Abstract
Background: Tumor ablation is often employed for unresectable colorectal liver metastases. However, no survival benefit has ever been demonstrated in prospective randomized studies. Here, we investigate the long-term benefits of such an aggressive approach.
Methods: In this randomized phase II trial, 119 patients with unresectable colorectal liver metastases (n < 10 and no extrahepatic disease) received systemic treatment alone or systemic treatment plus aggressive local treatment by radiofrequency ablation ± resection. Previously, we reported that the primary end point (30-month overall survival [OS] > 38%) was met. We now report on long-term OS results. All statistical tests were two-sided. The analyses were according to intention to treat.
Results: At a median follow up of 9.7 years, 92 of 119 (77.3%) patients had died: 39 of 60 (65.0%) in the combined modality arm and 53 of 59 (89.8%) in the systemic treatment arm. Almost all patients died of progressive disease (35 patients in the combined modality arm, 49 patients in the systemic treatment arm). There was a statistically significant difference in OS in favor of the combined modality arm (hazard ratio [HR] = 0.58, 95% confidence interval [CI] = 0.38 to 0.88, P = .01). Three-, five-, and eight-year OS were 56.9% (95% CI = 43.3% to 68.5%), 43.1% (95% CI = 30.3% to 55.3%), 35.9% (95% CI = 23.8% to 48.2%), respectively, in the combined modality arm and 55.2% (95% CI = 41.6% to 66.9%), 30.3% (95% CI = 19.0% to 42.4%), 8.9% (95% CI = 3.3% to 18.1%), respectively, in the systemic treatment arm. Median OS was 45.6 months (95% CI = 30.3 to 67.8 months) in the combined modality arm vs 40.5 months (95% CI = 27.5 to 47.7 months) in the systemic treatment arm.
Conclusions: This phase II trial is the first randomized study demonstrating that aggressive local treatment can prolong OS in patients with unresectable colorectal liver metastases.
Surgery is the gold standard of treatment in patients with resectable colorectal liver metastases, with reported five-year survival rates ranging from 40% to 60% (1–3). Only 20% to 30% of patients with CRC metastases confined to the liver are candidates for surgery (2,4). In others, extensive tumor burden within the liver or poor anatomical position of the tumors close to critical vascular or biliary structures precludes resection. In these patients, systemic therapy is offered with the goal of improving survival or potentially converting patients into resection candidates (5,6). Although the outcome of systemic therapy is still being improved and promising biological agents are being incorporated into treatment protocols, the realistic goal of systemic treatment remains palliative (7–10). It is for this reason that more aggressive local therapeutic approaches are being pursued in patients with unresectable colorectal liver metastases.
Radiofrequency ablation (RFA) is a treatment modality that is being increasingly used (11). In patients with unresectable colorectal liver metastases, total tumor clearance from the liver can often still be obtained by RFA or a combination of RFA plus resection (12–14). The efficacy of this approach is controversial because data on the effect on overall survival compared with the standard of care, systemic treatment, are lacking (11–18). To deliver compelling evidence on the beneficial effect of such an aggressive approach, a European intergroup randomized phase III study (European Organisation for Research and Treatment of Cancer 40004 CLOCC trial, ClinicalTrials.gov, No. NCT00043004) was initiated. The trial was designed with overall survival (OS) as the primary end point.
Patients with unresectable colorectal liver metastases were randomly assigned to systemic treatment alone (standard arm) or systemic treatment plus local treatment by RFA with or without additional resection (experimental arm). Because of slow accrual, the study was amended to a randomized phase II trial. Previously published results of this phase II study showed that the primary end point, being a 30-month overall survival (OS) rate greater than 38% in the combined modality arm, was met (61.7%) (19).
At the time of primary analysis with a median follow-up time of 4.4 years, median progression-free survival (PFS) was statistically significantly different between both arms, being 16.8 months in the combined modality arm and 9.9 months in the systemic treatment–only arm (P = .025) (19). After an extended follow-up of 9.7 years, we now report on the definitive impact on overall survival.
Methods
Study Design and Patients
Patients with unresectable colorectal liver–limited metastases were randomly assigned to systemic treatment alone (standard arm) or systemic treatment plus local treatment by RFA ± resection (experimental arm). The primary end point of this phase II study was a 30-month OS rate higher than 38% in the combined modality arm. Using a Fleming design, 76 patients were required in the experimental arm to reject a 30-month OS rate of 38% or lower under the alternative hypothesis of a 30-month OS rate of 53% with 90% power, using a one-sided test with a type I error of 10%.
Secondary end points were progression-free survival (PFS), overall survival (OS), and health-related quality of life. From April 2002, patients were recruited from 22 centers in Europe. The trial was prematurely closed for poor accrual because of physician’s preferences in treatment modalities in June 2007, with 60 patients in the experimental arm and 59 patients in the control arm.
Eligible patients were age 18 to 80 years with a World Health Organization performance of less than 2 and who presented with nonresectable colorectal liver metastases without extrahepatic disease. Nonresectability was defined as no possibility to completely resect all tumor lesions, as judged by a multidisciplinary team with at least a hepatobiliary surgeon and radiologist on board. Patients were eligible only when all liver lesions could be fully treated by either RFA alone or combined treatment that consisted of resection of resectable lesions and RFA of the remaining unresectable lesions. To allow complete treatment of all liver lesions, the number of liver metastases had to be less than 10. Full inclusion and exclusion criteria have been previously reported (19). The trial was approved by the medical ethics committees of all participating centers. Written informed consent was obtained from all patients prior to random assignment. Random assignment (1:1) was done at the EORTC headquarters with the minimization technique and was stratified according to center, previous systemic treatment for liver metastases, previous adjuvant treatment, and route of random assignment (before or during surgery).
Procedures
Patients assigned to the combined modality therapy received complete treatment of all liver metastases either by RFA alone or by RFA in combination with resection. The optimal strategy to obtain adequate local treatment was decided upon by the hepatobiliary surgeon and the multidisciplinary team. RFA procedures were carried out according to the guidelines of the manufacturer of the ablation device used during open surgery, laparoscopically, or percutaneously. Quality control for RFA and surgery required specialized liver surgeons and radiologists to assess the suitability of patients for ablation and full documentation of the lesions treated. From April 2002 to October 2005, systemic treatment in both study arms consisted of 5-FU/LV/oxaliplatin. After October 2005, bevacizumab was added when it became accepted as the standard of care in most participating centers. Treatment of 5-FU/LV/oxaliplatin consisted of the FOLFOX 4 regimen while bevacizumab was administered at 5 mg/kg body weight once every two weeks. Detailed systemic treatment regimens were reported previously (19).
In the systemic treatment arm only, no additional local treatment options were allowed except for resection when unresectable disease was converted to resectable disease by systemic treatment. In both study arms, treatment was started within four weeks of random assignment and systemic treatment after RFA was planned within four to eight weeks after the procedure.
Systemic treatment in both arms was administered for six months unless there was disease progression or unacceptable toxicity. After protocol treatment, any further systemic treatment was at the discretion of the multidisciplinary team.
Patients were assessed for PFS and OS every six weeks during protocol treatment, every three months after treatment for a period of two years, and every six months thereafter. Disease progression was assessed using contrast-enhanced CT scan by the local radiologist according to RECIST 1.0. Recurrence at the RFA site was defined by the appearance on CT imaging of one or more new lesions along the margin of the ablated lesion or at least a 20% increase in the longest diameter of the RFA-treated lesion.
Statistical Analysis
Follow-up duration was computed from the time of random assignment to the date of last follow-up. Patients who died were censored at the date of death. PFS was defined as the time interval between the date of random assignment and the date of progression (or recurrence) of the disease or death, whichever occurred first. OS was defined as the time interval between the date of random assignment and the date of death. Patients who were still event free at the last visit were censored at the date of last follow-up.
The updated analyses of PFS and OS are intent-to-treat analyses. Overall PFS and OS were estimated by the Kaplan-Meier method and compared by a two-sided log-rank test. The level of statistical significance was set to .05.
Additional sensitivity univariate analyses of OS, adjusting for baseline factors, that is, number of liver metastases (≤4 vs >4) and synchronicity (synchronous vs metachronous), were performed to correct for a potential prognostic effect on the results. OS was compared between arms using a two-sided log-rank test stratified for the baseline factor. Possible heterogeneity of the results in these subgroups was tested by means of a Cochran’s Q test. A graphical display of the results is provided using Forest plots. To determine any possible influence of secondary treatments on OS, survival duration after initial disease progression was analyzed in progressive patients (for whom death was not the first recorded event, 55 and 43 patients in the systemic treatment and in the combined modality arms, respectively). Survival duration after initial disease progression was computed as the time interval between the date of first progression and the date of death. Patients who were still alive at the last visit were censored at the date of last follow-up. Survival duration after initial disease progression was estimated by the Kaplan-Meier method and compared between treatment arms by a two-sided log-rank test.
The analysis of the time to hepatic progression and to extrahepatic progression was performed using the competing risk methodology in the intent-to-treat population as exploratory analyses. The cumulative incidence of the event of interest (including the occurrence of the event and a simultaneous progression at another site) was estimated and compared by means of a Gray test (20). In these analyses, death in absence of hepatic or extrahepatic progression, respectively, was considered the only competing risk.
Results
A total of 119 patients were randomly assigned to either systemic treatment alone or combined modality treatment (systemic plus local treatment). Patient and tumor characteristics appeared balanced between both arms (Table 1).
Table 1.
Baseline characteristics
| Patient and tumor characteristics | Local plus systemic treatment (n = 60) | Systemic treatment (n = 59) |
|---|---|---|
| No. (%) | No. (%) | |
| Age, y | ||
| Median (range) | 64 (31–79) | 61 (38–79) |
| Sex | ||
| Male | 37 (61.7) | 42 (71.2) |
| Female | 23 (38.3) | 17 (28.8) |
| WHO performance status | ||
| 0 | 47 (78.3) | 47 (79.7) |
| 1 | 13 (21.7) | 12 (20.3) |
| No. of liver metastases | ||
| 1 | 15 (25.0) | 7 (11.9) |
| 2 | 6 (10.0) | 4 (6.8) |
| 3 | 8 (13.3) | 7 (11.9) |
| 4 | 9 (15.0) | 8 (13.6) |
| 5 | 6 (10.0) | 10 (16.9) |
| 6 | 3 (5.0) | 9 (15.3) |
| 7 | 6 (10.0) | 8 (13.6) |
| 8 | 3 (5.0) | 2 (3.4) |
| 9 | 4 (6.7) | 4 (6.8) |
| Median | 4.0 | 5.0 |
| Synchronicity of liver metastases | ||
| Metachronous metastases | 37 (61.7) | 31 (52.5) |
| Synchronous metastases* | 23 (38.3) | 28 (47.5) |
| Time from surgery for primary cancer to random assignment, d | ||
| Median (range) | 290 (28–1802) | 308 (30–2754) |
| T stage of primary cancer | ||
| pT2 | 9 (15.0) | 4 (6.8) |
| pT3 | 42 (70.0) | 48 (81.4) |
| pT4 | 9 (15.0) | 6 (10.2) |
| Unknown | 0 (0.0) | 1 (1.7) |
| N stage of primary cancer | ||
| pN0 | 17 (28.3) | 21 (35.6) |
| pN1 | 22 (36.7) | 24 (40.7) |
| pN2 | 20 (33.3) | 12 (20.3) |
| Unknown | 1 (1.7) | 2 (3.4) |
| Adjuvant chemotherapy for primary cancer† | ||
| No | 50 (83.3) | 49 (83.1) |
| Yes | 10 (16.7) | 10 (16.9) |
| Prior chemotherapy for metastatic disease† | ||
| No | 51 (85.0) | 51 (86.4) |
| Yes | 9 (15.0) | 8 (13.6) |
| Previous liver surgery for CRC metastases | ||
| No | 51 (85.0) | 49 (83.1) |
| Yes | 9 (15.0) | 10 (16.9) |
| Route of random assignment† | ||
| Before surgery | 46 (76.7) | 44 (74.6) |
| During surgery | 14 (23.3) | 15 (25.4) |
Liver metastases detected within three months after primary cancer diagnosis. CRC = colorectal cancer; WHO = World Health Organization.
Stratification factors.
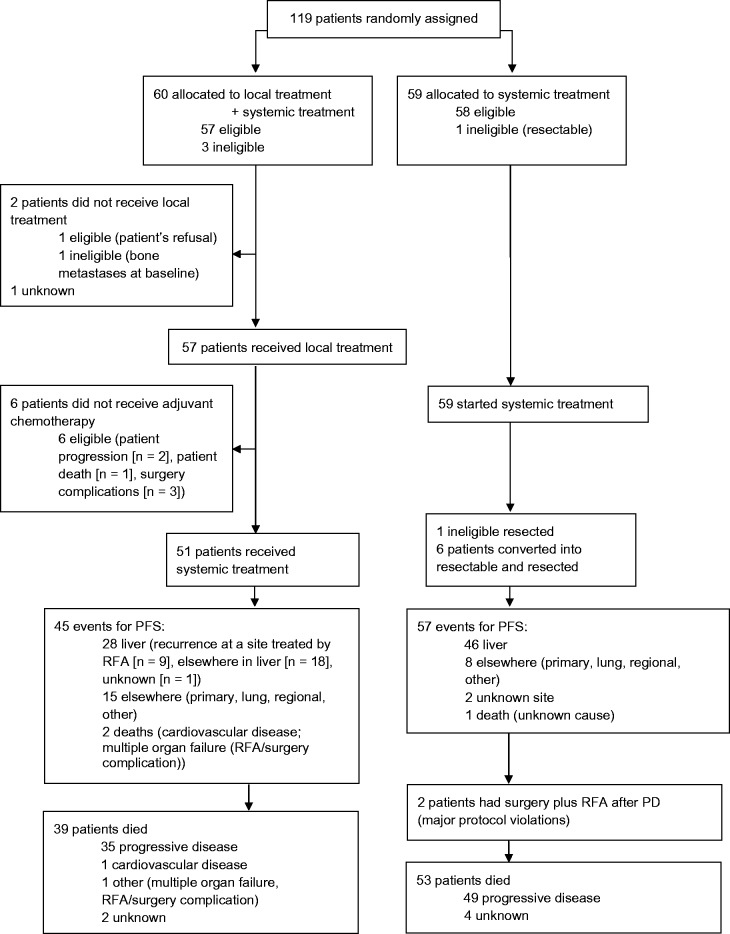
In the combined modality arm (n = 60), three patients were ineligible, two had more advanced disease than allowed per protocol, and one showed liver metastases that were considered resectable at baseline (Figure 1). Of the 60 patients randomly assigned to combined modality, two patients did not receive any local treatment because of patient refusal (n = 1) or ineligibility (n = 1); for one patient, no treatment data are available. Local treatment in the 57 remaining patients consisted of RFA only in 30 patients, RFA plus resection in 26 patients, and resection only in one patient (Table 2). In 51 patients, local treatment was combined with planned systemic treatment. Six patients did not receive any systemic treatment because of fast disease progression (n = 2), patient death (n = 1), or postoperative complications (n = 3).
Figure 1.
CONSORT flow diagram. PD = progressive disease; PFS = progression-free survival; RFA = radiofrequency ablation.
Table 2.
Local treatment received in the combined treatment arm
| Radiofrequency/surgery | Method |
Total (n = 57) | |
|---|---|---|---|
| RFA only (n = 30) | RFA plus resection* (n = 27) | ||
| No. (%) | No. (%) | No. (%) | |
| Means of radiofrequency administration | |||
| At laparotomy | 25 (83.3) | 26 (96.3) | 51 (89.5) |
| Laparascopically | 1 (3.3) | 0 (0.0) | 1 (1.8) |
| Percutaneously | 4 (13.3) | 0 (0.0) | 4 (7.0) |
| No RFA performed | 0 (0.0) | 1 (3.7)* | 1 (1.8) |
| Worst margin for resected† tumors per patient (n = 27), cm | |||
| ≥1 | NA | 10 (37.0) | – |
| <1 | NA | 16 (59.3) | – |
| Residual tumor | NA | 1 (3.7) | – |
| Worst margin for tumors treated by radiofrequency per patient (n = 56), cm | (n = 26) | (n = 56) | |
| ≥1 | 8 (26.7) | 5 (19.2) | 13 (23.2) |
| <1 | 16 (53.3) | 17 (65.4) | 33 (58.9) |
| No margin | 4 (13.3) | 1 (3.8) | 5 (8.9) |
| Unknown | 2 (6.7) | 3 (11.5) | 5 (8.9) |
| Treatment of at least one liver metastasis unsuccessful | |||
| No | 29 (96.7) | 26 (96.3) | 55 (96.5) |
| Yes | 1 (3.3)‡ | 1 (3.7) | 2 (3.5) |
One patient was ineligible; all lesions were resected at baseline, no RFA done. RFA = radiofrequency ablation.
Resection consisted of one segment or wedge resection(s) (n = 16) or resection of two or more liver segments (n = 11).
For this patient, one lesion could not be successfully treated by RFA because of its close proximity to the stomach.
In the systemic treatment arm (n = 59), all patients started systemic therapy. One patient was considered ineligible; this patient showed resectable disease on the initial CT scan and was resected after the start of systemic treatment. Six additional patients underwent liver resection as intended by the protocol because unresectable disease was converted by systemic treatment into resectable disease. In the systemic treatment arm, the median number of systemic treatment cycles was 10 (range = 1–12), in the combined modality arm 8.5 (range = 0–12).
After a similar long-term follow-up in both arms at a median of 9.7 years, 92 of 119 (77.3%) patients had died, 53 of 59 (89.8%) patients in the systemic treatment arm and 39 of 60 (65.0%) patients in the combined modality arm (Table 3). Nearly all patients died due to progressive disease (PD), 49 patients in the systemic treatment arm and 35 patients in the combined modality arm. Only five patients were lost to follow-up, two patients in the systemic treatment arm and three patients in the combined modality arm.
Table 3.
Site of first progression and main cause of death
| Disease and survival status | Local plus systemic treatment (n = 60) | Systemic treatment (n = 59) |
|---|---|---|
| No. (%) | No. (%) | |
| Site(s) of first progression | ||
| Any hepatic progression* | 28 (46.7) | 46 (78.0) |
| Site treated by radiofrequency | 9 (15.0) | |
| Extrahepatic only | 15 (25.0) | 8 (13.6) |
| Unknown site | 2 (3.4)† | |
| Death before first progression | 2 (3.3) | 1 (1.7) |
| Survival status | ||
| Lost to follow-up | 3 (5.0) | 2 (3.4) |
| Alive at last contact‡ | 18 (30.0) | 4 (6.8) |
| Dead | 39 (65.0) | 53 (89.8) |
| Main cause of death | ||
| Progressive disease | 35 (58.3) | 49 (83.1) |
| Cardiovascular disease | 1 (1.7) | 0 (0.0) |
| Other | 1 (1.7)§ | 0 (0.0) |
| Unknown | 2 (3.3) | 4 (6.8) |
Any hepatic progression with or without extrahepatic disease.
One patient in the systemic treatment arm died from progressive disease as first event.
All patients were followed for a minimum of 7.8 years.
Sepsis and multiple organ failure (radiofrequency ablation/surgery complication).
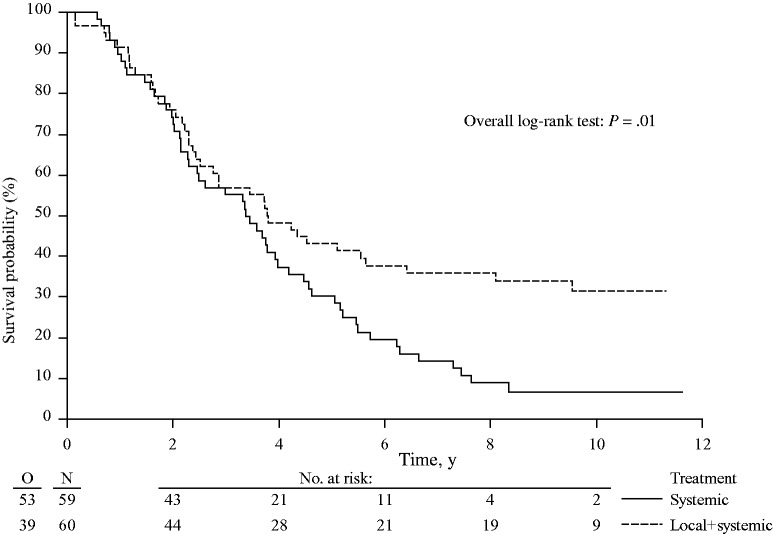
Patients in the combined modality arm had a statistically significantly longer OS as compared with the patients in the systemic treatment arm (HR = 0.58, 95% CI = 0.38 to 0.88, P = .01) (Figure 2). Three-, five-, and eight-year OS rates were 56.9% (95% CI = 43.3% to 68.5%), 43.1% (95% CI = 30.3% to 55.3%), and 35.9% (95% CI = 23.8% to 48.2%) in the combined modality arm and 55.2% (95% CI = 41.6% to 66.9%), 30.3% (95% CI = 19.0% to 42.4%), and 8.9% (95% CI = 3.3% to 18.1%) in the systemic treatment arm. The median overall survival was 45.6 months (95% CI = 30.3 to 67.8 months) for the combined modality arm and 40.5 months (95% CI = 27.5 to 47.7 months) for the systemic treatment arm.
Figure 2.
Kaplan-Meier curves for overall survival in patients with unresectable colorectal liver metastases treated by systemic treatment alone or combined modality treatment by systemic treatment plus aggressive local treatment by radiofrequency ablation ± resection (P = .01). P value was calculated using a two-sided log-rank test.
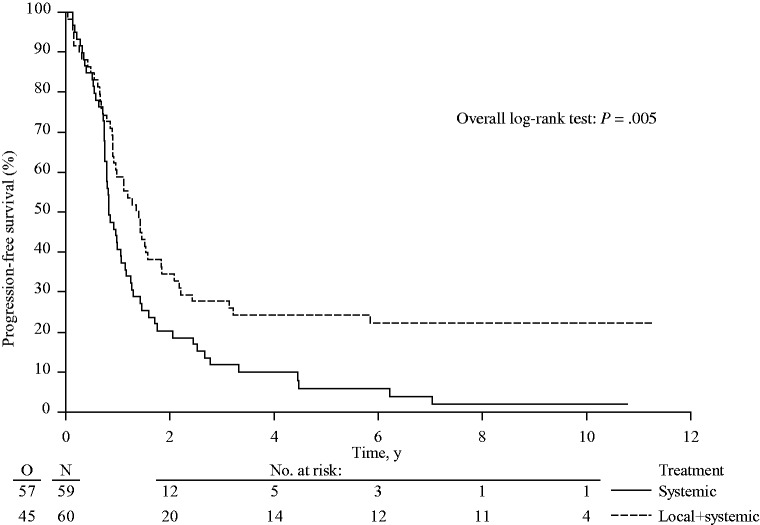
As previously reported, PFS was statistically significantly prolonged in the combined modality arm as compared with the systemic treatment arm (HR = 0.57, 95% CI = 0.38 to 0.85, P = .005) (Figure 3). Median PFS was improved from 9.9 months (95% CI = 9.1 to 12.9 months) to 16.8 months (95% CI = 11.0 to 21.9 months).
Figure 3.
Kaplan-Meier curves for progression-free survival in patients with unresectable colorectal liver metastases treated by systemic treatment alone or combined modality treatment by systemic treatment plus aggressive local treatment by radiofrequency ablation ± resection (P = .005). P value was calculated using a two-sided log-rank test.
After this long-term follow-up, 45 patients in the combined modality arm had recurrent disease or had died compared with 57 patients in the systemic treatment arm. In the combined modality arm, apart from the three patients who were lost to follow-up without progression, 12 patients did not experience any recurrence or death. The minimum follow-up in these 12 patients was 7.9 years. In the systemic treatment arm, one patient was lost to follow-up without progression and only one patient did not experience any progression after a follow-up duration of 10.8 years.
Three-, five-, and eight-year PFS rates in the combined modality arm were 27.7% (95% CI = 16.9% to 39.5%), 24.2% (95% CI = 14.1% to 35.7%), and 22.3% (95% CI = 12.7% to 33.7%), respectively (Figure 3). In the systemic treatment arm, three-, five-, and eight-year PFS rates were 11.9% (95% CI = 5.2% to 21.5%), 5.9% (95% CI = 1.6% to 14.4%), and 2.0% (95% CI = 0.2% to 9.0%), respectively. Among patients who experienced progression, six and three patients in the combined modality arm and in the systemic treatment arm, respectively, were still alive during the last follow-up. In the systemic treatment arm, the six patients who underwent liver resection because nonresectable disease was converted by systemic treatment into resectable disease all developed recurrence.
The liver as first site of recurrence (with or without extrahepatic disease) was observed in 28 of 60 (46.7%) patients in the combined modality arm and in 46 of 59 (78.0%) of those in the systemic treatment arm. In the combined modality arm, in 56 patients treated with radiofrequency ablation, nine patients (16.1%) experienced a first liver recurrence at a site treated by RFA. Extrahepatic progression only as first progression was observed in 25.0% of patients (15/60) in the combined modality arm and 13.6% of patients (8/59) who received systemic treatment alone (Table 3).
Furthermore, given the small sample size, it was impossible to completely eliminate the possibility of small imbalances in baseline characteristics, for example, in the number of lesions or synchronicity. To correct for a potential prognostic effect of baseline factors on the results, we conducted additional sensitivity analyses. The results of the sensitivity analyses of OS adjusting for the number of liver metastases (≤4 vs >4) and synchronicity (synchronous vs metachronous) show that the difference in OS between both arms remains statistically significant. No heterogeneity in the results in patients with four or fewer liver metastases vs more than four liver metastases or in patients with synchronous vs metachronous liver metastases was observed (Supplementary Figures 1 and 2, available online).
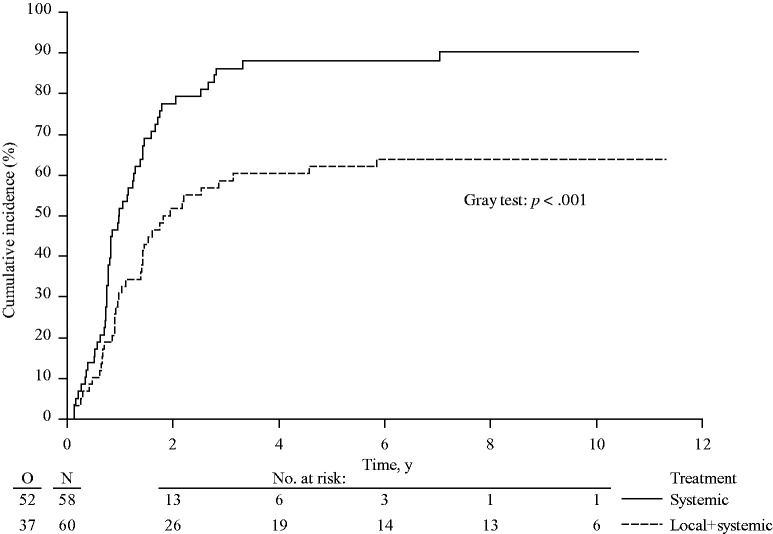
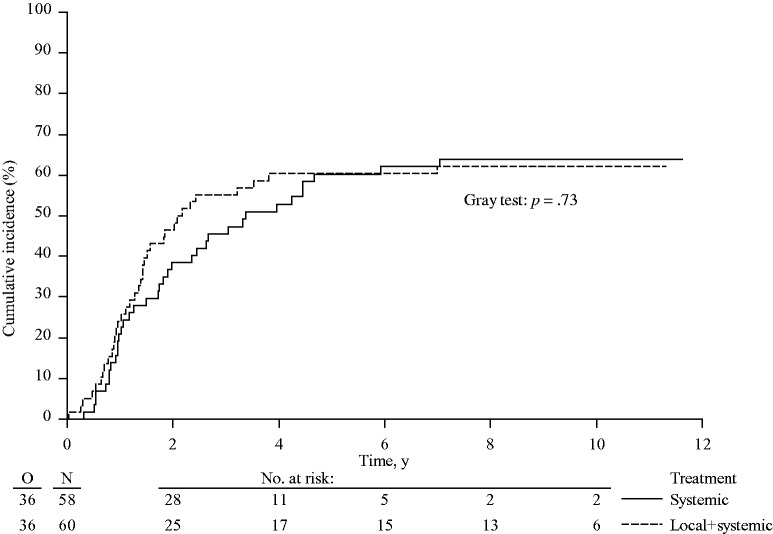
To determine any possible influence of secondary treatments on OS, survival after initial disease progression was analyzed. Median survival after disease progression was 21.0 months (95% CI = 16.2 to 30.5 months) in the systemic treatment arm only and 19.5 months (95% CI = 14.3 to 32.3 months) in the combined modality arm (HR = 0.86, 95% CI = 0.56 to 1.31, P = .48) (Supplementary Figure 3; Supplementary Table 1, available online). With death in absence of hepatic progression considered the only competing risk, the cumulative incidence of hepatic progressions at one, three, and five years were 31.0% (95% CI = 19.1% to 42.9%), 58.6% (95% CI = 45.9% to 71.3%), and 62.1% (95% CI = 49.6% to 74.6%) in the combined modality arm vs 51.7% (95% CI = 38.9% to 64.6%), 86.2% (95% CI = 77.3% to 95.1%), and 88.2% (95% CI = 79.8% to 96.6%) in the systemic treatment arm (P < .001) (Figure 4). The cumulative incidence of extrahepatic progressions at one, three, and five years was 24.1% (95% CI = 13.1% to 35.1%), 55.2% (95% CI = 42.4% to 68.0%), and 60.4% (95% CI = 47.8% to 72.9%) for the combined modality arm and 20.9% (95% CI = 10.4% to 31.5%), 45.5% (95% CI = 32.6% to 58.5%), and 60.2% (95% CI = 47.4% to 73.1%) for the systemic treatment arm (P = .73) (Figure 5). With regard to local recurrence after RFA, in total 11 lesions (in nine patients) out of 170 RFA-treated lesions showed a local recurrence at the RFA site as first progression. In addition, three lesions (in two patients) recurred after initial progression at another site.
Figure 4.
Cumulative incidence of hepatic progressions in patients with unresectable colorectal liver metastases treated by systemic treatment alone or combined modality treatment by systemic treatment plus aggressive local treatment by radiofrequency ablation ± resection (P < .001). P value was calculated using a two-sided Gray test.
Figure 5.
Cumulative incidence of extrahepatic progressions in patients with unresectable colorectal liver metastases treated by systemic treatment alone or combined modality treatment by systemic treatment plus aggressive local treatment by radiofrequency ablation ± resection (P = .73). P value was calculated using a two-sided Gray test.
Discussion
In this randomized phase II trial, we found that a combination of aggressive local treatment plus systemic treatment, as compared with systemic treatment alone, improved both progression-free survival and overall survival in patients with unresectable colorectal liver metastases. The addition of local treatment using RFA was clinically beneficial and was associated with a statistically significant improvement in overall survival (P = .01). Patients were followed for a minimum of 7.8 years, and only five patients (4.2%) were lost to follow-up. Almost all deaths were due to progressive disease.
The current analysis, after a median follow up time of 9.7 years, extends the initial analysis on the primary end point of 30-month overall survival. At 30 months, overall survival in the experimental arm (61.7%) and the control arm (57.6%) was comparable, both being higher than expected. At the time of original study design (late 1990s), figures on overall survival of patients with liver-limited colorectal metastases were scarce, which has led to a very conservative estimation of overall survival (21,22). At the time of the initial analysis, a statistically significant difference was observed for PFS between both treatment arms. Longer follow-up, as presented in this study, was needed before this statistically significant difference in PFS was translated into a statistically significant and clinically relevant difference in overall survival. The currently observed results on overall survival in the control arm correspond more closely to the recent studies in metastatic colorectal cancer (7–10,23–27).
This trial has limitations. This is a phase II trial with a sample size lower than the sample size initially planned for a phase III trial. A larger sample size would have offered better protection against possible risks of imbalances between treatment arms and a better reassurance on the external validity of the results. Also, overall survival was a secondary end point of the phase II trial. However, the patients’ baseline characteristics appeared well balanced between treatment arms, and despite the reduced power to detect a difference in long-term outcomes, statistical significance was reached reflecting the clear difference observed in OS between both arms.
Our results demonstrate for the first time in a randomized trial that aggressive local ablative treatment of colorectal liver metastases can improve overall survival. Even for resection of extensive colorectal liver metastases, such compelling evidence for survival benefit was still lacking. In the present study, we selected patients with colorectal liver metastases that were judged unresectable by experienced liver surgeons. Over 90% of the patients were included in tertiary referral centers. Patients were classified unresectable by the multidisciplinairy team either because of extensive bilobar involvement of the liver or unfavorable location of lesions that precluded hepatic resection as the sole treatment option. The latter also applied to some isolated lesions situated in the middle of the three hepatic veins involving their origin. The criteria on resectability as used in the present study still apply in the current era of advanced liver surgery for colorectal metastases. It is important, however, to highlight the role of specialized multidisciplinary teams and experienced liver surgeons in assessing the suitability of unresectable patients for RFA treatment.
The intention to treat all liver lesions underlines the curative intent of our approach. It should be stressed that to obtain complete tumor treatment a combined approach of RFA with resection was often required (45.6%). Using this approach, complete tumor treatment was achieved in all patients except two. In one patient undergoing resection residual tumor was found in the resection margin, and in one patient undergoing RFA treatment one lesion could not be successfully treated due to its close proximity to the stomach.
After eight years, 36% of the patients treated with the combined modality approach were still alive. This number approximates the 40% eight-year overall survival in the recently published Eloxatin Peri-Operative Chemotherapy (EPOC) study, in which patients with one to four resectable colorectal liver metastases (median number of lesions = 1) were treated by resection plus systemic treatment (28).
Data on progression-free survival after 9.7 years of median follow-up are consistent with earlier results published after a median follow up of 4.4 years (19). Initial analysis at that time showed a median PFS of 16.8 months (95% CI = 11.7 to 22.1 months) in the arm assigned to combination treatment and 9.9 months (95% CI = 9.3 to 13.7 months) in the arm assigned to systemic treatment only (HR = 0.63, 95% CI = 0.42 to 0.95, P = .025).
In the current analysis, we calculated the cumulative incidence of hepatic progression, which was statistically significantly different between both treatment arms. In contrast, the cumulative incidence of extrahepatic progression was similar for both treatment arms. These data stress the effect of aggressive liver treatment on PFS and ultimately on overall survival. While extrahepatic recurrence was not affected by local liver treatment as might be expected, the effect of maximal treatment of liver metastases translated into superior PFS for liver recurrence and into a better long-term overall survival.
In the systemic treatment arm, resection of liver metastases was allowed when unresectable disease was converted to resectable disease later in the treatment. Seven patients (11.9%) underwent resection, six per protocol, and one was resectable from the start of systemic treatment. Compared with figures in the literature for patients with liver-only disease, this percentage is relatively low and may reflect the unfavorable anatomic locations of the lesions at random assignment, which is not influenced by systemic treatment (5,6).
As reported earlier, the percentage of patients undergoing salvage treatment was comparable between both arms (19). The choice of salvage treatment, however, varied between both treatment arms. In the systemic treatment arm, a higher percentage of patients received systemic treatment, whereas in the combined modality arm salvage treatment more often consisted of local treatment by surgery or RFA. Because differences in overall survival reflect the differences in PFS, variation in salvage treatment did not seem to influence the ultimate course of the disease between both treatment arms. Indeed, further analysis showed that survival after recurrence was not statistically significantly different between both arms.
With the availability of local ablative techniques, the treatment options for surgeons in more advanced disease of colorectal liver metastases have increased. Despite the limitations of this study, these data strongly suggest that the combined modality of aggressive local tumor treatment in combination with systemic treatment can change the outcome of these patients considerably with a clear benefit on overall survival. This encourages the early integration of local ablative techniques alone or in combination with surgical resection in patients with unresectable colorectal liver metastases, as should be discussed in a multidisciplinary team setting.
Funding
The sponsor of this trial was the European Organisation for Research and Treatment of Cancer (EORTC). The trial was supported by a grant from Cancer Research UK through the Cancer Research Fund (C837/A3488) and Arbeitsgruppe Lebermetastasen und tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO), by grants from the Dutch Cancer Foundation (Commissie Klinisch Toegepast Onderzoek), by grant numbers 5U10 CA11488-32 through 5U10 CA011488-40 from the National Cancer Institute (Bethesda, MD), by a donation from the Kankerbestrijding/KWF from the Netherlands through the EORTC Charitable Trust, and by an unrestricted scientific grant from Sanofi-Aventis. Radionics, Radiotherapeutics, and Rita provided free RFA needles.
Notes
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The content of this report is solely the responsibility of the authors. Trial management and data analysis were undertaken at the EORTC Data Center (Brussels, Belgium) independently of any commercial interest. None of the authors has any conflict of interest.
TR was responsible for the design of the trial. TR, CP, FvC JP, IBR, JAL, and WB contributed patients to the study and reviewed the report. TR, MAL, MD, EVC, BN, and GP contributed to the trial management and reviewed the report. MM analyzed the trial data and contributed to the writing of the report together with TR and GF. All authors approved the final version of the report.
EORTC-intergroup trial 40004 participants: coauthors: T. Ruers, The Netherlands Cancer Institute, Antoni van Leeuwenhoek Ziekenhuis, Amsterdam, the Netherlands; F. Van Coevorden, The Netherlands Cancer Institute, Antoni van Leeuwenhoek Ziekenhuis, Amsterdam, the Netherlands; C. Punt, Amsterdam Medical Centre, University of Amsterdam, Amsterdam, the Netherlands; J. P. E. N. Pierie, Medisch Centrum Leeuwarden-Zuid, Leeuwarden, the Netherlands; I. Borel-Rinkes, Universitair Medisch Centrum, Academisch Ziekenhuis, Utrecht, the Netherlands; J. A. Ledermann, Cancer Research UK and UCL Cancer Trials Centre, University College Hospital, and UCL Hospitals, London, United Kingdom; G. Poston, Aintree University Hospital, Liverpool, United Kingdom; W. O. Bechstein, Frankfurt University Hospital and Clinics, Frankfurt Am Main, Germany; M. A. Lenz and M. Mauer, EORTC headquarters, Brussels, Belgium; G. Folprecht, University Hospital Carl Gustav Carus, University Cancer Center Dresden, Germany; E. Van Cutsem, University Hospital Gasthuisberg, Leuven, Belgium; M. Ducreux, Institut Gustave Roussy, Villejuif, France; B. Nordlinger, Ambroise Paré, Assistance Publique Hôpitaux de Paris, Boulogne-Billancourt, France.
Investigators who participated through the collaborative groups: V. J. Verwaal, The Netherlands Cancer Institute, Antoni van Leeuwenhoekziekenhuis, Amsterdam, the Netherlands; T. Gruenberger, Medical University Vienna, General Hospital, Vienna, Austria; J. Klaase, Medisch Spectrum Twente, Enschede, the Netherlands; S. Falk, University Hospitals Bristol NHS Foundation Trust, Bristol Haematology and Oncology Centre, Bristol Avon, United Kingdom; J. Wals, Atrium Medisch Centrum, Heerlen, the Netherlands; R. L. Jansen, Akademisch Ziekenhuis Maastricht, the Netherlands; P. Lindnér, Sahlgrenska Sjukhuset, Goteborg, Sweden; S. Mulier, Cliniques Universitaires de Mont Godinne, Yvoir, Belgium; K. Bosscha, Jeroen Bosch Ziekenhuis, 'S Hertogenbosch, the Netherlands; D. Jaeck, Hôpital Universitaire Hautepierre, Strasbourg, France; J. P. Arnaud, Centre Hospitalier Universitaire d’Angers, France; D. Smith, Clatterbridge Centre for Oncology NHS Trust, Wirral Merseyside, United Kingdom; D. Sherlock, North Manchester General Hospital, Manchester, United Kingdom; B. Ammori, Manchester Royal Infirmary, Manchester, United Kingdom; A. Gillams, UCL Hospitals, London, United Kingdom; M. El-Serafi, National Cancer Institute, Cairo, Egypt; B. Glimelius, Karolinska University Hospital Solna, Stockholm, Sweden; P. Hellman, Akademiska Sjukhuset, Uppsala, Sweden.
We thank all patients who consented to enter the study. This study was undertaken by the European Organization for Research and Treatment of Cancer Gastro-Intestinal Tract Cancer Group (EORTC GITCG) in collaboration with National Cancer Research Institution Colorectal Study Group (NCRI CCSG) and was partly managed by the Cancer Research UK and UCL Cancer Trial Centre in collaboration with EORTC. We acknowledge Carmela Caballero, surgeon and Junior Clinical Research Physician at EORTC Headquarters, for reviewing the manuscript.
Supplementary Material
References
- 1.House MG, Ito H, Gönen M, et al. Survival after hepatic resection for metastatic colorectal cancer: Trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210(5):744–752, 752–755. [DOI] [PubMed] [Google Scholar]
- 2.Hackl C, Neumann P, Gerken M, et al. Treatment of colorectal liver metastases in Germany: A ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable livermetastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–1215. [DOI] [PubMed] [Google Scholar]
- 4.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann Surg. 2004;240(4):644–657; discussion 657–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol. 2010;11(1):38–47. [DOI] [PubMed] [Google Scholar]
- 7.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. [DOI] [PubMed] [Google Scholar]
- 8.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563–572. [DOI] [PubMed] [Google Scholar]
- 9.Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–1315. [DOI] [PubMed] [Google Scholar]
- 10.Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): A phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843–1852. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria—a 10-year update. Radiology. 2014;273(1):241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gervais DA, Goldberg SN, Brown DB, et al. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol. 2009;20(suppl 7):S342–S347. [DOI] [PubMed] [Google Scholar]
- 13.Wong SL, Mangu PB, Choti MA, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28(3):493–508. [DOI] [PubMed] [Google Scholar]
- 14.Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010;33(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirocchi R, Trastulli S, Boselli C, et al. Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Database Syst Rev. 2012;(6):CD006317. [DOI] [PubMed] [Google Scholar]
- 16.Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: Factors affecting outcomes—a 10-year experience at a single center. Radiology. 2016;278(2):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hompes D, Prevoo W, Ruers T. Radiofrequency ablation as a treatment tool for liver metastases of colorectal origin. Cancer Imaging. 2011;11:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stang A, Fischbach R, Teichmann W, et al. A systematic review on the clinical benefit and role of radiofrequency ablation as treatment of colorectal liver metastases. Eur J Cancer. 2009;45(10):1748–1756. [DOI] [PubMed] [Google Scholar]
- 19.Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: A randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol. 2012;23(10):2619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 21.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22(2):229–237. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. [DOI] [PubMed] [Google Scholar]
- 23.Barone C, Nuzzo G, Cassano A, et al. Final analysis of colorectal cancer patients treated with irinotecan and 5-fluorouracil plus folinic acid neoadjuvant chemotherapy for unresectable liver metastases. Br J Cancer. 2007;97(8):1035–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alberts SR, Horvath WL, Sternfeld WC, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: A North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23(36):9243–9249. [DOI] [PubMed] [Google Scholar]
- 25.Ychou M, Viret F, Kramar A, et al. Tritherapy with fluorouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX): A phase II study in colorectal cancer patients with non-resectable liver metastases. Cancer Chemother Pharmacol 2008;62(2):195–201. [DOI] [PubMed] [Google Scholar]
- 26.Saltz L, Badarinath S, Dakhil S. et al. Phase III trial of cetuximab, bevacizumab, and 5-fluorouracil/leucovorin vs FOLFOX-bevacizumab in colorectal cancer. Clin Colorectal Cancer. 2012;11(2):101–111. [DOI] [PubMed] [Google Scholar]
- 27.Venook AP, Niedzwiecki D, Lenz HJ, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). ASCO Annual Meeting Abstracts. J Clin Oncol. 2014;32(18). [Google Scholar]
- 28.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–1215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.