Abstract
Background:
The neurovascular plasticity of hippocampus is an important theory underlying major depression. Ketamine as a novel glutamatergic antidepressant drug can induce a rapid antidepressant effect within hours. In a mechanistic proof of this concept, we examined whether ketamine leads to an increase in synaptogenesis and vascularization within 24 hours after a single injection in a genetic rat model of depression.
Methods:
Flinders Sensitive Line and Flinders Resistant Line rats were given a single intraperitoneal injection of ketamine (15 mg/kg) or saline. One day later, their behavior was evaluated by a modified forced swim test. Microvessel length was evaluated with global spatial sampling and optical microscopy, whereas the number of asymmetric synapses was quantified through serial section electron microscopy by using physical disector method in the CA1.stratum radiatum area of hippocampus.
Results:
The immobility time in the forced swim test among Flinders Sensitive Line rats with ketamine treatment was significantly lower compared with Flinders Sensitive Line rats without treatment. The number of nonperforated and perforated synapses was significantly higher in the Flinders Sensitive Line-ketamine vs the Flinders Sensitive Line-vehicle group; however, ketamine did not induce a significant increase in the number of shaft synapses. Additionally, total length of microvessels was significantly increased 1 day after ketamine treatment in Flinders Sensitive Line rats in the hippocampal subregions, including the CA1.stratum radiatum.
Conclusion:
Our findings indicate that hippocampal vascularization and synaptogenesis is co-regulated rapidly after ketamine, and microvascular elongation may be a supportive factor for synaptic plasticity and neuronal activity. These findings go hand-in-hand with the behavioral observations, where ketamine acts as a potent antidepressant.
Keywords: antidepressant, ketamine, hippocampus, synaptic plasticity, vascularization
Significance Statement
Preclinical studies on the mechanisms underlying the antidepressant effect of NMDAR antagonists such as ketamine suggest that the glutamate system is a key target for developing novel antidepressant drugs with fast onset of action and broader efficacy. It has been suggested that differences in the onset of action between traditional antidepressant agents and ketamine could be due to the differences in the initiation time of synaptogenesis. However, to our knowledge, none of the studies has made a quantitative analysis of synaptogenesis in the hippocampus after ketamine treatment. Moreover, it has been reported that one of the main consequences of chronic stress is impairment of hippocampal vascular supply. Thus, in the present study, we tested the hypothesis whether changes in synaptogenesis and vascularization of hippocampus contribute to the rapid antidepressant effect of ketamine.
Introduction
Major depressive disorder is a heterogenic and complex psychiatric disease with high morbidity and mortality (suicide) rates worldwide (Vos et al., 2012). Despite progress in treatment of major depression, 2 main challenges remain unsolved. First, patients with treatment-resistant depression do not achieve complete clinical remission despite optimized antidepressant treatment; second, it usually lasts at least 2 weeks between the administration of conventional antidepressant drugs and clinical improvement, which increases the risk for patients with suicidal ideation in this time frame (Rush et al., 2011). The evidence related to the effect of chronic administration of monoaminergic antidepressant drugs like imipramine or citalopram on the N-methyl-D-aspartate (NMDA) receptors, suggesting that NMDA receptor antagonists can mimic the therapeutic effects of traditional antidepressant treatment (Boyer et al., 1998). Preclinical studies on the mechanisms underlying the antidepressant effect of NMDAR antagonists such as ketamine suggest that the glutamate system is a key target for developing novel antidepressant drugs with fast onset of action and broader efficacy (Covvey et al., 2012; Murrough, 2012; Hasselmann, 2014). Recently, a growing number of clinical studies indicate that ketamine improves depressive symptoms quickly, within hours (Salvadore et al., 2009; Larkin and Beautrais, 2011; Ghasemi et al., 2014; Drewniany et al., 2015). Notwithstanding, a growing number of studies also point to many different mechanisms of action of ketamine, but it is still not completely clear. It is well known that abnormality in cellular integrity, structure, and function of brain regions, especially the limbic structure hippocampus, is involved in the pathophysiology of depression and the action of current antidepressant treatments. Structural neurovascular plasticity of hippocampus is one of the important mechanisms that has been extensively studied in relation to major depression and the function of antidepressant drugs (Dranovsky and Hen, 2006; Chen et al., 2008, 2010; Leuner and Gould, 2010). It has been documented that neuroplasticity refers to the alteration in neural synapses and pathways in response to changes in behavior and environmental stressors (Pascual-Leone et al., 2011). Thereby, hippocampus has the crucial role in response to the stressful life events and environmental factors by structural and functional modifying (Pittenger and Duman, 2008). It has been suggested that differences in the onset of action between traditional antidepressant agents and ketamine could be due to the differences in the initiation time of synaptogenesis (S. W. Tang et al., 2012). Consistent with this evidence, recent preclinical studies have indicated a rapid enhancement of synaptic structure and function in cortical regions after ketamine treatment (Li et al., 2010, 2011). However, to our knowledge, none of the studies have made a quantitative analysis of synaptogenesis in the hippocampus after ketamine treatment. Vascular plasticity is another important structural mechanism regulating transport of oxygen and nutrients for neuronal metabolism. It has been reported that one of the main consequences of chronic stress is impairment of hippocampal vascular supply (Czeh et al., 2010), and electroconvulsive seizure (ECS) treatment has a counteracting effect on the vascular density in hippocampal subregions (Newton et al., 2006). Accordingly, identification of hippocampal substructures involved in the fast antidepressant action of ketamine could give us more insight about the mechanisms and targets underlying the antidepressant effect of ketamine and help us developing better antidepressant drugs. Thus, in the present study we tested the hypothesis whether changes in synaptogenesis and vascularization of hippocampus contribute to the rapid antidepressant effect of ketamine.
Materials and Methods
Animals
A total of 24 male Flinders Sensitive Line (FSL; a highly validated genetic animal model of depression with good face, construct, and predictive validities) (Overstreet, 1993; Wegener et al., 2012) and Flinders Resistant Line (FRL) rats as control group were used in this study. Flinders rats were bred at Translational Neuropsychiatry Unit, Aarhus University Hospital. The average age of the animals was 95 days. Animal care was carried out in accordance with the guidelines issued by the Danish committee on animal ethics (permission id 2012-15-2934-00254). Rats were pairwise housed in groups of 2, and the room temperature was maintained at 20°C to 22°C with a normal 12-h-light/-dark cycle and free access to food and water.
Treatments
A single (15 mg/kg) dose of S-Ketamine hydrochloride (5 mg/mL, Pfizer, ATC-code N01AX14) from the local hospital pharmacy was injected i.p. in 12 rats (6 animals in the FSL group and 6 animals in the FRL group) (Muller et al., 2013), and 12 animals (FSL=6 and FRL=6) received a single i.p. injection of saline. Twenty-four hours after treatment, animals were transcardially perfused as described below.
Behavioral Testing
Depression-like behavior of FSL/FRL model rats and the fast antidepressant-like effect of a single dose of ketamine 1 day after administration were evaluated by applying a modified forced swim test (FST) (Slattery and Cryan, 2012). In the modified FST, rats were placed individually 23.5 hours after a single i.p. ketamine injection in clear glass cylinders (60 cm high, 24 cm in diameter) that were filled with water to a height of 40 cm. The temperature of water was meticulously maintained at 25°C ± 1°C. At the end of the test session, the animals were removed from the cylinders; they were dried softly with a cloth towel and placed in a warm environment before putting them back in the home cages. The main behavioral parameters, mobility and floating, were monitored by applying a video camera for 7 minutes. The 7-minute session was divided into 7 parts (1 minute in each), and the predominant behavior in each 5-second period of a minute was recorded. All investigations were performed by the same researcher, who was blinded about the identity of the rats.
Tissue Preparation
The animals were deeply anesthetized with an i.p. injection of pentobarbital sodium/lidocaine (Unikem) 1 day after ketamine injection. Afterwards, they were perfused transcardially with heparinized (10 U/mL) 0.9% saline (pH=7.3) for 4 minutes followed by ice cold 2.5% glutaraldehyde in 4% paraformaldehyde (pH=7.2–7.4) for 7 minutes. Brains were removed, split into 2 hemispheres, stored in the same fixative, and post fixed at 4°C until they were cut. Left or right hippocampus was selected randomly and isolated. The natural curvature of the hippocampal CA1 region was straightened out manually along the septotemporal axis and embedded in 5% agar to cut coronally perpendicular to its longest dorsoventral axis at 65-μm thickness on a vibratome 3000 (Vibratome).
The selection of the first section for each series was done randomly by using a random table. In this study, 2 sets of sections were chosen based on a systematic sampling principle and a section sampling fraction of 1/12. One set of coronal hippocampal sections was used for quantification of the volume of hippocampal subregions, length density, and total length of microvessels under light microscopy (LM) and the second one was used for estimating the number of synapses by electron microscopy (EM). The set of tissue sections for LM was mounted on gelatin-coated slides and Nissl stained with a 0.25% thionin solution (thionin, Sigma T3387).
Electron Microscopy
Tissue sections for quantifying the number of synapses using EM were post fixed for 1 hour in 0.6% osmium tetroxide and embedded in TAAB 812 Epon. For the analysis of synapses, the Epon blocks were trimmed and 16 ultrathin serial sections (65 nm) were cut from their surface. The main reason for selecting the CA1.SR subregion of hippocampus for studying the synaptogenesis after ketamine treatment is the high percentage (94.7%) of excitatory glutamatergic synapses in this area. Moreover, LTP in the Schaffer collateral pathway in the CA1 stratum radiatum (CA1.SR) area of the hippocampus is NMDA receptor-dependent.
Stereological Estimation of Volume of CA1.SR and Molecular Layer of Dentate Gyrus (MDG)
For unbiased volume estimation of 2 hippocampal subregions including CA1.SR and MDG, the Cavalieri estimator was applied under light microscopy (Gundersen et al., 1988) using a 10× lens (Olympus, Splan, N.A. 0.45) at 456× magnification.
The following formula was used for calculation the volume of hippocampal subregions:
where ΣP is the total number of the points hitting the structure of interest, (a/p) is the area per test point (0.0073 mm2), t is the section thickness (65 µm), and SSF is the section sampling fraction (1/12).
Quantification of Vascular Parameters in the Hippocampal CA1.SR and MDG Subregions
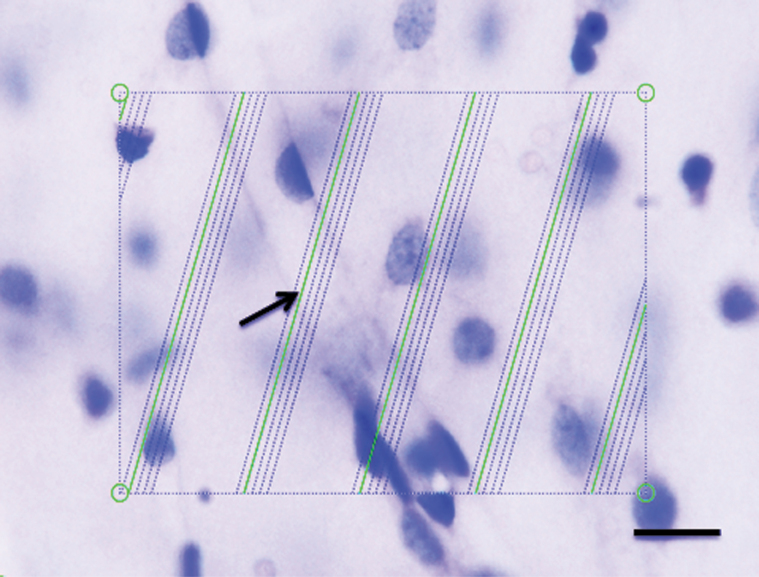
Quantification of the length density (Lv) and total length (L) of microvessels was performed with the aid of the global spatial sampling method (Larsen et al., 1998) in CA1.SR and MDG areas. Briefly, the regions of interest were delineated by a 4× objective on 65-µm-thick coronal thionin-stained sections. Afterwards, a 60× oil immersion lens (Olympus, Plan Apochromat, N.A. 1.35) was used to quantify the length of microvessels. Within a 3-dimensional sampling box, isotropic virtual planes with a fixed plane separation distance (d=25 µm) were overlaid systematically on CA1.SR and MDG areas, and the total number of intersections between the virtual planes and the microvessels was counted to estimate the length density of microvessels (Figure 1). The counting frame area was 7200 µm2 and the box height was 20 µm with a top guard zone of 5 µm. The x-y steps between sampling areas were 200 µm × 200 µm, obtaining counts of ~300 microvessel intersections per region per animal.
Figure 1.
Estimation of the length of microvessels in a 65-µm-thick vibratome section stained with thionin and visualized on a light microscope with a 60× objective oil immersion lens. Green test lines are superimposed on the live image by newCAST software, and they represent the intersection between isotropic virtual planes and the focal plane. Whenever the microvessels were in focus and virtual planes intersect them, they were counted. One microvessel is intersecting a virtual plane (arrow). Scale bar = 10 µm.
Estimates of microvessel length density within the CA1.SR and MDG subfields were performed using the following equation:
where Lv (microvessels) is the length density of microvessels in the region of interest; is the sum of intersections between test lines and microvessels; p (box) is the number of box corners =4; avg a(plane) is the average of plane area= 5760 µm2; and is sum of the box corners hitting the regions of interest.
Total length of microvessels was estimated with the following equation:
is the total microvessel length and is the total volume of the sampled hippocampal subregion. Microvessel was defined as a vessel with a 1-celled wall and a diameter ≤10 µm.
Sampling of CA1.SR Area for Stereological Estimation of the Number of Synapses
The CA1 pyramidal cell layer is located between the CA2 (small subfield with higher densely packed cells than CA1) and the subiculum (the area with the lower cell density and larger neurons than those found in CA1). Total length of CA1 stratum pyramidal was measured along the CA2-subicular (CA2-SUB) axis on the 65-µm-thick Nissl-stained sections under LM. In each animal the length of CA1 was divided into 6 uniform intervals, and 6 trapezoid-shaped areas along the CA2-SUB axis were cut with a diamond knife at these intervals after random placement within the first interval. Serial ultrathin (60–80 nm) sections were cut from each trapezoid-shaped area. The mean section thickness was 73.2 nm according to the Small’s method of minimal folds (Williams, 1981).
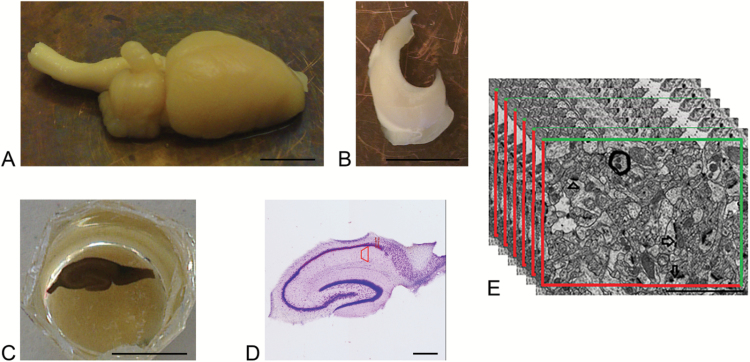
A FEI Morgagni transmission electron microscope was used for capturing electron micrographs of the trapezoid-shaped area of CA1.SR. Electron micrographs were taken with a digital camera (SISIII Mega-View digital camera, Olympus Soft Imaging Solutions) at an initial magnification of 10500× and digitally increased to a final magnification of 23850×. At the final magnification, all types of excitatory synapses were visualized clearly and the synapse number density was analyzed using iTEM software (Olympus Soft Imaging Solutions) (Figure 2).
Figure 2.
Schematic illustration of tissue processing of sections for studying under electron microscopy. (A) Rat brain hemisphere, scale bar 5.5 = mm. (B) Isolated rat hippocampus, scale bar 5.5 = mm. (C) Epon block containing osmium tetroxide stained hippocampal section of thickness 65 µm, scale bar = 5.5 mm. (D) 65-µm-thick-Nissl stained vibratome section with trapezoid-shaped area of interest, scale bar = 0.55 mm. (E) Disector electron micrographs from CA1.SR hippocampal subregion with the 2D unbiased counting frame for counting the number of perforated spine synapses (arrow), nonperforated spine synapses (arrowhead). Scale bar = 2 µm.
Unbiased Stereological Method for Quantification of Different Synapse Types in CA1.SR
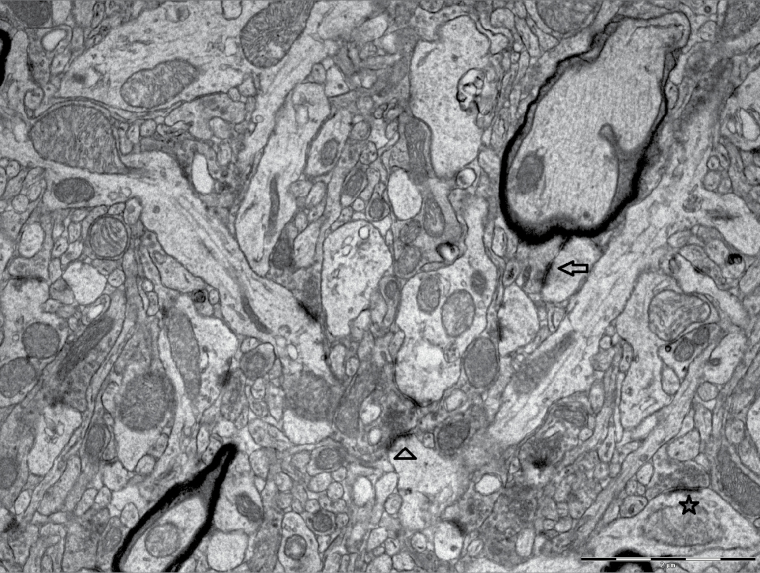
Synapses were recognized according to their ultrastructural features (presynaptic axonal terminal that contains vesicles, synaptic cleft, and the postsynaptic density [PSD]) and were classified into symmetric and asymmetric synapses. However, in this study, we perused merely asymmetric synapses. The asymmetric synapses were grouped into 2 types: spine (axospinous) and shaft (axodendritic) synapses. Furthermore, spine synapses were subdivided into perforated (discontinuous PSD) and nonperforated (continuous PSD) synapses (Figure 3).
Figure 3.
Perforated (arrow), nonperforated (arrowhead) spine, and shaft (star) synapses in electron micrograph from CA1 stratum radiatum subfield of hippocampus. Scale bar = 2 µm.
Unbiased estimates of number of different morphological types of asymmetric synapses in the CA1.SR subregion of hippocampus was done by the physical disector method (Sterio, 1984) modified from previous studies (Y. Tang et al., 2001). Each disector is consisted of 2 nonoverlapping electron micrographs from 2 adjacent ultra-thin sections, a reference section and a look-up section. Each of the 2 micrographs was superimposed by an unbiased counting frame, and PSD used as a counting unit. The number of synaptic profiles was counted only if their PSD profiles were presented either fully or partly within an unbiased counting frame in the reference section micrograph without intersecting the exclusion lines.
The numerical density of synapse in the CA1.SR subregion of hippocampus for each animal (Nv) was calculated by the following formula:
where the total number of synapses is sampled by the disector; is the disector volume.
The volume of the disector was estimated by applying the following formula:
where is the sum of areas of all 2-dimensional unbiased counting frames and is the height of the disector (the mean section thickness).
The total number of synapses for each animal was calculated by applying this formula:
where is the total volume of CA1.SR and is the numerical density of synapses in hippocampal CA1.SR.
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics 22 program and graphs were created by the use of Sigmaplot 12.5 (SYSTAT, San Jose, CA).
Test for rapid effect of a single ketamine injection on behavioral and neurostructural parameters between 4 groups of animals was made using 2-way ANOVA with the posthoc Tukey’s test. Dependent variables were tested for normality using Q-Q plots and histograms. Variance homogeneity was checked by Levene’s test. The coefficient of error for all structural variable measurements was estimated according to the Gundersen paper (Gundersen et al., 1999). The CV of the estimates was computed as the SD/mean. Testing the correlation between different structural parameters of hippocampus and immobility behavior was performed by 2-tailed Pearson analysis. A 2-tailed probability level of P<.05 was used as the significance level and a nonsignificant trend was based on .05 ≤ P ≤ .10.
Results
Rapid Effect of Ketamine on the Behavioral Results of FST
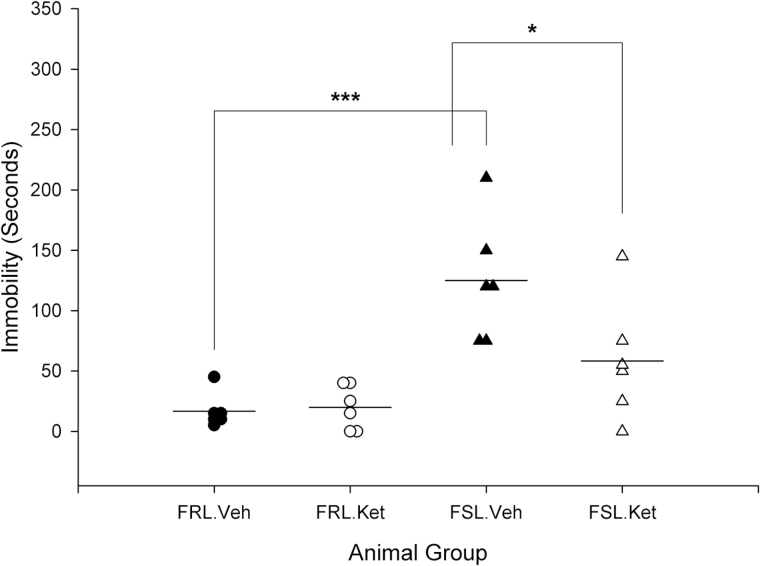
Results of the fast effect of ketamine on the immobility behavior in the FST revealed a significant strain × treatment interaction (F3,20=5.25; P=.03) with a significant influence of strain (F3,20=23.07; P<.001). FSL vehicle rats were significantly more immobile than FRL vehicle rats (P=.000). One day after ketamine treatment, immobility behavior significantly decreased in FSL rats (P=.02), while it did not change significantly in FRL rats (P>.05) (Figure 4).
Figure 4.
Fast effect of a single i.p. ketamine injection on immobility behavior in forced swim test (FST) in Flinders Sensitive Line (FSL) and Flinders Resistant Line (FRL) rats. *P<.05 and ***P<.001.
Open Field Test
Previously, our group published the results of open field test on the FSL rats. They found that ketamine treatment did not change locomotor activity in FSL rats [F(3,28)=0.864, P=.47] (Liebenberg et al., 2015).
Rapid Effect of Ketamine on the Number of Asymmetric Synapses in CA1.SR Area of Hippocampus
The number of nonperforated synapses in the CA1.SR area of hippocampus was significantly influenced by strain (F3,20=4.78; P=.041) and ketamine treatment (F3,20=5.01; P=.037). We found a significant difference in the number of nonperforated synapses between FSL vehicle and FRL vehicle rats (P=.043). However, there was no significant difference in perforated or shaft synapse number between FSL vehicle and FRL vehicle rats (P>.05). The number of nonperforated synapses increased notably in FSL rats 1 day after ketamine treatment (P=.040). Interestingly, ketamine treatment had a significant influence on the number of perforated synapses (F3,20=10.41; P=.004) and 1 day after a single ketamine injection, a significant alteration in the number of perforated synapses was revealed in FSL rats (P=.033). In the FRL group, neither nonperforated nor perforated synapses changed significantly 1 day after ketamine treatment (P>.05) (Table 1).
Table. 1.
The rapid effect of ketamine on the number of different types of asymmetric synapses in CA1.SR subfield of hippocampus
| FRL-Veh | FRL-Ket | FSL-Veh | FSL-Ket | FSL-Veh vs FRL-Veh |
FSL- Ket vs FSL-Veh |
FRL-Ket vs FRL-Veh |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (CV) | CE | Mean (CV) | CE | Mean (CV) | CE | Mean (CV) | CE | P | P | P | |
| No. of nonperforated synapses (109) | 10.3 (0.12) | 0.10 | 10.5 (0.12) | 0.12 | 7.8 (0.06) | 0.10 | 10.3 (0.21) | 0.10 | .043 | .040 | .994 |
| No. of perforated synapses (109) | 0.91 (0.17) | 0.17 | 1.17 (0.17) | 0.15 | 0.96 (0.19) | 0.15 | 1.44 (0.30) | 0.16 | .993 | .033 | .442 |
| No. of Shaft synapses (109) | 1.96 (0.40) | 0.16 | 1.92 (0.35) | 0.17 | 1.11 (0.40) | 0.17 | 1.72 (0.57) | 0.16 | .247 | .519 | 1.000 |
Rapid Effect of Ketamine on the Hippocampal Vascularization
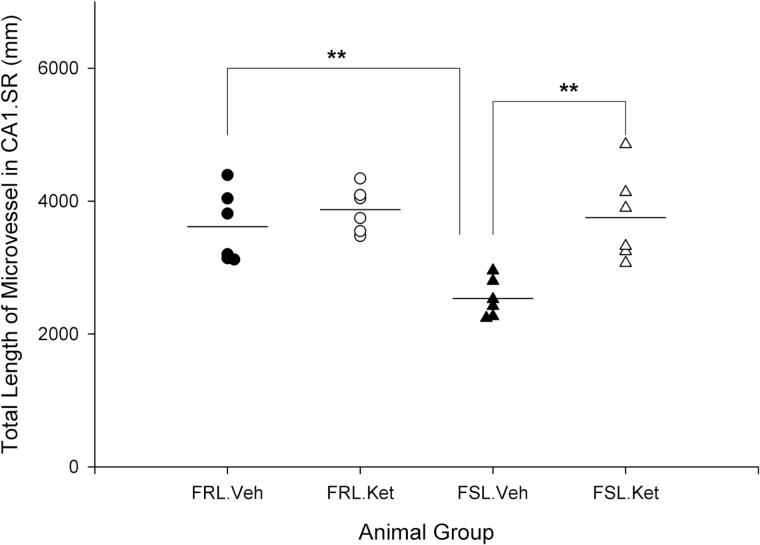
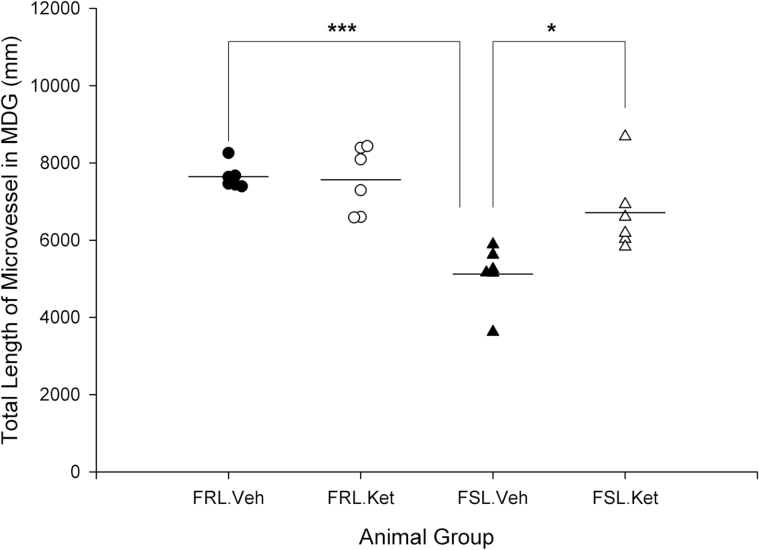
In this study, the length density and total length of microvesssels were quantitatively assessed in 2 hippocampal subregions (CA1.SR and MDG). A significant effect of strain × treatment interaction was observed on the length density of microvessels in MDG layer (F3,20 =5.42; P=.031). The length density of microvessels in both CA1.SR and MDG layers was significantly different between FSL vehicle rats and the FRL vehicle group (P=.043, P=.018). Overall, no significant increase in length density of microvessels was seen 1 day after ketamine treatment in FSL and FRL rats (P>.05). Two-way ANOVA analysis showed a significant effect of strain (F3,20=9.11; P=.007), ketamine treatment (F3,20=13.77; P=.001), and strain × treatment interaction (F3,20=5.87; P=.025) on the total length of microvessels in CA1.SR. Microvessels in the CA1.SR were significantly longer in FRL vehicle rats in comparison with FSL vehicle and in FSL ketamine rats vs the FSL vehicle group, respectively (P=.005; P=.002) (Figure 5). Similarly, total length of microvessels in MDG was significantly higher in FRL vehicle rats vs the FSL vehicle group and rapidly increased after ketamine treatment in FSL rats (P=.000; P=.012) (Figure 6). Furthermore, a negative correlation was found between the total length of microvessels (in both CA1.SR and MDG) and the duration of immobility behavior in the FST (r=-0.50, P=.012; r=-0.56, P=.004). Moreover, total length of microvessels in CA1.SR was correlated with the number of nonperforated synapses in this area of hippocampus (r=0.65, P=.000).
Figure 5.
Total length of microvessels in CA1.SR area of hippocampus in male Flinders Sensitive Line (FSL) and Flinders Resistant Line (FRL) rats 1 day after a single ketamine injection, **P<.01.
Figure 6.
Total length of microvessels in molecular layer of dentate gyrus (MDG) area of hippocampus in male Flinders Sensitive Line (FSL) and Flinders Resistant Line (FRL) rats 1 day after a single ketamine injection, *P<.05 and ***P<.001.
Rapid Effect of Ketamine on the Volume of Hippocampal Subregions
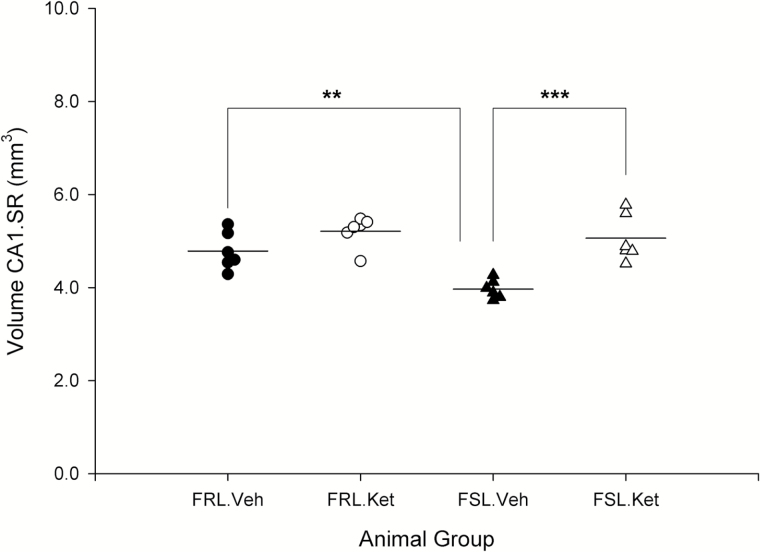
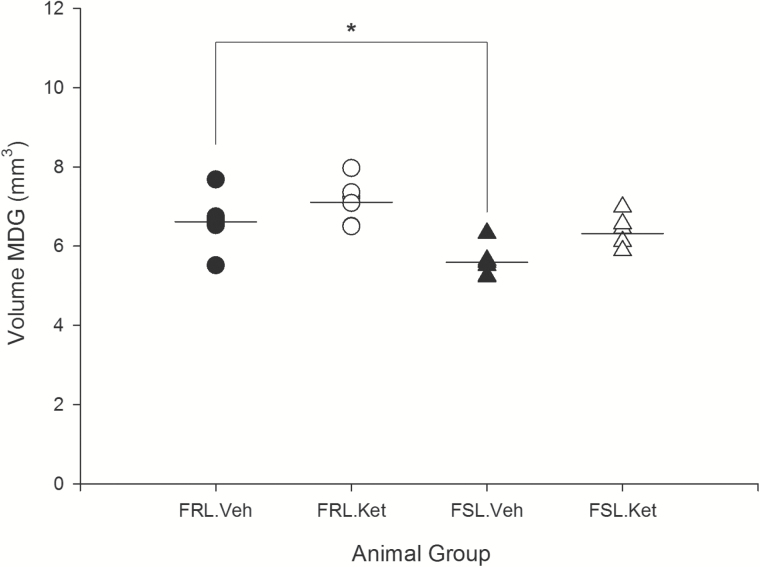
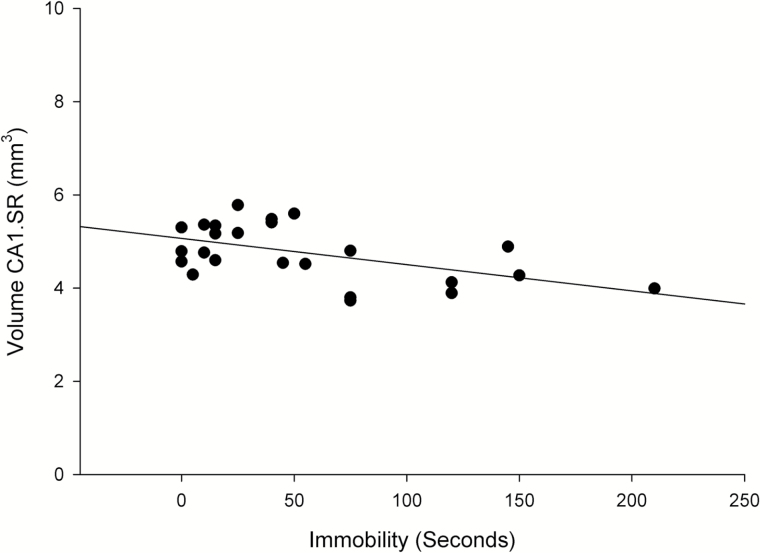
The volume of CA1.SR and MDG significantly influenced by strain (F3,20=9.82 and 17.48; P=.005, P=.000) and a rapid effect of ketamine treatment (F3,20=24.44 and 7.82; P=.000, P=.011). Moreover, a significant strain×ketamine-treatment interaction effect was observed on the volume of CA1.SR (F3,20=4.72; P=.042). There was a remarkable difference in the volume of CA1.SR and MDG between FRL vehicle and FSL vehicle, respectively (P=.006; P=.016). Our results demonstrated that the volume of the hippocampal CA1.SR subregion rapidly increased in FSL (depressed) rats after ketamine treatment (P=.000) (Figure 7). However, in the control group, changes in the volume of CA1.SR area were not significant (P>.05). Furthermore, the volume of MDG did not change significantly after ketamine treatment in both FSL and FRL rats (P>.05) (Figure 8). These data demonstrated a substantial negative correlation between the duration of immobility behavior in the FST and the volume of CA1.SR and MDG subregions of hippocampus (CA1.SR: r=-0.52, P=.008; MDG: r=-0.614, P=.001) (Figure 9).
Figure 7.
Volume of CA1.SR area of hippocampus in male Flinders Sensitive Line (FSL) and Flinders Resistant Line (FRL) rats 1 day after a single ketamine or saline administration, **P<.01, ***P<.001.
Figure 8.
Volume of the molecular layer of dentate gyrus (MDG) area of hippocampus in male Flinders Sensitive Line (FSL) and Flinders Resistant Line (FRL) rats 1 day after a single ketamine or saline administration, *P < .05.
Figure 9.
A significant negative correlation between the duration of immobility behavior in forced swim test (FST) and the volume of CA1.SR subregion of hippocampus (r=-0.52, P=.008).
Discussion
The etiology of major depressive disorder is complex and includes genetic and environmental factors with the neurobiological consequences involving structural and functional disruption of the brain. Numerous evidences indicate that disruption of hippocampal neuroplasticity contributes to the pathophysiology of depression and the action of antidepressant drugs. In the present study, we have selected hippocampus for studying the rapid effect of ketamine on the neurovascular plasticity due to simple and highly characteristic structural arrangement and more specifically, a critical role of hippocampus in the pathophysiology of depression (Nibuya et al., 1995; Mongeau et al., 1997; Cole et al., 2011). Results of the present study support the notion that ketamine rapidly induces synaptogenesis and vascularization of hippocampus. These structural changes mimic the alteration of neurovascular plasticity of hippocampus following chronic treatment with traditional antidepressant drugs (Chen et al., 2008, 2010). These findings go hand-in-hand with the behavioral observations, where ketamine acts as a fast and potent antidepressant without negative effect on locomotion (Liebenberg et al., 2015). Regarding the type of ketamine, it has been known that the affinity of S-ketamine is greater than R-ketamine. However, one rodent study showed that R-ketamine is more potent in antidepressant effect compared with S-ketamine (Zhang et al., 2014). Interestingly, a recent clinical study demonstrated the rapid antidepressant effect following low-dose S-ketamine administration (0.2 and 0.4 mg/kg), and they suggested that the lower dose will have less side effect with the same efficacy (Singh et al., 2016).
Fast Effect of Ketamine on Hippocampal Synaptic Plasticity
The observed hippocampal synaptic deficit in FSL rats was concordant with the abnormalities seen in major depression (Pittenger and Duman, 2008). It seems that hippocampus is hypoactive in major depression and ketamine has the rapid converse effect on this neuronal impairment. Preclinical studies provide evidence that the behavioral symptoms of depression are related to alteration of spine synapses in the hippocampus (C. H. Duman and Duman, 2015). We found that the number of nonperforated spine synapses in the hippocampus of depressed rats was significantly lower than in the control group. Qiao et al. (2014) demonstrated that in the chronic unpredictable mild stress animal model of depression, synaptic transmission in hippocampal CA1-CA3 synapses and the density of dendritic spines in CA1 and CA3 pyramidal neurons were decreased, and this impairment of synaptic plasticity was accompanied by a reduction of hippocampal brain-derived neurotrophic factor.
Earlier findings indicate that following 5 days of treatment with fluoxetine in ovariectomized female rats, synaptogenesis was induced in the CA1 subfield of hippocampus (Hajszan et al., 2005). Our results showed rapid increase in the number of spine (nonperforated and perforated) synapses 1 day after a single ketamine injection in FSL rats. Dendritic spines are dynamic structures, and making of new dendritic spines and changes in spine size and shape occurs rapidly after stimulation in adulthood (Bonhoeffer and Yuste, 2002). Accordingly, one speculation for the potency of ketamine for rapid synaptogenesis particularly in the CA1.SR area of mature hippocampus is stimulation of creating new postsynaptic spines together with remodeling of previous single axonal buttons to multiple synapse boutons (Yankova et al., 2001). Formerly, it was shown that the majority of axonal varicosities/boutons (68%) in the CA1.SR area of hippocampus forms a single synaptic connection and only 19% forms multiple synapses with 2 to 4 postsynaptic densities (Shepherd and Harris, 1998).
Reports indicate substantial correlation between the synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor level as one of the main involved receptors in synaptic plasticity and the size of the PSD in glutamatergic synapses in the CA1.SR area of hippocampus, whereby there is a lack of AMPA receptor in glutamatergic synapses with a small PSD in the CA1.SR area of hippocampus (Nusser et al., 1998; Mateos et al., 2007). Notably, it has been suggested that the rapid antidepressant action of ketamine is dependent on glutamate-AMPA receptor activation (R. S. Duman et al., 2012). Recently, it was discovered that one of the ketamine metabolites (2R,6R)-hydroxynorketamin enantiomer is the main required factor for the rapid antidepressant effect of ketamine. More interestingly, the main function of this component is activating AMPA receptor instead of inhibiting of NMDA receptor. Additionally, ketamine-related side effect was not observed following (2R,6R)-hydroxynorketamin treatment. Therefore, they concluded that antidepressant effect of ketamine is independent of NMDA receptor antagonism (Zanos et al., 2016). Accordingly, our results converge on the hypothesis that 1 day after ketamine treatment, the number of perforated synapses with a large size of PSD and possibly higher level of AMPA receptors was remarkably increased. The possible explanation for rapid increasing in the number of nonperforated synapses after ketamine treatment could be stimulating the synaptic remodeling by transforming shaft synapses into the spine synapses and the splitting of large perforated synapses into 2 or more nonperforated synapses (Popov et al., 2004).
Fast Effect of Ketamine on Hippocampal Vascularization
A significant correlation between depression and cardiovascular diseases (high incidence of cardiovascular diseases in depressed patients and cardiovascular dysfunction as a predisposing factor for depression) has been documented (Malhotra et al., 2000). Therefore, vascular dysfunction has been suggested as one of the mechanisms underlying the pathophysiology of depression. Additionally, counteracting effect of antidepressant treatments on the vascular alteration of hippocampus has been reported previously (Carney and Freedland, 2003; Camus et al., 2004). In one study in 2006, vascular density of 2 hippocampal subfields (dentate gyrus and the stratum lacunosum moleculare) was quantified following ECS treatment in male Sprague-Dawley rats. The results of this study showed only a 6% increase in the vascular density of dentate gyrus. However, the vascular density of the stratum lacunosum moleculare area was increased by 20% to 30%. They concluded that one of the consequences of vascular dysfunction is abnormality of the function of trophic factors as important factors for neuronal and glial normal function, and improvement of brain microcirculation by ECS could have a protective effect on the neuronal and glial structure and function (Newton et al., 2006). Moreover, one of the regulator factors for generation, differentiation, and maturation of newborn neurons in the DG is the interaction between neurons and the surrounding microenvironments including blood vessels. It has been indicated that the vascular endothelial growth factor (VEGF) is a critical mediator of both neurogenesis and angiogenesis in the adult brain (Udo et al., 2008). In one study in 2009, the role of VEGF in the action of fluoxetine was investigated, and they concluded that VEGF is an important factor for the behavioral effects of fluoxetine by showing that chronic treatment with fluoxetine stimulates the expression of VEGF in both neurons and endothelial cells in the hippocampus (Greene et al., 2009). Indeed, it has been shown that brain areas with large amounts of neuropil have a higher metabolic demand and consequently, need a higher density of capillaries (Klein et al., 1986). Based on our findings, there is a reason to believe that ketamine induces a rapid increase in the vascular network of hippocampus and then potentially may improve neuronal and glial function.
Fast Effect of Ketamine on Hippocampal Subregions Volume
Previously, neuroimaging studies demonstrated a significant hippocampal volume reduction in patients suffering from depression (Kempton et al., 2011; Cobb et al., 2013; Alves et al., 2014) and the counteracting effect of traditional antidepressant treatments on the reduced volume of hippocampus (Czeh et al., 2001). In this study, we found a significant decrease in the volume of CA1.SR area of hippocampus in FSL (depressed) rats. Recently, Abdallah et al. (2015) examined correlation between the rapid antidepressant effect of ketamine and hippocampal volume alteration in patients with MDD by magnetic resonance imaging before and 24 hours after a single dose of ketamine administration. They found that a smaller size of hippocampus was correlated with an enhanced rapid curative effect of ketamine (Abdallah et al., 2015). In the present study, we observed profound enhancement in the volume of CA1.SR, 24 hours after a single ketamine injection in depressed rats. It is well known that CA1.SR contains mainly dendritic arborizations, vessels, and glia cells. Perhaps these 3 structures are the main determinants for the alteration of the volume of this layer. Intriguingly, our results support the hypothesis that a change in the vascularization of hippocampus is one of the possible mechanisms related to the rapid changes in the volume of hippocampus after ketamine treatment.
Conclusion
Our findings related to the rapid synaptic and vascular alteration of hippocampus 1 day after a single ketamine injection provides a possible structural basis for explaining the fast onset of action of ketamine as a novel glutamatergic antidepressant drug and microvascular elongation may be a supportive factor for increased synaptic plasticity and neuronal activity.
Statement of Interest
None.
Acknowledgments
We are grateful to Herdis Krunderup and Lone Lysgaard for their excellent EM technical assistance and Anette Larsen for helping to prepare good-quality images. This work was supported by the Lundbeck Foundation and AU-IDEAS Initiative (eMOOD).
The Center for Stochastic Geometry and Advanced Bioimaging is supported by the Villum Foundation. Maryam Ardalan was supported by the Lundbeck Foundation.
References
- Abdallah CG, Salas R, Jackowski A, Baldwin P, Sato JR, Mathew SJ. (2015) Hippocampal volume and the rapid antidepressant effect of ketamine. J Psychopharmacol 29:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves GS, Carvalho AF, Sudo FK, Oertel-Knochel V, Knochel C, de Carvalho Lde A, Laks J, Engelhardt E, Pantel J. (2014) Structural neuroimaging findings in major depressive disorder throughout aging: a critical systematic review of prospective studies. CNS Neurol Disord Drug Targets 13:1846–1859. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T, Yuste R. (2002) Spine motility. Phenomenology, mechanisms, and function. Neuron 35:1019–1027. [DOI] [PubMed] [Google Scholar]
- Boyer PA, Skolnick P, Fossom LH. (1998) Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J Mol Neurosci 10:219–233. [DOI] [PubMed] [Google Scholar]
- Camus V, Kraehenbuhl H, Preisig M, Bula CJ, Waeber G. (2004) Geriatric depression and vascular diseases: what are the links? J Affect Disord 81:1–16. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE. (2003) Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry 54:241–247. [DOI] [PubMed] [Google Scholar]
- Chen F, Madsen TM, Wegener G, Nyengaard JR. (2008) Changes in rat hippocampal CA1 synapses following imipramine treatment. Hippocampus 18:631–639. [DOI] [PubMed] [Google Scholar]
- Chen F, Madsen TM, Wegener G, Nyengaard JR. (2010) Imipramine treatment increases the number of hippocampal synapses and neurons in a genetic animal model of depression. Hippocampus 20:1376–1384. [DOI] [PubMed] [Google Scholar]
- Cobb JA, Simpson J, Mahajan GJ, Overholser JC, Jurjus GJ, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA. (2013) Hippocampal volume and total cell numbers in major depressive disorder. J Psychiatr Res 47:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J, Costafreda SG, McGuffin P, Fu CH. (2011) Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J Affect Disord 134:483–487. [DOI] [PubMed] [Google Scholar]
- Covvey JR, Crawford AN, Lowe DK. (2012) Intravenous ketamine for treatment-resistant major depressive disorder. Ann Pharmacother 46:117–123. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. (2001) Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A 98:12796–12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Abumaria N, Rygula R, Fuchs E. (2010) Quantitative changes in hippocampal microvasculature of chronically stressed rats: no effect of fluoxetine treatment. Hippocampus 20:174–185. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. (2006) Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry 59:1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewniany E, Han J, Hancock C, Jones RL, Lim J, Nemat Gorgani N, Sperry JK, 3rd, Yu HJ, Raffa RB. (2015) Rapid-onset antidepressant action of ketamine: potential revolution in understanding and future pharmacologic treatment of depression. J Clin Pharm Ther 40:125–130. [DOI] [PubMed] [Google Scholar]
- Duman CH, Duman RS. (2015) Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett 601:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. (2012) Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 62:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi M, Kazemi MH, Yoosefi A, Ghasemi A, Paragomi P, Amini H, Afzali MH. (2014) Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Res 215:355–361. [DOI] [PubMed] [Google Scholar]
- Greene J, Banasr M, Lee B, Warner-Schmidt J, Duman RS. (2009) Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacol 34:2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. (1988) The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96:857–881. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. (1999) The efficiency of systematic sampling in stereology--reconsidered. J Microsc 193:199–211. [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. (2005) Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci 21:1299–1303. [DOI] [PubMed] [Google Scholar]
- Hasselmann HW. (2014) Ketamine as antidepressant? Current state and future perspectives. Curr Neuropharmacol 12:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, Williams SC. (2011) Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry 68:675–690. [DOI] [PubMed] [Google Scholar]
- Klein B, Kuschinsky W, Schrock H, Vetterlein F. (1986) Interdependency of local capillary density, blood flow, and metabolism in rat brains. Am J Physiol 251:H1333–1340. [DOI] [PubMed] [Google Scholar]
- Larkin GL, Beautrais AL. (2011) A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol 14:1127–1131. [DOI] [PubMed] [Google Scholar]
- Larsen JO, Gundersen HJ, Nielsen J. (1998) Global spatial sampling with isotropic virtual planes: estimators of length density and total length in thick, arbitrarily orientated sections. J Microsc 191:238–248. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E. (2010) Structural plasticity and hippocampal function. Annu Rev Psychol 61:111–140, C111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenberg N, Joca S, Wegener G. (2015) Nitric oxide involvement in the antidepressant-like effect of ketamine in the Flinders sensitive line rat model of depression. Acta Neuropsychiatr 27:90–96. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Tesar GE, Franco K. (2000) The relationship between depression and cardiovascular disorders. Curr Psychiatry Rep 2:241–246. [DOI] [PubMed] [Google Scholar]
- Mateos JM, Luthi A, Savic N, Stierli B, Streit P, Gahwiler BH, McKinney RA. (2007) Synaptic modifications at the CA3-CA1 synapse after chronic AMPA receptor blockade in rat hippocampal slices. J Physiol 581:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeau R, Blier P, de Montigny C. (1997) The serotonergic and noradrenergic systems of the hippocampus: their interactions and the effects of antidepressant treatments. Brain Res Brain Res Rev 23:145–195. [DOI] [PubMed] [Google Scholar]
- Muller HK, Wegener G, Liebenberg N, Zarate CA, Jr., Popoli M, Elfving B. (2013) Ketamine regulates the presynaptic release machinery in the hippocampus. J Psychiatr Res 47:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW. (2012) Ketamine as a novel antidepressant: from synapse to behavior. Clin Pharmacol Ther 91:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Girgenti MJ, Collier EF, Duman RS. (2006) Electroconvulsive seizure increases adult hippocampal angiogenesis in rats. Eur J Neurosci 24:819–828. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. (1995) Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15:7539–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. (1998) Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron 21:545–559. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. (1993) The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev 17:51–68. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, Bashir S, Vernet M, Shafi M, Westover B, Vahabzadeh-Hagh AM, Rotenberg A. (2011) Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr 24:302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacol 33:88–109. [DOI] [PubMed] [Google Scholar]
- Popov VI, Davies HA, Rogachevsky VV, Patrushev IV, Errington ML, Gabbott PL, Bliss TV, Stewart MG. (2004) Remodelling of synaptic morphology but unchanged synaptic density during late phase long-term potentiation (LTP): a serial section electron micrograph study in the dentate gyrus in the anaesthetised rat. Neuroscience 128:251–262. [DOI] [PubMed] [Google Scholar]
- Qiao H, An SC, Ren W, Ma XM. (2014) Progressive alterations of hippocampal CA3-CA1 synapses in an animal model of depression. Behav Brain Res 275:191–200. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, Warden D, Morris DW, Luther JF, Husain MM, Cook IA, Shelton RC, Lesser IM, Kornstein SG, Wisniewski SR. (2011) Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry 168:689–701. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr., Manji HK. (2009) Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry 65:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GMG, Harris KM. (1998) Three-dimensional structure and composition of CA3 -> CA1 axons in rat hippocampal slices: Implications for presynaptic connectivity and compartmentalization. J Neurosci 18:8300–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, Drevets WC, Van Nueten L. (2016) Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry 80:424–431. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF. (2012) Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc 7:1009–1014. [DOI] [PubMed] [Google Scholar]
- Sterio DC. (1984) The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134:127–136. [DOI] [PubMed] [Google Scholar]
- Tang SW, Helmeste D, Leonard B. (2012) Is neurogenesis relevant in depression and in the mechanism of antidepressant drug action? A critical review. World J Biol Psychiatry 13:402–412. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, De Groot DM, Gundersen HJ. (2001) Total regional and global number of synapses in the human brain neocortex. Synapse 41:258–273. [DOI] [PubMed] [Google Scholar]
- Udo H, Yoshida Y, Kino T, Ohnuki K, Mizunoya W, Mukuda T, Sugiyama H. (2008) Enhanced adult neurogenesis and angiogenesis and altered affective behaviors in mice overexpressing vascular endothelial growth factor 120. J Neurosci 28:14522–14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, et al. (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener G, Finger BC, Elfving B, Keller K, Liebenberg N, Fischer CW, Singewald N, Slattery DA, Neumann ID, Mathe AA. (2012) Neuropeptide S alters anxiety, but not depression-like behaviour in Flinders Sensitive Line rats: a genetic animal model of depression. Int J Neuropsychopharmacol 15:375–387. [DOI] [PubMed] [Google Scholar]
- Williams M. (1981) Section of determined thickness for use in stereological estimations on cells. Stereol Jugosl 3:369–374. [Google Scholar]
- Yankova M, Hart SA, Woolley CS. (2001) Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. P Natl Acad Sci USA 98:3525–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Jr, Gould TD. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K. (2014) R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116:137–141. [DOI] [PubMed] [Google Scholar]