Abstract
Background:
Similar to the N-methyl-D-aspartate receptor antagonist ketamine, the metabotropic glutamate 2/3 receptor antagonist, MGS0039, shows antidepressant effects. However, there are no reports comparing these 2 compounds in the social defeat stress model of depression.
Methods:
We examined the effects of MGS0039 (1 mg/kg) and ketamine (10 mg/kg) on depression-like behavior in susceptible mice after repeated social defeat stress. Protein levels of brain-derived neurotrophic factor, TrkB, phospho-TrkB, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (GluA1), postsynaptic density protein 95, and dendritic spine density in selected brain regions were measured.
Results:
In the tail suspension and forced swimming tests, both MGS0039 and ketamine significantly attenuated the increased immobility time observed in susceptible mice, compared with vehicle-treated animals, 1 or 2 days after a single dose of drug. In the sucrose preference test, both compounds significantly improved the reduced preference typically seen in susceptible mice at 3 to 7 days after a single dose of drug. Western-blot analyses showed that similar to ketamine, MGS0039 significantly attenuated the reduced brain-derived neurotrophic factor, phospho-TrkB/TrkB ratio, GluA1 and postsynaptic density protein 95 seen in the prefrontal cortex, dentate gyrus, and CA3 of the hippocampus from susceptible mice, 8 days after a single dose. Again, in a similar manner to ketamine, MGS0039 significantly attenuated the reduction of spine density in the prelimbic regions of the medial prefrontal cortex, dentate gyrus, and CA3 of the hippocampus, but not infralimbic regions of the medial prefrontal cortex and CA1, in susceptible mice 8 days after a single dose. In contrast, neither drug elicited an effect on altered brain-derived neurotrophic factor-TrkB signaling, GluA1, and postsynaptic density protein 95 levels and did not increase spine density observed in the nucleus accumbens of susceptible mice.
Conclusions:
Similar to ketamine, MGS0039 shows rapid and sustained antidepressant effects in the social defeat stress model. Long-lasting synaptogenesis in the prelimbic regions of medial prefrontal cortex, dentate gyrus, and CA3 might be implicated in this sustained antidepressant effect.
Keywords: antidepressant, ketamine, metabotropic glutamate 2/3 (mGlu2/3) receptor antagonist, social defeat stress model, BDNF-TrkB signaling, synaptogenesis.
Significance Statement
The development of new antidepressants effective in the treatment of resistant depression is urgently required. Rapid and sustained antidepressant actions of the N-methyl-D-aspartate receptor antagonist ketamine are the most important discovery in half a century of depression research. Similar to ketamine, the metabotropic glutamate 2/3 receptor antagonist MGS0039 shows rapid and sustained antidepressant effects in the social defeat stress model. Long-lasting synaptogenesis in the medial prefrontal cortex and hippocampus might be implicated in the sustained antidepressant effect of MGS0039. This study suggests that metabotropic glutamate 2/3 receptor antagonists would be potential therapeutic drugs for depression.
Introduction
Depression is one of the most prevalent psychiatric disorders and the leading cause of disability worldwide. According to the World Health Organization, more than 350 million individuals of all ages suffer from depression (World Health Organization, 2016). Although current antidepressants are generally effective, the full therapeutic benefit of these therapies may require several weeks to bring about changes in mood and/or behavior. Additionally, approximately one-third of patients with depression fail to respond to current pharmacotherapy. Therefore, the development of new antidepressants effective in the treatment of resistant depression is urgently required (Chaki and Fukumoto, 2015; Hashimoto, 2015; Monteggia and Zarate, 2015).
The N-methyl-D-aspartate (NMDA) receptor antagonist ketamine shows rapid and sustained antidepressant effects in depressed patients, including those with treatment-resistant disease (Berman et al., 2000; Zarate et al., 2006; Aan Het Rot et al., 2012; Singh et al., 2016). Recent meta-analyses indicated that ketamine exhibited a greater potency of antidepressant effects compared with other NMDA receptor antagonists (Newport et al., 2015; Kishimoto et al., 2016). In addition, others have reported rapid and long-lasting antidepressant-like effects for ketamine in animal models of depression (Yilmaz et al., 2002; Maeng et al., 2008; Li et al., 2011; Ma et al., 2013; Yang et al., 2015a, 2016b; Zhang et al., 2015b; Sun et al., 2016). Accumulating evidence suggests that metabotropic glutamate 2/3 (mGlu2/3) receptor antagonists, such as MGS0039 and LY341495, evoke antidepressant-like activities in rodents (Chaki et al., 2004; Karasawa et al., 2005; Yoshimizu et al., 2006; Pa1ucha-Poniewiera et al., 2010; Koike et al., 2011; Dwyer et al., 2012; Ago et al., 2013; Koike and Chaki, 2014; Fukumoto et al., 2016; Witkin et al., 2016). Furthermore, mGlu2/3 receptor antagonists have been reported to share similar efficacy in rodents and neural mechanisms with ketamine (Koike et al., 2013a, 2013b), suggesting that mGlu2/3 receptor antagonists may have ketamine-like antidepressant actions. Although there is a report showing rapid and sustained antidepressant effects for LY341495 in a chronic unpredictable stress model (Dwyer et al., 2013), these effects have not been fully addressed in the context of synaptic plasticity.
The purpose of this study was to compare the rapid and sustained antidepressant actions of MGS0039 and ketamine in the social defeat stress model. Additionally, we examined the effects of MGS0039 and ketamine on brain-derived neurotrophic factor (BDNF) – TrkB signaling, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (GluA1), postsynaptic density protein 95 (PSD-95), and dendritic spine density in brain regions thought to be implicated in ketamine’s antidepressant effects (Autry et al., 2011; Duman and Aghajanian, 2012; Dwyer and Duman, 2013; Lepack et al., 2014; Zhou et al., 2014; Ohgi et al., 2015; Yang et al., 2015a, 2016b; Zhang et al., 2015b; Li et al., 2016; Björkholm and Monteggia, 2016).
Materials and Methods
Animals
Male adult C57BL/6 mice, aged 8 weeks (body weight 20–25 g, Japan SLC, Hamamatsu, Japan) and male adult CD1 (ICR) mice, aged 13 to 15 weeks (body weight >40 g, Japan SLC) were used for the social defeat stress model. Animals were housed under controlled temperatures and 12-h-light/-dark cycles (lights on between 7:00 am and 7:00 pm) with ad libitum food (CE-2; CLEA Japan, Tokyo, Japan) and water. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Chiba University Institutional Animal Care and Use Committee.
Drugs
MGS0039, synthesized at Taisho Pharmaceutical Co. Ltd. (Saitama, Japan), was dissolved in 1/15 M phosphate buffer (pH 8.0). Ketamine hydrochloride, prepared from Ketalar (Daiichi Sankyo Pharmaceutical, Tokyo, Japan) was dissolved in 1/15 M phosphate buffer (pH 8.0). The doses of MGS0039 (1.0 mg/kg) and ketamine (10 mg/kg) were used as previously reported (Chaki et al., 2004; Ago et al., 2013; Yang et al., 2015a; Zhang et al., 2015b).
Social Defeat Stress Model
The procedure of social defeat stress was performed as previously reported (Golden et al., 2011; Zhao et al., 2013; Yang et al., 2015a, 2016b; Zhang et al., 2015b). Every day the C57BL/6 mice were exposed to a different CD1 aggressor mouse for 10 minutes total for 10 days. When the social defeat session ended, the resident CD1 mouse and the intruder mouse were housed in one-half of the cage separated by a perforated Plexiglas divider to allow visual, olfactory and auditory contact for the remainder of the 24-hour period. At 24 hours after the last session, all mice were housed individually. On day 11, a social avoidance test was performed to identify subgroups of mice that were susceptible and unsusceptible to social defeat stress. This was accomplished by placing mice in an interaction test box (42 × 42 cm) with an empty wire-mesh cage (10 × 4.5 cm) located at one end. The movement of the mice was tracked for 2.5 minutes, followed by 2.5 minutes in the presence of an unfamiliar aggressor confined in the wire-mesh cage. The duration of the subject’s presence in the interaction zone (defined as the 8-cm-wide area surrounding the wire-mesh cage) was recorded by a stopwatch. The interaction ratio was calculated as the time spent in an interaction zone with an aggressor/time spent in an interaction zone without an aggressor. An interaction ratio of 1 was set as the cutoff: mice with scores <1 were defined as susceptible to social defeat stress and those with scores ≥1 were defined as unsusceptible (Zhao et al., 2013). Only susceptible mice were used in the experiments.
Behavioral Tests
On day 12, vehicle (10 mL/kg), MGS0039 (1.0 mg/kg), or ketamine (10 mg/kg) was injected i.p. (Figure 1a). All behavioral tests were performed in the following order: locomotion (30 minutes after injection), tail suspension test (TST) (24 hours after injection), forced swimming test (FST) (48 hours after injection), and 1% sucrose preference test (SPT) (3 and 7 days after injection) (Figure 1a). Mice were put into the test room 30 minutes before the behavioral tests. All tests were performed in a quiet room. After finishing all tests, mice were returned to the breeding room. The FST, TST, and SPT in mice are the most widely used behavioral assays for detecting potential antidepressant-like activity (Cryan and Holmes, 2005; Yang et al., 2015a; Zhang et al., 2015a; 2015b; Dong et al., 2016; Ren et al., 2016).
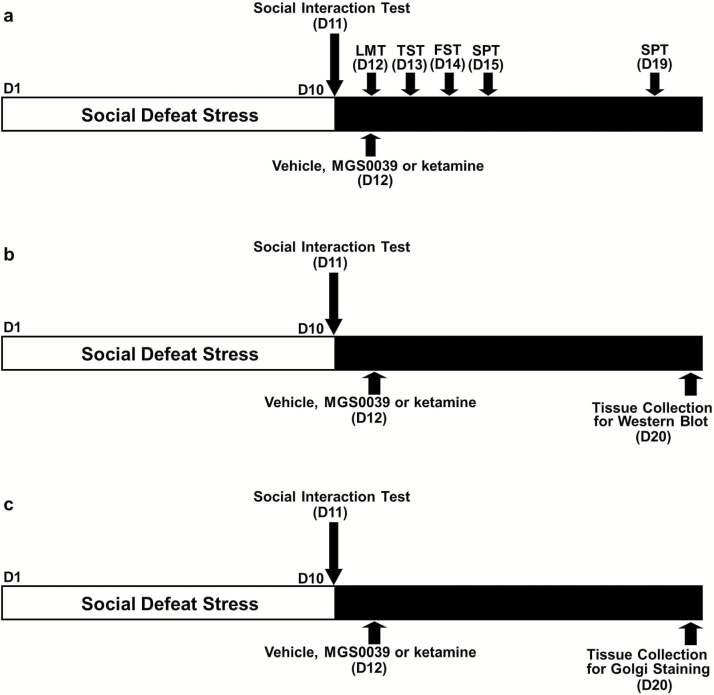
Figure 1.
Schedule of social defeat stress, treatment, and behavioral tests.
a: The schedule of social defeat stress (10 days), drug treatment, and behavioral tests. Social defeat stress was performed from day 1 to day 10. Social interaction test was performed on day 11. Vehicle (10 mL/kg), MGS0039 (1 mg/kg), or ketamine (10 mg/kg) was administered i.p into susceptible mice on day 12. Locomotion test (LMT), tail suspension test (TST), and forced swim test (FST) were performed 0.5, 24, and 48 hours after a single injection, respectively. A 1% sucrose preference test (SPT) was performed 3 day (day 15) and 7 days (day 19) after a single injection. (b) The schedule of social defeat stress (10 days), drug treatment, and collection brain samples for western blot. Social interaction test was performed on day 11. Vehicle (10 mL/kg), MGS0039 (1 mg/kg), or ketamine (10 mg/kg) was administered i.p into susceptible mice on day 12. Brain samples were collected on day 20. (c) The schedule of social defeat stress (10 days), drug treatment, and collection brain samples for Golgi staining. Social interaction test was performed on day 11. Vehicle (10 mL/kg), MGS0039 (1 mg/kg), or ketamine (10 mg/kg) was administered i.p into susceptible mice on day 12. Brain samples were collected on day 20. Ket, ketamine; MGS, MGS0039; Veh, vehicle.
Locomotion
Locomotion was measured using the SCANET MV-40 (MELQUEST Co., Ltd., Toyama, Japan). This system consists of a black square cage (565 × 565 mm) equipped with 2 crossing sensor frames of 72 (x axis) × 72 (y axis) pairs of near-infrared beam sensors, spaced 6 mm apart and at right angles to each other. The beam sensors thus form 2 parallel horizontal grids set at different heights. A transparent Plexiglas cage (560× 560 × 330 mm) for the mouse was centered inside the system. Each pair of sensors was scanned every 0.1 seconds to detect animal movement. Two different variables of horizontal movements could be monitored by the lower sensors: small horizontal movements of 12 mm or more (M1; 1 U=6 mm) and large horizontal movements of 60 mm or more (M2; 1 U = 6 mm). The locomotion was performed under house lighting, and the movement of the cumulative distance was recorded for 60 minutes. The cage was cleaned between testing session.
TST
The mice were taken from their home cage, and a small piece of adhesive tape was placed approximately 2 cm from the tip of the tail. A single hole was punched in the tape and the mice were hung individually on a hook. The immobility time of each mouse was recorded for 10 minutes. All mice were videoed, and then the immobility time of tail suspension was scored by a skilled observer who used stopwatch. Mice were considered immobile only when they hung passively and completely motionless.
FST
The mice were placed individually in a cylinder (diameter: 23 cm, height: 31 cm) containing 15 cm of water maintained at 23 ± 1°C. The animals were not able to touch the bottom of the cylinder. Animals were tested in an automated forced-swim apparatus by the SCANETMV-40 (MELQUEST Co., Ltd., Toyama, Japan). The cylinder was placed in a box with infrared cell sensors on the walls to detect swimming activity. The software set up a rectangle that circumscribed the body of an animal every 0.3 seconds. If the animals were out of the rectangle (i.e., a part of the animal body was detected outside the rectangle) 0.3 seconds after setting up the rectangle, the software counted as “movement (swimming)” in that period. Immobility was defined as such if the animal stayed within the same rectangle 0.3 seconds after setting up the rectangle. Immobility time was calculated from activity time as (total) − (active) time by using analysis software of the apparatus. Cumulative immobility time was scored for 6 minutes during the test.
SPT
Mice were exposed to water and 1% sucrose solution for 48 hours, followed by 4 hours of water and food deprivation and 1 hour exposure to 2 identical bottles: one was water and the other was 1% sucrose solution. The bottles containing water and sucrose were weighed before and at the end of this period and the sucrose preference was determined.
Western-Blot Analysis
Eight days after vehicle (10 mL/kg), MGS0039 (1.0 mg/kg), or ketamine (10 mg/kg) injection, the brain samples of prefrontal cortex (PFC), nucleus accumbens (NAc), dentate gyrus (DG), and CA1 and CA3 of the hippocampus were collected, since these brain regions are implicated in depression-like phenotype as described previously (Yang et al., 2015a, 2016b; Zhang et al., 2015a; Ren et al., 2016) (Figure 1b). Basically, tissue samples were homogenized in Laemmli lysis buffer. Aliquots (10 μg) of protein were measured using the DC protein assay kit (Bio-Rad, Hercules, CA) and incubated for 5 minutes at 95°C, with an equal volume of 125 mM Tris/HCl, pH 6.8, 20% glycerol, 0.1% bromophenol blue, 10% β-mercaptoethanol, and 4% sodium dodecyl sulfate and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, using 10% mini-gels (Mini-PROTEAN TGX Precast Gel; Bio-Rad). Proteins were transferred onto polyvinylidene difluoride membranes using a Trans Blot Mini Cell (Bio-Rad). For immunodetection, the blots were blocked with 2% bovine serum albumin in Tris buffered saline + 0.1 % Tween 20 (TBST) for 1 hour at room temperature (RT) and kept with primary antibodies overnight at 4°C. The following primary antibodies were used: BDNF (1:1000, Santa Cruz Biotechnology, Inc.), phosphorylated-TrkB (Tyr 706) (1:200, Santa Cruz Biotechnology), and TrkB (80E3) (1:1000, Cell Signaling Technology), AMPA glutamate receptor 1 (GluA1) (1 μg/mL, Abcam, Cambridge, MA), and PSD-95 (1 μg/mL, Invitrogen, Carlsbad, CA). The next day, the blots were washed 3 times in TBST and incubated with horseradish peroxidase-conjugated anti-rabbit antibody (1:5000) for 1 hour at RT. After 3 final washes with TBST, the bands were detected using enhanced chemiluminescence plus the Western Blotting Detection system (GE Healthcare Bioscience). The blots then were washed 3 times in TBST and incubated with the primary antibody directed against β-actin. Images were captured with a Fuji LAS3000-mini imaging system (Fujifilm, Tokyo, Japan) and immunoreactive bands were quantified.
Golgi Staining
Golgi staining was performed using the FD Rapid Golgi Stain TM Kit (FD Neuro Technologies, Inc., Columbia, MD) following the manufacturer’s instructions. Eight days after a single administration of vehicle (10 mL/kg), MGS0039 (1.0 mg/kg), or ketamine (10 mg/kg), mice were deeply anesthetized with sodium pentobarbital, and brains were removed from the skull and rinsed in double distilled water (Figure 1c). Brains were immersed in the impregnation solution, made by mixing equal volumes of Solution A and B, overnight and then stored in fresh solution for 2 weeks in the dark. Brains were transferred into Solution C overnight and then stored in fresh solution at 4°C for 1 week in the dark. Coronal brain sections (100-μm thickness) were cut on a cryostat (3050S, Leica Microsystems AG, Wetzlar, Germany) with the chamber temperature set at -20°C. Each section was mounted in Solution C on saline-coated microscope slides. After absorption of excess solution, sections were dried naturally at RT. Dried sections were processed following the manufacturer’s instructions. Briefly, images of dendrites within DG, CA1, and CA3 of the hippocampus, mPFC and NAc were captured using a 100× objective with a Keyence BZ-9000 Generation microscope (Osaka, Japan). Spines were counted along DG, CA1, CA3, mPFC, and NAc dendrites starting from their point of origin from the primary dendrite, as previously reported (Yang et al., 2015a, 2016b; Zhang et al., 2015a). For spine density measurements, all clearly evaluable areas containing 50 to 100 μm of secondary dendrites from each imaged neuron were used. To determine relative spine density, spines on multiple dendritic branches from a single neuron were counted to obtain an average spine number per 10 μm. For spine number measurements, only spines that emerged perpendicular to the dendritic shaft were counted. Three neurons per section, 3 sections per animal, and 6 animals were analyzed. The average value for each region in each individual was obtained. These individual averages were then combined to yield a grand average for each region.
Statistical Analysis
The data show as the mean ± SEM. Analysis was performed using PASW Statistics 20 (formerly SPSS Statistics; SPSS). Comparisons between groups were performed using the 1-way ANOVA followed by posthoc Tukey test. P <.05 was considered statistically significant.
Results
MGS0039 Shows Rapid-Acting and Long-Lasting Antidepressant Effects as Ketamine in the Social Defeat Stress Model
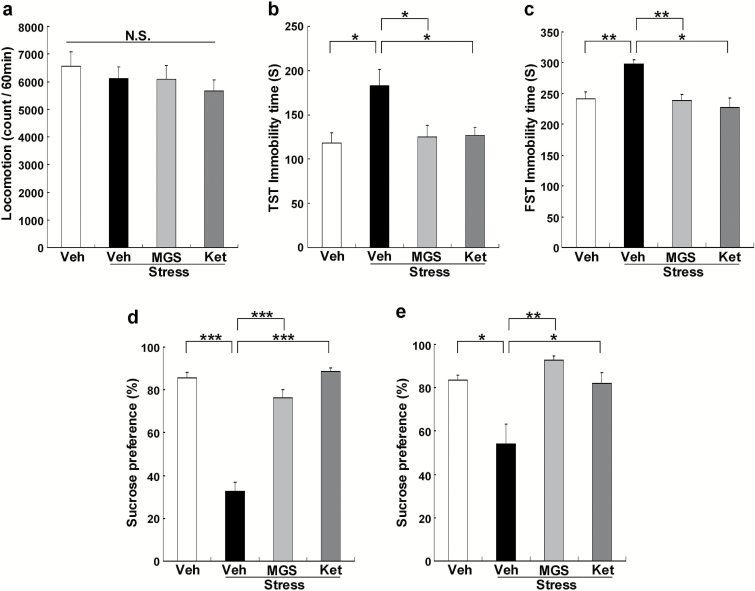
Ketamine produces rapid and long-lasting antidepressant effects in the chronic mild stress model (Li et al., 2011; Ma et al., 2013; Liu et al., 2016; Sun et al., 2016) and social defeat stress model (Yang et al., 2015a, 2016b; Zhang et al., 2015b). We examined whether MGS0039 showed rapid-acting and long-lasting antidepressant effects in the social defeat stress model. Vehicle, MGS0039 (1.0 mg/kg), or ketamine (10 mg/kg) was injected i.p. into the susceptible mice after social defeat stress (Figure 1a). Locomotion showed no difference (F3, 35 = 1.240, P = .311) among the 4 groups (Figure 2a). In the TST and FST, MGS0039 and ketamine significantly attenuated an increased immobility time in the susceptible mice (Figure 2b-c). One-way ANOVA detected statistical significance in both the TST and FST (TST: F3, 35 = 4.661, P = .008; FST: F3, 35 = 8.315, P < .001) among the 4 groups (Figure 2b-c). In the SPT, preference of mice after an injection of MGS0039 or ketamine was significantly higher (e: day 15, F3, 35 = 60.378, P < .001, f: day 19, F3, 35 = 5.511, P = .004) than that of the vehicle-treated group (Figure 2d-e). These behavioral data suggest that MGS0039 promotes rapid and long-lasting antidepressant effects in the social defeat stress model, consistent with ketamine’s antidepressant-like effect.
Figure 2.
Antidepressant effects of MGS0039 and ketamine in the social defeat mice. (a) Locomotion test (LMT). (b) Tail suspension test (TST). (c) forced swim test (FST). (d-e) 1% sucrose preference test (SPT). The values represent the mean ± SEM (n = 9). *P < .05, **P < .01, ***P < .001 compared with the vehicle + stress group. Ket, ketamine; MGS, MGS0039; Veh, vehicle.
Effects of MGS0039 and Ketamine on Alterations in BDNF-TrkB Signaling and Synaptogenesis in the Selected Brain Regions
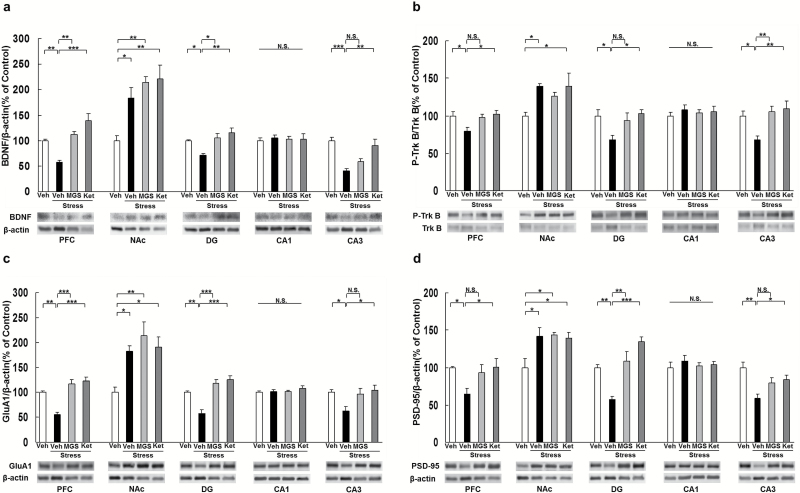
We performed western-blot analyses of BDNF, TrkB, phosphorylated TrkB (p-TrkB), GluA1, and PSD-95 in selected brain regions (PFC, NAc, DG, CA1, and CA3 of the hippocampus) 8 days after a dose of vehicle (10 mL/kg), MGS0039 (1.0 mg/kg), or ketamine (10 mg/kg) (Figure 1b). Social defeat stress significantly decreased levels of BDNF protein in the PFC, DG, and CA3, but not CA1, while significantly increased BDNF protein in the NAc. MGS0039 significantly attenuated reduced levels of BDNF protein in the PFC and DG of susceptible mice after social defeat stress, although ketamine significantly attenuated reduced levels of BDNF protein in the PFC, DG, and CA3 (Figure 3a). In contrast, 2 compounds did not alter the increased BDNF levels in the NAc (Figure 3a). One-way ANOVA detected statistical significance in BDNF protein (PFC: F3, 23 = 18.190, P < .001; NAc: F3, 23 = 9.129, P = .001; DG: F3, 23 = 8.274, P = .001; CA1: F3, 23 = 0.086, P = .967; CA3: F3, 23 = 12.408, P < .001) among the 4 groups (Figure 3a).
Figure 3.
Effects of MGS0039 and ketamine on the brain-derived neurotrophic factor (BDNF) and TrkB phosphorylation in the brain regions. (a) Expression of BDNF in the brain regions. (b) Ratio of phosphorylated TrkB (p-TrkB) to total TrkB in the brain regions. (c) Expression of GluA1 in the brain regions. (d) Expression of postsynaptic density protein 95 (PSD95) in the brain regions. The value was expressed as a percentage of that of control mice. The values represent the mean ± SEM (n = 5 or 6). *P < .05, **P < .01, ***P < .001 compared with the vehicle + stress group. Ket, ketamine; MGS, MGS0039; Veh, vehicle.
To clarify the role of TrkB phosphorylation in the action of MGS0039 and ketamine, we performed western-blot analyses of TrkB and p-TrkB, an activated form of TrkB, in samples from PFC, NAc, DG, CA1, and CA3. Social defeat stress significantly decreased the ratio of p-TrkB/TrkB in the PFC, CA3, and DG, but not CA1, while significantly increased the ratio of p-TrkB/TrkB in the NAc. MGS0039 significantly attenuated the reduced ratio of p-TrkB/TrkB in the CA3 of susceptible mice after social defeat stress, although ketamine significantly attenuated the reduced ratio of p-TrkB/TrkB in the PFC, DG, and CA3 (Figure 3b). One-way ANOVA revealed statistical significance in p-TrkB/TrkB (PFC: F3, 23 = 4.360, P=.016; NAc: F3, 23 = 3.715, P = .028; DG: F3, 23 = 4.168, P = .020; CA1: F3, 23 = 0.337, P = .799; CA3: F3, 23 = 6.889, P = .002) among the 4 groups (Figure 3b). In contrast, 2 compounds did not alter the increased ratio of p-TrkB/TrkB in the NAc. These findings suggest that BDNF–TrkB signaling in the PFC, CA3, and DG of hippocampus might be involved in the antidepressant mechanisms of MGS0039 and ketamine.
Proteins such as GluA1, a subtype of the AMPA receptor, and PSD-95 are markers for synaptogenesis (Duman and Aghajanian, 2012; Ohgi et al., 2015). Social defeat stress significantly decreased the levels of GluA1 in PFC, DG, and CA3, but not CA1, while significantly increased GluA1 protein in the NAc. Furthermore, MGS0039 significantly attenuated the reduction of GluA1 in the PFC and DG, although ketamine significantly attenuated the reduction of GluA1 in the PFC, DG, and CA3 (Figure 3c). One-way ANOVA revealed statistical significance in GluA1 protein (PFC: F3, 23 = 22.322, P < .001; NAc: F3, 23 = 6.834, P = .002; DG: F3, 23 = 17.683, P < .001; CA1: F3, 23 = 0.944, P = .438; CA3: F3, 23 = 4.496, P = .014) among the 4 groups (Figure 3c). In contrast, MGS0039 and ketamine did not alter the increased levels of GluA1 in NAc (Figure 3c).
Social defeat stress significantly decreased the levels of PSD-95 in the PFC, DG, and CA3, but not CA1, while significantly increased PSD-95 protein in the NAc. Furthermore, MGS0039 significantly attenuated the reduction of PSD-95 in the PFC and DG, although ketamine significantly attenuated the reduction of PSD-95 in the PFC, DG, and CA3 (Figure 3d). One-way ANOVA detected statistical significance in PSD-95 protein (PFC: F3, 23 = 3.611, P = .031; NAc: F3, 23 = 5.232, P = .008; DG: F3, 23 = 15.554, P < .001; CA1: F3, 23 = 0.428, P = .735; CA3: F3, 23 = 7.270, P = .002) among the 4 groups (Figure 3d). In contrast, MGS0039 and ketamine did not alter the increased levels of PSD-95 in NAc (Figure 3d).
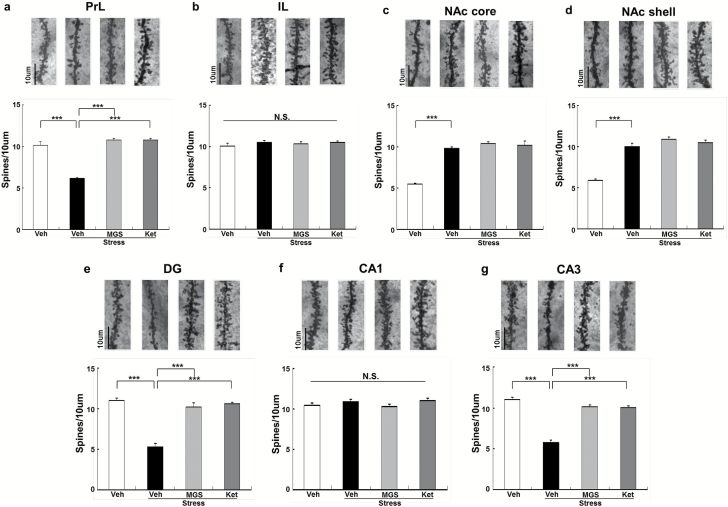
Effects of MGS0039 and Ketamine on Alterations in Dendritic Spine Density in the Selected Brain Regions After Social Defeat Stress
Social defeat stress causes alterations in dendritic spine density in the medial PFC (mPFC), CA3 and DG of the hippocampus, and NAc (Jiang et al., 2015; Zhang et al., 2015b; Yang et al., 2015a, 2016b). In this study, we examined whether MGS0039 (1.0 mg/kg) and ketamine (10 mg/kg) could affect alterations in the dendritic spines of the prelimbic (PrL) and infralimbic (IL) regions of mPFC, shell and core of the NAc, and DG, CA1, and CA3 of the hippocampus after social defeat stress. We found significant differences in the PrL of mPFC, NAc core, NAc shell, DG and CA3, but not IL of mPFC and CA1 (Figure 4). Social defeat stress significantly decreased dendritic spine density in the PrL of mPFC, DG, and CA3. Both MGS0039 and ketamine significantly attenuated the reduced spine density seen in the PrL, DG, and CA3 from mice with depression-like phenotype, 8 days after a single dose (Figure 4a, e, g). In contrast, social defeat stress significantly increased dendritic spine density in the NAc core and NAc shell. Neither MGS0039 nor ketamine altered spine density in the NAc of susceptible mice (Figure 4c-d). One-way ANOVA showed statistical significance for all of the examined regions (PrL: F3, 23 = 64.64, P < .001; IL: F3, 23 = 0.528, P = .668; NAc core: F3, 23 = 68.45, P < .001; NAc shell: F3, 23 = 62.44, P < 0.001; DG: F3, 23 = 59.75, P < .001; CA1: F3, 23 = 1.652, P = .209; CA3: F3, 23 = 77.53, P < .001). These findings suggest the role of the PrL of mPFC, DG, and CA3, but not IL of mPFC, NAc, and CA1, in the mechanistic action of MGS0039 and ketamine.
Figure 4.
Effects of MGS0039 and ketamine on alterations in dendritic spine density in the brain regions after social defeat stress. (a) Prelimbic region (PrL) of medial prefrontal cortex (mPFC). (b) Infralimbic region (IL) of mPFC. (c) nucleus Accumbens (NAc) core. (d) NAc shell. (e) Dentate gyrus (DG). (f) CA1. (g) CA3. Scale bar = 10 μm. Values represent the mean ± SEM (n = 6). Representative photomicrographs of Golgi-Cox stained pyramidal neurons in the selected brain regions from animals of each group. ***P < .001 compared with the vehicle + stress group. Ket, ketamine; MGS, MGS0039; Veh, vehicle.
Discussion
In this study, we found that similar to ketamine, a single dose of MGS0039 promoted rapid and prolonged antidepressant effects in the social defeat stress model. Interestingly, it evoked an antidepressant response 7 days after a single dose in a comparable manner to ketamine (Zhang et al., 2015b). Previous studies showed that, 7 days after a single dose, ketamine could ameliorate anhedonia in rodents under the chronic mild stress model (Li et al., 2011; Ma et al., 2013). Therefore, it is noteworthy that these rapid and long-lasting antidepressant effects for MGS0039 in the social defeat stress model bear similarity to the therapeutic effects of ketamine in chronic mild stress or social defeat stress models (Li et al., 2011; Ma et al., 2013; Yang et al., 2015a, 2016b; Zhang et al., 2015b). Since ketamine also shows rapid and sustained antidepressant actions in patients with treatment-resistant depression, it is feasible that MGS0039 could promote analogous actions in depressed patients.
Because MGS0039 has been reported to be a selective mGlu2/3 receptor antagonist (Chaki et al., 2004) and to exhibit both antidepressant and anxiolytic effects (Chaki et al., 2004; Yoshimizu et al., 2006) at a dose used in this study, the effect of MGS0039 is mediated through blockade of mGlu2/3 receptor. Moreover, since MGS0039 did not alter general behavioral including locomotion at this dose, it is unlikely that the effect of MGS0039 is attributable to change in general behaviors.
We previously reported conspicuous reductions in the level of BDNF in the PFC, DG, and CA3, but not CA1, of mice susceptible to social defeat stress (Yang et al., 2015a, 2016; Zhang et al., 2015b) and learned helplessness rats (Shirayama et al., 2015; Yang et al., 2015a, 2016a). In this study, we also noted reduced levels of BDNF in the PFC, DG, CA3, but not CA1, of susceptible mice after social defeat stress, but increased BDNF levels in the NAc (Zhang et al., 2015b; Shirayama et al., 2015; Yang et al., 2015a). Furthermore, we identified that social defeat stress caused decreased phosphorylation of TrkB in the PFC, DG, and CA3 and increased phosphorylation in the NAc. Thus, it is probable that social defeat stress attenuated BDNF–TrkB signaling in PFC and hippocampus (DG and CA3) but increased signals in the NAc, resulting in depression-like behavior in rodents. Compellingly similar to ketamine, MGS0039 significantly lessened the reduction of BDNF in the PFC, DG, and CA3, although it had no effect on the increased BDNF levels seen in the NAc of susceptible mice. Given the role of BDNF–TrkB signaling in the depression-like phenotype (Nestler et al., 2002; Duman and Monteggia, 2006; Hashimoto, 2010, 2013), just like ketamine, MGS0039 may promote rapid and sustained antidepressant effects by normalizing BDNF levels in the PFC and hippocampus (DG and CA3). This assumption is supported by a previous report that the mGlu2/3 receptor antagonist, LY341495, exerts its antidepressant effect through stimulation of TrkB signaling (Koike et al., 2013a).
Tracking dendritic morphology, we found opposing changes in spine density between the mPFC, hippocampus, and NAc after social defeat stress. The reduced spine density found in the PrL of mPFC, DG, and CA3 matches the findings seen in rodents under the unpredictable chronic mild stress, inflammation, and learned helplessness models (Li et al., 2011; Shirayama et al., 2015; Zhang et al., 2015a, 2015b; Yang et al., 2015b, 2016b). In contrast, we found increased spine density in the NAc of depressed mice after social defeat stress, consistent with results from the inflammation and learned helplessness models (Zhang et al., 2015a; Yang et al., 2015b, 2016b). Moreover, in keeping with ketamine, MGS0039 attenuated the reduced spine density observed in the PrL of mPFC, DG, and CA3, although it had no impact on the increased spine density found in the core and shell of the NAc. Considering the function of synaptogenesis in the action of antidepressants (McEwen, 2007; Duman and Aghajanian, 2012; Ohgi et al., 2015), it is likely that the PrL region of the mPFC, DG, and CA3 of the hippocampus are involved in this drug’s action. It should be noted that another mGlu2/3 receptor antagonist, LY341495, elevated synthesis of synaptic proteins in the PFC (Dwyer et al., 2012), strengthening the argument that increased synaptogenesis in the PFC may underpin the antidepressant actions of mGlu2/3 receptor antagonists. In addition, local injection of LY341495 into the mPFC exerts antidepressant effects (Fukumoto et al., 2016), indicating the role of the mPFC in the antidepressant action of mGlu2/3 receptor antagonists.
In conclusion, this study shows that like ketamine, MGS0039 can produce rapid and sustained antidepressant effects in the social defeat stress model. Furthermore, it is likely that increased synaptogenesis in the PrL of mPFC and DG and CA3 of the hippocampus after a single dose of MGS0039 may promote this prolonged antidepressant response. Therefore, mGlu2/3 antagonists such as MGS0039 can act as rapid and sustained therapeutic agents for patients with depression, although efficacy and safety of mGlu2/3 receptor antagonists need to be proven in a clinical setting.
Statement of Interest
Dr. Hashimoto is an inventor on a filed patent application on “The use of R-ketamine in the treatment of psychiatric diseases” by Chiba University. Dr. Hashimoto has received research support from Dainippon Sumitomo, Mochida, Otsuka, and Taisho. Dr. Shigeyuki Chaki is an employee of Taisho Pharmaceutical Co., Ltd., Japan. All other authors state no conflicts of interest.
Acknowledgments
The authors thank Professor Toshinori Nakayama (Department Of Immunology, Graduate School of Medicine, Chiba University) for the use of a Keyence BZ-9000 Generation II microscope.
This study was supported by Taisho Pharmaceutical Co., Ltd., (Tokyo, Japan) and the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and development, AMED (to K.H.). Dr. Chao Dong was supported by Uehara Memorial Foundation (Tokyo, Japan). Dr. Wei Yao was supported by Ishidsu Shun Memorial Scholarship (Tokyo, Japan). Dr. Qian Ren and Dr. Chun Yang were supported by Research Fellowship for Young Scientists of the Japan Society for the Promotion of Science. Ms. Ma was supported by Leading Graduate School at Chiba University.
References
- Aan Het Rot M, Zarate CA, Jr, Charney DS, Mathew SJ. (2012) Ketamine for depression: where do we go from here? Biol Psychiatry 72:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ago Y, Yano K, Araki R, Hiramatsu N, Kita Y, Kawasaki T, Onoe H, Chaki S, Nakazato A, Hashimoto H, Baba A, Takuma K, Matsuda T. (2013) Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology 65:29–38. [DOI] [PubMed] [Google Scholar]
- Autry AE Adachi M Nosyreva E Na ES Los MF Cheng PF, Kavalali ET Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM Cappiello A Anand A Oren DA Heninger GR, Charney DS Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:e351–e354. [DOI] [PubMed] [Google Scholar]
- Björkholm C, Monteggia LM. (2016) BDNF - a key transducer of antidepressant effects. Neuropharmacology 102:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S, Fukumoto K. (2015) Potential of glutamate-based drug discovery for next generation antidepressants. Pharmaceuticals (Basel) 8:590–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S Yoshikawa R Hirota S Shimazaki T Maeda M Kawashima N Yoshimizu T Yasuhara A Sakagami K, Okuyama S Nakanishi S Nakazato A (2004) MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology 46:457–467. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. (2005) The ascent of mouse: advances in modeling human depression and anxiety. Nat. Rev. Drug Discov 4:775–790. [DOI] [PubMed] [Google Scholar]
- Dong C, Zhang JC, Yao W, Ren Q, Yang C, Ma M, Han M, Saito R, Hashimoto K. (2016) Effects of escitalopram, R-citalopram, and reboxetine on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav 144:7–12. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. [DOI] [PubMed] [Google Scholar]
- Dwyer JM, Duman RS. (2013) Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol Psychiatry 73:1189–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Lepack AE, Duman RS. (2012) mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Int J Neuropsychopharmacol. 15:429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Lepack AE, Duman RS. (2013) mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J Mol Psychiatry 1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S. (2016) The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology 41:1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, III, Berton O, Russo SJ. (2011) A standard protocol for repeated social defeat stress in mice. Nat. Protoc. 6:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. (2010) Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin Neurosci 64:341–357. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. (2013) Sigma-1 receptor chaperone and brain-derived neurotrophic factor: emerging links between cardiovascular disease and depression. Prog Neurobiol 100:15–29. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. (2015) Inflammatory biomarkers as differential predictors of antidepressant response. Int J Mol Sci 16:7796–7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa J, Shimazaki T, Kawashima N, Chaki S. (2005) AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res 1042:92–98. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, Correll CU. (2016) Single-dose infusion ketamine and non-ketamine N-methyl-D-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 46:1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Chaki S. (2014) Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res. 271:111–115. [DOI] [PubMed] [Google Scholar]
- Koike H, Fukumoto K, Iijima M, Chaki S. (2013. a) Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav Brain Res 238:48–52. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. (2011) Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 224:107–111. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. (2013. b) Effects of ketamine and LY341495 on the depressive-like behavior of repeated corticosterone-injected rats. Pharmacol Biochem Behav 107:20–23. [DOI] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. (2014) BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 18:pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WX Wang J Xie ZM Xu N Zhang GF Jia M Zhou ZQ, Hashimoto K Yang JJ (2016) Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology (Berl) 233:405–415. [DOI] [PubMed] [Google Scholar]
- Ma XC, Dang YH, Jia M, Ma R, Wang F, Wu J, Gao CG, Hashimoto K. (2013) Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice. PLoS One 8:e56053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352. [DOI] [PubMed] [Google Scholar]
- McEwen BS. (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Zarate C., Jr (2015) Antidepressant actions of ketamine: from molecular mechanisms to clinical practice. Curr Opin Neurobiol 30:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. (2002) Neurobiology of depression. Neuron 34:13–25. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB; APA Council of Research Task Force on Novel Biomarkers and Treatments (2015) Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 172:950–966. [DOI] [PubMed] [Google Scholar]
- Ohgi Y, Futamura T, Hashimoto K. (2015) Glutamate signaling in synaptogenesis and NMDA receptors as potential therapeutic targets for psychiatric disorders. Curr Mol Med 15:206–221. [DOI] [PubMed] [Google Scholar]
- Pa1ucha-Poniewiera A, Wieronska JM, Branski P, Stachowicz K, Chaki S, Pilc A. (2010) On the mechanism of the antidepressant-like action of group II mGlu receptor antagonist, MGS0039. Psychopharmacology (Berl) 212:523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q, Ma M, Ishima T, Morisseau C, Yang J, Wagner KM, Zhang JC, Yang C, Yao W, Dong C, Han M, Hammock BD, Hashimoto K. (2016) Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc Natl Acad Sci U S A 113:E1944–E1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Yang C, Zhang JC, Ren Q, Yao W, Hashimoto K. (2015) Alterations in brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain regions of a learned helplessness rat model and the antidepressant effects of a TrkB agonist and antagonist. Eur. Neuropsychopharmacol 25:2449–2458. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L. (2016) A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry 173:816–826. [DOI] [PubMed] [Google Scholar]
- Sun HL, Zhou ZQ, Zhang GF, Yang C, Wang XM, Shen JC, Hashimoto K, Yang JJ. (2016) Role of hippocampal p11 in the sustained antidepressant effect of ketamine in the chronic unpredictable mild stress model. Transl Psychiatry 6:e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B, Rasmussen K, Rorick-Kehn LM. (2016) The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther 358:71–82. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2016) Depression. Fact sheet No. 369/April 2016 Available at http://www.who.int/mediacentre/factsheets/fs369/en/index.html. Accessed September 16, 2016.
- Yang B Yang C Ren Q Zhang JC Chen QX Shirayama Y, Hashimoto K (2016. a) Regional differences in the expression of brain-derived neurotrophic factor (BDNF) pro-peptide, proBDNF and preproBDNF in the brain confer stress resilience. Eur Arch Psychiatry Clin Neurosci DOI: 10.1007/s00406-016-0693-6. [DOI] [PubMed] [Google Scholar]
- Yang B, Zhang JC, Han M, Yao W, Yang C, Ren Q, Ma M, Chen QX, Hashimoto K. (2016. b) Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 233:3647–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K. (2015. a) R-ketamine: a rapid-acting and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K. (2015. b) Regional differences in brain derived neurotrophic factor levels and dendritic spine density confer resilience to inescapable stress. Int J Neuropsychopharmacol 18:pyu121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yilmaz A, Schulz D, Aksoy A, Canbeyli R. (2002) Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav 71:341–344. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Shimazaki T, Ito A, Chaki S. (2006) An mGluR2/3 antagonist, MGS0039, exerts antidepressant and anxiolytic effects in behavioral models in rats. Psychopharmacology (Berl) 186:587–593. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C, Li SX, Shirayama Y, Hashimoto K. (2015. a) Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol 18:pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC Yao W Dong C Yang C Ren Q Ma M Han M, Hashimoto K (2015. b) Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 232:4325–4335. [DOI] [PubMed] [Google Scholar]
- Zhao T, Huang GB, Muna SS, Bagalkot TR, Jin HM, Chae HJ, Chung YC. (2013) Effects of chronic social defeat stress on behavior and choline acetyltransferase, 78-kDa glucoseregulated protein, and CCAAT/enhancer-binding protein (C/EBP) homologous protein in adult mice. Psychopharmacology (Berl) 228:217–230. [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. (2014) Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 29:419–423. [DOI] [PubMed] [Google Scholar]