Abstract
Background
Nutritional exposures during in utero development may have long-lasting consequences for postnatal renal health. Animal studies suggest that specifically maternal dietary protein intake during pregnancy influences childhood kidney function.
Objective
We examined the associations of total, animal and vegetable maternal protein intake during pregnancy with kidney volume and function in school-age children.
Design
This study was performed in 3,650 pregnant women and their children participating in a population-based cohort study from early life onwards. First trimester energy adjusted maternal protein intake was assessed with a food frequency questionnaire. At the child’s age of 6 years, we assessed kidney volume, estimated glomerular filtration rate (eGFR) using serum creatinine and cystatin C levels, and microalbuminuria using urine albumin-creatinine ratios.
Results
First trimester maternal total protein intake was associated with a higher childhood creatinine-based eGFR (0.06 (95%CI 0.01, 0.12) ml/min/1.73m2 per gram of protein intake). This association was mainly driven by vegetable protein intake (0.22 (95%CI 0.10, 0.35) ml/min/1.73m2 per gram of vegetable protein intake). These associations were not explained by protein intake in early childhood. First trimester maternal protein intake was not significantly associated with childhood kidney volume, cystatin C-based eGFR or the risk of microalbuminuria.
Conclusion
Our findings suggest that higher total and vegetable, but not animal, maternal protein intake during first trimester of pregnancy is associated with a higher eGFR in childhood. Further follow-up studies are needed to investigate whether maternal protein intake in early pregnancy also affects the risk of kidney diseases in later life.
Introduction
Maternal nutritional exposures during pregnancy may persistently affect offspring kidney development (1). Results from the Dutch Famine Study suggest that maternal exposure to extreme famine in mid-gestation is linked to an increased risk of microalbuminuria in their adult offspring (2). The mechanisms by which suboptimal maternal nutrition affects offspring kidney development may include smaller kidneys with a reduced number of nephrons, which in turn leads to glomerular hyperfiltration and sclerosis (3). These adaptations predispose individuals to subsequent development of higher blood pressure, impaired kidney function and end-stage kidney disease in adulthood (3). Next to the evidence from the Dutch Famine Study, not much is known about more common and contemporary adverse nutritional exposures during pregnancy that influence kidney function in the offspring. Animal studies suggest that low maternal protein intake during pregnancy leads to a lower number of nephrons in their offspring and may hence impact the risk of renal disease and hypertension in later life (1, 4, 5). Nine-months old lambs whose mothers were fed with a 50% nutrient restricted diet during early-mid pregnancy, had fewer renal glomeruli compared to those whose mothers had a normal diet (4). Also, studies showed that a low protein diet in pregnant rats results in a nephron deficit in the offspring at birth which extends into postnatal life (5). Observational studies in human adults show that high dietary protein is associated with progression of renal disease (6). Interestingly, studies in adults also suggest different effects of animal versus vegetable protein on kidney health (7, 8).
Therefore, we examined, in a population-based prospective cohort study among 3,650 mothers and children, the associations of first-trimester maternal protein intake during pregnancy with child’s kidney outcomes at the age of 6 years. Main kidney outcomes were kidney volume, eGFR based on serum creatinine and cystatin C levels and microalbuminuria.
Methods
Subjects
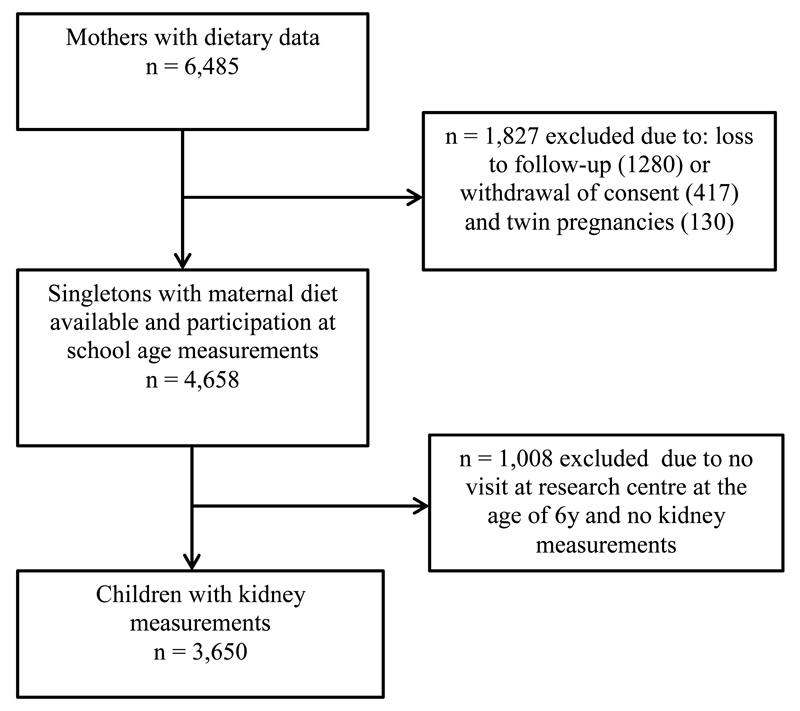
This study was embedded in the Generation R Study, a population-based prospective cohort study from fetal life onward in Rotterdam, the Netherlands (9). The study was conducted according to the guidelines of the Helsinki Declaration and approved by the Medical Ethics Committee of Erasmus Medical Center, Rotterdam. Written informed consent was obtained from all mothers. Enrolment in the study was aimed at early pregnancy, but was allowed until the birth of the child. Information about maternal diet and other lifestyle-related variables during pregnancy was collected at enrolment. At the age of 6 years, all participating children and their mothers were invited to a dedicated research center, to participate in detailed hands-on measurements. Of the 4,658 singleton live-born children with maternal nutritional data available and who participated in the study during childhood, 3,650 (78%) children attended the follow-up visit at the age of 6 years with successful measurements on kidney volume and function (Figure 1).
Figure 1.
Flow-chart of the study participants
Maternal dietary assessment
Maternal dietary intake was assessed at enrollment (median 13.5 weeks of gestation, 95% range 9.8-22.9) using a modified version of the validated semi-quantitative food frequency questionnaire (FFQ) of Klipstein-Grobusch et al. (10). The FFQ considered food intake over the previous 3 months, which mostly covered dietary intake in the first trimester of pregnancy. The FFQ consisted of 293 items structured according to the meal pattern. Questions included consumption frequency, portion size, preparation method, and additions. We estimated portion sizes by using Dutch household measures and photographs of foods that showed different portion sizes. We used the Dutch Food-Composition Table 2006 to calculate average daily intakes of total energy, carbohydrates, fat, and of total, animal, and vegetable protein (11). For this study we split protein into animal (from e.g. meat, fish, and dairy) and vegetable protein (from e.g. grains, nuts, and legumes).
Kidney outcome assessments
Children’s kidney outcomes were assessed at a median age of 6.1 years (95% range 5.6 to 7.3) in a dedicated research center in the Sophia Children’s Hospital in Rotterdam by well-trained staff.
Our main kidney outcomes were combined kidney volume, estimated glomerular filtration rate (eGFR) and microalbuminuria. Combined kidney volume is a structural developmental outcome. Post-mortem studies showed that kidney volume is correlated with the number of nephrons. We used eGFR, calculated from blood creatinine and cystatin C levels as main measure of kidney function: eGFR is a general marker of glomerular filtration used in both clinical practice and population based cohort studies. Microalbuminuria is an established predictor of chronic kidney disease and end-stage renal disease.
Kidney volume was measured with ultrasound, using an ATL-Philips HDI 5000 instrument (Seattle, WA, USA), equipped with a 2.0-5.0MHz curved array transducer. We identified the left and right kidney in the sagittal plane along its longitudinal axis. We performed measurements of maximal bipolar kidney length, width and depth. Kidney width and depth were measured at the level of the hilum. The cross-sectional area in which the kidney appeared symmetrically round at its maximum width was used. Kidney volume was calculated using the equation for a prolate ellipsoid: volume (cm3) = 0.523 x length (cm) x width (cm) x depth (cm) (12). Combined kidney volume was calculated by summing right and left kidney volume. We previously reported good intra-observer and inter-observer correlation coefficients (13).
Non-fasting blood samples were drawn by antecubital venipuncture. Blood samples were collected in plasma Li-heparin and K2-EDTA tubes and temporally stored at the research center in a fridge for a maximum of 4 hours. Twice per day the samples were transported in cool boxes to a dedicated laboratory facility (STAR-MDC, Rotterdam, the Netherlands). After transport, blood samples were centrifuged for 10 minutes and stored at -80 °C at one location in the STAR-MDC laboratory. Samples were transported on dry ice to the Erasmus Medical Centre where creatinine concentrations were measured with enzymatic methods and cystatin C levels with a particle enhanced immunoturbidimetric assay (using Cobas 8000 analyzers, Roche, Almere, the Netherlands). Quality control samples demonstrated intra-assay and inter-assay coefficients of variation ranging from 0.69 to 1.57%, and 0.87 to 2.40%, respectively.
Estimated glomerular filtration rate (eGFR) was calculated according to the revised Schwartz 2009 formula: eGFRcreat = 36.5 * (height (cm) / serum creatinine (µmol/l)) (14). Additionally, we estimated the glomerular filtration rate using Zappitelli’s formula based on cystatin C levels: eGFRCyst = 75.94 / [CysC1.17] (15). Urine creatinine (µmol/l) and urine albumin (µg/l) levels were determined with a Beckman Coulter AU analyzer, creatinine levels were measured with the Jaffe reaction. In line with clinical cut-offs, microalbuminuria was defined as an albumin-creatinine ratio between 2.5 and 25 mg/mmol for boys and between 3.5 and 25 mg/mmol for girls (16).
Covariates
Information on maternal age, ethnicity, educational level, household income, smoking during pregnancy, alcohol usage during pregnancy, and folic acid supplementation were obtained using questionnaires (9). We classified maternal ethnicity into 6 categories (Dutch, Turkish, Moroccan, Surinamese or Dutch Antillean, other western, and other non-western) (17). Maternal pre-pregnancy height and weight were self-reported and pre-pregnancy body mass index (BMI) was calculated (kg/m2). Information on the presence of pre-pregnancy comorbidities (defined as the occurrence of high cholesterol, diabetes, hypertension) was available from a questionnaire administered in the first trimester. Information on child’s sex, birthweight and gestational age was available from medical records and hospital registries. Sex and gestational age specific SD scores for birth weight were calculated using existing reference data (18). Information on breastfeeding was obtained from postnatal questionnaires (19). Child protein intake at the age of 1 year was measured with a validated semi-quantitative FFQ in a subgroup of 2,193 children (20). At the age of 6 years, child height was determined in standing position to the nearest millimeter without shoes by a Harpenden stadiometer (Holtain Limited, Dyfed, U.K.). Weight was measured using a mechanical personal scale (SECA, Almere, the Netherlands). We calculated BMI (kg/m2), and body surface area (BSA) (m2) (using DuBois formula BSA = weight (kg)0.425x height (cm)0.725x 0.007184) (21). Time spent watching television or using a computer at the age of 6 years was assessed with a questionnaire (22).
Statistical analysis
We adjusted protein intake for energy intake using the nutrient residual method (23). For interpretation, the predicted protein intake for the mean energy intake (2,073 kcal/d) was added to the residuals as a constant (23). Protein intake was analyzed as a continuous variable. We used multivariable linear regression models to assess associations of total, animal, and vegetable protein intake with kidney volume, creatinine, cystatin C and eGFR. For these models, we examined whether the residuals were normally distributed using normal probability plots, whether the variance of the residuals was homoscedastic and whether the regression models were linear. We assessed associations of protein intake with risk of microalbuminuria with multivariable logistic models. All models were adjusted for child’s age and sex (basic model). Analyses with vegetable protein intake were additionally adjusted for animal protein intake and vice versa. The adjusted models were further controlled for maternal characteristics and socio-demographic factors (maternal age, BMI before pregnancy, gestational weight gain, gestational age at intake, ethnicity, and education), maternal lifestyle factors (smoking, and alcohol consumption during pregnancy, folic acid supplement use during pregnancy), pre-pregnancy comorbidities and child characteristics (birthweight adjusted for gestational age, breastfeeding, body surface area at 6 years visit, and screen time at the age of 6 years). Covariates were included in the regression models based on previous literature or a change of >10% in effect estimates. To assess whether the associations were different by maternal ethnicity, child sex, birthweight, gestational age, or BSA of the child at the age of 6 years, we evaluated the statistical interaction by adding the product term of the covariate and total protein intake to the models. To examine whether the associations of maternal protein intake with kidney outcomes could be explained by later diet of the child, we performed a sensitivity analysis for a subgroup of our cohort in which information was available about protein intake at the age of 1 year. Also, we performed a sensitivity analysis including Dutch women only (n=2,332). To prevent bias associated with missing data, we used multiple imputations (n=5) for covariates with missing values on the basis of the correlation of missing variables with other participant characteristics, according to the Markov Chain Monte Carlo method (24). The amount of missing values ranged from 2.2% to 16.1%. Because we found similar results, we report the pooled results. Subjects characteristics before and after imputation are shown in Supplementary Table 1. Statistical analyses were performed using the Statistical Package of Social Sciences version 21.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of the mothers and their children are presented in Table 1. Mean (±SD) maternal energy intake was 2,073 kcal per day (±543). Mean protein intake in these mothers was 76.6 g (±20.4), which provided on average 15% of total energy intake. Animal protein intake provided 9% of total energy intake, and vegetable protein intake provided 6%. No differences were observed in maternal energy and protein intake between mothers of children with and without kidney follow up measurements (data not shown).
Table 1. Subject characteristics (N = 3,650) 1.
| Maternal characteristics | |
| Maternal age (y) | 31.1 (4.7) |
| Gestational age at intake (weeks) | 13.5 (9.8, 22.9) |
| Maternal body mass index at enrolment | 22.6 (18.4, 34.4) |
| Nulliparous (%) | 59.4 |
| Education level (%) | |
| - No higher education | 44.9 |
| - Higher education | 55.1 |
| Ethnicity (%) | |
| - Dutch | 63.9 |
| - Turkish | 5.0 |
| - Moroccan | 3.5 |
| - Surinamese or Dutch Antilles | 7.8 |
| - Other western | 12.5 |
| - Other non-western | 6.7 |
| Household income (%) | |
| - < 1400 euro | 13.4 |
| - 1400-2200 euro | 14.5 |
| - > 2200 euro | 60.7 |
| Smoking during pregnancy (%) | |
| - Never | 76.0 |
| - Until pregnancy was known | 9.6 |
| - Continued | 14.2 |
| Alcohol during pregnancy (%) | |
| - Never | 37.9 |
| - Until pregnancy was known | 14.5 |
| - Continued | 47.3 |
| Folic acid supplements use (%) | |
| - No | 22.4 |
| - Start 1st to 10 weeks | 31.1 |
| - Start periconceptional | 45.8 |
| Pre-pregnancy comorbidities (%) | 1.6 |
| Maternal diet | |
| Total energy intake (kcal) | 2,073 (543) |
| Protein (g/d)2 | |
| - Total | 76.6 (20.4) |
| - Animal | 46.9 (15.4) |
| - Vegetable | 29.9 (9.2) |
| Protein intake (E%) | 15.0 (2.5) |
| Carbohydrate intake (E%) | 48.5 (6.3) |
| Fat intake (E%) | 36.3 (5.5) |
| Infant characteristics | |
| Girls (%) | 50.1 |
| Dutch ethnicity (%) | 65.8 |
| Gestational age at birth (wk) | 40.1 (36.1, 42.4) |
| Birth weight (g) | 3,469 (538) |
| Breastfeeding (%) | |
| - Exclusive ≥ 4 months | 25.5 |
| - Partial ≥ 4 months | 64.7 |
| - Never or ≤ 4 months | 9.9 |
| Child protein intake at 1 y (g/d) | 25.1 (29.4) |
| Child characteristics at 6 y visit | |
| Age (y) | 6.1 (5.6, 7.3) |
| Height (cm) | 119.1 (5.6) |
| Weight (kg) | 22.9 (3.8) |
| Body mass index (kg/m2) | 16.1 (1.7) |
| Body surface area (m2) | 0.90 (0.08) |
| Screen time (hour/day) | 1.3 (0.3, 4.6) |
| Kidney volume (cm3) | 119 (23) |
| Creatinine (µmol/l) | 37.1 (5.4) |
| Cystatin C (µg/l) | 784 (83) |
| eGFRcreat ( ml/min/1.73m2) | 120 (16) |
| eGFRcyst C ( ml/min/1.73m2) | 103 (15) |
| Microalbuminuria (%) | 7.2 |
Values are percentages for categorical variables, means (SD) for continuous variables with a normal distribution, or medians (95% range) for continuous variables with a skewed distribution. Values are based on imputed data.
Abbreviations: eGFRcreat, estimated glomerular filtration rate based on creatinine levels; eGFRcyst C, estimated glomerular filtration rate based on cystatin C levels.
unadjusted for energy intake
Table 2 shows that in the multivariable adjusted models higher maternal total protein intake in the first trimester was associated with higher childhood eGFRcreat (0.06 (95%CI 0.01, 0.12) ml/min/1.73m2 per gram of protein intake). Stronger associations were observed for first trimester maternal vegetable protein intake with childhood eGFRcreat (0.22 (95%CI 0.10, 0.35) ml/min/1.73m2 per gram of vegetable protein intake). Maternal animal protein intake was not significantly associated with childhood eGFRcreat. Furthermore maternal protein intake was not significantly associated with kidney volume, eGFRcyst C or risk of microalbuminuria. In line with our eGFR findings, first trimester maternal total protein intake and vegetable protein intake were associated with lower levels of childhood creatinine (-0.02 (95%CI -0.04, -0.01) µmol/l and (-0.07 (95%CI -0.11, -0.03) µmol/l per gram of total and vegetable protein intake), but not with cystatin C (Supplementary Table 2).
Table 2. Associations of maternal protein intake during pregnancy with childhood kidney outcomes (N = 3,650)1.
| Kidney volume | eGFRcreat | eGFRcyst C | Microalbuminuria | |
|---|---|---|---|---|
| Difference (95% Confidence Interval) in cm3 | Difference (95% Confidence Interval) in ml/min/1.73 m2 | Difference (95% Confidence Interval) in ml/min/1.73 m2 | Odds ratio (95% Confidence Interval) | |
| n = 3,344 | n = 2,494 | n = 2,500 | n = 3,515 | |
| Basic model2 | ||||
| Total protein intake (g)5 | 0.09 (0.06, 0.11)4 | 0.08 (0.06, 0.11)4 | 0.04 (-0.01, 0.09) | 1.00 (0.99, 1.01) |
| Animal protein intake (g)5 | 0.08 (0.05, 0.10)4 | 0.06 (0.04, 0.09)4 | 0.04 (-0.01, 0.09) | 1.00 (0.99, 1.01) |
| Vegetable protein intake (g)5 | 0.18 (0.12, 0.23)4 | 0.28 (0.23, 0.33)4 | 0.03 (-0.08, 0.14) | 1.00 (0.99, 1.01) |
| Multivariable adjusted model3 | ||||
| Total protein intake (g)5 | 0.03 (-0.03, 0.08) | 0.06 (0.01, 0.12)4 | 0.03 (-0.03, 0.08) | 1.00 (0.99, 1.01) |
| Animal protein intake (g)5 | 0.02 (-0.04, 0.08) | 0.05 (-0.01, 0.10) | 0.03 (-0.03, 0.08) | 1.00 (0.99, 1.01) |
| Vegetable protein intake (g)5 | 0.05 (-0.80, 0.18) | 0.22 (0.10, 0.35)4 | 0.01(-0.11, 0.13) | 1.00 (0.97, 1.02) |
Values are based on multivariable linear or logistic regression models and reflect differences or odds ratios and 95% confidence intervals in kidney volume and function measures per gram increase of protein intake. Basic model2 is adjusted for child’s sex and age at 6 year visit. Multivariable adjusted model3 is adjusted for child’s sex, age at 6 year visit, maternal characteristics (age, body mass index before pregnancy, weight gain during pregnancy, gestational age at intake), socio-demographic factors (ethnicity, education), maternal lifestyle (smoking, and alcohol consumption during pregnancy, folic acid intake during pregnancy), pre-pregnancy comorbidities, and child characteristics (birthweight adjusted for gestational age, breastfeeding, body surface area, and screen time at the age of 6y). Models with animal protein intake were additionally adjusted for vegetable protein intake and vice versa.
p < 0.05.
Protein intakes are energy-adjusted using the nutrient residual method.
Abbreviations: eGFRcreat, estimated glomerular filtration rate based on creatinine levels; eGFRCyst C, estimated glomerular filtration rate based on cystatin C levels.
After additional adjustment for child protein intake at the age of 1 year, the multivariable associations of maternal protein intake with kidney outcomes did not change (Supplementary Table 3). We did not observe significant interactions between maternal total protein intake and sex, birthweight, gestational age, maternal ethnicity or child BSA in the models with kidney outcomes. Results of the sensitivity analyses in Dutch mothers only are presented in Supplementary Tables 4 showing similar patterns as in the full group, however in this group we also observe an association between higher animal protein intake and a higher eGFRcyst C.
Discussion
In this large population-based prospective cohort study, we observed that a higher maternal intake of total and vegetable protein, but not animal protein, during first trimester of pregnancy is associated with higher eGFRcreat, but not with kidney size, eGFRcyst C or microalbuminuria in school-age children. The observed differences in eGFR related to maternal protein intake with eGFR were small. These differences may be without clinical consequence at an individual level, but may be relevant on a population level.
Historical cohort studies suggest that adult offspring of mothers who were exposed to severe undernutrition during their pregnancy have increased risk of cardiovascular and renal disease (25). During the winter 1944-1945 the western part of the Netherlands was struck by a period of severe food scarcity, where the daily rations dropped to 400-800 calories (26). Follow up studies among adults whose mothers were exposed to the famine during their pregnancy, showed an increased risk for having microalbuminuria in adults whose mothers were exposed to the famine during mid-gestation (2). Blood pressure was also higher in adults whose pregnant mothers where exposed to the famine. The variation in blood pressure depended on the timing mothers were exposed to the famine, with strongest associations on blood pressure for famine during late gestation (26). Rooseboom et al. postulated that it might be the macronutrient composition rather than the quantity of a pregnant woman’s diet that affects the child’s blood pressure in later life (26). Blood pressure was especially higher in adults whose mothers ate small amounts of protein in relation to carbohydrate during the third trimester of pregnancy (27). Interestingly, a study in Aberdeen showed a higher blood pressure in adults who were exposed to a low animal protein and corresponding high carbohydrate diet in utero (28).
The mechanisms by which maternal undernutrition affects kidney development in the offspring may include developmental adaptations leading to smaller kidneys with a reduced number of nephrons, which in turn lead to glomerular hyperfiltration and sclerosis (3). Increased filtration through each glomerulus leads to hypertrophy and hyperfiltration injury, which is marked by the onset of microalbuminuria, and may eventually lead to a reduction in renal function (3, 29, 30). The renal effects of undernutrition depend upon its timing during gestation and the nutrients balance (2).
Thus far, not much is known about common, contemporary nutritional exposures during pregnancy that affect offspring kidney health. Studies in animals have shown that undernutrition, mainly protein restriction, of pregnant rats raises blood pressure in the offspring permanently (31, 32). Also, maternal protein restriction in rats influences on offspring kidney structure and function, it promotes a reduction in nephron number that is associated with reduction of GFR in postnatal life (33). In the same population-based cohort as the current study, we did not observe any association between maternal protein intake during the first trimester of the pregnancy with blood pressure in 6 years old children (34).
In the current study, we observed that first trimester maternal protein intake was positively associated with eGFRcreat in 6 years old children. Our results are in line with results from studies performed in rats (5) and sheep (35). We did not find an association of maternal first trimester protein intake with childhood microalbuminuria. It may be that the effects of impaired kidney growth on microalbuminuria may not be detectable during childhood, but may become evident later in life. Fetal adverse exposures can be compensated for many years before the adverse outcomes are present (36).
In our study population, associations with eGFR were stronger for vegetable than for animal protein intake during pregnancy. Previous studies in adults also reported different associations for animal versus vegetable protein on kidney health (7, 8). A mechanism through which animal and vegetable protein may differentially affect eGFR is via differences in amino acid composition. Experimental studies have shown that different types of amino acids have different renal impact (37). However further studies need to explore the mechanisms underlying the associations of specifically vegetable proteins with childhood kidney function outcomes.
Nephrogenesis requires a fine balance of many factors that can be disturbed by intrauterine growth restriction, leading to a low nephron endowment (38). Since nephrogenesis continues until 36 weeks of gestation and largely ceases thereafter, adverse exposures during this critical period may lead to impaired kidney development (39, 40). A previous study suggested that adult hypertension programmed by maternal exposure to a low protein diet is linked to marked changes in the renal expression of the glucocorticoid receptor, 11β-HSD2, and components of the renin-angiotensin system. Also, protein restriction during pregnancy could affect the growth hormone-insulin-like growth factor and the prostaglandins axis in the offspring (35). Welham et al. suggested that maternal diet programs the embryonic kidney, altering cell turnover and gene expression at a time when nephrons and glomeruli have yet to form (41).
Some methodological issues need to be discussed. A major strength of our study is the prospective design from fetal life onwards within a large population-based cohort. Our analyses were based on 3,650 mother-child pairs. We used FFQs to assess maternal diet during the first pregnancy trimester. Although the FFQ yielded valid estimates of nutrient intakes when validated against 3-days 24-hours recalls, measurement error may still have occurred. One of the limitations in our study is that the FFQ was validated only in Dutch women. However, a sensitivity analysis in Dutch mothers only revealed similar results as in the whole group. Unfortunately, we did not have information about child protein intake at a later stage in childhood. In a subgroup of children of the present study group (n = 2,193) we had dietary data at the age of 1 year. When we adjusted our models additionally for child protein intake at the age of 1 year, the regression coefficients remained similar in this subgroup, suggesting that child protein intake at the age of 1 year does not affect the association between maternal protein intake and childhood.
We performed detailed measurements of childhood kidney outcomes. As nephron number cannot be studied in vivo, we used kidney size as a measure of kidney development employing ultrasound as a reliable method to measure kidney volume (13). Kidney size is also correlated with the number of glomeruli and can be used in epidemiological studies as a measure of kidney development (42). Glomerular enlargement due to hyperfiltration may also increase kidney volume (43). The estimation of GFR in children remains challenging. Blood creatinine is most commonly used to calculate eGFR. We used the Schwartz formula based on creatinine levels and height, which was previously validated in a pediatric population (14). In addition to blood creatinine levels, we also measured blood cystatin C levels to calculate eGFR based on cystatin C levels using Zappitelli’s formula (15, 44). Interestingly, in our population, maternal protein intake was associated with creatinine-based eGFR, but not with cystatin-based eGFR. Our results are in line with those from a trial in adults with chronic kidney disease, in which protein intake affected creatinine but not cystatin C levels (45). Further studies are needed to evaluate the mechanism explaining these differences. Microalbuminuria was evaluated using urine albumin-creatinine ratio from a random urine sample (46). Finally, although we performed adjustment for a large number of potential maternal and childhood confounders, residual confounding by other lifestyle factors, might still be present. Residual confounding may also be present because of measurement error in several unhealthy life style behaviors such as underreporting of smoking and alcohol consumption. Since the main outcomes were correlated, we did not adjust for multiple comparisons. However, we may have observed false positive associations due to the multiple tests that were performed.
Conclusion
In conclusion, results from this large prospective study suggests that higher first trimester maternal intake of total and vegetable protein is associated with a higher eGFR in their children at school-age. These findings are important from an etiological perspective. Further studies are needed to investigate the underlying mechanisms. Although longitudinal studies suggest that risks factors for kidney diseases track from childhood to adulthood, follow up studies are needed to explore whether protein intake in pregnancy affects risk of kidney diseases in adulthood.
Supplementary Material
Acknowledgements
We gratefully acknowledge the contribution of participating mothers, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
Financial support: This phase of the Generation R Study was supported by the Erasmus MC, Erasmus University Rotterdam, the Netherlands, Organization for Health Research and Development (ZonMw) and an unrestricted grant from NutsOhra. KM has been financially supported through Erasmus Mundus Western Balkans (ERAWEB). TV, EHH and OHF work in ErasmusAGE, a center for aging research across the life course funded by an AXA Research Fund, Metagenics Inc. and Nestlé Nutrition (Nestec Ltd.). The funders had no role in the design of the study; the data collection and analyses; the interpretation of data; the preparation and review of the manuscript; or the decision to submit the manuscript.
Abbreviations
- BMI
body mass index
- BSA
body surface area
- CI
confidence interval
- eGFRcreat
estimated glomerular filtration rate based on creatinine levels
- eGFRCyst C
estimated glomerular filtration rate based on cystatin C levels
- FFQ
food frequency questionnaire
Footnotes
Potential competing interests: None
Author contributions
The authors’ contributions to this study were as follows: KM, TV and VWVJ designed the research project; VWVJ and AH were involved in the design and planning of the study and data collection; KM and TV conducted the analyses; VWVJ, EHH, and OHF provided consultation regarding the analyses and interpretation of the data; KM, TV and VWVJ wrote the paper; KM and VWVJ had primary responsibility for the final content. All authors critically reviewed and approved the final manuscript.
Disclosure
None.
References
- 1.Langley-Evans SC, Langley-Evans AJ, Marchand MC. Nutritional programming of blood pressure and renal morphology. Arch Physiol Biochem. 2003;111:8–16. doi: 10.1076/apab.111.1.8.15136. [DOI] [PubMed] [Google Scholar]
- 2.Painter RC, Roseboom TJ, van Montfrans GA, Bossuyt PM, Krediet RT, Osmond C, Barker DJ, Bleker OP. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J Am Soc Nephrol. 2005;16:189–94. doi: 10.1681/ASN.2004060474. [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis. 1994;23:171–5. [PubMed] [Google Scholar]
- 4.Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol. 2005;565:137–47. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64:965–74. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 6.Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460–7. doi: 10.7326/0003-4819-138-6-200303180-00009. [DOI] [PubMed] [Google Scholar]
- 7.Kontessis P, Jones S, Dodds R, Trevisan R, Nosadini R, Fioretto P, Borsato M, Sacerdoti D, Viberti G. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 1990;38:136–44. doi: 10.1038/ki.1990.178. [DOI] [PubMed] [Google Scholar]
- 8.Nettleton JA, Steffen LM, Palmas W, Burke GL, Jacobs DR., Jr Associations between microalbuminuria and animal foods, plant foods, and dietary patterns in the Multiethnic Study of Atherosclerosis. Am J Clin Nutr. 2008;87:1825–36. doi: 10.1093/ajcn/87.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaddoe VWV, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC, van der Lugt A, Mackenbach JP, Moll HA, Raat H, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27:739–756. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- 10.Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, Geleijnse JM, Hofman A, Grobbee DE, Witteman JC. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr. 1998;52:588–96. doi: 10.1038/sj.ejcn.1600611. [DOI] [PubMed] [Google Scholar]
- 11.Heppe DH, Steegers EA, Timmermans S, Breeijen H, Tiemeier H, Hofman A, Jaddoe VW. Maternal fish consumption, fetal growth and the risks of neonatal complications: the Generation R Study. Br J Nutr. 2011;105:938–49. doi: 10.1017/S0007114510004460. [DOI] [PubMed] [Google Scholar]
- 12.Geelhoed JJM, Taal HR, Steegers EAP, Arends LR, Lequin M, Moll HA, Hofman A, van der Heijden AJ, Jaddoe VWV. Kidney growth curves in healthy children from the third trimester of pregnancy until the age of two years. The Generation R Study. Pediatr Nephrol. 2010;25:289–298. doi: 10.1007/s00467-009-1335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geelhoed JJ, Kleyburg-Linkers VE, Snijders SP, Lequin M, Nauta J, Steegers EA, van der Heijden AJ, Jaddoe VW. Reliability of renal ultrasound measurements in children. Pediatr Nephrol. 2009;24:1345–53. doi: 10.1007/s00467-009-1148-3. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New Equations to Estimate GFR in Children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zappitelli M, Parvex P, Joseph L, Paradis G, Grey V, Lau S, Bell L. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006;48:221–30. doi: 10.1053/j.ajkd.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 16.Donaghue KC, Chiarelli F, Trotta D, Allgrove J, Dahl-Jorgensen K, International Society for P, Adolescent D ISPAD Clinical Practice Consensus Guidelines 2006-2007. Microvascular and macrovascular complications. Pediatr Diabetes. 2007;8:163–70. doi: 10.1111/j.1399-5448.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 17.Statistiek CBvd. Immigrants in the Netherlands 2004. Netherland: Statistics Netherlands; 2004. [Google Scholar]
- 18.Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C, Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977-1981) Acta Paediatr Scand. 1991;80:756–62. doi: 10.1111/j.1651-2227.1991.tb11945.x. [DOI] [PubMed] [Google Scholar]
- 19.Durmus B, van Rossem L, Duijts L, Arends LR, Raat H, Moll HA, Hofman A, Steegers EAP, Jaddoe VWV. Breast-feeding and growth in children until the age of 3 years: the Generation R Study. British Journal of Nutrition. 2011;105:1704–1711. doi: 10.1017/S0007114510005374. [DOI] [PubMed] [Google Scholar]
- 20.Kiefte-de Jong JC, de Vries JH, Bleeker SE, Jaddoe VW, Hofman A, Raat H, Moll HA. Socio-demographic and lifestyle determinants of 'Western-like' and 'Health conscious' dietary patterns in toddlers. Br J Nutr. 2013;109:137–47. doi: 10.1017/S0007114512000682. [DOI] [PubMed] [Google Scholar]
- 21.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303–11. discussion 312–3. [PubMed] [Google Scholar]
- 22.American Academy of PEDIATRICS. Children, adolescents, and television. Pediatrics. 2001;107:423–6. doi: 10.1542/peds.107.2.423. [DOI] [PubMed] [Google Scholar]
- 23.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roseboom TJ, Painter RC, van Abeelen AF, Veenendaal MV, de Rooij SR. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70:141–5. doi: 10.1016/j.maturitas.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Roseboom TJ, van der Meulen JH, Ravelli AC, van Montfrans GA, Osmond C, Barker DJ, Bleker OP. Blood pressure in adults after prenatal exposure to famine. J Hypertens. 1999;17:325–30. doi: 10.1097/00004872-199917030-00004. [DOI] [PubMed] [Google Scholar]
- 27.Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185:93–8. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 28.Campbell DM, Hall MH, Barker DJ, Cross J, Shiell AW, Godfrey KM. Diet in pregnancy and the offspring's blood pressure 40 years later. Br J Obstet Gynaecol. 1996;103:273–80. doi: 10.1111/j.1471-0528.1996.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 29.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85–93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- 30.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. J Am Soc Nephrol. 2001;12:1315–25. [Google Scholar]
- 31.Langley-Evans SC, Phillips GJ, Jackson AA. In utero exposure to maternal low protein diets induces hypertension in weanling rats, independently of maternal blood pressure changes. Clin Nutr. 1994;13:319–24. doi: 10.1016/0261-5614(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 32.Langley-Evans SC. Intrauterine programming of hypertension in the rat: nutrient interactions. Comp Biochem Physiol A Physiol. 1996;114:327–33. doi: 10.1016/0300-9629(96)00018-7. [DOI] [PubMed] [Google Scholar]
- 33.Hall SM, Zeman FJ. Kidney function of the progeny of rats fed a low protein diet. J Nutr. 1968;95:49–54. doi: 10.1093/jn/95.1.49. [DOI] [PubMed] [Google Scholar]
- 34.van den Hil LCL, Taal HR, de Jonge LL, Heppe DHM, Steegers EAP, Hofman A, van der Heijden AJ, Jaddoe VWV. Maternal first-trimester dietary intake and childhood blood pressure: the Generation R Study. British Journal of Nutrition. 2013;110:1454–1464. doi: 10.1017/S0007114513000676. [DOI] [PubMed] [Google Scholar]
- 35.Brennan KA, Olson DM, Symonds ME. Maternal nutrient restriction alters renal development and blood pressure regulation of the offspring. Proc Nutr Soc. 2006;65:116–24. doi: 10.1079/pns2005484. [DOI] [PubMed] [Google Scholar]
- 36.Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? Lancet. 2002;360:659–65. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 37.Lee KE, Summerill RA. Glomerular filtration rate following administration of individual amino acids in conscious dogs. Q J Exp Physiol. 1982;67:459–65. doi: 10.1113/expphysiol.1982.sp002661. [DOI] [PubMed] [Google Scholar]
- 38.Schreuder M, Delemarre-van de Waal H, van Wijk A. Consequences of intrauterine growth restriction for the kidney. Kidney Blood Press Res. 2006;29:108–25. doi: 10.1159/000094538. [DOI] [PubMed] [Google Scholar]
- 39.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 1991;64:777–84. [PubMed] [Google Scholar]
- 41.Welham SJ, Riley PR, Wade A, Hubank M, Woolf AS. Maternal diet programs embryonic kidney gene expression. Physiol Genomics. 2005;22:48–56. doi: 10.1152/physiolgenomics.00167.2004. [DOI] [PubMed] [Google Scholar]
- 42.Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21:898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 43.Boubred F, Buffat C, Feuerstein JM, Daniel L, Tsimaratos M, Oliver C, Lelievre-Pegorier M, Simeoni U. Effects of early postnatal hypernutrition on nephron number and long-term renal function and structure in rats. Am J Physiol Renal Physiol. 2007;293:F1944–9. doi: 10.1152/ajprenal.00141.2007. [DOI] [PubMed] [Google Scholar]
- 44.Bacchetta J, Cochat P, Rognant N, Ranchin B, Hadj-Aissa A, Dubourg L. Which creatinine and cystatin C equations can be reliably used in children? Clin J Am Soc Nephrol. 2011;6:552–60. doi: 10.2215/CJN.04180510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tangri N, Stevens LA, Schmid CH, Zhang YL, Beck GJ, Greene T, Coresh J, Levey AS. Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int. 2011;79:471–7. doi: 10.1038/ki.2010.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Jong PE, Curhan GC. Screening, monitoring, and treatment of albuminuria: Public health perspectives. J Am Soc Nephrol. 2006;17:2120–6. doi: 10.1681/ASN.2006010097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.