-5-HT accumulated in the tissue, dismount the mucosa from the slider, wash it once with ice-cold KRB buffer, and place it in glass culture tubes.
Incubate the mucosa in 0.5 mL of 10% KOH overnight at 37 °C. Measure the radioactivity in 150 µL aliquots of the lysates (in triplicates) using a liquid scintillation counter.
Use aliquots (3 - 5 µL) of the lysates to measure protein concentration using the Bradford method. NOTE: 10 mL of incubation medium containing radioactive serotonin is used as a standard. 5-HT retained in the tissue is expressed as pmol/mg protein/min.
Representative Results
Immunostaining in 3D Caco-2 cysts is shown in Figure 1. A representative image of an XY-plane showing well-demarcated lumen in the 3D cyst stained with phalloidin actin is depicted in Figure 1A. The side facing the lumen denotes the apical side. Epithelial cells cultured in a 3D environment result in a robust epithelial phenotype. Different Caco-2 cysts on day 12, when actin and SERT were co-stained, are shown in Figure 1B. In this representation of an XY-plane (different from the plane shown in Figure 1A), SERT staining is visible, predominantly in the luminal membrane, along with sub-apical staining. Figure 2 shows that SERT protein levels are higher in 3D cysts as compared to Caco-2 cells grown as monolayers on the 12th day postplating. These data suggest that the 3D culture of Caco-2 cells may trigger signaling events that mimic native epithelia and result in increased SERT expression. Figure 3A depicts the general overview and operation of an Ussing chamber system and shows the mounted small intestine in the pins of the aperture. This technique allows for the evaluation of the activity of epithelial transporters in the native intestine. The advantage of the Ussing chamber approach is the ability to assess the function of transporters expressed on the luminal (mucosa) or basolateral (serosal) side. Figure 4 demonstrates increased 5-HT Jm-s flux (A) and increased 5-HT accumulation in the ileal mucosa (B) in response to TGF-β. Since TGF-β1 increased the movement of radiolabeled 5-HT from the mucosal to serosal side, as represented by the Jm-s, these data are indicative of an increase in 5-HT uptake from the luminal membrane of ileal epithelial cells. This is further evident from an increase in the accumulation of radiolabeled 5-HT in the cell, which was sensitive to inhibition by fluoxetine, reflecting the activity of SERT expressed on the luminal membrane.
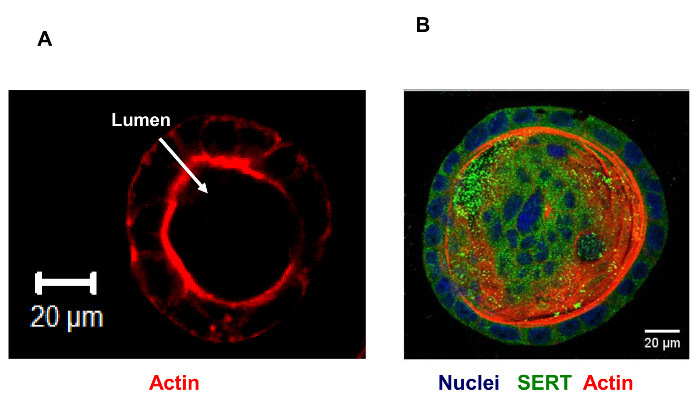 Figure 1:Caco-2 Grown on a Gelatinous Protein Mixture at 12 d Showing Distinct Lumen. Red: actin. For the immunostaining of Caco-2 cysts grown in 3D, cells were cultured in an 8-well chambered slide and stained with phalloidin (A), as described previously by Debnath et al.9. For immunostaining the 3D Caco-2 culture (B), cysts were fixed in 2% PFA for 30 min, treated with PBS-glycine, and permeabilized with 0.5% Triton-X-100 for 15 min at RT. After blocking, the cysts were incubated in SERT antibody (1:100) overnight at 4 °C. They were then washed and incubated with fluorescence-conjugated secondary antibodies (1:200) and phalloidin (1:200) for 45 min at RT. Finally, they were mounted in antifade containing DAPI. Images were captured using a confocal microscope. Scale bar: 20 µm. Please click here to view a larger version of this figure.
Figure 1:Caco-2 Grown on a Gelatinous Protein Mixture at 12 d Showing Distinct Lumen. Red: actin. For the immunostaining of Caco-2 cysts grown in 3D, cells were cultured in an 8-well chambered slide and stained with phalloidin (A), as described previously by Debnath et al.9. For immunostaining the 3D Caco-2 culture (B), cysts were fixed in 2% PFA for 30 min, treated with PBS-glycine, and permeabilized with 0.5% Triton-X-100 for 15 min at RT. After blocking, the cysts were incubated in SERT antibody (1:100) overnight at 4 °C. They were then washed and incubated with fluorescence-conjugated secondary antibodies (1:200) and phalloidin (1:200) for 45 min at RT. Finally, they were mounted in antifade containing DAPI. Images were captured using a confocal microscope. Scale bar: 20 µm. Please click here to view a larger version of this figure.
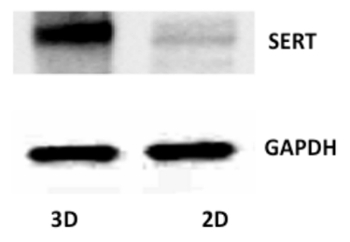 Figure 2:SERT Protein Expression in Caco-2 Cells Grown in 2D and 3D. Lysates from Caco-2 cells cultured on a plastic support and Caco-2 3D spheres were resolved by SDS-PAGE blotted onto nitrocellulose membranes and analyzed with the SERT antibody and GAPDH. Please click here to view a larger version of this figure.
Figure 2:SERT Protein Expression in Caco-2 Cells Grown in 2D and 3D. Lysates from Caco-2 cells cultured on a plastic support and Caco-2 3D spheres were resolved by SDS-PAGE blotted onto nitrocellulose membranes and analyzed with the SERT antibody and GAPDH. Please click here to view a larger version of this figure.
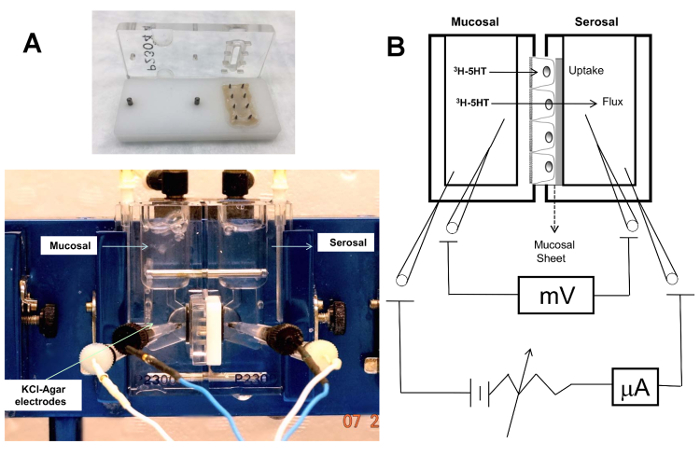 Figure 3:(A) The Ussing chamber and "slider" with the pins and aperture showing a mounted mouse small intestine. Slider aperture area = 0.30 cm2. The slider fits into the two halves of the Ussing chamber, which are compartmentalized. Electrical parameters, such as transepithelial voltage potential (Vt), is measured by a potentiometer and electrodes (typically calomel half-cells or Ag-AgCl are connected by salt bridges to each chamber half). Salt bridges are composed of 3% agar melted in the physiological buffer (e.g., KBR) used for bathing the mucosa to avoid alterations in the ionic composition of the solutions. (B) Schematic of the principle of Ussing chamber. The stripped intestinal mucosa was mounted in the two halves of the Ussing chamber separating the mucosal and serosal chambers. Radioactive tracer was added to the mucosal side; the uptake was measured as the amount of radioactivity incorporated by the tissue, normalized to the total protein content. The flux was evaluated by measuring the radioactivity in the serosal chamber over a period of time. The electrodes shown in the schematic allow for the simultaneous monitoring of electrical parameters, the including current, voltage, and resistance. Please click here to view a larger version of this figure.
Figure 3:(A) The Ussing chamber and "slider" with the pins and aperture showing a mounted mouse small intestine. Slider aperture area = 0.30 cm2. The slider fits into the two halves of the Ussing chamber, which are compartmentalized. Electrical parameters, such as transepithelial voltage potential (Vt), is measured by a potentiometer and electrodes (typically calomel half-cells or Ag-AgCl are connected by salt bridges to each chamber half). Salt bridges are composed of 3% agar melted in the physiological buffer (e.g., KBR) used for bathing the mucosa to avoid alterations in the ionic composition of the solutions. (B) Schematic of the principle of Ussing chamber. The stripped intestinal mucosa was mounted in the two halves of the Ussing chamber separating the mucosal and serosal chambers. Radioactive tracer was added to the mucosal side; the uptake was measured as the amount of radioactivity incorporated by the tissue, normalized to the total protein content. The flux was evaluated by measuring the radioactivity in the serosal chamber over a period of time. The electrodes shown in the schematic allow for the simultaneous monitoring of electrical parameters, the including current, voltage, and resistance. Please click here to view a larger version of this figure.
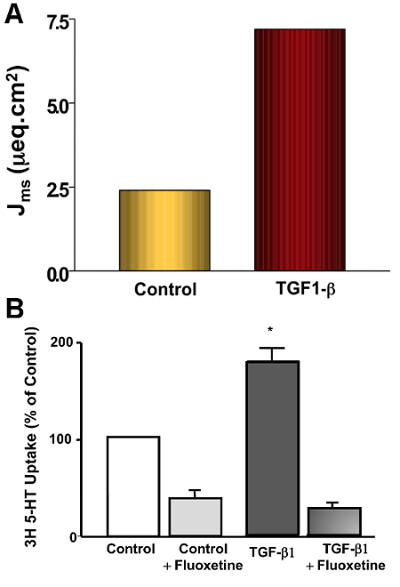 Figure 4:Measurement of SERT Function in Ileal Mucosa Stripped of the Seromuscular Layer.Ex vivo effects of short-term TGF-β1 treatment on SERT function in the mouse ileum, demonstrated through the measurement of 3[H]-5-HT uptake and 3[H]-5-HT fluxes using the Ussing chamber. TGF-β1 treatment (10 ng/mL, 1 h) on the basolateral side significantly increased SERT function, as evidenced by the increased Na+-sensitive mucosal-to-serosal flux (Jm-s) (A) and 3[H]-5-HTuptake (B). Fluoxetine treatment of the ileal tissue inhibited TGF-β1-stimulated 5-HT uptake. Fluoxetine-sensitive uptake was increased 2.5-fold by TGF-β1 compared to the control. Results are represented as the mean SEM. A one-way ANOVA Tukey's Test was used for the statistical analysis. Calculation of Jm-s flux: [(CPMe - CPMs) x Dilution factor] / [CPMstd / (Vstd x conc. of substrate) x t x a]. CPMe: counts per min at the end of the flux measurement; CPMs: counts per min at the start of the flux measurement; CPMstd: counts per min of standard (taken from the mucosal reservoir); Vstd: volume of the CPMstd; t: time of flux in h; a: area of the aperture (0.3 cm2). Please click here to view a larger version of this figure.
Figure 4:Measurement of SERT Function in Ileal Mucosa Stripped of the Seromuscular Layer.Ex vivo effects of short-term TGF-β1 treatment on SERT function in the mouse ileum, demonstrated through the measurement of 3[H]-5-HT uptake and 3[H]-5-HT fluxes using the Ussing chamber. TGF-β1 treatment (10 ng/mL, 1 h) on the basolateral side significantly increased SERT function, as evidenced by the increased Na+-sensitive mucosal-to-serosal flux (Jm-s) (A) and 3[H]-5-HTuptake (B). Fluoxetine treatment of the ileal tissue inhibited TGF-β1-stimulated 5-HT uptake. Fluoxetine-sensitive uptake was increased 2.5-fold by TGF-β1 compared to the control. Results are represented as the mean SEM. A one-way ANOVA Tukey's Test was used for the statistical analysis. Calculation of Jm-s flux: [(CPMe - CPMs) x Dilution factor] / [CPMstd / (Vstd x conc. of substrate) x t x a]. CPMe: counts per min at the end of the flux measurement; CPMs: counts per min at the start of the flux measurement; CPMstd: counts per min of standard (taken from the mucosal reservoir); Vstd: volume of the CPMstd; t: time of flux in h; a: area of the aperture (0.3 cm2). Please click here to view a larger version of this figure.
Discussion
Fully differentiated Caco-2 cell monolayers have been used extensively as polarized epithelial monolayers to study intestinal transport10,11,12,13,14,15. However, to mimic the physiological organization of intestinal epithelial cells, there has been considerable interest in the development of a Caco-2 culture in 3D. A number of 3D cell culture models for intestinal epithelial cells have been recently developed that mimic native cell-cell and cell-extracellular matrix (ECM) interactions with greater physiological relevance than 2D systems16. Thus, ECM-based natural hydrogels containing common components (such as laminin, collagen IV, and heparin sulfate proteoglycan) are extensively used for the generation of 3D cell cultures16.
The protocol described here demonstrates the suitability of 3D Caco-2 cells grown in gelatinous protein mixture (an ECM-based natural hydrogel extracted from Engelbreth-Holm-Swarm (EHS) mouse tumors) for epithelial transport research. Within 10 days, the cells start to form a hollow lumen surrounded by a layer of cells and show polarity, with distinct apical and basolateral domains. The cell density at the time of seeding on a gelatinous protein mixture is critical for the formation of regular cysts with central lumen. For staining experiments, we added 4,000 cells/well of an 8-chamber slide (well surface area: 0.7 cm2). Seeding cells at a higher density results in irregular spheres. The 3D Caco-2 cysts at 10 - 12 days show polarity and more differentiation than 2D Caco-2 cells grown on transwells17 (unpublished observations). We have shown a marked increase in the expression of SERT mRNA and protein levels as compared to fully differentiated 2D monolayers. Also, utilizing these methods, we have shown that TGF-TGF-β1 increases the surface levels of SERT, as shown by immunofluorescence staining15.
Interestingly, recent studies have demonstrated that 3D Caco-2 cells are more responsive to pathophysiological stimuli because of the presence of critical signaling components, such as TNF receptor 2 and MyD88, and represent a better model than the conventional 2D system17. However, one issue that limits the use of 3D Caco-2 in epithelial transport research is the lack of the different cell types and the complexity that are found in the native intestine. In this regard, intestinal organoids (also known as enteroids/colonoids) of human or mouse origin represent a state-of-the-art model containing all different cell types of native epithelium to study the transport of ions, nutrients, or drugs. As compared to organoids, well-differentiated 3D Caco-2 cysts represent one type of epithelial cells (i.e. absorptive enterocytes) and are certainly suitable for studies focusing on the cellular and molecular mechanisms governing intestinal transporters. This is because the 3D Caco-2 cells are convenient to handle, are cost-effective, and are easy to manipulate, such as for transfections of plasmid DNA and siRNAs. Another limitation of 3D Caco-2 cells in epithelial transport research is the difficulty in accessing the lumen. Agents that bind to luminal membrane receptors or to infectious agents that access the epithelium from the luminal side may be challenging to study in this system. This limitation can be overcome by microinjecting the agents in the lumen, as has been done previously in cases of human intestinal organoid culture for C. difficile infections18. It is, however, important that studies in in vitro cell culture models are validated in the native intestine using in vivo or ex vivo approaches.
Over the last several decades, the Ussing chamber has contributed greatly to the recognition of the functional or regulatory properties of many epithelial transporters19. Here, we show the suitability of the Ussing chamber approach to measure 5-HT flux and 5-HT uptake in mouse intestinal mucosa devoid of the seromuscular layer15. TGF-β1 treatment of the ileal mucosa on the basolateral side (10 ng/mL, 1 h) significantly increased SERT function, as evidenced by increased Na+-sensitive mucosal-to-serosal flux (Jm-s) and 3H-5-HTuptake. Fluoxetine treatment of the ileal tissue inhibited the TGF-β1-mediated stimulation of 5-HT uptake.
Critical steps of this technique include mounting the tissue on the slide aperture as an intact layer (without any tearing) in order to achieve accurate measurements. Appropriate surgical tools, such as ball scissors, thin-tip forceps, and feather surgical blades, are important for the appropriate handling and stripping of tissue.
This method is applicable to study the function of other epithelial transporters, such as electrolyte transporters (chloride transporters, Na+/H+ exchangers, nutrient transporters, etc.). Specialized uses of the Ussing chamber-such as the pH stat technique, to measure transepithelial bicarbonate secretion, and the isotopic flux method, to measure net secretion or absorption of substrates-have been reviewed comprehensively by Dr. Clarke et al.8. They extensively reviewed the application of Ussing chambers to the study of nutrient transport19. Mini Ussing chambers have also been used in clinically relevant situations, such as to measure transport function in biopsy samples20. The major advantage of the Ussing chamber technique over the everted sac method is the ability to simultaneously measure the electrical and transport parameters of intact, polarized intestinal epithelium.
One concern with the Ussing chamber method is the limited viability and optimal function of an ex vivo intestinal preparation. Upon removal from the animal, the ex vivo intestinal preparation may only last for up to 3 h in an Ussing chamber19. Thus, this method may have limitations in testing the effect of agents for longer time periods. In such cases, treatment can be performed in vivo and then isolated intestines from control and treatment groups can be used to measure the transport parameters with the Ussing method. In all cases, the magnitude of the Gt (or Rt) can be a real-time indicator of viability and can be useful in discriminating between the specific and nonspecific effects of tested reagents.
In summary, the methods described here can facilitate our understanding of the cellular and molecular mechanisms governing the function and regulation of epithelial transporters in healthy and diseased conditions, such as intestinal inflammation, infectious diarrhea, malabsorption, or metabolic disorders.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank Mr. Christopher Manzella, MD/PhD student in the RKG laboratory, for helping to edit and proofread our manuscript.
References
- Simon-Assmann P, Turck N, Sidhoum-Jenny M, Gradwohl G, Kedinger M. In vitro models of intestinal epithelial cell differentiation. Cell Biol Toxicol. 2007;23(4):241–256. doi: 10.1007/s10565-006-0175-0. [DOI] [PubMed] [Google Scholar]
- Shen C, Meng Q, Zhang G. Design of 3D printed insert for hanging culture of Caco-2 cells. Biofabrication. 2014;7(1):015003. doi: 10.1088/1758-5090/7/1/015003. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183(4):625–633. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel F, Monteiro R, Lemos C. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT) J Pharmacol Exp Ther. 2003;306(1):355–362. doi: 10.1124/jpet.103.049668. [DOI] [PubMed] [Google Scholar]
- Gill RK, et al. Function, expression, and characterization of the serotonin transporter in the native human intestine. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G254–G262. doi: 10.1152/ajpgi.00354.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaili A, et al. Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology. 2009;137(6):2074–2083. doi: 10.1053/j.gastro.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade PR, et al. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16(7):2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296(6):G1151–G1166. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30(3):256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Vedeler A, Pryme IF, Hesketh JE. Insulin induces changes in the subcellular distribution of actin and 5'-nucleotidase. Mol Cell Biochem. 1991;108(1):67–74. doi: 10.1007/BF00239543. [DOI] [PubMed] [Google Scholar]
- Botvinkin AD, Selimov MA, Zgurskaia GN, Kulikov LG, Aksenova TA. [Detection of rabies virus antibodies in human blood serum by an immunoenzyme method] Vopr Virusol. 1986;31(3):333–334. [PubMed] [Google Scholar]
- Woods GL, Mills RD. Effect of dexamethasone on detection of herpes simplex virus in clinical specimens by conventional cell culture and rapid 24-well plate centrifugation. J Clin Microbiol. 1988;26(6):1233–1235. doi: 10.1128/jcm.26.6.1233-1235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase AO. Acyclovir for shingles. Br Med J (Clin Res Ed. 1987;295(6603):926–927. doi: 10.1136/bmj.295.6603.926-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill PF, Churchill SA, Martin MV, Guengerich FP. Characterization of a rat liver cytochrome P-450UT-H cDNA clone and comparison of mRNA levels with catalytic activity. Mol Pharmacol. 1987;31(2):152–158. [PubMed] [Google Scholar]
- Steiner M, Tateishi T. Distribution and transport of calcium in human platelets. Biochim Biophys Acta. 1974;367(2):232–246. doi: 10.1016/0005-2736(74)90046-7. [DOI] [PubMed] [Google Scholar]
- McClelland M, Nelson M, Cantor CR. Purification of Mbo II methylase (GAAGmA) from Moraxella bovis: site specific cleavage of DNA at nine and ten base pair sequences. Nucleic Acids Res. 1985;13(20):7171–7182. doi: 10.1093/nar/13.20.7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee Ishita, K A, Gill Ravinder K, Alrefai Waddah A, Dudeja Pradeep K. 3D Cell Culture of CaCo2: A Better Model to Study the Functionality and Regulation of Intestinal Ion Transporters. Gastroenterology. 2014;146(5, Supplement 1):S-654. [Google Scholar]
- Fagg RH, Hyslop NS. Isolation of a variant strain of foot-and-mouth disease virus (type O) during passage in partly immunized cattle. J Hyg (Lond) 1966;64(4):397–404. doi: 10.1017/s0022172400040699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilkina NP. [The aspects of the problem of systemic vasculitis open to discussion]) Ter Arkh. 1989;61(12):102–106. [PubMed] [Google Scholar]
- Derichs N, et al. Intestinal current measurement for diagnostic classification of patients with questionable cystic fibrosis: validation and reference data. Thorax. 2010;65(7):594–599. doi: 10.1136/thx.2009.125088. [DOI] [PubMed] [Google Scholar]


