Abstract
Background:
Brain-derived neurotrophic factors are known to be related to the psychopathology of major depressive disorder. However, studies focusing on drug-naïve first-episode patients are still rare.
Methods:
Over a 6-year period, we examined the serum brain-derived neurotrophic factors levels in patients with first-episode drug-naïve major depressive disorder and compared them with sex-matched healthy controls. We also investigated the relationships between serum brain-derived neurotrophic factors levels, suicidal behavior, and Hamilton Depression Rating Scale scores before and after a 4-week antidepressant treatment.
Results:
The baseline serum brain-derived neurotrophic factors levels of 71 patients were significantly lower than those of the controls (P=.017), and the Hamilton Depression Rating Scale scores in 71 patients did not correlate with brain-derived neurotrophic factor levels. Brain-derived neurotrophic factor levels were significantly lower in 13 suicidal major depressive disorder patients than in 58 nonsuicidal major depressive disorder patients (P=.038). Among 41 followed-up patients, there was no alteration in serum brain-derived neurotrophic factors levels after treatment with antidepressants (P=.126). In receiver operating characteristic curve analysis of using pretreatment brain-derived neurotrophic factors to estimate the response to treatment, the area under the curve was 0.684. The most suitable cut-off point was 6.1 ng/mL (sensitivity=78.6%, specificity = 53.8%).
Conclusions:
Our data support the serum brain-derived neurotrophic factor levels in patients with drug-naïve first-episode major depressive disorder were lower than those in the healthy controls, and patients with pretreatment brain-derived neurotrophic factors >6.1 ng/mL were more likely to be responders. Although the relationship of our results to the mechanism of drug action and pathophysiology of depression remains unclear, the measure may have potential use as a predictor of response to treatment. In the future, it needs a large sample to prove these results.
Keywords: brain-derived neurotrophic factor, drug-naïve, first episode, suicide, major depressive disorder, antidepressant
Significance Statement
The serum brain-derived neurotrophic factor levels in patients with first-episode drug-naïve major depressive disorder were lower than those in the healthy controls. In addition, brain-derived neurotrophic factor levels were significantly lower in suicidal major depressive disorder patients than in nonsuicidal major depressive disorder patients. There was no alteration in serum brain-derived neurotrophic factors levels after treatment with antidepressants.
Introduction
The neurotrophin hypothesis depicts major depressive disorder (MDD) as being caused by deviant neurogenesis in brain regions that govern memory and emotion (Duman et al., 1997). On the basis of this hypothesis, a stress-induced decrease in expression of brain-derived neurotrophic factor (BDNF) resulted in deviant neurogenesis. Furthermore, the neurotrophin hypothesis posits that antidepressants exerted the efficacy by means of promoting BDNF expression and thereby restore deviant neuronal plasticity (Duman and Monteggia, 2006; Park and Poo, 2013). Karege et al. (2002) demonstrated low serum BDNF levels in depressed patients compared with healthy controls and negative correlation between serum BDNF and depression severity. Shimizu et al. (2003) found serum BDNF increased after antidepressant treatment. During the last few years, there have been multiple attempts to replicate these findings. Most, but not all studies of the patients with MDD reveal that BNDF levels increase after antidepressant treatment (Brunoni et al., 2008; Sen et al., 2008; Molendijk et al., 2014; Polyakova et al., 2015). Noticeable variation in outcomes across studies should be kept in mind while interpreting these data (Piccinni et al., 2008; Basterzi et al., 2009; Matrisciano et al., 2009; Elfving et al., 2012). The inconsistent findings could plausibly be attributed to the heterogeneous patient populations, small sample sizes lacking statistical power, and confounders such as administration of antidepressants, age at onset, or different clinical profiles. Moreover, a striking question is whether alterations in BDNF levels are confined to certain types of antidepressants or BDNF levels are correlated with improvement in clinical depressive symptoms.
In previous studies, lower levels of BDNF correlated with more severe clinical symptoms (Shimizu et al., 2003; Gonul et al., 2005). However, systemic reviews have failed to demonstrate these findings (Molendijk et al., 2014).
Studies focusing on the association of BDNF with suicide were scarce. Previous data indicated reduced messenger RNA levels of BDNF and BDNF levels in the postmortem hippocampus and prefrontal cortex of suicide victims who were drug-free compared with nonsuicide controls, regardless of diagnosis (Dwivedi et al., 2003; Karege et al., 2005). Kim et al. (2007) also demonstrated that reduction of BDNF level is associated with suicidal behavior in major depression. These results supported that BDNF played a pivotal role in the pathophysiology of suicidal behavior.
In this study, we wanted to ascertain whether there is a difference in serum BDNF levels between patients with drug-naïve first-episode MDD and healthy subjects, so we tried to investigate the serum BDNF levels of MDD patients for the following variables: with/without a suicide behavior and family tendency. We also aimed to investigate the effects of antidepressants on serum BDNF levels in patients with drug-naïve first-episode MDD. Finally, we examined whether the serum BDNF levels are associated with the severity of clinical symptoms.
Materials and Methods
Subjects
We aimed to investigate the serum BDNF levels in patients with first-episode drug-naïve MDD compared with sex-matched healthy controls using naturalistic study by clinical observation during a 6-year period (December 2003 to June 2006, August 2007 to November 2008, June 2011 to December 2013). All participants were recruited at Chang Gung Memorial Hospital in Kaohsiung, Taiwan. Patients with first-episode drug-naïve MDD were evaluated by the same psychiatrist utilizing the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1997). The severity of depression was assessed utilizing the 17-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960) by the same psychiatrist. If the score was <20 at baseline, patients would be excluded from the study. Participants were defined as individuals who had a suicidal behavior (defined as self-directed injury with intent to end one’s life) just before being recruited to our study and needed medical or psychiatric management. Family tendency was defined as >1 first-degree relatives of patients having a history of MDD.
The healthy controls, enrolled from the medical staff and students at Chang Gung Memorial Hospital in Kaohsiung, were screened by the same psychiatrist using the Chinese Health Questionnaire-12 (Chong and Wilkinson, 1989) or the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1997) to rule out present and past major and minor mental illnesses (schizophrenia, affective disorder, personality disorder, alcohol abuse/dependence, anxiety disorder, and illegal substance use disorder). Some data had been published (Huang et al., 2008). In this study, we focused on patients with first-episode drug-naïve MDD and increased the sample size. All patients and healthy controls received blood pressure, routine blood tests, chest X-ray, and electrocardiogram to exclude the subjects with any chronic medical illness including liver, kidney, lung, heart, and metabolic diseases. They had neither allergic reactions nor acute infections.
Assessment of Clinical Situation and Treatment
Among the followed-up patients, we investigated the association between body mass indices (BMI), duration of illness, education, HDRS scores, and serum BDNF levels before and after a 4-week antidepressant treatment. Depressive patients were administered only one kind of antidepressant. The antidepressants involved escitalopram (dose range: 10-20 mg/d), fluoxetine (dose range: 20-40 mg/d), mirtazapine (dose range: 30–60 mg/d), paroxetine (dose range: 20–40 mg/d), or venlafaxine (dose range: 75–225 mg/d). Applied treatment preparations/dosages were not changed during the follow-up period. Besides, patients were permitted simultaneously taking benzodiazepines (i.e., alprazolam 1.5 mg/d or lorazepam 3 mg/d) or hypnotics (i.e., zolpidem 10-20 mg/d) but could not take antidepressant drugs or mood stabilizers. Response to treatment was defined as at least 50% improvement in HDRS scores.
The study was approved by the Chang Gung Memorial Hospital Institutional Review Board. All subjects enrolled in the study gave written informed consent for their participation in the study.
Laboratory Analysis
Venous blood (5 mL) of the patients and healthy controls was collected and serum BDNF levels were measured by an ELISA Kit (BDNF Emax Immunoassay System, Promega Co.). Absorbencies were detected using a microtiter plate reader (absorbency at 450 nm). The intra-assay and inter-assay variations were both <10%.
Statistical Analysis
The participants were sorted into different diagnostic groups (i.e., patients with MDD vs healthy controls) or clinical phenotypes (i.e., those who had and those who did not have a suicide behavior, family tendency). Parameters, including age, BMI, and education of the patient and control groups were compared using Student’s t tests. Previous studies indicated that parameters such as ages or genders have specific influences on circulating and stored BDNF levels in peripheral blood (Elfving et al., 2012). Consequently, BDNF levels of the patient and control groups were compared using an ANCOVA with age and gender adjustments for group mean differences in different groups and gender. Within the patient group, the relationship of BDNF levels, age, sex, BMI, education, age at onset, HDRS scores, suicide behavior, and family tendency was evaluated by means of Pearson’s correlation test.
Pretreatment and posttreatment serum BDNF levels of the followed-up patients were evaluated using the paired t test. The estimation validity of pretreatment BDNF for the response to treatment was examined using receiver operating characteristic (ROC) analyses with calculations of the area under the ROC curve, sensitivity, and specificity.
All results are presented as means ± SD. Data analysis was performed using IBM SPSS Statistics 12. Two-tailed significance values were used and significance levels were set at 0.05.
Results
Demographic Data
In all, 71 patients with drug-naïve first-episode MDD and 71 sex-matched healthy controls were recruited. Table 1 exhibits the serum BDNF levels and demographic data of all participants. The healthy controls had significantly more years of education than the patients (16.0±2.7 years vs 10.3±2.7 years; df=140, P<.001), and there were significant educational differences between the male subgroups (16.7±3.5 years vs 10.5±2.7 years; df=28, P<.001) and female subgroups (15.8 ± 2.5 years vs 10.3±2.8 years; df=110, P<.001). The healthy controls were significantly younger than the patients (33.3 ±5.4 years vs 37.4±10.5 years; df =105.0, P=.005), and there were significant age differences between the male subgroups (35.7±6.0 years vs 43.5 ± 9.9 years; df = 23.2, P=.015) and female subgroups (32.7 ± 5.1 years vs 35.7 ± 10.1 years; df = 81.6, P=.048). There was significant difference in BMI only between the male subgroups (Table 1).
Table 1.
Serum BDNF Levels and Demographic Data of All Participants
| Diagnostic groups | Age (y) | BMI (kg/m 2) | Education (y) | Age of onset | 17-item HDRM at baseline | Serum BDNF levels (ng/mL) | |
|---|---|---|---|---|---|---|---|
| T |
PATIENTS
(n=71) |
37.4 ± 10.5 | 21.9 ± 3.8 | 10.3 ± 2.7 | 36.3 ± 10.7 | 33.2 ± 4.4 | 10.0 ± 7.0 |
|
Healthy controls
(n=71) |
33.3 ± 5.4 | 22.8 ± 4.0 | 16.0 ± 2.7 | NA | 13.3 ± 7.8 | ||
| P value | .005* | .189 | <.001* | .017* | |||
| M |
PATIENTS
(n=15) |
43.5 ± 9.9 | 22.9 ± 3.7 | 10.5 ± 2.7 | 42.9 ± 9.7 | 34.3 ± 4.2 | 8.2 ± 6.3 |
|
Healthy controls
(n=15) |
35.7 ± 6.0 | 25.8 ± 3.3 | 16.7 ± 3.5 | NA | 13.7 ± 7.8 | ||
| P value | .015* | .027* | <.001* | .100 | |||
| F |
PATIENTS
(n=56) |
35.7 ± 10.1 | 21.6 ± 3.8 | 10.3 ± 2.8 | 34.6 ± 10.3 | 32.9 ± 4.4 | 10.5 ± 7.1 |
|
Healthy controls
(n=56) |
32.7 ± 5.1 | 21.9 ± 3.8 | 15.8 ± 2.5 | NA | 13.2 ± 7.8 | ||
| P value | .048* | .678 | <.001* | .071 | |||
Abbreviations: BDNF, brain-derived neurotrophic factor; BMI, body mass index; F, female; 17-item HDRS, 17-item Hamilton Depression Rating Scale; M, male; PATIENTS, Patients with drug-naïve first-episode major depressive disorder. T, total.
*P < .05, comparison using Student’s t tests.
In the patient group, no parameters, including age, gender, BMI, education, age at onset, suicidal behavior, family tendency, and HDRS scores significantly correlated with BDNF levels (all P>.05) using Pearson’s correlation. But there was a trend in negative associations between BDNF levels and suicide behavior (γ = -0.211, P=.077)
BDNF Levels in Patients and Healthy Controls
The ANCOVAs were constructed with diagnostic groups as the independent variables and BDNF levels as dependent variables with ages as the covariates. The ANCOVAs with age adjustment showed that depressive patients had significantly lower BDNF levels than healthy controls (F=5.859, P=.017), and age was not a significant covariate (P=.512). There was a trend towards lower BDNF levels in depressive women (F =3.334, P=.071), but not in depressive men (F = 2.896, P=.100).
Table 2 exhibited the serum BDNF levels in depressive patients with different clinical phenotypes. Using ANCOVAs with age and gender adjustments, we found that patients who had a suicide behavior had lower serum BDNF protein levels than those who did not (F=4.460, P=.038). However, no significant difference was elicited between serum BDNF levels and family tendency (F=1.836, P=.180).
Table 2.
Serum BDNF Levels and Demographic Data of 71 Patients with Clinical Phenotypes
| Items | Age (y) | Sex a | BMI (kg/m 2) | Education (y) | Age of onset | 17-item HDRM at baseline | Serum BDNF levels (ng/mL) |
|---|---|---|---|---|---|---|---|
| Suicide or not | |||||||
| Suicide (n=13) |
32.8 ± 9.6 | 0.08 ± 0.28 | 20.7 ± 4.2 | 11.1 ± 3.1 | 32.1 ± 10.0 | 33.8 ± 4.3 | 6.9 ± 4.8 |
| Nonsuicide (n=58) |
38.4 ± 10.4 | 0.24 ± 0.43 | 22.2 ± 3.7 | 10.1 ± 2.7 | 37.3 ± 10.7 | 33.0 ± 4.4 | 10.7 ± 7.2 |
| P value | .079 | .097 | .212 | .269 | .115 | .539 | .038* |
| Family tendency | |||||||
| Family history (n=8) |
34.4 ± 10.5 | 0.25 ± 0.46 | 20.2 ± 2.6 | 11.5 ± 1.7 | 33.1 ± 11.1 | 34.1 ± 5.2 | 13.2 ± 7.4 |
| No family history (n=63) |
37.8 ± 10.5 | 0.21 ± 0.41 | 22.1 ± 3.9 | 10.2 ± 2.8 | 36.7 ± 10.7 | 33.0 ± 4.3 | 9.6 ± 6.9 |
| P value | .392 | .780 | .170 | .196 | .375 | .513 | .180 |
Abbreviations: BDNF, brain-derived neurotrophic factor; BMI, body mass index; 17-item HDRS, 17-item Hamilton Depression Rating Scale.
aFemale = 0. Male = 1.
*P <. 05, comparison using Student’s t tests.
BDNF Levels Before and After Antidepressant Treatment
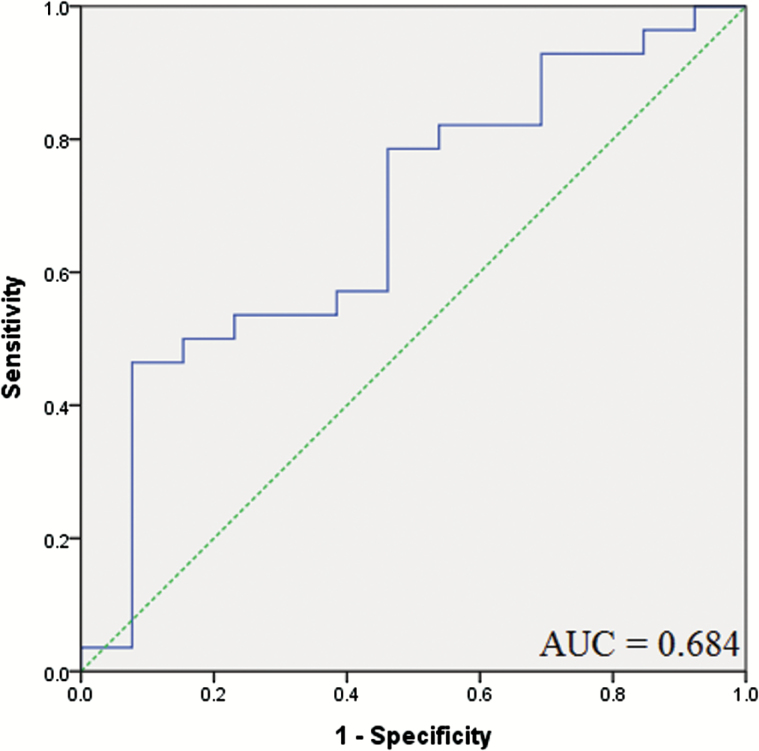
Of 71 patients with drug-naïve first-episode MDD, only 41 patients received antidepressant treatment during a period of 4 weeks and completed the assessments of HDRS, including 21 patients took fluoxetine, 2 for escitalopram, 7 for paroxetine, 8 for venlafaxine, and 3 for mirtazapine. Table 3 exhibits the demographic, clinical characteristics and BDNF levels. Using the paired t test, the serum BDNF levels of the 41 followed-up patients were not significantly elevated (10.7 ± 6.9 ng/mL vs 12.9±11.9 ng/mL; P=.126). In addition, there are no significantly elevated BDNF levels in the 28 responsive patients after antidepressant treatment (12.0 ± 7.0 ng/mL vs 15.3±13.3 ng/mL; P=.113). In ROC curve analysis using pretreatment BDNF to estimate the response to treatment, the area under the curve was 0.684 (standard error =0.091, 95%CI=0.507-0.862) (Figure 1). The most suitable cut-off point of pretreatment BDNF level discriminating response to treatment from nonresponse to treatment was 6.1 ng/mL. At this cut-off point, the sensitivity was 78.6% and specificity was 53.8%.
Table 3.
Demographics, Clinical Characteristics,and BDNF Levels of the 41Followed-Up Patients
| Items | Age | Baseline BMI (kg/m 2) | Duration of illness (y) | BDNF protein (ng/mL) at baseline | BDNF protein (ng/mL) at endpoint | Pair t value | P value | 17-Item HDRM at baseline | 17-Item HDRM at endpoint |
|---|---|---|---|---|---|---|---|---|---|
|
Total
(n=41) |
38.1 ± 10.3 | 22.0 ± 3.9 | 1.2 ± 1.9 | 10.7 ± 6.9 | 12.9 ± 11.9 | -1.564 | .126 | 34.5 ± 4.4 | 11.5 ± 11.4 |
| M (n=11) | 42.6 ± 10.6 | 23.1 ± 3.5 | 0.7 ± 1.0 | 9.2 ± 7.2 | 8.5 ± 5.5 | 0.885 | .397 | 35.6 ± 4.0 | 8.2 ± 12.2 |
| F (n=30) | 36.5 ± 9.9 | 21.6 ± 4.0 | 1.4 ± 2.1 | 11.2 ± 6.8 | 14.5 ± 13.2 | -1.751 | .091 | 34.1 ± 4.5 | 12.7 ± 11.1 |
|
Responder
(n=28) |
37.9 ± 10.0 | 22.3 ± 3.6 | 1.2 ± 1.8 | 12.0 ± 7.0 | 15.3 ± 13.3 | -1.640 | .113 | 34.0 ± 4.3 | 4.3 ± 4.0 |
| M (n=9) | 42.9 ± 10.8 | 22.2 ± 3.2 | 0.8 ± 1.1 | 10.4 ± 7.4 | 9.2 ± 5.8 | 1.248 | .247 | 34.4 ± 3.1 | 2.9 ± 3.7 |
| F (n=19) | 35.6 ± 9.0 | 22.3 ± 3.9 | 1.4 ± 2.1 | 12.7 ± 6.8 | 18.1 ± 15.0 | -1.919 | .071 | 33.7 ± 4.8 | 4.9 ± 4.0 |
|
Non-responder
(n=13) |
38.5 ± 11.4 | 21.4 ± 4.4 | 1.3 ± 2.2 | 7.8 ± 6.1 | 7.7 ± 5.4 | 0.288 | .779 | 35.8 ± 4.6 | 27.0 ± 4.1 |
| M (n=2) | 41.5 ± 13.4 | 27.1 ± 0.9 | 0.5 ± 0.4 | 3.6 ± 1.7 | 4.9 ± 2.0 | -5.801 | .109 | 41.0 ± 2.8 | 32.0 ± 0.0 |
| F (n=11) | 38.0 ± 11.7 | 20.4 ± 3.9 | 1.4 ± 2.4 | 8.6 ± 6.3 | 8.2 ± 5.7 | 0.621 | .548 | 34.8 ± 4.2 | 26.2 ± 3.7 |
BMI, body mass index; BDNF, brain-derived neurotrophic factor; 17-item HDRS, 17-item Hamilton Depression Rating Scale.
Figure 1.
Receiver operating characteristic curve (ROC) using pretreatment BDNF to estimate the response to treatment in 41 follow-up patients. Response to treatment was defined as at least 50% improvement in HDRS scores. The area under the ROC curve was 0.684.
Discussion
The principal findings in this study, which focused on patients with drug-naïve first-episode MDD, are as follows: (1) there were decreased serum BDNF levels in patients with MDD, (2) BDNF levels were significantly lower in suicidal MDD patients than nonsuicidal MDD patients, (3) there was no alteration in serum BDNF levels after treatment with antidepressants, and (4) patients with pretreatment BDNF >6.1 ng/mL were more likely to be responders.
BDNF Levels in Patients and Healthy Controls
Investigating BDNF alterations in drug-naïve first-episode patients is a pivotal step to identify the role of BDNF in MDD pathophysiology, as patients are not influenced by medication. Most studies recruited MDD patients who were drug free for only 4 weeks or less from the study entry (Brunoni et al., 2008) for the purpose of minimizing medication effects. Considering a lot of data revealed a reversal of decreased BDNF levels to baseline levels after antidepressant treatment in patients with MDD (Aydemir et al., 2005; Gervasoni et al., 2005), we could not entirely exclude the repeated effects of antidepressants on BDNF levels. Furthermore, repetitive administration of antidepressant raises BDNF mRNA levels in the rat hippocampus and cerebral cortex (Rogoz et al., 2005).
We demonstrated that the serum BDNF levels in patients with first-episode drug-naïve MDD were significantly lower than in healthy controls and HDRS scores did not correlate with BDNF levels, which were in line with the results of recent meta-analyses (Brunoni et al., 2008; Sen et al., 2008; Molendijk et al., 2014; Polyakova et al., 2015). Although the above results need replication in independent studies with larger samples, we supposed that BDNF can play a key role in eliciting the dynamic neurobiological and clinical alterations observed in an early depressive phase.
Another important finding in this study is that serum BDNF levels in MDD patients who had suicidal behavior are lower than those in MDD patients who did not have suicidal behavior. One interpretation for the low BDNF levels in suicidal patients is that impaired serotonin function in suicidal depression would induce downregulation of BDNF expressions (Keller et al., 2010). Serotonin and BDNF have been linked to regulate neurogenesis, synaptic plasticity, and neuronal survival, and these two signals coregulate one another (Mattson et al., 2004). As a result, aberrant serotonin signaling could reduce expression of BDNF in suicidal depression and lead to deficits in neurons (van Heeringen and Mann, 2014). This concept is associated with the “stress and diathesis” model of suicide in which the risk for suicidal behaviors is ascertained not only by a psychiatric illness (the stressor) but also by a diathesis (Mann et al., 1999).
BDNF Levels Before and After Antidepressant Treatment
The results of our study on serum BDNF levels of patients with drug-naïve first-episode MDD did not agree with the concept that antidepressants exerted their effects by alterations in neurotrophins, and patients with pretreatment BDNF >6.1 ng/mL were more likely to be responders. Whether the BDNF levels alter significantly after antidepressant treatment or stay unchanged has been a matter of much debate, with discordant data (Brunoni et al., 2008; Sen et al., 2008; Molendijk et al., 2014; Polyakova et al., 2015). The reasons for these conflicting findings include antidepressant metabolic polymorphisms and other confounding factors, including differences in patient characteristics (such as duration of antidepressants intake, different clinical profiles, and sample size) and the tested materials (serum vs plasma). The real impact of various antidepressants on BDNF levels still needs further investigation.
Study Limitations
Though there was a decrease in BDNF levels among patients with first-episode drug-naïve MDD patients with suicidal behavior, the main limitation of this study is that BDNF levels were measured in serum, thus exhibiting an indirect assessment of brain BDNF levels. The true level of brain BDNF expression and its influence on different mental illnesses needs further study to reach stronger conclusions. Secondly, the ELISA kit utilized in this study is unfortunately not able to discriminate between isoforms of BDNF (pro-BDNF and mature BDNF). The third limitation of this study was the fact that the size of our sample was still small; however, drug-naive first-episode patients with MDD are pragmatically difficult to investigate. A replication study with a larger sample size is required. The fourth limitation was that not all the patients took the same antidepressants, and we could not distinguish the effects of the different antidepressants (escitalopram, fluoxetine, paroxetine, mirtazapine, and venlafaxine) on serum BDNF levels due to the small sample size of the patient group.
Conclusion
Our study showed the serum BDNF levels of patients with drug-naïve first-episode MDD were lower than those of healthy controls, and BDNF levels were significantly lower in suicidal MDD patients than in nonsuicidal MDD patients. There was no alteration in serum BDNF levels after treatment with antidepressants, and patients with pretreatment BDNF above 6.1 ng/mL were more likely to be responders. Although the relationship of our results to the mechanism of drug action and pathophysiology of depression remains unclear, the measure may have potential use as a predictor of response to treatment. In the future, studies with larger sample sizes are needed to investigate the relationships between BDNF levels, various antidepressants, and clinical symptoms.
Statement of Interest
None
Acknowledgments
The authors thank the medical staff at Kaohsiung Chang Gung Memorial Hospital for their continued support and services. This work was funded by grant numbers CMRPG860491, NSC94-2314-B-182A-208, NSC96-2314-B-182A-091, NSC99-2628-B-182A-065-MY2, and MOST103-2314-B182A-012 in Taiwan.
References
- Aydemir O, Deveci A, Taneli F. (2005) The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry 29:261–265. [DOI] [PubMed] [Google Scholar]
- Basterzi AD, Yazici K, Aslan E, Delialioglu N, Tasdelen B, Tot Acar S, Yazici A. (2009) Effects of fluoxetine and venlafaxine on serum brain derived neurotrophic factor levels in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 33:281–285. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. (2008) A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol 11:1169–1180. [DOI] [PubMed] [Google Scholar]
- Chong MY, Wilkinson G. (1989) Validation of 30- and 12-item versions of the Chinese Health Questionnaire (CHQ) in patients admitted for general health screening. Psychol Med 19:495–505. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. (1997) A molecular and cellular theory of depression. Arch Gen Psychiatry 54:597–606. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. (2003) Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 60:804–815. [DOI] [PubMed] [Google Scholar]
- Elfving B, Buttenschon HN, Foldager L, Poulsen PH, Andersen JH, Grynderup MB, Hansen AM, Kolstad HA, Kaerlev L, Mikkelsen S, Thomsen JF, Borglum AD, Wegener G, Mors O. (2012) Depression, the Val66Met polymorphism, age, and gender influence the serum BDNF level. J Psychiatr Res 46:1118–1125. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. (1997) User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-CV). Washington, DC: American Psychiatric Press. [Google Scholar]
- Gervasoni N, Aubry JM, Bondolfi G, Osiek C, Schwald M, Bertschy G, Karege F. (2005) Partial normalization of serum brain-derived neurotrophic factor in remitted patients after a major depressive episode. Neuropsychobiology 51: 234–238. [DOI] [PubMed] [Google Scholar]
- Gonul AS, Akdeniz F, Taneli F, Donat O, Eker C, Vahip S. (2005) Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci 255:381–386. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TL, Lee CT, Liu YL. (2008) Serum brain-derived neurotrophic factor levels in patients with major depression: effects of antidepressants. J Psychiatr Res 42:521–525. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. (2002) Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res 109:143–148. [DOI] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. (2005) Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res 136:29–37. [DOI] [PubMed] [Google Scholar]
- Keller S, Sarchiapone M, Zarrilli F, Videtic A, Ferraro A, Carli V, Sacchetti S, Lembo F, Angiolillo A, Jovanovic N, Pisanti F, Tomaiuolo R, Monticelli A, Balazic J, Roy A, Marusic A, Cocozza S, Fusco A, Bruni CB, Castaldo G, Chiariotti L (2010) Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Arch Gen Psychiatry 67:258–267. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee HP, Won SD, Park EY, Lee HY, Lee BH, Lee SW, Yoon D, Han C, Kim DJ, Choi SH. (2007) Low plasma BDNF is associated with suicidal behavior in major depression. Prog Neuropsychopharmacol Biol Psychiatry 31:78–85. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM. (1999) Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry 156:181–189. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Bonaccorso S, Ricciardi A, Scaccianoce S, Panaccione I, Wang L, Ruberto A, Tatarelli R, Nicoletti F, Girardi P, Shelton RC. (2009) Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. J Psychiatr Res 43:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. (2004) BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 27:589–594. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. (2014) Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry 19:791–800. [DOI] [PubMed] [Google Scholar]
- Park H, Poo MM. (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14:7–23. [DOI] [PubMed] [Google Scholar]
- Piccinni A, Marazziti D, Catena M, Domenici L, Del Debbio A, Bianchi C, Mannari C, Martini C, Da Pozzo E, Schiavi E, Mariotti A, Roncaglia I, Palla A, Consoli G, Giovannini L, Massimetti G, Dell’Osso L. (2008) Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord 105:279–283. [DOI] [PubMed] [Google Scholar]
- Polyakova M, Stuke K, Schuemberg K, Mueller K, Schoenknecht P, Schroeter ML. (2015) BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord 174:432–440. [DOI] [PubMed] [Google Scholar]
- Rogoz Z, Skuza G, Legutko B. (2005) Repeated treatment with mirtazepine induces brain-derived neurotrophic factor gene expression in rats. J Physiol Pharmacol 56:661–671. [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. (2008) Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry 64:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. (2003) Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatr 54:70–75. [DOI] [PubMed] [Google Scholar]
- van Heeringen K, Mann JJ. (2014) The neurobiology of suicide. Lancet Psychiatry 1:63–72. [DOI] [PubMed] [Google Scholar]