Abstract
Background:
Chronic abuse of heroin leads to long-lasting and complicated cognitive impairment. Dopamine receptors are critically involved in the impulsive drug-driven behavior and the altered attention, processing speed, and mental flexibility that are associated with higher relapse rates. However, the effects of the different dopamine receptors and their possible involvement in heroin-induced cognitive impairment remain unclear.
Methods:
The 5-choice serial reaction time task was used to investigate the profiles of heroin-induced cognitive impairment in mice. The expression levels of dopamine D1- and D2-like receptors in the prefrontal cortex, nucleus accumbens, and caudate-putamen were determined. The effects of dopamine receptors on heroin-induced impulsivity in the 5-choice serial reaction time task were examined by agonist/antagonist treatment on D1 or D3 receptor mutant mice.
Results:
Systemic heroin administration influences several variables in the 5-choice serial reaction time task, most notably premature responses, a measure of motor impulsivity. These behavioral impairments are associated with increased D1 receptor and decreased D3 receptor mRNA and protein levels in 3 observed brain areas. The heroin-evoked increase in premature responses is mimicked by a D1 agonist and prevented by a D1 antagonist or genetic ablation of the D1 receptor gene. In contrast, a D3 agonist decreases both basal and heroin-evoked premature responses, while genetic ablation of the D3 receptor gene results in increased basal and heroin-evoked premature responses.
Conclusions:
Heroin-induced impulsive behavior in the 5-choice serial reaction time task is oppositely modulated by D1 and D3 receptor activation. The D1 receptors in the cortical-mesolimbic region play an indispensable role in modulating such behaviors.
Keywords: dopamine receptor, heroin, cognition, impulsivity
Significance Statement
Repeated heroin administration attenuates attention and increases impulsivity, which is accompanied by increased expression of D1 receptors and decreased expression of D3 receptors in the brain dopaminergic system. The heroin-induced impulsivity behavior may be modulated by the µ-opioid receptor-mediated disinhibition of dopamine neurons and further acts through the activation of both D1 and D3 receptors. The D1 receptor in the cortical-mesolimbic dopaminergic system is indispensable for modulating impulsive behavior.
Introduction
The chronic abuse of addictive substances, such as heroin, may lead to long-lasting and complicated impairment in the cognitive function of individuals (van Holst and Schilt, 2011). Such cognitive impairment potentially contributes to the burden of clinical treatment, either by requiring additional rehabilitation for cognitive deficits that impair daily function or by strengthening the drug-seeking urge through ancillary effects on behavior (Ornstein et al., 2000).
Heroin not only impairs impulse control, including a compromised ability to exert control over drug demands or to inhibit impulsive drug-driven behavior, but also alters attention, processing speed, and mental flexibility in ways associated with higher relapse rates (Passetti et al., 2008). In the past few years, studies of the acute and chronic effects of heroin on humans have shown a cluster of cognitive, behavioral, and physiological symptoms. For example, heroin-dependent participants showed a considerable attentional bias for heroin cues (Franken et al., 2003). Heroin also has a negative effect on impulse control, while attention and mental flexibility/abstract reasoning ability are not affected (Pau et al., 2002). Furthermore, heavier use of heroin has been shown to be associated with a greater likelihood of cognitive impairment (Zhong et al., 2015). However, inconsistent findings have been observed (Harty et al., 2011; Zhai et al., 2015), and there have been relatively few detailed investigations of the neuropsychological changes associated with long-term heroin use, particularly when comparing profiles of cognitive impairment in the same study.
There is substantial evidence that the corticostriatal system, particularly dopaminergic transmission, is a common neurobiological substrate for the cognitive processes expressed by decision-making, inhibitory control, attentional regulation, assigning reward, and motivation valence (Taylor et al., 2013). The mesolimbic and corticolimbic distributions of dopamine and opiate receptors might be expected to lead to different patterns of cognitive impairment among opiate abusers (Joyce and Meador-Woodruff, 1997). Consequently, the chronic abuse of opiates may lead to neuroadaptive changes in dopaminergic terminal regions such as the nucleus accumbens (NAc), caudate-putamen (CPu), and prefrontal cortex (PFC), leading to disruptive cognitive and behavioral patterns. For example, neuroimaging studies have demonstrated that dopamine depletion impairs PFC-NAc functional connectivity, thus reducing the control of attention and cognitive flexibility (Nagano-Saito et al., 2008). Dopamine receptors have been shown to be involved in the reinstatement of drug seeking both following reexposure to the previously self-administered drug (priming-induced reinstatement) and exposure to cues previously associated with drug administration (cue-induced reinstatement) (Shalev et al., 2002; Shaham et al., 2003; Bossert et al., 2005). Intracranial infusion of SCH-23390, an antagonist for dopamine D1-like receptors (including D1 and D5 receptors), into the prelimbic cortex potently and dose dependently attenuated heroin seeking in response to either cue presentations or a priming dose of heroin, suggesting that D1 receptors regulate prefrontal pathways necessary for the reinstatement of heroin seeking (See, 2009). Attenuated reinstatement of heroin seeking was observed in rats injected with SCH-23390 into the NAc shell, suggesting that dopamine transmission in the NAc through the D1-like receptors plays a critical role in the drug reinforcement and motivational drive that promotes drug-seeking behavior (Tobin et al., 2013). Studies also revealed an attenuation of drug seeking following systemic administration of the D3 receptor antagonist, SB-277011A (Xi et al., 2004). Furthermore, activation of the D2-like receptors in the NAc exerts divergent effects on impulsivity and locomotor activity in high- and low-impulsivity rats (Moreno et al., 2013). While there is currently considerable debate on the effects of a specific dopamine receptor on differential cognitive modulation in heroin addicts (J. H. Liu et al., 2015b), there is a lack of experimental evidence addressing the expression of D1- and D2-like receptors and their sustained neurocognitive consequences after a protracted period of heroin administration.
The present study aimed to examine the comparative effects of dopamine D1- and D2-like receptors on heroin-induced cognitive impairment. For this purpose, 4 experiments were included: (1) the 5-choice serial reaction time task (5-CSRTT) was used to investigate the profiles of heroin-induced cognitive impairment. This task measures different types of performance that include aspects of attention, motivation, compulsiveness (perseveration), and impulsivity (premature responses) and that depend on neural systems including the PFC and striatum (Robbins, 2002). (2) The difference between D1- and D2-like receptor expressions after repeated heroin was examined by Western blot and quantitative RT-PCR. (3) The comparative effects of D1 and D3 receptors on heroin-induced impulsivity in the 5-CSRTT were examined using selective agonist/antagonist administration. (4) The interaction between heroin-induced impulsive actions and dopamine receptors was further investigated by using the D1 or D3 receptor knockout mice.
Materials and Methods
Animals
A total of 152 age-matched C57BL/6J male mice (8 weeks old, weighing 20~22 g on arrival) were housed under humidity- (50±5%) and temperature-controlled (22±3°C) conditions. All training and testing were conducted during the light phase (lights on from 7:00 am to 7:00 pm). All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals (NIH Publication No. 90-23), and the experimental protocols were approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University.
Drugs and Antibodies
Heroin (First Pharmaceutical Factory of Shenyang, Shenyang, China), the selective dopamine D1 receptor agonist SKF-38393, D1 receptor antagonist SCH-23390, and D3 receptor agonist PD-128907 (Sigma-Aldrich) were dissolved in 0.9% saline to obtain the required final concentration. The doses were based on previous reports (Cote and Kuzhikandathil, 2014; Sheng et al., 2015). Rabbit polyclonal antibodies against dopamine D1 (ab20066), D5 (ab40656), D2 (ab21218), D3 (ab42114), and D4 (ab135978) receptor were purchased from Abcam Technology. The mouse monoclonal antibodies against GAPDH and the horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies were purchased from Santa Cruz Technology.
5-CSRTT Training and Testing
Mice were trained on the 5-CSRTT as described previously with minor modifications (Finlay et al., 2015; see the supplementary Materials for detailed information.
Quantitative RT-PCR and Western Blot
Brain punches of the PFC, NAc, and CPu were collected and processed for RNA and protein extraction as previously described (Wang et al., 2015). The specific primers used in the qRT-PCR are shown in supplementary Table 1. See the supplementary Materials for detailed information.
Experiment 1: Effects of Systemic Heroin Administration on Cognitive Behaviors and Dopamine Receptor Expression
Four groups of mice (n=6/group) were trained on the 5-CSRTT until they attained ≥80% accuracy and ≤20% omissions under the 0.8-second stimulus duration condition for 3 consecutive days. They were then injected with heroin (1.0, 2.0, or 5.0 mg/kg) or saline i.p. 30 minutes before 5-CSRTT testing. The drug administration and 5-CSRTT testing proceeded for 10 consecutive days. After the completion of the test, the mice were killed immediately, and their brains were quickly removed and frozen on dry ice. The mRNA and protein expression levels of dopamine receptors in the PFC, NAc, and CPu were examined by RT-PCR and Western blot.
Experiment 2: Effects of Systemic Dopamine D1 and D3 Receptor Agonists on Cognitive Behaviors
A separate cohort of mice was randomly assigned to 3 groups (n=6/group) and trained on the 5-CSRTT until their accuracy was ≥80% and omissions were ≤20% under the 0.8-second stimulus duration. They were then treated with SKF-38393 (10 mg/kg), PD-128907 (0.05 mg/kg), or saline 30 minutes before 5-CSRTT testing. The drug administration and 5-CSRTT testing proceeded for 10 consecutive days.
Experiment 3: Effects of Dopamine D1 and D3 Receptor Activation/Inhibition on Heroin-Induced Behaviors
Mice were trained on the 5-CSRTT to the basal level (n=6/group). They were then treated with SKF-38393 (10 mg/kg), SCH-23390 (0.05 mg/kg), PD-128907 (0.05 mg/kg), or saline 15 minutes before the 2.0-mg/kg heroin treatment. All drugs were administered i.p., and the 5-CSRTT testing proceeded for 10 consecutive days.
Experiment 4: Effects of Heroin Administration on Impulsive Actions in Dopamine D1 or D3 Receptor Knockout Mice
Dopamine D1 (D1-/-) and D3 (D3-/-) receptor mutant mice were generated by Xu et al. (1994, 1997). Confirmation of genetic deletion, the D1 and D3 mutation was accomplished by Southern blot of tail DNA. C57BL/6 wild-type (WT) littermates, D1-/- and D3-/- mice were randomly assigned to 4 groups (n=6/group) and trained on the 5-CSRTT until the WT mice acquired a stable basal level. They were then i.p. treated with 0.05 mg/kg SCH23390 (15 minutes before heroin or saline injection), 2.0 mg/kg heroin, or saline 30 minutes before 5-CSRTT testing. The drug administration and 5-CSRTT testing proceeded for 10 consecutive days.
Statistical Analyses
All statistical analyses were performed using GraphPad Prism ver. 6.07 (GraphPad Software Inc.). The results are presented as the means±SEM. Behavioral data were analysed using repeated-measures 2-way ANOVA with time as a within-subjects factor and treatment as a between-subjects factor. For the mRNA results, 1-way ANOVA was used to determine the effect of heroin on dopamine receptor expression in different brain regions. Posthoc Dunnett’s comparisons were used where appropriate. For the analysis of Western-blot results, unpaired t tests were applied to measure the difference between heroin and saline groups. The values of the saline control groups were all set as 1. The other columns represent the fold change relative to the saline control group. Statistical significance was set at P < .05.
Results
Effects of Systemic Heroin Administration on 5-CSRTT Performance
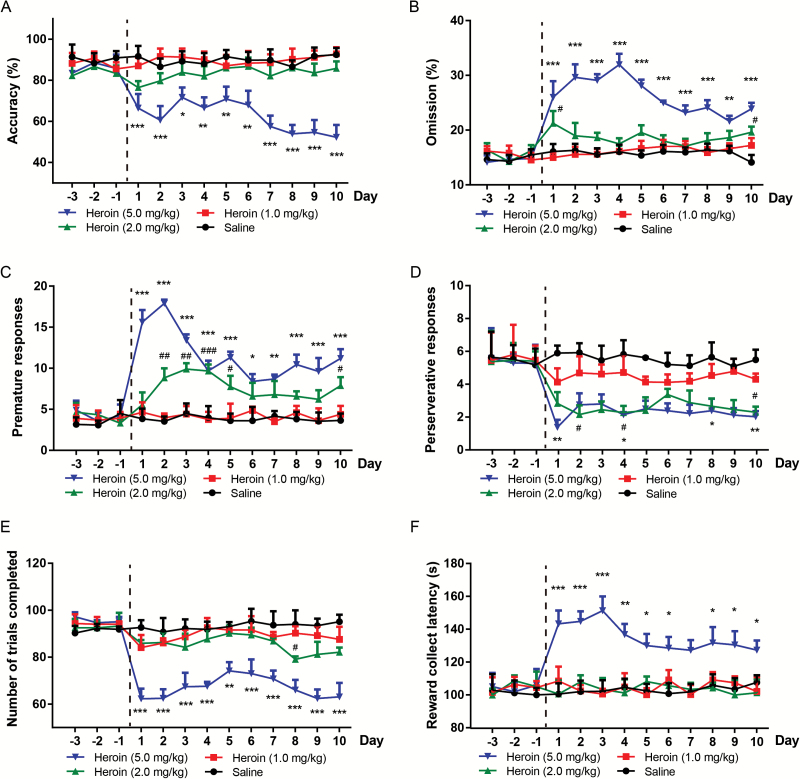
Following the acquisition of baseline performance, repeated heroin produced a significant decrease in accuracy% (dose-effect: F3, 200 = 73.66, P<.0001; Figure 1A) and an increase in omission% (dose-effect: F3, 200=129.6, P<.0001) (Figure 1B). The premature responses (dose-effect: F3, 200=107.4, P<.0001; Figure 1C) were increased, while the perseverative responses (dose-effect: F3, 200=47.06, P<.0001; Figure 1D) and trials completed (dose-effect: F3, 200=79.37, P<.0001; Figure 1E) were significantly decreased. In addition, the 5.0 mg/kg heroin produced an increase in feeder latency (dose-effect: F3, 200=556.62, P<.0001) (Figure 1F). The final 5-CSRTT performance of mice was analyzed by multiple comparisons. Five mg/kg heroin decreased attentional performance as measured by accuracy% (P<.0001) and omission% (P<.0001). Both 2.0 and 5.0 mg/kg heroin induced significant increases in premature responses (P<.0001). Meanwhile, the perseverative responses were attenuated by 2.0 and 5.0 mg/kg heroin (P<.01). Notably, the number of trials completed (P<.0001) and the feeder latency (P<.05) were significantly affected by 5.0 mg/kg heroin. Because 1.0 mg/kg heroin did not significantly affect the cognitive behaviors, while 5.0 mg/kg of heroin reduced motivation and exerted a strong sedation effect, 2.0 mg/kg of heroin was selected for the subsequent experiments.
Figure 1.
Effects of systemic heroin on 5-choice serial reaction time task (5-CSRTT) performance. Mice were administered i.p. with 1.0, 2.0, and 5.0 mg/kg heroin or saline. The 5-CSRTT testing was performed from day 1 to day 10. The values represent the mean ± SEM. All groups are compared with the saline group. For the 5.0-mg/kg heroin group, *P<.05, **P<.01, ***P<.0001; for the 2.0-mg/kg heroin group, #P<.05.
Dopamine Receptor Expression following Systemic Heroin Administration
Brain regions where tissue punches were performed are illustrated in Figure 2A. Figure 2B shows the relative mRNA levels of dopamine receptors in the PFC. One-way ANOVA revealed a significant effect of repeated heroin on the mRNA expression of dopamine D1 (F3, 20=12.82, P<.0001), D5 (F3, 20 = 4.73, P<.05), D2 (F 3, 20 = 3.92, P<.05), and D3 (F3, 20 =7.24, P<.01) receptors. In general, higher doses of heroin induced higher levels of D1 and D5 mRNA as well as lower levels of D2 and D3 receptors. In NAc (Figure 2C), D1 (F 3, 20=10.97, P<.0001) but not D5 (F 3, 20=0.369, P=.783) mRNA was increased by heroin. Meanwhile, the mRNA levels of D2 (F 3, 20=6.42, P<.01) and D3 (F 3, 20=6.02, P<.05) were decreased. As shown in Figure 2D, the mRNA expression levels of D1 (F 3, 20 = 7.34, P<.0001) and D5 (F 3, 20=5.22, P<.05) were increased and D3 (F3, 20=4.94, P<.05) were decreased in the CPu. No effect of heroin was found on D2 (F3, 20=0.214, P=.884), or D4 (F3, 20 = 0.093, P=.96) mRNA expression.
Figure 2.
Effects of systemic heroin on the expression of dopamine receptors. Mice were administered i.p. with 2.0 mg/kg heroin or saline. (A) Schematic representation showing the approximate location of the brain regions excised (blue area) and used for analysis. The relative fold change in dopamine receptor mRNA levels in the (B) prefrontal cortex (PFC), (C) nucleus accumbens (NAc), and (D) caudate putamen (CPu) are analyzed. The mRNA levels of the saline group are set as 1. All groups are compared with the saline group. (E) Representative blots for dopamine receptor expression in those brain regions following repeated 2.0 mg/kg heroin. Sal, saline group; Her, 2.0 mg/kg heroin group. (E) Quantitative analysis of Western-blot results; unpaired t test was applied to measure difference between heroin and saline groups. The data of saline groups were set as 1. All values represent the mean ± SEM. *P<.05, **P<.01, ***P<.0001.
We further examined the protein expression of these receptors by Western blot (Figure 2E) in those brain regions following repeated 2.0-mg/kg heroin treatments. Quantitative analysis for the Western-blot results (Figure 2F) showed that D1 expression levels in the 2.0-mg/kg heroin group were significantly increased compared with their saline controls in the PFC, NAc, and CPu (all P<.0001). Conversely, significant decreases of the D3 receptor expression levels in the 2.0-mg/kg heroin group were observed in all 3 brain regions (all P<.05). No obvious alteration of the expression of other dopamine receptors was found in 3 brain regions except for a decrease of D2 receptor in the PFC (P<.05).
Effects of Systemic Dopamine D1 and D3 Receptor Agonists on 5-CSRTT Performance
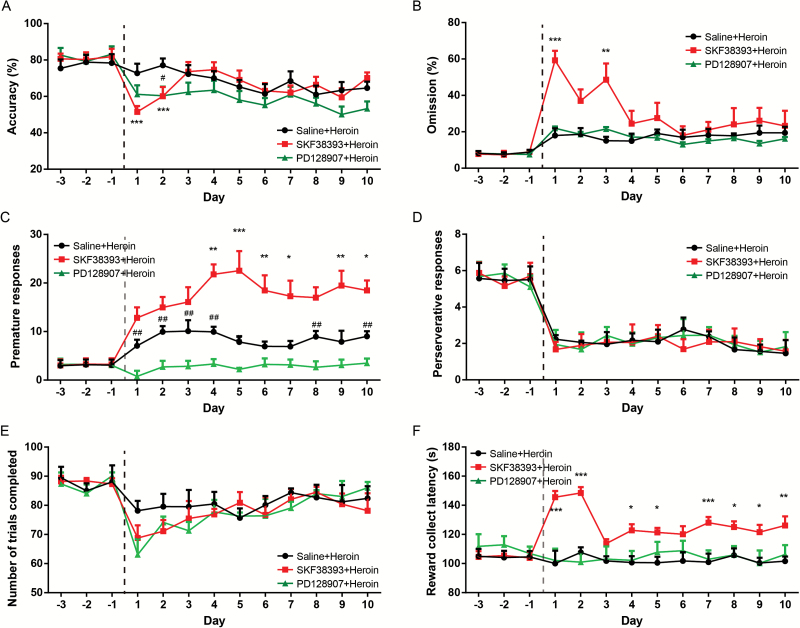
Three groups (n=6/group) of mice were trained on the 5-CSRTT until they achieved stable baseline performance. SKF-38393 increased omission% (drug-effect: F1, 100 = 668.2, P<.0001) but not accuracy% (drug-effect: F1, 100 = 2.472, P = .1197; Figure 3A-B), suggesting a partially attenuated attentional performance. There was a significant effect of time on accuracy% (time-effect: F9, 100=11.77, P<.0001) during the SKF-38393 administration period. Posthoc analysis revealed a sharp decrease in accuracy% in the first 2 days of SKF-38393 administration (P<.0001 and <.05, respectively) and a rapid return to baseline that might have resulted from adaptation. Premature responses were increased by SKF-38393 (drug-effect: F1, 100=164.9, P<.0001) (Figure 3C). No effect of SKF-38393 was found on perseverative action (drug-effect: F1, 100=0.3783, P =.5399) (Figure 3D). In addition, SKF-38393 induced a transient decrease in the number of trials completed (drug-effect: F1, 100=3.539, P<.05; time-effect: F9, 100=15.96, P<.0001; Figure 3E) and a persistent increase in the feeder latency (F1, 100=96.28, P<.0001; Figure 3F), suggesting subdued motivation.
Figure 3.
Effects of systemic administration of dopamine D1 and D3 receptor agonists on 5-choice serial reaction time task (5-CSRTT) performance. Mice were administered i.p. with SKF-38393 (10.0 mg/kg), PD-128907 (0.05 mg/kg), or saline. The 5-CSRTT testing consisted of 10 consecutive days. The values represent the mean ± SEM. All groups are compared with the saline group. For SKF-38393 group, *P<.05, **P<.01, ***P<.0001; for PD-128907 group, #P<.05, ##P<.01, ###P<.0001.
Compared with the effects of SKF-38393, the D3 receptor agonist PD-128907 affected attentional parameters in a different pattern. The accuracy% showed a persistent decrease (drug-effect: F1, 100 = 303.0, P<.0001; Figure 3A), whereas the omission% only increased on the first day (drug-effect: F1, 100 = 3.514, P=.0638; time-effect: F9, 100 = 4.786, P<.0001) (Figure 3B). Furthermore, repeated PD-128907 decreased the premature responses (drug-effect: F1, 100=92.61, P<.0001) (Figure 3C). The perseverative responses were not affected (drug-effect: F1, 100 = 3.273, P=.2734) (Figure 3D) during the experimental period. Repeated PD-128907 also decreased the motivational performances as measured by the trials completed (drug-effect: F1, 100=53.06, P<.0001; Figure 3E) and the feeder latency (drug-effect: F1, 100 = 78.15, P<.0001) (Figure 3F).
Dopamine D1 and D3 Receptor Activation on Heroin-Induced 5-CSRTT Performance
Because dopamine D1 and D3 receptor activation exerted complex effects on attentional and impulsive behavior, we further investigated whether the activation of these receptors contributed to the heroin-induced effects on 5-CSRTT performance. There was a significant effect of treatment on the attentional parameters, including accuracy% (drug-effect: F1, 100=12.55, P<.01; Figure 4A) and omission% (drug-effect: F1, 100=28.15, P<.0001; Figure 4B). However, no difference in accuracy% and omission% was found in the final 5-CSRTT tests, suggesting an adaptive process. In the SKF-38393/heroin combination treatment group, the premature responses (drug-effect: F1, 100 = 91.17, P<.0001; Figure 4C) were increased compared with heroin alone. The cognitive flexibility was not altered by the SKF-38393/heroin combination (drug-effect on the perseverative response: F1, 100=0.157, P=.693) (Figure 4D). No significant effect of treatment on the number of trials has been found (Figure 4E). The feeder latency was significantly affected by the SKF-38393/heroin combination treatment (drug-effect: F1, 100=142.3, P<.0001) (Figure 4F).
Figure 4.
Effects of dopamine D1 and D3 receptor activation on heroin-induced 5-choice serial reaction time task (5-CSRTT) performance. Mice were treated with SKF-38393 (10.0 mg/kg) and PD-128907 (0.05 mg/kg) or saline 15 minutes before the 2.0-mg/kg heroin treatment. All drugs were administered i.p., and the 5-CSRTT testing proceeded for 10 consecutive days. The values represent the mean ± SEM. All groups are compared with the saline+heroin group. For the SKF-38393+heroin group, *P<.05, **P<.01, ***P<.0001; for PD-128907+heroin group, #P<.05, ##P<.01, ###P<.0001.
In contrast to the heroin alone group, the accuracy% was slightly decreased by the combination treatment compared with heroin alone (drug-effect: F1, 100=24.21, P<.0001). The premature responses were significantly affected by the PD-128907/heroin treatment (drug-effect: F1, 100=102.5, P<.0001). Other performance measures reflecting attentional performance, cognitive flexibility, and motivation were not affected by the combination treatment (drug-effect in omission%: F1, 100=0.4681, P=.4954; perseverative responses: F1, 100=0.004, P=.95; trials completed: F1, 100 = 3.719, P = .0656; feeder latency: F1, 100 = 0.4681, P = .4954).
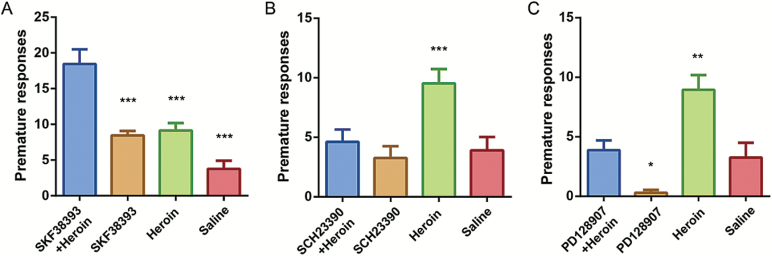
These results suggested that impulsive action was the most affected behavior by the agonists/heroin combination treatments, and the activation of dopamine D1 and D3 receptors seem to have opposite effects. In another cohort of mice, we further determined the premature responses following SKF-38393/SCH-23390/PD-128907 with or without heroin. Analysis revealed that the premature responses in the SKF-38393/heroin group were increased compared with both the SKF-38393 and heroin-alone groups (both P<.0001) (Figure 5A). As SKF-38393 enhanced heroin-induced impulsivity, we further examined whether the D1 antagonist could block this effect. As shown in Figure 5B, no difference was found between the SCH-23390 and saline mice (P=.952). However, the premature responses were significantly decreased in the SCH23390/heroin group compared with heroin alone (P<.0001). There was no difference between the SCH-23390/heroin group and the saline group (P=.939), indicating that SCH-23390 fully antagonized the effects of heroin. The PD-128907/heroin decreased premature responses significantly compared to heroin alone (P<.01) (Figure 5C). Moreover, the premature responses in the PD-128907/heroin group were increased compared with the PD-128907 group (0.3 ± 0.24, P<.05), further suggesting that heroin-induced impulsive action was blocked by PD-128907.
Figure 5.
Effect of SKF-38393, SCH-23390, and PD-128907 on heroin-induced impulsive actions. Trained mice were treated with SKF-38393 (10 mg/kg), SCH-23390 (0.05 mg/kg), PD-128907 (0.05 mg/kg), or saline, respectively, 15 minutes before the 2.0-mg/kg heroin treatment. All drugs were administered i.p., and 5-CSRTT testing consisted of 10 consecutive days. Data are the means ± SEM of the premature responses on the final 5-CSRTT test day. In each panel, all groups are compared with (A) SKF-39392+heroin, (B) SCH-23390+heroin or (C) PD-128907+heroin, respectively. *P<.05, **P<.01, ***P<.0001.
Effects of Heroin Administration on Impulsive Actions in D1-/- or D3-/- Mice
The basal performances for WT, D1-/-, and D3-/- mice are shown in Table 1. The accuracy% was significantly decreased while omission% was increased in D1-/- mice, indicating attenuated attentional function. The basal premature responses, perseverative responses, and number of trials completed were all significantly attenuated in D1-/- mice. These results may reflect that the D1 receptor affects cognitive behaviors comprehensively and is playing crucial roles in attention, impulsivity, and compulsivity. However, compared with WT mice, only accuracy% was decreased in D3-/- mice. Moreover, D3-/- mice showed increased basal premature responses and decreased perseverative responses and number of trials completed. The omission% and feeder latency were intact in D3-/- mice.
Table 1.
5-CSRTT Baseline Performance in WT, D1-/-, and D3-/- Mice
| WT | D1 -/- | D3 -/- | ANOVA | |
|---|---|---|---|---|
| Accuracy (%) | 92.1 ± 3.24 | 14.56 ± 2.11** | 56.47 ± 12.02*** | F2, 33 = 28.35, P < 0.0001 |
| Omission (%) | 16.44 ± 2.1 | 75.21 ± 6.46*** | 18.22 ± 2.89 | F2, 33 = 63.66, P < 0.0001 |
| Premature response | 4.25 ± 0.78 | 0.21 ± 0.07*** | 13.31 ± 0.66*** | F2, 33 = 128.7, P < 0.0001 |
| Perseverative response | 7.15 ± 1.38 | 0.38 ± 0.14*** | 1.1 ± 0.22*** | F2, 33 = 21.03, P < 0.0001 |
| Trials completed | 94.4 ± 4.2 | 11.34 ± 2.87*** | 72.34 ± 4.11* | F2, 33 = 129.8, P < 0.0001 |
| Feeder latency (s) | 1.24 ± 0.17 | 5.46 ± 1.14*** | 1.17 ± 0.13 | F2, 33 = 63.63, P < 0.0001 |
Data is showed in means±SEM. One-way ANOVA followed by Dunnett’s posthoc test used to analyze difference between strains. *P < .05, **P < .01, ***P < .0001, compared with WT mice.
In the following experiment, we focused on the effect of heroin administration on impulsive actions in D1-/- or D3-/- mice. As shown in Figure 6A, after 3 days of 5-CSRTT training under baseline conditions, WT and D1-/- mice showed stable premature nose pokes. The average number of premature nose pokes in D1-/- mice was significantly decreased compared with WT mice. Two-way ANOVA revealed a significant effect of both heroin (as the between-groups factor, F 3, 280 = 350.1, P<.0001) and time (F 13, 280 = 2.699, P<.01), as well as an interaction between them (F 39, 280=2.481, P<.0001). As expected, premature responses were increased in WT mice following the repeated heroin. However, it is surprising that D1-/- mice barely showed any premature responses either in the 5-CSRTT training sessions or after repeated heroin. To further dissect the impact of heroin and D1 receptor on impulsivity, the premature responses on day 10 were analyzed (Figure 6B). There were significant effects of both heroin (F 1, 20=53.59, P<.0001) and genotype (F 1, 20=275.2, P<.0001). Posthoc analysis showed a significant decrease in D1-/- mice regardless of whether they were injected with heroin or saline (both P<.0001).
Figure 6.
Effects of heroin administration on impulsive actions in D1-/- or D3-/- mice. Mice were administered i.p. with 2.0 mg/kg heroin or saline from day 1 to 10. The values represent the mean ± SEM. In A and C, all groups are compared with the WT-saline group. For the WT-heroin group, *P<.05, **P<.01, ***P<.0001. For the D1-/- (or D3-/-)-saline group, ##P<.01, ###P<.0001. For the D1-/- (or D3-/-)-heroin group, ##P<.01, ###P<.0001. In B and D, ***P<.0001 was compared with the WT-saline group, with ###P<.0001 between the indicated groups. In E and F, all groups are compared with the saline-treated group, *P<.05, **P<.01, ***P<.0001.
In contrast to D1-/- mice, the baseline premature nose pokes in D3-/- mice were obviously increased compared to WT mice (Figure 6C). There was a significant effect of both treatment (F 3, 280=1074, P<.0001) and time (F 13, 280 = 18.03, P<.0001) as well as an interaction between them (F 39, 280=5.728, P<.0001). In the final 5-CSRTT test, there was a significant increase in the WT/heroin, D3-/-/saline, and D3-/-/heroin mice (all P<.0001) compared with the WT/saline controls. It is worth noting that D3-/-/heroin mice showed more premature responses than their saline counterparts (P<.0001), suggesting an enhancement of impulsive actions following repeated heroin in the absence of D3 receptors.
Our results suggest that the D1 and D3 receptors oppositely regulate impulsivity, and the D1 receptor may have an essential “gateway” function in impulsive behavior. To better understand the role of D1 and D3 receptors in impulsivity, an experiment with the D1 receptor antagonist SCH23390 with or without heroin was performed with the D3-/- mice. Repeated measurement 2-way ANOVA revealed significant effects of both treatment (F 3, 280 = 390, P<.0001; Figure 6E) and time (F 13, 280 = 4.471, P<.0001) as well as an interaction between them (F 39, 280 = 9.043, P<.0001). In the final 5-CSRTT test, as expected, SCH23390 decreased the number of premature nose pokes (P<.01; Figure 6F) in the D3-/- mice while heroin significantly increased them (P<.0001). However, when pretreated with the SCH23390, the heroin-induced increase of premature nose pokes was completely blocked (saline vs SCH23390+heroin, P<.05; SCH23390 vs SCH23390+heroin, P = .857).
Discussion
The main findings of this study demonstrate divergent functional roles of the dopamine receptors in the modulation of heroin-induced cognitive impairment in mice and provide additional support for the idea that dopamine receptor dysfunction, especially D1 and D3 receptors, is associated with extreme-impulsivity endophenotypes on the 5-CSRTT.
Attentional function is often severely disrupted in many neuro-psychiatric disorders, including schizophrenia (Laurent et al., 1999), attention deficit/hyperactivity disorder (Asherson, 2005), depression (Asherson et al., 2014), and substance abuse. A greater percentage of individuals with attention deficit disorder reported previous use of alcohol, cannabis, and cocaine compared with those individuals who screened negative for attention deficit disorder (Vingilis et al., 2014). We reported that the attentional parameters were significantly affected by 2.0 and 5.0 mg/kg heroin. A decrease in accuracy, especially accompanied by an increase in omissions, usually reflects a deficit in attention. Of course, such a pattern can also result from noncognitive disruptions, such as sedation, locomotor impairment, or reduced motivation. Subsequent inspection of the total number of trials completed and the feeder latency suggested that the 5.0 but not 2.0 mg/kg heroin induced significant motivational deficits or locomotor impairment, and therefore, 2.0 mg/kg heroin was used in the following experiments.
Impulsivity is defined in part as the tendency to act prematurely and without adequate foresight into the consequences of the behavior (Evenden, 1999). One form of impulsivity, called impulsive action, occurs when an individual is unable to withhold or refrain from making an inappropriate or premature response (Pattij and Vanderschuren, 2008). This is different from impulsive choice that is exemplified by, for example, behavior that results in the delivery of a small immediate reward, rather than a better reward for which the individual must wait for a period of time (Moeller et al., 2001). In the present study, the observation that heroin robustly increased premature responses extends the previously reported acute effects of psychostimulants such as amphetamine (Harrison et al., 1997), cocaine (Paine et al., 2003), and nicotine (Kirshenbaum et al., 2011) on impulsive action. It is interesting that in the present study, perseverative responses in mice were attenuated by the repeated heroin administration (2.0 and 5.0 mg/kg). There is evidence that increased compulsiveness is closely related with the development and relapse of heroin addiction in both heroin abusers and animals (Ornstein et al., 2000; Belin et al., 2008). However, it should be considered that, although some of the measures in the 5-CSRTT often co-vary in certain circumstances, they sometimes dissociate and appear to depend on different processes, probably under the control of separable neural mechanisms (Robbins, 2002). An acutely high dose of morphine (6.0 mg/kg) was found to increase impulsive behavior in the 5-CSRTT and reduce the number of perseverative responses following the correct choice and before food reward collection (Pattij et al., 2009). This finding supports our observation and suggests certain beneficial effects of opiates on aspects of compulsive behavior. Moreover, in the present study, a high dose of morphine (5 mg/kg) also strongly decreased the response accuracy, increased the number of omissions, and lengthened response latencies; therefore, these effects on perseverative responding may also due to nonspecific drug effects on motor behavior.
While there is evidence that µ-opioid receptor mediates the disinhibition of dopamine neuron activity and is thus involved in psychopathological disorders in individuals (Spanagel et al., 1992), the dopaminergic system, especially dopamine receptors, can clearly be expected to play critical roles in heroin-induced cognitive impairment. Opiates differentially enhance dopamine transmission in the NAc (Muller and Unterwald, 2005), CPu (Gao et al., 2013), and PFC (C. Liu et al., 2015a). Previous research showed that the detrimental effects of psychostimulants on impulsivity depend heavily on dopamine receptor activation (van Gaalen et al., 2006). Moreover, variation in dopamine receptor expression has been linked to individual differences in impulsive behavior. Prolonged use or withdrawal from opiates could result in altered availability of D1- and D2-like receptors in the brain (Cosgrove, 2010; Hou et al., 2014). In our study, increases of D1/5 as well as decreases of D2/3 receptors were observed following repeated heroin. Specifically, the most significant changes were observed in D1 and D3 receptors within all 3 observed brain regions. Thus, we speculated that the altered function of D1 and D3 receptors might play a leading role in the effects of repeated heroin.
The results from experiments 2 and 3 revealed that the systemic activation of D1 or D3 receptors via their selective agonists led to divergent effects on impulsive actions. SKF-38393 robustly increased premature responses, whereas PD-128907 reduced such responses. The evidence indicated that the D1 receptor in the medial PFC is positively correlated with impulsive choice in rats (Loos et al., 2010). Blockade of D1 receptor in the core, but not the shell, of the NAc reduced impulsivity in the 5-CSRTT (Pattij et al., 2007), whereas the administration of SKF-38393 in the core had the opposite effect (Pezze et al., 2007). By contrast, striatal D2/3 receptor availability is negatively correlated with impulsive action in rodents (Laughlin et al., 2011) and with impulsive choice in humans (Ghahremani et al., 2012). D2/3 receptor agonist in humans attenuates the impulsive actions observed in stimulant-dependent individuals (Ersche et al., 2011). These studies were consistent with our observations. The apparent opposite contribution of the D1 and D3 receptors to impulsivity might reflect their general proposed roles in reward-directed behaviors, with D1 receptor activation thought to represent the positive value of actions and generate a response bias towards actions of higher value, while D3 receptor activation applies the “brake” on that goal-directed selection (Tai et al., 2012). In addition, perseverative nose pokes were unaffected following both treatments, suggesting that the D1 and D3 receptors have specific functions in modulating impulsive actions and attentional and motivational performance rather than compulsive aspects of performance (Tai et al., 2012). It is worth noting that repeated administration of the SCH23390 had no effect of premature responses, while many studies reported that decreased premature responding has been observed following administration of the D1 receptor antagonists. For example, on a simple reaction time task, decreased premature responses have been reported following acute treatment with SCH23390 (0.025~0.2 mg/kg, i.p.), raclopride (0.05~0.8 mg/kg, i.p.), and haloperidol (0.05~0.4 mg/kg, i.p.) (Marrow et al., 1993). However, D1 receptor antagonists have also been reported to have null effects on premature responding in response to SCH23390 (5, 10, 20 µg/kg, s.c.) (Amalric et al., 1993). Neither impulsive nor perseverative responding was affected following intra-NAc infusions of SCH23390 (1~100 ng/side), while SKF38393 increased impulsivity (5 μg/side) (Pezze et al., 2007). These discrepant results might reflect differences in the animal strain used; the specifics of the task, such as the delay after the onset of the stimulus, the behavior performed (e.g., nose poke vs lever press); or the administration paradigm of these compounds. In the present study, mice were repeatedly i.p. administered with antagonists 15 min before the 2.0-mg/kg heroin treatment for 10 consecutive days, while most of the previous studies used a single acute administration, which may not reflect the adaptive changes on cognition. In addition, SCH23390 fully antagonized the heroin-induced increase in premature responses, further proving the effectiveness of this drug.
In experiment 4, we found that D1-/- mice demonstrated hardly any premature responses, even after repeated heroin administration. This is presumably because the D1 receptor is essential to the expression of impulsivity endophenotypes and serves as a “gateway.” The experiment with SCH23390 with or without heroin in the D3-/- mice demonstrated that SCH23390 fully blocked premature responses even when the D3 receptor-mediated inhibition is lacking in D3-/- mice. This result provided important evidence that further supports our hypothesis that the enhancement of impulsive actions by heroin was fully blocked when this D1 receptor-regulated “gateway” was unavailable. Recently, evidence has shown that both the behavioral responses to reward-related cue and the specific NAc neuronal firing promoting such responses depend on dopamine release in those neurons (Nicola et al., 2004). Moreover, in medium spiny neurons, the stimulation of D1 receptor showed a dual effect: both depolarizing the cell to the firing threshold and decreasing the efficacy of the weakest synaptic inputs (O’Donnell, 2003). Thus, the diminished impulsive actions of D1-/- mice may be one behavioral correlate of this physiological mechanism. On the other hand, D3 receptor knockout resulted in elevated impulsivity, which was augmented by repeated heroin. Considering the general behavioral inhibitory effect of the D2-like receptors, the deletion of D3 receptor might represent the absence of an important way to negatively modulate impulsive actions.
Special attention should be paid to the methods of drug administration used in the present study. Investigator administration is a relatively high throughput method used to examine differences caused by acute or chronic drug exposure at varying time points after treatment (Renthal and Nestler, 2009). Self-administration paradigms, while labor-intensive, are better models of addiction and permit the study of rodents that, similar to humans, choose to take cocaine or another drug of abuse. Both of these paradigms have been used extensively to identify transient and long-lasting changes induced by drugs of abuse in mesolimbic and corticolimbic regions (Freeman et al., 2008). Because of the characteristics and operation principles of the 5-CSRTT, the 10% milk solution was provided as the only source of liquids for the animal to keep strong motivation to complete the task (Supplemental Materials). Thus, in the present study, a schedule of repeated heroin administration was used to examine the possible interaction between heroin-induced cognitive impairment and activation of dopamine receptors in mice. However, it should be noted that the human studies of drug abusers and much of the experimental evidence involves self-administration of opioids or psychostimulants. There are specific differences between the investigator-administration of a drug and drug self-administration, and our data shall take this into consideration for better interpretation and understanding.
Taken together, we demonstrated that heroin-induced cognitive impairment in the 5-CSRTT, including the attention, motivation, compulsivity, and most importantly impulsivity, were influenced by both the D1 and D3 receptors. Although in the present study, brain region drug infusion was not performed to examine the brain region-specific function of D1/D3 receptors in impulsivity, the results of systemic application of D1 and D3 receptor agonists/antagonist and the results of D1-/- and D3-/- mice on premature responses were robust and consistent, which supported the notion that impulsive behavior is critically and oppositely modulated by D1 and D3 receptor activation. Moreover, the D1 receptors in the cortical-mesolimbic region play an indispensable role in modulating such behaviors.
Funding
This work was supported by the National Science Foundation of China (81571858 to Y.Z. and 81501636 to Y.W.) and the Postdoctoral Science Foundation of China (2015M582673 to Y.W.).
Statement of Interest
None.
Supplementary Material
References
- Amalric M, Berhow M, Polis I, Koob GF. (1993) Selective effects of low-dose D2 dopamine receptor antagonism in a reaction-time task in rats. Neuropsychopharmacology 8:195–200. [DOI] [PubMed] [Google Scholar]
- Asherson P. (2005) Clinical assessment and treatment of attention deficit hyperactivity disorder in adults. Expert Rev Neurother 5:525–539. [DOI] [PubMed] [Google Scholar]
- Asherson P, Young AH, Eich-Hochli D, Moran P, Porsdal V, Deberdt W. (2014) Differential diagnosis, comorbidity, and treatment of attention-deficit/hyperactivity disorder in relation to bipolar disorder or borderline personality disorder in adults. Curr Med Res Opin 30:1657–1672. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320:1352–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. (2005) Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol 526:36–50. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP. (2010) Imaging receptor changes in human drug abusers. Curr Top Behav Neurosci 3:199–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote SR, Kuzhikandathil EV. (2014) In vitro and in vivo characterization of the agonist-dependent D3 dopamine receptor tolerance property. Neuropharmacology 79:359–367. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Abbott S, Craig KJ, Muller U, Suckling J, Ooi C, Shabbir SS, Clark L, Sahakian BJ, Fineberg NA, Merlo-Pich EV, Robbins TW, Bullmore ET. (2011) Response perseveration in stimulant dependence is associated with striatal dysfunction and can be ameliorated by a D(2/3) receptor agonist. Biol Psychiatry 70:754–762. [DOI] [PubMed] [Google Scholar]
- Evenden JL. (1999) Varieties of impulsivity. Psychopharmacology 146:348–361. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Dunham GA, Isherwood AM, Newton CJ, Nguyen TV, Reppar PC, Snitkovski I, Paschall SA, Greene RW. (2015) Effects of prefrontal cortex and hippocampal NMDA NR1-subunit deletion on complex cognitive and social behaviors. Brain Res 1600:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, Stam CJ, Hendriks VM, van den Brink W. (2003) Neurophysiological evidence for abnormal cognitive processing of drug cues in heroin dependence. Psychopharmacology 170:205–212. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, Morgan D, Roberts DC, Vrana KE. (2008) Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology 33:1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Li Y, Zhu N, Brimijoin S, Sui N. (2013) Roles of dopaminergic innervation of nucleus accumbens shell and dorsolateral caudate-putamen in cue-induced morphine seeking after prolonged abstinence and the underlying D1- and D2-like receptor mechanisms in rats. J Psychopharmacol 27:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, Poldrack RA, London ED. (2012) Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci 32:7316–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. (1997) Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology 133:329–342. [DOI] [PubMed] [Google Scholar]
- Harty SC, Whaley JE, Halperin JM, Ranaldi R. (2011) Impulsive choice, as measured in a delay discounting paradigm, remains stable after chronic heroin administration. Pharmacol Biochem Behav 98:337–340. [DOI] [PubMed] [Google Scholar]
- Hou H, Wang C, Jia S, Hu S, Tian M. (2014) Brain dopaminergic system changes in drug addiction: a review of positron emission tomography findings. Neurosci Bull 30:765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JN, Meador-Woodruff JH. (1997) Linking the family of D2 receptors to neuronal circuits in human brain: insights into schizophrenia. Neuropsychopharmacology 16:375–384. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AP, Jackson ER, Brown SJ, Fuchs JR, Miltner BC, Doughty AH. (2011) Nicotine-induced impulsive action: sensitization and attenuation by mecamylamine. Behav Pharmacol 22:207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin RE, Grant TL, Williams RW, Jentsch JD. (2011) Genetic dissection of behavioral flexibility: reversal learning in mice. Biol Psychiatry 69:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent A, Saoud M, Bougerol T, d’Amato T, Anchisi AM, Biloa-Tang M, Dalery J, Rochet T. (1999) Attentional deficits in patients with schizophrenia and in their non-psychotic first-degree relatives. Psychiatry Res 89:147–159. [DOI] [PubMed] [Google Scholar]
- Liu C, Fang X, Wu Q, Jin G, Zhen X. (2015. a) Prefrontal cortex gates acute morphine action on dopamine neurons in the ventral tegmental area. Neuropharmacology 95:299–308. [DOI] [PubMed] [Google Scholar]
- Liu JH, Zhong HJ, Dang J, Peng L, Zhu YS. (2015. b) Single-nucleotide polymorphisms in dopamine receptor D1 are associated with heroin dependence but not impulsive behavior. Gene Mol Res 14:4041–4050. [DOI] [PubMed] [Google Scholar]
- Loos M, Pattij T, Janssen MC, Counotte DS, Schoffelmeer AN, Smit AB, Spijker S, van Gaalen MM. (2010) Dopamine receptor D1/D5 gene expression in the medial prefrontal cortex predicts impulsive choice in rats. Cereb Cortex 20:1064–1070. [DOI] [PubMed] [Google Scholar]
- Marrow L, Overton P, Clark D. (1993) Disruption of conditioned reaction time performance by dopamine receptor antagonists in the rat. Behav Pharmacol 4:15–28. [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. (2001) Psychiatric aspects of impulsivity. Am J Psychiatry 158:1783–1793. [DOI] [PubMed] [Google Scholar]
- Moreno M, Economidou D, Mar AC, Lopez-Granero C, Caprioli D, Theobald DE, Fernando A, Newman AH, Robbins TW, Dalley JW. (2013) Divergent effects of D(2)/(3) receptor activation in the nucleus accumbens core and shell on impulsivity and locomotor activity in high and low impulsive rats. Psychopharmacology 228:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DL, Unterwald EM. (2005) D1 dopamine receptors modulate deltaFosB induction in rat striatum after intermittent morphine administration. J Pharmacol Exp Ther 314:148–154. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. (2008) Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci 28:3697–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. (2004) Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol 91:1840–1865. [DOI] [PubMed] [Google Scholar]
- O’Donnell P. (2003) Dopamine gating of forebrain neural ensembles. Eur J Neurosci 17:429–435. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. (2000) Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology 23:113–126. [DOI] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. (2003) Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res 147:135–147. [DOI] [PubMed] [Google Scholar]
- Passetti F, Clark L, Mehta MA, Joyce E, King M. (2008) Neuropsychological predictors of clinical outcome in opiate addiction. Drug Alcohol Depend 94:82–91. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. (2008) The neuropharmacology of impulsive behavior. Trends Pharmacol Sci 29:192–199. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM. (2007) Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology 191:587–598. [DOI] [PubMed] [Google Scholar]
- Pattij T, Schetters D, Janssen MC, Wiskerke J, Schoffelmeer AN. (2009) Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology 205:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau CW, Lee TM, Chan SF. (2002) The impact of heroin on frontal executive functions. Arch Clin Neuropsychol 17:663–670. [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, Robbins TW. (2007) Differential roles of dopamine D1 and D2 receptors in the nucleus accumbens in attentional performance on the five-choice serial reaction time task. Neuropsychopharmacology 32:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. (2009) Histone acetylation in drug addiction. Semin Cell Dev Biol 20:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. (2002) The 5-choice serial reaction time task: behavioral pharmacology and functional neurochemistry. Psychopharmacology 163:362–380. [DOI] [PubMed] [Google Scholar]
- See RE. (2009) Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol 12:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168:3–20. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42. [DOI] [PubMed] [Google Scholar]
- Sheng ZF, Cui XY, Cui SY, Yu B, Zhang XQ, Li SJ, Cao Q, Huang YL, Xu YP, Song JZ, Ding H, Lin ZG, Yang G, Zhang YH. (2015) Involvement of adrenoceptors, dopamine receptors and AMPA receptors in antidepressant-like action of 7-O-ethylfangchinoline in mice. Acta Pharmacol Sin 36:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 89:2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. (2012) Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci 15:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SB, Lewis CR, Olive MF. (2013) The neurocircuitry of illicit psychostimulant addiction: acute and chronic effects in humans. Subst Abuse Rehabil 4:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin S, Sedki F, Abbas Z, Shalev U. (2013) Antagonism of the dopamine D1-like receptor in mesocorticolimbic nuclei attenuates acute food deprivation-induced reinstatement of heroin seeking in rats. Eur J Neurosci 37:972–981. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ. (2006) Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology 187:73–85. [DOI] [PubMed] [Google Scholar]
- van Holst RJ, Schilt T. (2011) Drug-related decrease in neuropsychological functions of abstinent drug users. Curr Drug Abuse Rev 4:42–56. [DOI] [PubMed] [Google Scholar]
- Vingilis E, Mann RE, Erickson P, Toplak M, Kolla NJ, Seeley J, Jain U. (2014) Attention deficit hyperactivity disorder, other mental health problems, substance use, and driving: examination of a population-based, representative Canadian sample. Traffic Inj Prev 15:S1–9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lai J, Cui H, Zhu Y, Zhao B, Wang W, Wei S. (2015) Inhibition of histone deacetylase in the basolateral amygdala facilitates morphine context-associated memory formation in rats. J Mol Neurosci 55:269–278. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr., Hagan JJ, Heidbreder CA, Gardner EL. (2004) Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology 176:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, Tonegawa S. (1994) Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell 79:945–955. [DOI] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S. (1997) Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron 19:837–848. [DOI] [PubMed] [Google Scholar]
- Zhai T, Shao Y, Chen G, Ye E, Ma L, Wang L, Lei Y, Chen G, Li W, Zou F, Jin X, Li SJ, Yang Z. (2015) Nature of functional links in valuation networks differentiates impulsive behaviors between abstinent heroin-dependent subjects and nondrug-using subjects. NeuroImage 115:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N, Yuan Y, Chen H, Jiang H, Du J, Sun H, Hao W, Zhao M. (2015) Effects of a randomized comprehensive psychosocial intervention based on cognitive behavioral therapy theory and motivational interviewing techniques for community rehabilitation of patients with opioid use disorders in Shanghai, China. J Addict Med 9:322–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.