Abstract
Background:
Early exposure to enriched environments has been shown to decrease the locomotor effects induced by repeated injections of cocaine and modify basal and cocaine-induced total protein levels of the transcription factor ΔFosB in the whole striatum of mice. In this study, we aimed at characterizing whether the profile of ΔFosB accumulation induced by enriched environments and cocaine would be similar or different in terms of brain areas and cell type.
Methods:
We used mice expressing the eGFP protein in D1 receptor positive (D1R(+)) neurons to determine whether Δ FosB induced by enriched environment or cocaine injections (5×15 mg/kg) would occur in selective subpopulations of neurons in several subregions of the striatum and prefrontal cortex.
Results:
We found that: (1) exposure to enriched environment reduces cocaine-induced locomotor activation, confirming our previous findings; (2) exposure to enriched environment by itself increases the accumulation of Δ FosB mostly in D1R(-) cells in the shell part of the nucleus accumbens and dorsal striatum, whereas in the nucleus accumbens core, Δ FosB accumulates in both D1R(+) and D1R(-) neurons; (3) in standard environment mice, cocaine induces accumulation of Δ FosB selectively in D1R(+) cells in the nucleus accumbens, dorsal striatum, and infralimbic cortex; and (4) the effects of enriched environments and cocaine on accumulation of Δ FosB were reciprocally blocked by their combination.
Conclusions:
Altogether, these results suggest that the enriched environment-induced reduction in behavioral effects of cocaine might result from 2 distinct effects on ΔFosB in striatal medium-sized spiny neurons belonging to the direct and indirect pathways.
Keywords: enriched environment, cocaine, ΔFosB, medium-sized spiny neurons, behavioral sensitization
Significance Statement
Here, we show that the preventive effects of environmental enrichment on the development of behavioral sensitization are associated with cell-specific alterations in ΔFosB levels in the striatum. Contrary to cocaine that increases ΔFosB levels in neurons expressing dopamine D1 receptors, which constitute the direct pathway, environmental enrichment increases them in neurons lacking D1 receptors, which constitute the indirect pathway. Moreover, environmental enrichment inhibits cocaine-induced locomotor activation and increases in ΔFosB levels. Therefore, by strengthening the indirect pathway, environmental enrichment opposes the addiction-related effects of cocaine. These results shed light on the neuroadaptations induced by environmental enrichment that participate in its beneficial effects. In addition, these results highlight the importance of cell-specific neuroadaptations that can lead to opposite functional effects.
Introduction
Acute injection of cocaine increases locomotor activity in animals, and this effect sensitizes after repeated injections of the drug. This phenomenon is termed behavioral sensitization (Kalivas and Stewart, 1991), and it has been shown to be long-lasting (Vanderschuren and Kalivas, 2000) and to be associated with long-term neuroadaptations in the brain (Pierce and Kalivas, 1997). One of the molecular mechanisms involved in the behavioral sensitization is the accumulation of the transcription factor ΔFosB in the striatum (Chen et al., 1997). Compared with other members of the Fos family protein, whose expression is rather transient, the expression of ΔFosB is more stable (Chen et al., 1997) and, because of its stability, ΔFosB accumulates in several brain regions, including the prefrontal cortex and striatum, thought to be involved in habit formation after chronic administration of cocaine (Hope et al., 1994; Nestler et al., 2001; McClung et al., 2004; Perrotti et al., 2008). For this reason, ΔFosB is often considered as a molecular switch for addiction (Nestler et al., 2001).
In the striatum, most of the neurons are GABAergic medium-sized spiny neurons (MSNs) (95% in rodents) (Kawaguchi, 1997; Bolam et al., 2000; Tepper and Bolam, 2004; Bertran-Gonzalez et al., 2010). These neurons can be mainly classified in 2 subtypes of neurons according to the dopaminergic receptor subtype that they express. One subpopulation of MSNs expresses the D1 receptor (D1R) subtype and corresponds to neurons projecting directly to midbrain regions, thus constituting the direct pathway (Le Moine et al., 1991; Cerovic et al., 2013; Gangarossa et al., 2013). The second subpopulation of MSNs expresses the D2 receptor (D2R) subtype and corresponds to neurons projecting to the same midbrain regions through other structures such as the globus pallidus pars externa or the subthalamic nucleus, thus constituting the indirect pathway (Le Moine et al., 1990; Cerovic et al., 2013; Gangarossa et al., 2013). It is thought that these MSNs subtypes and their parallel pathways exert complementary, and sometimes opposite, actions on behaviors controlled by the cortico-striatal system (Gerfen and Surmeier, 2011). By use of optogenetic approaches, it was shown that activation of the direct pathway, corresponding to D1R(+) cells, increases locomotion and produces reinforcement, whereas activation of the indirect pathway, corresponding to D2R(+) cells, increases freezing behavior and does not produce reinforcement (Kravitz et al., 2010, 2012). In addition, the indirect pathway seems to play a role in aversive behavior (Hikida, et al., 2010), and strengthening of this pathway promotes resilience to cocaine compulsive use (Bock et al., 2013), whereas its inactivation increases the behavioral sensitization induced by amphetamine (Ferguson et al., 2011). Finally, activation of neurons of the direct pathway increases the rewarding effects of cocaine, whereas activation of neurons of the indirect pathway decreases them (Lobo et al., 2010). Importantly, the reactivity to cocaine of each subtype of neurons of the striatum is also different. In fact, the D1R(+) MSNs are selectively activated in response to acute cocaine, as attested by the activation of the ERK pathway in these neurons (Bertran-Gonzalez et al., 2008), which is in agreement with the major involvement of the D1R subtype in the increased expression of immediate early genes induced by cocaine (Moratalla et al., 1996; Lee et al., 2006). After chronic cocaine, accumulation of ΔFosB is mostly observed in MSNs of the direct pathway in the nucleus accumbens (NAc) and dorsal striatum (DSt) (Lobo et al., 2013). Interestingly, the inducible overexpression of ΔFosB selectively in D1R(+) MSNs of the striatum in transgenic mice seems to reproduce cocaine’s long-term effects such as increased sensitivity to the locomotor-activating and rewarding effects of drugs (Kelz et al., 1999; McClung et al., 2004); these mice also show an increased motivation for cocaine in a progressive ratio schedule of self-administration (Colby et al., 2003). These results suggest a major involvement of neurons of the direct pathway in behavioral and neuronal adaptations induced by drugs.
Environmental enrichment (EE) has been shown to reduce the behavioral and neurobiological effects of drugs such as cocaine (Solinas et al., 2009) and heroin (El Rawas et al., 2009), but not methamphetamine (Thiriet et al., 2011). In particular, exposing mice to enriched conditions during adolescence reduces the activating effects of cocaine in a behavioral sensitization protocol compared with controls animals housed in standard environments (SE) (Solinas et al., 2009). In parallel to these behavioral effects, EE was found to produce drastic changes in the accumulation of ΔFosB induced by cocaine in the striatum. In fact, EE by itself increased levels of ΔFosB in striatum (Solinas et al., 2009; Venebra-Munoz et al., 2014; Zhang et al., 2014) and prefrontal cortex (Lehmann and Herkenham, 2011; Venebra-Munoz et al., 2014). In addition, ΔFosB levels were comparable in EE mice injected repeatedly with cocaine and control SE animals injected with saline, suggesting that, in contrast to what is observed in SE mice, cocaine promotes degradation of ΔFosB protein in EE mice (Solinas et al., 2009; Zhang et al., 2014). Although previous studies suggest a role for ΔFosB in the decreased behavioral effects of cocaine found in EE mice, it is not clear how similar increases in striatal ΔFosB levels could lead to opposite effects. A recent study by Lobo and colleagues (2013) has started to provide insights into these apparent paradoxical effects. In that study, it was shown that exposure to EE, in contrast to what is observed in response to cocaine, increased the expression of ΔFosB in both D1R(+) and D2R(+) cells in NAc (shell and core) and DSt, suggesting that the neurons targeted by cocaine and by EE are not the same. However, they did not compare the accumulation of ΔFosB in response to cocaine as a function of differential environmental conditions.
In this study, we investigated whether the pattern of ΔFosB accumulation in response to chronic cocaine differs in EE compared with SE mice. We used transgenic mice that express the enhanced green fluorescent protein (eGFP) specifically in DR1(+) cells, which allowed for dissociation of the direct dopamine pathway from the indirect pathway. Mice were housed in EE or SE from weaning to adulthood and then underwent a behavioral sensitization protocol (5 injections of 15 mg/kg cocaine every second day). On the day after the last injection, brains were obtained for immunohistochemistry staining. We investigated ΔFosB accumulation in different subregions of the striatum and prefrontal cortex, as these regions play role in behavioral sensitization and addiction (Vanderschuren and Kalivas, 2000).
Materials and Methods
Subjects
BAC heterozygous Drd1a-eGFP mice generated by GENSAT (Gene Expression Nervous System Atlas) at Rockefeller University (New York, NY) backcrossed with C57BL/6 line (Gong et al., 2007) were used in this study (male genitors were kindly provided by Drs D. Hervé and J. A. Girault). In these mice, the expression of the eGFP protein is driven by the D1R gene regulatory elements to identify cells expressing the D1R subtype, which are labeled in green. Mice were housed in a temperature-controlled environment on a 12-h-light/-dark cycle with the lights on from 7:00 am to 7:00 pm and had ad libitum access to food and water. All experiments were conducted during the light phase, were in accordance to European Union directives (2010/63/EU) for the care of laboratory animals, and were approved by the local ethical committee (COMETHEA no. 02469-01).
Housing Environmental Conditions
After weaning (3 weeks of age), mice were randomly divided into 2 different housing environmental conditions: SE or EE. SE cages were common housing cages (25×20×15 cm) and EE cages consisted of larger (60×38×20 cm) cages containing a running wheel and a small plastic house, and 4 toys that were changed once a week with new toys of different shapes and colors. For both SE and EE conditions, mice were housed in groups of 4 for 2 to 3 months before the start of the behavioral experiments.
Locomotor Activity and Behavioral Sensitization Procedure
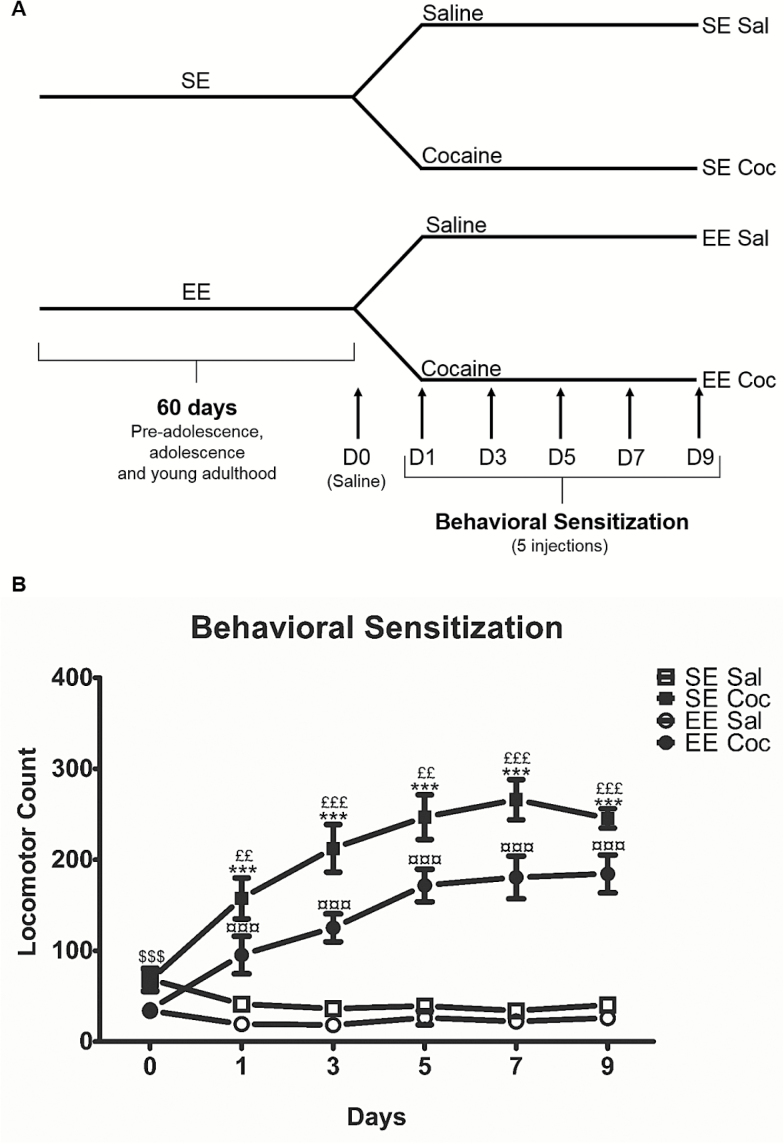
Horizontal locomotion was measured by the number of beam crossings in motor chambers (19×11×14 cm) (www.imetronic.com) connected to a computer (Solinas et al., 2009). A schematic representation of the protocol used for the behavioral sensitization is presented in Figure 1A. On the first day (day 0), all mice were injected with saline and placed in the locomotor chamber for 60 minutes to evaluate their basal locomotor activity. The next day, mice were placed again for 30 minutes in the locomotor chamber for habituation. After this period, one-half of the mice were injected with cocaine (15 mg/kg, i.p.) and the other one-half with saline (NaCl 0.9 g/L, i.p.), and they were immediately placed back in the same locomotor chamber for a 60-minute period during which their locomotor activity was measured. Then, every second day (5 injections in total), mice were submitted to the same protocol (Figure 1A). Four groups of animals were obtained (n=15–16/group): SE Sal, SE Coc, EE Sal, and EE Coc.
Figure 1.
Behavioral sensitization to cocaine in D1R-eGFP mice reared in standard (SE) or enriched (EE) environments. (A) Schematic representation of the experimental design used for the behavioral sensitization protocol. (B) Development of behavioral sensitization to cocaine (15 mg/kg, i.p.). Mice exposed to SE and EE develop behavioral sensitization, that is, cocaine-induced locomotor activity increases over days, but EE consistently show reduced locomotor response to cocaine compared with SE. We used Fisher’s protected least-squares difference posthoc test: $$$ P<.001 indicates different responses to saline of SE and EE mice on day 0; ***P<.001 indicates difference between SE Sal and SE Coc groups; ¤¤¤ P<.001 indicates difference between EE Sal and EE Coc groups and ££ P<.01 and £££ P<.001 indicates difference between SE Coc and EE Coc groups. Results represent means ± SEM from 15 to 16 mice.
Immunohistochemistry
Tissue Preparation
Eighteen to 20 hours after the last injection of cocaine or saline, mice were rapidly and deeply anaesthetized with pentobarbital (500 mg/kg, i.p., Sanofi-Aventis). We chose this time interval of brain collection to ensure that we are only detecting ΔFosB protein after chronic cocaine administrations and not other forms of FosB (Hope et al., 1994; McClung et al., 2004; Perrotti et al., 2008). Mice were then transcardially perfused with 4% paraformaldehyde dissolved in 0.1 M sodium phosphate buffer (pH 7.4). Brains were then removed and postfixed in 4% paraformaldehyde for 4 hours and stored in 30% sucrose at 4°C until sectioning. All serial brain sections (40 μm) were then cut using a freezing microtome (Leica RM2145, www.leica-microsystems.com) and stored in cryo-protective solution (glycerol 20%, DMSO 2%, NaCl 0.9%, PB 0.1 M) at −20°C until processed for immunolabeling.
Immunolabeling
Free-floating sections obtained from 8 to 10 mice of each group (SE Sal, SE Coc, EE Sal, and EE Coc) were processed for ΔFosB and eGFP protein detection. Sections taken from the prefrontal cortices to the striatum (+1.98 to +1.1 mm from bregma) were submitted to immunolabeling. Sections were initially washed extensively in phosphate buffered saline (PBS) (3×10 minutes) and incubated for 2 hours in blocking solution (PBS containing 3% bovine serum albumin [Sigma-Aldrich] and 0.3% Triton X-100). Subsequently, sections were incubated for 24 hours at room temperature with 2 primary antibodies diluted in the blocking solution: a rabbit monoclonal anti-FosB antibody (1:2000, 14695S, Cell Signaling) and a goat polyclonal anti-eGFP antibody (1:2000, Ab6673, Abcam) to amplify the eGFP signal. Although the anti-FosB antibody recognizes both FosB and ΔFosB proteins, previous studies showed that the expression of other Fos and Fra proteins disappears by 18 hours after the last cocaine administration, because they are rapidly degraded, unlike FosB, which is more stable and accumulates in brain areas after cocaine chronic administration (Hope et al., 1994; McClung et al., 2004; Perrotti et al., 2008). This is in agreement with our previous results using western-blot approaches (Solinas et al., 2009). Sections were then washed in PBS (3×10 minutes) and incubated for 45 minutes at room temperature with secondary antibodies diluted in the blocking solution: donkey anti-rabbit coupled to AlexaFluor555 (1:500, A31572, Life Technologies) and a donkey anti-goat coupled to AlexaFluor488 (1:500, A11055, Life Technologies). Sections were rinsed in PBS (2×10 minutes) and with a phosphate buffer (0.1 M, 10 minutes). Finally, sections were mounted onto gelatin-coated slides, dried, and dehydrated before cover slipping. Negative control sections were incubated in blocking solution without primary antibody (not shown).
Analysis of the Density of ∆FosB(+) Cells
The colocalization of ∆FosB labeling with the eGFP labeling (i.e., D1R(+) neurons) in the same cell was evaluated using confocal microscopy (confocal laser-scanning microscope FV1000, Olympus). For quantification of ∆FosB(+) cells, images were acquired with the Axio Imager M2 microscope with Apotome.2 (Carl Zeiss) (×20). The number of cells expressing ∆FosB was quantified using Image J software in 7 regions of the brain bilaterally. Two to 3 sections were analyzed for each animal and for each region, including the prelimbic cortex (PrL) and the infralimbic cortex (IL) (corresponding to sections from +1.98 to +1.70 mm from bregma), the anterior cingulate cortex (ACC), the NAc (shell and core), and the DSt, medial and lateral parts (corresponding to sections from +1.42 to +1.10 mm from bregma). We initially counted the total number of ∆FosB(+) cells in each brain area (see supplementary Figure 2 for delineation) to evaluate any changes in their density expressed as cells/mm2. To this extent, we fixed an arbitrary threshold using slides from a saline animal; this threshold corresponds to the half value of the maximal red signal. Cells with mean intensity above this threshold were considered ∆FosB(+) in sections from all other animals blindly counted. We then counted the number of ∆FosB(+) cells in which labeling corresponding to the eGFP protein was also detected in order to determine whether ∆FosB accumulates selectively in D1R(+) cells, in D1R(-) cells, or in both. In the striatum, MSNs correspond to about 95% of all neurons; consequently, we could consider that the cells without eGFP labeling in which ∆FosB accumulates are probably MSNs expressing the D2R subtype; however, to be more accurate, we described them as D1R(-) cells.
Statistical Analysis
All results are presented as group means (±SEM). Differences in behavioral activity and in the number of cells expressing ∆FosB between groups were assessed by 1-, 2-, or 3-way ANOVA. Results showing significant overall changes were subjected to Fisher protected least-squares difference posthoc test. Differences were considered significant when P<.05.
Results
EE Reduces the Sensitivity to the Locomotor Effects Induced by Repeated Injections of Cocaine in Drd1a-eGFP Mice
Prior to the first cocaine injection, basal levels of locomotor activity were higher in SE mice compared with EE mice (P<.001). Further, as previously reported in C57BL/6 (Bezard et al., 2003), the first injection of cocaine (15 mg/kg, i.p.) produced significantly higher locomotor activation (40%, P<.01) in the SE group compared with the EE group in BAC Drd1a-eGFP mice (Figure 1B). With repeated administrations, both SE Coc and EE Coc groups developed significant sensitization; for both groups the effect of cocaine increased upon repeated administration (Figure 1B). The relative amplitude of the sensitization as measured by the mean of the activities at days 7 and 9 (corresponding to the maximal response to cocaine) over the activity at day 1 was similar in both SE and EE mice (supplementary Figure 1). However, the locomotor effects of cocaine were consistently lower (about 40%) in EE compared with SE mice (Figure 1B) (3-way ANOVA, EE, F(1,57)=16.02, P<.01; treatment effect, F(1,57)=197.83, P<.0001; day effect, F(4, 228)=15.83, P<.0001; environment×treatment interaction, F(1,57)=6.73, P<.05; treatment×day interaction, F(4,228)=14.85, P<.0001]. These results provide additional evidence, using transgenic mice, that EE exposure can reduce the activating effects of cocaine in a behavioral sensitization protocol.
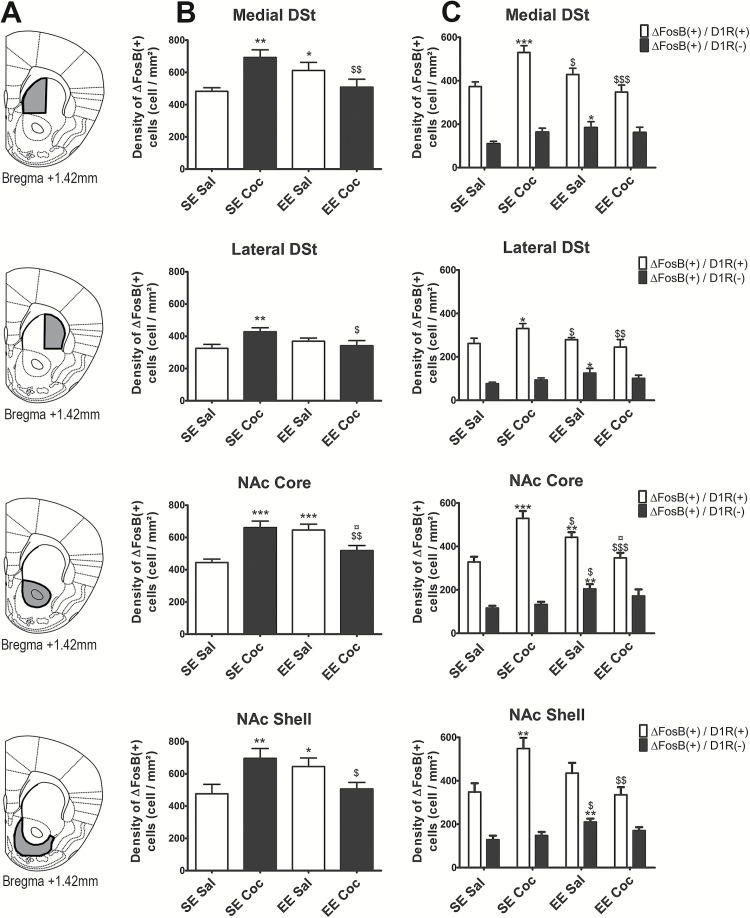
∆FosB Accumulation in Subregions of the Striatum after Chronic Cocaine in SE and EE Mice
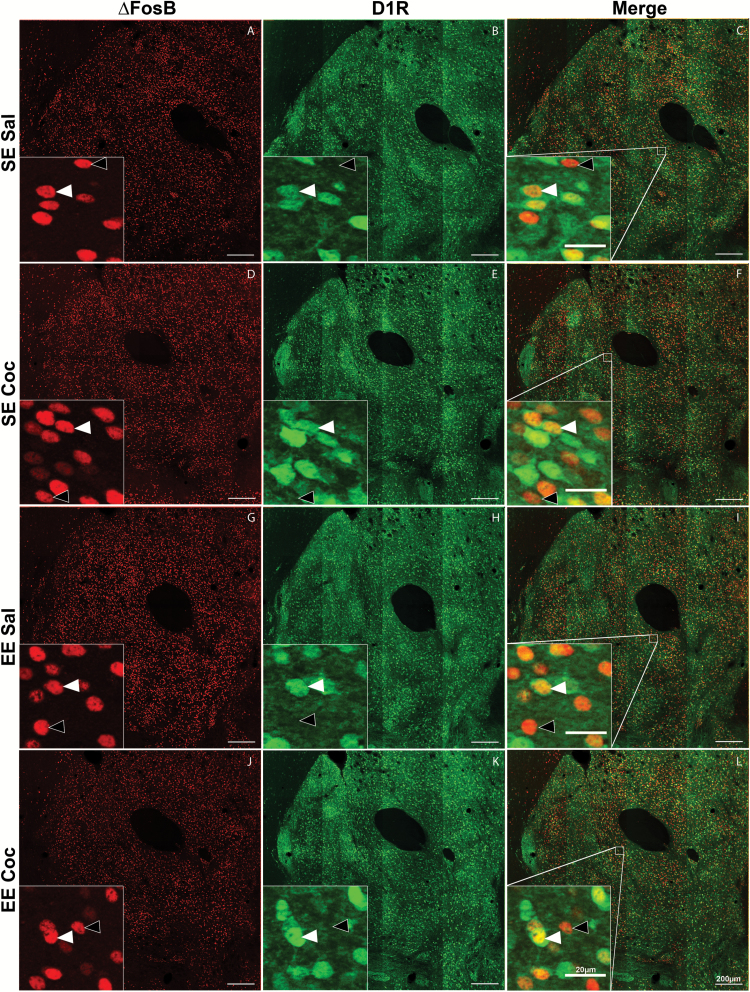
Figure 2 illustrates the labeling obtained in NAc core with antibodies against the eGFP protein (green labeling) (Figure 2, middle and right column), which allowed identifying the cells expressing D1R and against ∆FosB protein (red labeling) (Figure 2, left and right column) in mice of the 4 groups: SE Sal (Figure 2A-C), SE Coc (Figure 2D-F), EE Sal (Figure 2G-I), and EE Coc (Figure 2J-L). Consistent with a previous report (Lobo et al., 2013), exposure to EE or injections of cocaine did not alter the number of D1R(+) cells (data not shown). The number of ∆FosB(+) cells and ∆FosB/D1R(+) cells was quantified in 4 regions of the striatum: medial DSt, lateral DSt, NAc shell, and NAc core (Figure 3A, left to right). The delimitations of the surface in which cells were counted for each brain area are presented in Figure 3A and supplementary Figure 2. In saline controls, exposure to EE increased the density of total ∆FosB(+) cells (+21%, P<.05 in medial DSt; +26%, P<.05 in NAc shell; and +31%, P<.001 in NAc core), but not in lateral DSt compared with SE saline controls (Figure 3B). When we looked at specific cell type, this effect was mainly observed for D1R(-) cells (+35%, P<.05 in lateral DSt; +40%, P<.05 in medial DSt; +38%, P<.01 in NAc shell), except for NAc core, in which the increase was observed both in D1R(+) cells (+25%, P<.01) and D1R(-) cells (+43%, P<.01). In SE mice, we found a significant cocaine-induced increase in the density of total ∆FosB(+) cells in all these striatal regions (Figure 3B) (+30%, P<.001 in medial DSt; +24%, P<.001 in lateral DSt; +31%, P<.001 in NAc shell; and +32%, P<.01 in NAc core). In contrast to the effects of EE, however, repeated administration of cocaine in SE mice increased ∆FosB expression selectively in D1R(+) neurons (Figure 3C) (+29%, P<.001 in medial DSt; +24%, P<.05 in lateral DSt; +36%, P<.01 in NAc shell; and +37%, P<.001 in NAc core). Finally, in EE mice, cocaine did not increase the density of ∆FosB(+) cells in any of the striatal regions investigated, and it also reversed the accumulation of ∆FosB observed in EE control mice (Figure 3B). It should be noted that in all groups of mice, the density of ∆FosB/D1R(+) cells was always higher than the density of ∆FosB/D1R(-) cells (mean difference for all groups: +175%, P<.0001 in medial DSt; +178%, P<.0001 in lateral DSt; +160%, P<.0001 in NAc shell; and +166%, P<.0001 in NAc core).
Figure 2.
Representative examples of immunostaining obtained at the level of NAc core in standard environment (SE) Sal, SE Coc, enriched environment (EE) Sal, and EE Coc groups of D1R-eGFP mice. (A, D, G, and J) Immunolabeling against ΔFosB in red; (B, E, H, and K) immunolabeling against enhanced green fluorescent protein (eGFP) in green; (C,F, I, and L) the merge of the 2 labeled in orange. Orange cells correspond to cells that expressed both ΔFosB and eGFP. Images were taken at the level of Bregma +1.42 mm. Scale bars = 200 µm. The insets in each image show enlargement of regions indicated by open squares on images C, F, I, and L. White filled arrowheads point to ΔFosB(+)/D1R(+) cells and unfilled arrowheads point to ΔFosB(+)/D1R(-) cells. Scale bar in insets = 20 µm.
Figure 3.
Density of ΔFosB(+) cells in the striatum of standard environment (SE) Sal, SE Coc, enriched environment (EE) Sal, and EE Coc mice and characterization of the phenotype of these cells (D1 receptor [D1R](+) or D1R(-)). (A) Delimitations of the 4 striatal subregions (medial dorsal striatum [DSt], lateral DSt, nucleus accumbens [NAc] core and shell) used for quantification. (B) Density of ΔFosB(+) cells in each subregion. (C) Density of ΔFosB(+)/D1R(+) cells (white bars) and ΔFosB(+)/D1R(-) cells (black bars) in each subregion. Cocaine treatment significantly increased the density of ΔFosB(+)/D1R(+) MSNs in the 4 regions. EE significantly increased the density of ΔFosB(+)/D1R(-) cells in the medial and lateral DSt and NAc shell. In the NAc core, EE significantly increased the density of both ΔFosB/D1R(+) in and ΔFosB(+)/D1R(-) cells. The density of ΔFosB/D1R(+) and ΔFosB(+)/D1R(-) cells were similar in EE Coc and SE Sal animals. Results are expressed as means±SEM (n=8–10/group). We used Fisher’s protected least-squares difference posthoc test: * P<.05, ** P<.01, and *** P<.001, difference compared with SE Sal; $ P<.05, $$ P<.01, and $$$ P<.001, difference compared with SE Coc; ¤ P<.05, difference compared with EE Sal.
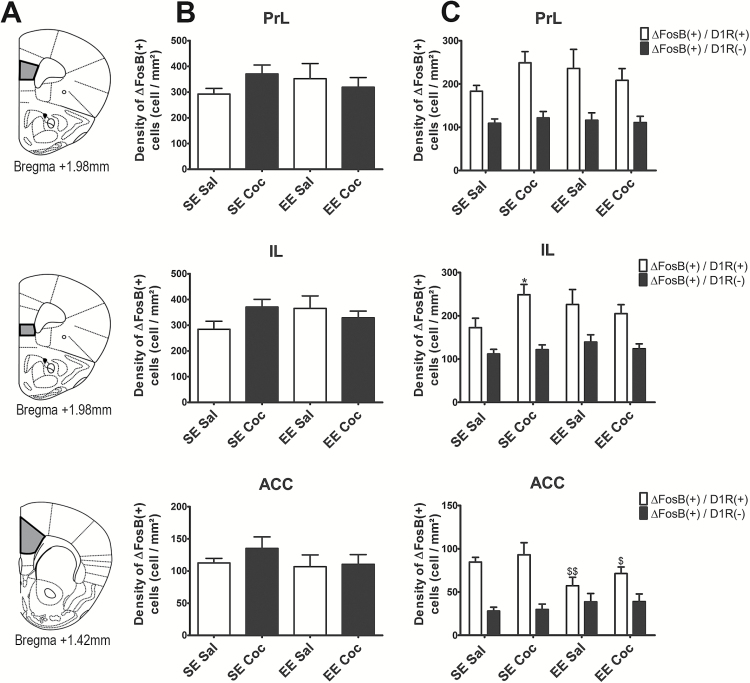
∆FosB Accumulation in Subregions of the Prefrontal Cortex after Chronic Cocaine in SE and EE Mice
Figure 4A presents the different cortical subareas (PrL, IL, and ACC) in which expression of ∆FosB(+) cells was quantified. The delimitations of the surface in which cells were counted for each brain area are presented in Figure 4A. We found no effect of the rearing environment or of repeated administration of chronic cocaine on the global density of ∆FosB(+) cells across groups (Figure 4B). However, in the IL, we found an increase in the density of ∆FosB/D1R(+) in SE mice exposed to repeated cocaine (+30%, P<.05) and this effect was blunted by EE (Figure 4C). Interestingly, in the ACC, EE decreased the density of ∆FosB/D1R(+) cells (-37%, P<.01), but did not change the density of ∆FosB/D1R(-) cells (a significant DA subtype × environment interaction (F(1,58)= 10.27, P<.01) (Figure 4C). As found in the striatal subregions, the density ∆FosB/D1R(+) cells was always higher than the density of ∆FosB/D1R(-) cells in all groups (mean difference for all groups: +128%, P<.0001 in PrL; +71%, P<.0001 in IL; +128%, P<.0001 in ACC).
Figure 4.
Density of total ΔFosB(+) cells in the prefrontal cortex of standard environment (SE) Sal, SE Coc, enriched environment (EE) Sal, and EE Coc mice and characterization of the phenotype of these cells (D1 receptor [D1R](+) or D1R(-)). (A) Illustrations of the 3 cortical subregions (prelimbic [PrL], infralimbic [IL], and the anterior cingulate [ACC]) used for quantification. (B) Density of ΔFosB(+) cells in each subregion. (C) Density of ΔFosB(+)/D1R(+) cells (white bars) and ΔFosB(+)/D1R(-) cells (black bars) in each subregion. Cocaine treatment did not significantly modulate the overall density of ΔFosB(+) cells in the 3 regions; however, it increased the density of ΔFosB(+)/D1R(+) cells in the IL of SE mice, an effect blunted in EE mice. EE significantly decreased the density ΔFosB(+)/D1R(+) cells in the ACC, independently of cocaine treatment. Results are expressed as means ± SEM (n=8–10/group). We used Fisher’s protected least-squares difference posthoc test: * P<.05, difference compared with SE Sal; $ P<.05 and $$ P<.01, difference compared with SE Coc.
Discussion
The striatum is a relatively heterogenous brain region that anatomically and functionally appears to have a gradient from the more ventral and medial part (NAc shell), passing from intermediate zones (NAc core and medial DSt) to the more dorsal and lateral part (lateral DSt) that are respectively involved in limbic, associative, and sensorimotor functions (Le Moal and Simon, 1991; Voorn et al., 2004; Ikemoto et al., 2015). In this study, we found that the profile of the effects of cocaine and EE were relatively similar in the 4 regions of the striatum analyzed, but the magnitude of these effects was larger in the ventral compared with dorsal areas (Figure 3B). This is consistent with the general finding that the ventral striatum is more sensitive to external events such as drug self-administration (Perrotti et al., 2008) and environmental manipulations (Lobo et al., 2013).
In the present study, we found that chronic cocaine increases the levels of ΔFosB in all striatal regions of SE mice, an effect that is likely due to the repeated overstimulation of dopaminoceptive neurons secondary to cocaine-induced increases of extracellular dopamine (Chen et al., 1997; Pontieri et al., 1995). Consistent with previous findings (Lobo et al., 2013), cocaine-induced accumulation of ΔFosB was selective for neurons expressing the D1 subtype of dopamine receptors in all striatal regions. These findings are also consistent with previous studies demonstrating that acute cocaine induces phosphorylation of several intracellular signaling proteins restricted to D1R(+) MSNs (Bateup et al., 2008; Bertran-Gonzalez et al., 2008) and that the expression of Fos-related protein in response to cocaine depends on the D1R subtype (Moratalla et al., 1996; Lee et al., 2006). This is also in agreement with previous work showing that ΔFosB accumulation in D1R(+) MSNs plays a major role in cocaine reward (Kelz et al., 1999; Colby et al., 2003; Grueter et al., 2013) and that the activation of D1R(+) cells in the striatum produces reinforcing effects (Kravitz et al., 2012; Ikemoto et al., 2015). Therefore, the accumulation of ΔFosB in the D1R(+) cells in the striatum may represent a long-lasting adaptation that strengthens the direct pathway and increases the addiction-inducing effects of repeated injections of cocaine.
Consistent with previous studies (Solinas et al., 2009; Lobo et al., 2013), EE by itself increased ΔFosB levels in the striatum. Interestingly, in contrast to what was found with cocaine, especially in the NAc Shell, ΔFosB levels were selectively increased in D1R(-) cells, which are likely D2R(+) MSNs. This finding contrasts with the study by Lobo et al. (2013), who reported that enrichment resulted in nonselective increased ΔFosB levels in both D1R(+) and D2R(+) MSNs (Lobo et al., 2013). The reason for this discrepancy is not clear, but it could be due to differences in EE conditions such as the length of exposure to EE (4 weeks for Lobo et al. and 8 weeks in our study), the age of the animals (around postnatal day 50 for Lobo et al. and around postnatal day 78 in our study), and differences in the behavioral manipulation (injections in the home-cage for Lobo et al. and in the locomotor activity apparatus in our study). These experimental differences can also explain the fact that whereas in the paper by Lobo et al. D1R(+) and D2R(+) show similar levels of expression of ΔFosB under basal condition, we found significantly more ΔFosB/D1R(+) than ΔFosB/D1R(-) cells. Regardless of these differences, the general finding in both Lobo et al. (2013) and the current report is that EE produced qualitatively different effects on striatal ΔFosB expression compared with the selective cocaine-induced increase ΔFosB levels in D1R MSNs. Stimulation of D2R(+) MSNs neurons appears to have effects that are opposite to stimulation of D1R(+) MSNs neurons; for example, in contrast to D1R stimulation, D2R stimulation produces aversive effects (Kravitz et al., 2012; Ikemoto et al., 2015). Therefore, elevated levels of ΔFosB in D2R(+) MSNs may strengthen this pathway and oppose cocaine’s reinforcing effects. Indeed, it has been shown that activation of D2R(+) MSNs reduces the reinforcing effects of cocaine (Bock et al., 2013). These observations may explain the decreased locomotor reactivity to cocaine observed in EE mice (present study and Bezard et al., 2003) as well as the reduced locomotor effects induced by repeated cocaine administrations we found in EE mice (present study and Solinas et al., 2009).
Consistent with previous findings in the whole striatum (Solinas et al., 2009; Venebra-Munoz et al., 2014; Zhang et al., 2014), we found that: (1) exposure to EE prevented cocaine-induced increases in ΔFosB levels in the NAc shell and core and in the medial DSt and (2) striatal ΔFosB levels were reduced in cocaine-treated EE mice compared with saline-treated EE mice. Thus, the inability of cocaine to increase ΔFosB levels in the striatum of EE mice may explain the reduced behavioral effects (present study and Solinas et al., 2009) and reward (Solinas et al., 2009) produced by cocaine in these mice. Conversely, cocaine reversed the accumulation of ΔFosB induced by EE in D1R(-) MSNs neurons. Thus, EE and cocaine appear to antagonize each other’s effects. Similarly, Robinson and Kolb (2004) found that both cocaine and EE increased dendritic arborization in the striatum but that previous exposure to cocaine prevented the ability of EE to produce this effect (Robinson and Kolb, 2004).
We also investigated the effects of cocaine and EE on ΔFosB levels in 3 different subregions of the medial prefrontal cortex: the PrL, the IL, and the ACC, which are all involved in behavioral sensitization to cocaine (Schmidt et al., 1999; Tzschentke and Schmidt, 1999). In our study, cocaine produced a significant increase in ΔFosB levels in D1R(+) but not in D1R(-) neurons in the IL, and EE did not increase ΔFosB levels but decreased them in the ACC regardless of cocaine treatment. Previous studies (Perrotti et al., 2008; Winstanley et al., 2009) found increases in ΔFosB levels in different subregions of the prefrontal cortex in response to cocaine (although their brain region distinctions were somewhat less precise than ours), but these increases were less pronounced than in the striatum. The fact that we were unable to detect significant cocaine-induced increases in ΔFosB levels in cortical regions may be related to the fact that our protocol of cocaine exposure was less intense than those used in these studies (Perrotti et al., 2008; Winstanley et al., 2009). D1R(+) neurons have been shown to be expressed in cortical layers II, V, and VI (Vincent et al. 1993, 1995; Gaspar et al., 1995) and in both nonpyramidal (Vincent et al. 1993, 1995) and pyramidal neurons (Seong and Carter, 2012) that project to several brain regions, including the cortex itself, striatum, thalamus (Gaspar et al., 1995), and amydgala (Land et al., 2014). Future studies will be needed to identify the cortical cell type in which ΔFosB levels were affected by cocaine and EE and to characterize their functional consequences on behavior.
Compared with the striatum, little is known about the consequences of increased ΔFosB in IL on cocaine addiction, and therefore, the interpretation of these findings is not straightforward. In fact, only one study investigated the effects of EE on basal ΔFosB levels in the cortex and found an increase in IL, PrL, and ACC (Lehmann and Herkenham, 2011). In contrast, in our study, EE did not affect ΔFosB levels in the IL and PrL, and it even decreased ΔFosB levels in the ACC. These differences may be due to differences in the protocol of EE exposure, such as the duration and the age of mice, as well as the fact that Lehman and Herkenham (2011) housed control animals singly, whereas they were housed in groups of 4 in the current study. However, ΔFosB increases have been reported after exposure to stressors such as social defeat (Hinwood et al., 2011; Lehmann and Herkenham, 2011; Vialou et al., 2014) and after opiate sensitization (Kaplan et al., 2011), which could suggest that this neuroadaptation participates in a negative emotional state that can render individuals vulnerable to drug addiction. Therefore, it is of interest that exposure to EE prevents this accumulation of ΔFosB and probably the establishment of such a negative emotional state. Future studies are needed to determine whether increases of ΔFosB levels in IL participate in the development and maintenance of addiction-related behaviors.
In conclusion, our study shows that both cocaine and EE increases ΔFosB levels in the striatum, especially in the NAc. However, their profile of ΔFosB induction is very different, and indeed opposite. Our results suggest that EE-induced increases in the levels of ΔFosB in putative D2R(+) neurons of the striatum may result in potentiation of the indirect dopamine pathway and ultimately in the blunting of effects of cocaine that work through the direct dopamine pathway. Furthermore, we found that EE increases in ΔFosB levels in the striatum but not in the prefrontal cortex, suggesting the EE effects on ΔFosB are selective for this brain region. Finally, our study highlights and confirms the importance of investigation of cell-type specificity to better understand the consequences of different manipulations on brain neuroadaptations.
Statement of Interest
None.
Supplementary Material
Acknowledgments
We thank Drs D. Hervé and J. A. Girault for generously providing the BAC Drd1a-eGFP mice used to generate animals for this study. We thank A. Gaillard and S. Brot for assistance for the immunohistochemistry experiments and Pauline Belujon for comments on a previous version of this manuscript.
This work was supported by INSERM, University of Poitiers, Mission Interministérielle de la Lutte contre les Drogues et la Toxicomanie (MILDT-INSERM), and Région Poitou-Charentes. A. Lafragette is a recipient of a PhD fellowship from French ministry of research. M. T. Bardo was supported by the U.S. Public Health Service (grants nos. P50 DA 05312, R01 DA12964) and visiting professor grant from the “région Poitou-Charentes.”
References
- Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P. (2008) Cell type specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci 11:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. (2008) Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 28:5671–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. (2010) What is the degree of segregation between striatonigral and striatopallidal projections? Front Neuroanat 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Belin D, Duconger S, Jackson-Lewis V, Przedborski S, Piazza PV, Gross CE, Jaber M. (2003) Enriched environment confers resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J Neurosci 23:10999–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA. (2013) Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci 16:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. (2000) Synaptic organisation of the basal ganglia. J Anat 196:527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerovic M, d’Isa R, Tonini R, Brambilla R. (2013) Molecular and cellular mechanisms of dopamine mediated behavioral plasticity in the striatum. Neurobio Learn Mem 105:63–80. [DOI] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. (1997) Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci 17:4933–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. (2003) Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J Neurosci 23:2488–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M. (2009) Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology (Berl) 203:561–570. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. (2011) Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci 14:22–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa G, Espallergues J, de Kerchove d’Exaerde A, El Mestikawy S, Gerfen CR, Herve D, Girault JA, Valjent E. (2013) Distribution and compartmental organization of GABAergic medium sized spiny neurons in the mouse nucleus accumbens. Front Neural Circuits 7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. (1995) D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci 7:1050–1063. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. (2007) Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci 27:9817–9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. (2013) FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci U S A 110:1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. (2010) Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 66:896–907. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Tynan RJ, Day TA, Walker FR. (2011) Repeated social defeat selectively increases deltaFosB expression and histone H3 acetylation in the infralimbic medial prefrontal cortex. Cereb Cortex 21:262–271. [DOI] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. (1994) Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron 13:1235–1244. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Yang C, Tan A. (2015) Basal ganglia circuit loops, dopamine and motivation: a review and enquiry. Behav Brain Res 290:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev 16:223–244. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Leite-Morris KA, Fan W, Young AJ, Guy MD. (2011) Opiate sensitization induces FosB/DeltaFosB expression in prefrontal cortical, striatal and amygdala brain regions. PloS One 6:e23574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. (1997) Neostriatal cell subtypes and their functional roles. Neurosci Res 27:1–8. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr., Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. (1999) Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature 401:272–276. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. (2010) Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466:622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. (2012) Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature Neurosci 15:816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, Sarhan M, Guarnieri DJ, Deisseroth K, Aghajanian GK, DiLeone RJ. (2014) Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci 17:248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moal M, Simon H. (1991) Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev 71:155–234. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Normand E, Guitteny AF, Fouque B, Teoule R, Bloch B. (1990) Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proc Natl Acad Sci U S A 87:230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moine C, Normand E, Bloch B. (1991) Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci U S A 88:4205–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. (2006) Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A 103:3399–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M. (2011) Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci 31:6159–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. (2010) Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han MH, Dietz DM, Self DW, Hurd YL, Vialou Vet al. (2013) DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci 33:18381–18395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. (2004) DeltaFosB: a molecular switch for long-term adaptation in the brain. Mol Brain Res 132:146–154. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Vallejo M, Elibol B, Graybiel AM. (1996) D1-class dopamine receptors influence cocaine induced persistent expression of Fos-related proteins in striatum. Neuroreport 8:1–5. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. (2001) DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A 98:11042–11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin BR, Sim-Selley L, Bachtell RK, Self DW, Nestler EJ. (2008) Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse 62:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain research Brain Res Rev 25:192–216. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. (1995) Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A 92:12304–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. (2004) Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47:33–46. [DOI] [PubMed] [Google Scholar]
- Schmidt WJ, Tzschentke TM, Kretschmer BD. (1999) State-dependent blockade of haloperidol-induced sensitization of catalepsy by MK-801. Eur J Neurosci 11:3365–3368. [DOI] [PubMed] [Google Scholar]
- Seong HJ, Carter AG. (2012) D1 receptor modulation of action potential firing in a subpopulation of layer 5 pyramidal neurons in the prefrontal cortex. J Neurosci 32:10516–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, El Rawas R, Lardeux V, Jaber M. (2009) Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology 34:1102–1111. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. (2004) Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol 14:685–692. [DOI] [PubMed] [Google Scholar]
- Thiriet N, Gennequin B, Lardeux V, Chauvet C, Decressac M, Janet T, Jaber M, Solinas M. (2011) Environmental enrichment does not reduce the rewarding and neurotoxic effects of methamphetamine. Neurotox Res 19:172–182. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. (1999) Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci 11:4099–4109. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology 151:99–120. [DOI] [PubMed] [Google Scholar]
- Venebra-Munoz A, Corona-Morales A, Santiago-Garcia J, Melgarejo-Gutierrez M, Caba M, Garcia-Garcia F. (2014) Enriched environment attenuates nicotine self-administration and induces changes in DeltaFosB expression in the rat prefrontal cortex and nucleus accumbens. Neuroreport 25:688–692. [DOI] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M, Ku SM, Harrigan E, Winstanley CA, Joshi T, Feng J, Berton O, Nestler EJ. (2014) Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of DeltaFosB. J Neurosci 34:3878–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SL, Khan Y, Benes FM. (1993) Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci. 13:2551–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SL, Khan Y, Benes FM. (1995) Cellular colocalization of dopamine D1 and D2 receptors in rat medial prefrontal cortex. Synapse. 19:112–120. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. (2004) Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27:468–474. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Green TA, Theobald DE, Renthal W, LaPlant Q, DiLeone RJ, Chakravarty S, Nestler EJ. (2009) DeltaFosB induction in orbitofrontal cortex potentiates locomotor sensitization despite attenuating the cognitive dysfunction caused by cocaine. Pharmacol Biochem Behav 93:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Crofton EJ, Li D, Lobo MK, Fan X, Nestler EJ, Green TA. (2014) Overexpression of DeltaFosB in nucleus accumbens mimics the protective addiction phenotype, but not the protective depression phenotype of environmental enrichment. Front Behav Neurosci 8:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.