Abstract
Background:
Two open-label, randomized, parallel-arm studies compared pharmacokinetics, safety, and tolerability of aripiprazole once-monthly 400 mg following deltoid vs gluteal injection in patients with schizophrenia.
Methods:
In the single-dose study, 1 injection of aripiprazole once-monthly 400 mg in the deltoid (n=17) or gluteal (n=18) muscle (NCT01646827) was administered. In the multiple-dose study, the first aripiprazole once-monthly 400 mg injection was administered in either the deltoid (n=71) or gluteal (n=67) muscle followed by 4 once-monthly deltoid injections (NCT01909466).
Results:
After single-dose administration, aripiprazole exposure (area under the concentration-time curve) was similar between deltoid and gluteal administrations, whereas median time to maximum plasma concentration was shorter (7.1 [deltoid] vs 24.1 days [gluteal]) and maximum concentration was 31% higher after deltoid administration. In the multiple-dose study, median time to maximum plasma concentration for deltoid administration was shorter (3.95 vs 7.1 days), whereas aripiprazole mean trough concentrations, maximum concentration, and area under the concentration-time curve were comparable between deltoid and gluteal muscles (historical data comparison). Multiple-dose pharmacokinetic results for the major metabolite, dehydro-aripiprazole, followed a similar pattern to that of the parent drug for both deltoid and gluteal injection sites. Safety and tolerability profiles were similar after gluteal or deltoid injections. Based on observed data, minimum aripiprazole concentrations achieved by aripiprazole once-monthly 400 mg are comparable with those of oral aripiprazole 15 to 20 mg/d.
Conclusions:
The deltoid muscle is a safe alternative injection site for aripiprazole once-monthly 400 mg in patients with schizophrenia.
Keywords: aripiprazole once-monthly 400 mg, injection site, pharmacokinetics, antipsychotic, safety/tolerability
Significance Statement
The research presented here shows that the site of injection of aripiprazole once-monthly 400 mg (AOM 400), gluteal or deltoid muscle after multiple once-monthly doses, does not substantially affect its pharmacokinetics or tolerability and thus should not alter the effectiveness previously demonstrated in multiple controlled clinical trials. Pharmacokinetics and safety/tolerability of AOM 400 were assessed in 2 randomized trials and were also compared with historical data for gluteal injections. In the single-dose trial, patients received one injection of AOM 400 in either the gluteal or deltoid muscle. In the multiple-dose study, patients received their first injection in the gluteal or deltoid muscle, followed by 4 once-monthly injections in the deltoid muscle. These data indicate that the deltoid muscle is a safe alternative to the established gluteal injection site for AOM 400 in patients with schizophrenia.
Introduction
Schizophrenia is a chronic psychiatric disorder in which maintenance treatment with antipsychotic therapy is recommended for most patients to reduce the risk of relapse and avoid functional impairments (Kreyenbuhl et al., 2010; Caseiro et al., 2012; Hasan et al., 2013). Because adherence to antipsychotic therapy is important for avoiding relapse and rehospitalization (Law et al., 2008; Caseiro et al., 2012), long-acting injectable (LAI) antipsychotic formulations that allow for less frequent administration may improve patient adherence and outcomes (Kishimoto et al., 2013; Taylor and Olofinjana, 2014; Kane et al., 2015). For patients treated with LAI antipsychotics, the option to select between deltoid and gluteal administration sites can accommodate patient preferences. For example, in a crossover study of stable outpatients with schizophrenia, 77% of patients in the United States preferred deltoid injections, whereas 70% of patients in non-US countries preferred gluteal injections. Those who preferred deltoid administration considered it easier, less embarrassing or painful, and faster than gluteal administration; similarly, those who preferred gluteal injection also considered it easier and less painful than deltoid injections (Hough et al., 2009). Patients may also value the opportunity to select or alternate the administration site. In a survey of 60 patients with schizophrenia who were stabilized on an LAI antipsychotic administered at the gluteal site, 57% (n=34/60) of patients chose to switch to the deltoid injection site when given the choice in administration site (Heres et al., 2012). Common reasons noted for switching included less burden from undressing and being able to receive the injection while standing, whereas common reasons for not switching included fear of increased pain and potential dermal injection site reactions in a visible area of the body (Heres et al., 2012).
Aripiprazole once-monthly 400 mg is an LAI formulation of aripiprazole for the treatment of schizophrenia (Otsuka Pharmaceutical Europe Ltd., 2016a; Otsuka Pharmaceutical Co., Ltd., 2016b). The efficacy of aripiprazole may be mediated through partial agonistic activity at dopamine D2 and serotonin 5-HT1A receptors and antagonistic activity at 5-HT2A receptors (Burris et al., 2002). A 24-week, open-label, parallel-arm, multiple-dose study in adult patients with schizophrenia stabilized on oral aripiprazole (n=41) evaluated the pharmacokinetics (PK), safety, and tolerability of 3 different doses of aripiprazole once-monthly (200, 300, and 400 mg) with concomitant oral aripiprazole (10 mg/d) for the first 14 days following the initial gluteal injection (Mallikaarjun et al., 2013). The PK data and clinical studies supported 400 mg as the starting and maintenance dose of aripiprazole once-monthly gluteal injection (Raoufinia et al., 2015). The mean (SD) steady state maximum concentration (Cmax,ss) of aripiprazole once-monthly 400 mg (316 [160] ng/mL) (Mallikaarjun et al., 2013) was comparable with the Cmax of oral aripiprazole 20 to 30 mg/d (range, 393–452 ng/mL) (Mallikaarjun et al., 2004), and the trough steady-state concentration (Cmin,ss; mean [SD], 212 [113] ng/mL) (Mallikaarjun et al., 2013) was similar to the steady-state Cmin of oral aripiprazole 15 to 20 mg (mean, 214 ng/mL; interquartile range, 124–286 ng/mL) (Kirschbaum et al., 2008). Cmin and Cmax values for oral aripiprazole and aripiprazole once-monthly 400 mg were all within a conservatively estimated therapeutic window, 94.0 to 534.0 ng/mL. The therapeutic window represents the median estimated Cmin,ss for oral aripiprazole 10 mg/d (94.0 ng/mL) and the 75th percentile of the simulated Cmax,ss for oral aripiprazole 30 mg/d (534 ng/mL) based on population PK simulations of oral aripiprazole once-daily (Raoufinia et al., 2015). The current studies evaluated whether injection of aripiprazole once-monthly 400 mg in the deltoid muscle provided adequate aripiprazole concentrations throughout the 28-day dosing interval and determined the PK, safety, and tolerability of initiating aripiprazole once-monthly 400 mg at the deltoid site compared with the gluteal injection site. Further PK comparisons were made with historical gluteal injection site data in patients with schizophrenia who participated in earlier aripiprazole once-monthly 400-mg clinical studies (Kane et al., 2012; Mallikaarjun et al., 2013; Fleischhacker et al., 2014).
Methods
These were randomized, open-label, parallel-arm, multicenter phase 1 studies conducted in compliance with International Conference on Harmonisation Good Clinical Practice guidelines. The informed consent forms, protocols, and amendments for both trials were submitted to and approved by central institutional review boards (single-dose study, Quorum Review IRB, Seattle, WA; multiple-dose study, Schulman Associates IRB, Cincinnati, OH). All patients provided written informed consent before study enrollment. Consistent with ethical principles for the protection of human research subjects, no trial procedures were performed on trial candidates until written consent was obtained.
Patients
Inclusion and exclusion criteria were similar for the single- and multiple-dose studies. Male and female patients aged 18 to 64 years with body mass index 18 to 35 kg/m2 and a diagnosis of schizophrenia based on DSM-IV-TR criteria were eligible for study inclusion. Patients without a history of tolerating aripiprazole were excluded from the single-dose study. In the multiple-dose study, patients without a history of tolerating aripiprazole (based on investigator judgment) were required to establish tolerability ≥15 days before the first aripiprazole once-monthly 400-mg dose by taking oral aripiprazole 10 mg/d for 3 days. In addition, patients had to be stabilized (based on investigator judgment) for ≥14 days before the first dose of aripiprazole once-monthly 400 mg with 1 of the following oral antipsychotics: aripiprazole (only in the multiple-dose study), olanzapine, paliperidone, quetiapine, risperidone, or ziprasidone. Patients had to demonstrate prior response to an antipsychotic medication other than clozapine (i.e., not treatment resistant to antipsychotic therapy). Patients who had a DSM-IV-TR comorbid diagnosis of substance abuse or dependence within the 180 days before screening or a current DSM-IV-TR diagnosis other than schizophrenia, were at significant risk of committing suicide, were treated with concomitant psychotropic medications (i.e., other than their current antipsychotic medication), or were taking cytochrome P450 (CYP) 2D6 or CYP3A4 inhibitors or CYP3A4 inducers within the 14 days before study dosing were excluded. Use of the aforementioned CYP inhibitors or inducers was prohibited for the duration of the trial. In addition, genotyping was performed to assess the presence of CYP2D6 alleles and assign metabolism status.
Study Designs
In both studies, patients were randomized 1:1 to receive an injection of aripiprazole once-monthly 400 mg in either the deltoid or gluteal muscle; in the multiple-dose study, randomization applied to the first dose only (i.e., after the first dose, all patients received deltoid injections). Needle length and gauge sizes for gluteal administration were consistent with US and EU prescribing information: a 22-gauge, 1.5-inch needle was recommended for patients <200 lb (90.7 kg), and a 21-gauge, 2.0-inch needle was recommended for patients ≥200 lb (Otsuka Pharmaceutical Europe Ltd., 2016a; Otsuka Pharmaceutical Co., Ltd., 2016b). For deltoid administration, a 23-gauge, 1.0-inch needle was recommended for patients <200 lb, and a 22-gauge, 1.5-inch needle was recommended for patients ≥200 lb.
In the single-dose study (6 sites), patients received a single injection of aripiprazole once-monthly 400 mg on day 1 in either the deltoid or gluteal muscle and remained on their current atypical oral antipsychotic for the study duration. Investigators had the option of reducing the dose of the oral antipsychotic medication to the mid to lower range of recommended doses (as described in the prescribing information) during the first 5 weeks after aripiprazole once-monthly 400-mg administration. Investigators could also further adjust the dose of oral antipsychotic at their discretion and based on patients’ symptoms and the individual’s safety/tolerability profile. Patients could be treated in an inpatient psychiatric unit for up to 3 weeks after administration of aripiprazole once-monthly 400 mg at the investigator’s discretion and were then followed as outpatients for the remainder of the trial. Study duration ranged from 127 to 170 days (1–30 days for screening, up to 14 days for washout of prohibited medications, and 126 days for the PK blood sampling period).
In the multiple-dose study (11 sites), patients with no history of aripiprazole exposure were required to establish tolerability as described above. During the time that tolerability was established, patients continued treatment with their current antipsychotic, with dose adjustments permitted based on study investigators’ judgment. Likewise, patients continued to receive their current oral antipsychotic for 14 days following the first aripiprazole injection. To compare the safety and PK of aripiprazole once-monthly 400 mg by injection site, patients received their first injection in the deltoid or gluteal muscle according to the randomization plan. Thereafter, all patients received 4 monthly administrations of aripiprazole once-monthly 400 mg in the deltoid muscle (for a total of 5 once-monthly injections). Patients were allowed a 1-time dose reduction to 300 mg and a 1-time return to the 400-mg dose for safety and tolerability reasons. A minimum of 4 injections at the deltoid site were planned to achieve steady-state plasma concentrations of aripiprazole (Mallikaarjun et al., 2004).
Blood Sampling for PK Assessments
Blood samples in the single-dose study were collected predose on day 1 and postinjection through day 126 or until early termination (supplementary Figure 1). In the multiple-dose study, to better characterize the PK profiles, 3 study sites were designated as extensive sampling sites where frequent PK samples were drawn after the first and fifth injections. Patients at extensive sampling sites were required to remain in the inpatient clinical psychiatric unit for the first 21 days after the first and fifth injections and were otherwise outpatients. Patients at the 8 sparse sampling sites could be followed as inpatients for the first 14 days after the first injection and as outpatients for the remainder of the trial, based on investigator judgment. Patients returned to their study sites for scheduled safety assessments and PK samplings. Patients at sparse sampling sites had blood collected on day 1 (predose) and once weekly or monthly thereafter (supplementary Figure 1). At extensive sampling sites, blood samples were collected on day 1 (predose) and at 2- to 7-day intervals (some injections coinciding with sparse sampling days) until day 141 or early termination (supplementary Figure 1). All blood samples that were not drawn predose were collected between 8 am and 2 pm. All patients continued to take their current oral antipsychotic therapy during the 14 days after the first aripiprazole once-monthly injection. After 14 days, patients discontinued their oral antipsychotic for the remainder of the trial. At extensive PK sampling sites (supplementary Figure 1), aripiprazole was not permitted as the concomitant oral antipsychotic in an effort to accurately capture the PK profile of aripiprazole once-monthly 400 mg.
Safety Assessments
In both studies, safety variables included adverse events (AEs), clinical laboratory tests, vital signs, physical examinations, and electrocardiograms. Extrapyramidal symptoms (EPS) were measured using the Simpson-Angus Scale (Simpson and Angus, 1970), Barnes Akathisia Rating Scale (Barnes, 1989), and Abnormal Involuntary Movement Scale (Guy, 1976a). Suicidality was monitored with the Columbia Suicide Severity Rating Scale (C-SSRS) (Posner et al., 2009). Patients assessed injection site pain using a visual analog scale ranging from 0 mm (no pain) to 100 mm (unbearable pain) 30 minutes before injection and 1 hour postinjection for each injection in the multiple-dose study and on days 1, 14, and 28 in the single-dose study. Investigators assessed injection site reactions (localized pain, redness, swelling, and induration at the injection site) using a 4-point categorical scale ranging from absent to severe.
Bioanalysis
Aripiprazole and its major metabolite, dehydro-aripiprazole, were extracted from a 50.0-µL aliquot of plasma using a liquid-liquid extraction and were analyzed using a validated high-performance liquid chromatography with tandem mass spectrometry detection method; additional details are provided in the supplementary appendix.
PK Analyses
For calculation of descriptive statistics and PK analyses, concentration values below the quantitation limit (0.5 ng/mL) were set to 0. Plasma concentrations of aripiprazole were analyzed using standard noncompartmental methods (Jusko, 1992). Actual blood sampling times relative to the drug administration were used for plasma PK calculations. Values for Cmax, C28 (i.e., trough concentration), and time to maximum (peak) plasma concentration (tmax) were determined directly from the observed data. Area under the concentration-time curve (AUC) values AUCt, AUC28, and AUC∞ were estimated using the linear trapezoidal rule. The terminal-phase elimination rate constant (λz) was estimated by a log-linear regression of ≥3 nonzero concentrations. The elimination half-life (t1/2) was determined as (ln2)/λz. Phoenix WinNonlin (Pharsight Corporation, version 6.2) was used for the analyses. In the multiple-dose study, AUC, Cmax, and tmax following the first and fifth injections were determined only in patients from the extensive sampling sites. Summary statistics for trough (C28) concentrations included patients from extensive and sparse sampling sites; however, PK samples from patients who were taking oral aripiprazole during the first 2 weeks were excluded from C28 calculations. Moreover, patients who had dose reductions to 300 mg of aripiprazole once-monthly were excluded from all PK analyses.
For historical comparisons, mean aripiprazole concentration-time profiles were used together with Cmin,ss from an open-label, randomized, multiple-dose, 24-week study that assessed the PK and safety/tolerability of aripiprazole once-monthly 400-mg gluteal injections in patients with schizophrenia (Mallikaarjun et al., 2013). In that study, all patients received oral aripiprazole 10 mg for the first 14 days following the initial injection of aripiprazole once-monthly. Predose blood samples were collected weekly or biweekly throughout the study; after the final dose, blood samples were collected on days 1 to 5 and then at weeks 1, 1.5, 2, 3, and 4. Steady-state PK parameters were calculated for samples collected after the fifth and last aripiprazole once-monthly injection (Mallikaarjun et al., 2013).
Additional historical comparisons for Cmin,ss were drawn from 2 randomized, controlled, phase 3 maintenance studies with aripiprazole once-monthly 400-mg gluteal injections in patients with schizophrenia (Kane et al., 2012; Fleischhacker et al., 2014). Predose blood samples used for the steady-state trough comparison were drawn at week 8 of the double-blind maintenance treatment phase in the 52-week study and at week 12 of the double-blind, active-controlled phase in the 38-week study. Bioanalytic procedures for aripiprazole and dehydro-aripiprazole in the 52- and 38-week studies were similar to those described in the current study (see supplementary Appendix).
Efficacy Assessments
Maintenance of therapeutic effect was assessed as a secondary outcome in the multiple-dose study with the Positive and Negative Syndrome Scale (Kay et al., 1987) and the Clinical Global Impression-Severity of Illness (CGI-S) and CGI-Improvement (CGI-I) scales (Guy, 1976b).
Statistical Analyses
For the single-dose study, point estimates and 90% CIs for geometric mean ratios were calculated by using the antilog of the estimates of difference and 90% CIs derived from an ANOVA on the natural-log scale of Cmax, C28, AUCt, AUC28, and AUC∞. tmax values were compared using the Wilcoxon test. Sample sizes of 24 and 100 evaluable patients were planned for the single-dose and multiple-dose studies, respectively. Though formal power calculations were not performed, these numbers were considered sufficient to provide PK, safety, and tolerability data when evaluating a new site of administration. The dataset for the PK analyses consisted of all dosed patients who had evaluable PK parameters; missing data were not imputed. Analyses were conducted using the software program SAS version 9 (SAS Institute Inc.).
Baseline and demographic characteristics were summarized using descriptive statistics for all randomized patients. Safety data were summarized for all randomized patients who received ≥1 dose of aripiprazole once-monthly 400 mg, regardless of protocol violations. Missing data were imputed using the last observation carried forward method for the EPS scales.
Results
Patient Disposition and Characteristics
In the single-dose study, 52 patients were screened and 37 patients enrolled and were randomized to receive a single injection of aripiprazole once-monthly 400 mg in the deltoid (n=18) or gluteal (n=19) muscle. Two randomized patients discontinued before dosing (1 patient in the deltoid group withdrew consent and 1 patient in the gluteal group had an AE before dosing and was withdrawn by the investigator). After dosing, the most frequent reasons for study discontinuation were being lost to follow-up and patient withdrawn by investigator (Table 1). In the multiple-dose study, 204 patients were screened and 141 patients enrolled and were randomized to receive their first injection of aripiprazole once-monthly 400 mg in the deltoid (n=73) or gluteal (n=68) muscle. Of the 141 randomized patients, 51 were at extensive sampling sites (n=28 deltoid, n=23 gluteal) and 90 were at sparse sampling sites (n=45 deltoid, n=45 gluteal). Three randomized patients discontinued before the first injection; 3 patients met withdrawal criteria and 2 patients were withdrawn by the investigator. The most frequent reasons for study discontinuation were withdrawal of consent (n=20), loss to follow-up (n=12), and occurrence of treatment-emergent AE (n=7). In both studies, demographic and baseline characteristics were similar between deltoid and gluteal administration groups (Table 1). In the multiple-dose study, 97.1% of patients had no dose adjustment of aripiprazole once-monthly 400 mg; dose reduction to 300 mg was required for 2 patients in the deltoid/deltoid group starting with the second injection and for 2 patients in the gluteal/deltoid group (1 starting with the second injection and 1 starting with the fourth injection). A small number of patients in the 2 studies were determined to be CYP2 D6 poor metabolizers (single-dose study, n=1; multiple-dose study, n=4).
Table 1.
Patient Disposition and Characteristics
| Single-Dose Study | Multiple-Dose Study | |||||
|---|---|---|---|---|---|---|
| Deltoid (n= 18) | Gluteal (n = 19) | Total (n = 37) | Deltoid/Deltoid (n = 73) | Gluteal/Deltoid (n = 68) | Total (n = 141) | |
| Patient disposition, n | ||||||
| Screened | 52 | 204 | ||||
| Enrolled and randomized | 18 | 19 | 37 | 73 | 68 | 141 |
| Treated | 17 | 18 | 35 | 71 | 67 | 138 |
| Completed,a n (%) | 16 (88.9) | 14 (73.7) | 30 (81.1) | 50 (68.5) | 46 (67.6) | 96 (68.1) |
| Discontinued, n (%) | 2 (11.1) | 5 (26.3) | 7 (18.9) | 23 (31.5) | 22 (32.4) | 45 (31.9) |
| Lost to follow-up | 0 | 3 (15.8) | 3 (8.1) | 7 (9.6) | 5 (7.4) | 12 (8.5) |
| Adverse eventb | 0 | 0 | 0 | 4 (5.5) | 3 (4.4) | 7 (5.0) |
| Patient met withdrawal criteria | 0 | 0 | 0 | 3 (4.1) | 0 | 3 (2.1) |
| Investigator withdrew patient | 1 (5.6) | 2 (10.5) | 3 (8.1) | 0 | 2 (2.9) | 2 (1.4) |
| Patient withdrew consent | 1 (5.6) | 0 | 1 (2.7) | 9 (12.3) | 11 (16.2) | 20 (14.2) |
| Lack of efficacy | 0 | 0 | 0 | 0 | 1 (1.5) | 1 (0.7) |
| Demographics and baseline characteristics | ||||||
| Age, mean (SD), y | 43.1 (11.0) | 45.9 (7.3) | 44.5 (9.3) | 44.3 (9.6) | 43.5 (10.9) | 43.9 (10.2) |
| BMI, mean (SD), mg/kg2 | 27.6 (4.2) | 27.6 (4.7) | 27.6 (4.4) | 27.8 (4.4) | 28.3 (4.5) | 28.0 (4.4) |
| Men, n (%) | 12 (67.0) | 15 (79.0) | 27 (73.0) | 54 (74.0) | 55 (80.9) | 109 (77.3) |
| Race/ethnicity, n (%) | ||||||
| White | 5 (28.0) | 7 (37.0) | 12 (32.0) | 15 (20.5) | 16 (23.5) | 31 (22.0) |
| Black | 13 (72.0) | 12 (63.0) | 25 (68.0) | 57 (78.1) | 50 (73.5) | 107 (75.9) |
| Asian | 0 | 0 | 0 | 1 (1.4) | 1 (1.5) | 2 (1.4) |
| Native Hawaiian/Other Pacific Islander | 0 | 0 | 0 | 0 | 1 (1.5) | 1 (0.7) |
| Hispanic or Latino | 2 (11.0) | 1 (5.0) | 3 (8.0) | 4 (5.5) | 6 (8.8) | 10 (7.1) |
Abbreviation: BMI, body mass index.
a In the single-dose study, patients who completed the day 126 visit were defined as completers. In the multiple-dose study, patients who completed the day 141 visit were defined as completers.b In the multiple-dose study, 7 of 138 patients (5.1%) experienced a treatment-emergent AE that led to study discontinuation (4 in the deltoid/deltoid group, including 1 each with agitation, akathisia, psychotic disorder, and schizophrenia, and 3 in the gluteal/deltoid group, including 1 each with akathisia and psychotic disorder, and 1 patient with decreased weight, apathy, and decreased appetite. Percentages are based on the number of randomized patients.
PKs
Aripiprazole exposure (AUCt and AUC∞) was comparable between deltoid and gluteal administration groups in the single-dose study (Table 2), and dehydro-aripiprazole exposure was slightly higher with deltoid versus gluteal administration (supplementary Table 1). Mean (SD) aripiprazole plasma concentrations on day 28 after the dose (C28) were 103 (49.6) and 86.2 (37.5) ng/mL with deltoid and gluteal administration, respectively (Table 2). In the multiple-dose study, C28 concentrations after the first injection were 112 (50.1) and 79.0 (37.3) ng/mL after the deltoid and gluteal injections, respectively (data pooled between extensive and sparse sampling sites; Table 2); a similar pattern was noted with dehydro-aripiprazole (supplementary Table 1).
Table 2.
PK Parameters
| Single-Dose Study | Multiple-Dose Study | |||||
|---|---|---|---|---|---|---|
| Deltoid (n = 16) | Gluteal (n = 17) | GMR (90% CI) | Deltoid/Deltoid (n = 73) | Gluteal/Deltoid (n = 68) | GMR (90% CI) | |
| PK parameters after the first injectiona | ||||||
| Cmax, ng/mL | 170 (58.6) | 136 (70.3) | 1.31 (1.02–1.67) | 135 (57.8) n = 24 | 126 (88.6) n = 22 | 1.20 (0.90–1.60) |
| C28, ng/mL | 103 (49.6) | 86.2 (37.5) | 1.24 (0.94–1.63) | 112 (50.1) n = 51b | 79.0 (37.3) n = 52b | 1.43 (1.22–1.69)† |
| tmax,c days | 7.1 (4.0–63.1) | 24.1 (4.1–70.0)* | N/A | 14.3 (3.8–29.0) n = 24 | 13.9 (3.9–28.0) n = 22 | N/A |
| AUC28, ng•d/mL | 3120 (1150) | 2380 (1410) | 1.42 (1.06–1.91) | 2728 (1277) n = 21 | 2359 (1527) n = 20 | 1.24 (0.91–1.68) |
| AUCt, ng•d/mL | 7360 (2420) | 7340 (2360) | 0.99 (0.80–1.23) | N/A | N/A | N/A |
| AUC∞, ng•d/mL | 7590 (2590) | 7920 (2580) n = 14 | 0.95 (0.75–1.21) | N/A | N/A | N/A |
| t1/2, days | 17.8 (7.45) | 24.0 (7.36) n = 14 | N/A | N/A | N/A | N/A |
| PK parameters after the fifth injection | ||||||
| Combined Deltoid/Deltoid and Gluteal/Deltoid Groups d | ||||||
| Cmax,ss, ng/mL | N/A | 328 (133) n=39 |
||||
| Cmin,ss, ng/mL | N/A | 239 (133) n=86b |
||||
| AUCt, ng•d/mL | N/A | 7027 (2689) n=36 |
||||
| tmax,c days | N/A | 3.95 (0.00–27.87) n=39 |
||||
Abbreviations: AUC, area under the concentration-time curve; Cmax, maximum plasma concentration; Cmax,ss, Cmax at steady state; Cmin,ss, minimum plasma concentration at steady state; GMR, geometric mean ratio; N/A, not applicable; PK, pharmacokinetic; t1/2, elimination half-life; tmax, time to maximum (peak) plasma concentration.
*P<0.05; †P=0.0002.
a Unless otherwise noted, PK parameters for the multiple-dose study were assessed following the first and before the second injection of aripiprazole once-monthly 400 mg. Following the first injection, 19 patients were excluded from the PK analysis, because they received oral aripiprazole during the first 2 weeks; 1 patient who had a high baseline aripiprazole concentration and 2 patients who had no baseline PK data were also excluded. Two patients were excluded because of insufficient PK profiles. In addition, AUC28 and C28 were not reported for 11 patients, because the sampling time of their last measurable plasma concentration available before the second injection was not within 28 ± 2 days after the first injection.
b Sample size is larger because of inclusion of data from sparse sampling sites.
c Reported as median (minimum-maximum). All other parameters are reported as mean (SD).
d Following the fifth deltoid injection of aripiprazole once-monthly 400 mg in the deltoid/deltoid group and fourth deltoid injection in the gluteal/deltoid group.
In the single-dose study, deltoid administration of aripiprazole once-monthly 400 mg resulted in a median aripiprazole tmax of 7.1 days (range, 4.0–63.1) compared with 24.1 days (range, 4.1–70.0) with gluteal administration (P<.05, Table 2), and median dehydro-aripiprazole tmax was 22.0 (range, 6.1–63.1 days) for deltoid and 35.0 (range, 8.0–84.0 days) for gluteal administration (P>.05) (supplementary Table 1). In the multiple-dose study, median aripiprazole tmax during the first 28 days after the initial injection was 14.3 (range, 3.8–29.0) vs 13.9 (range, 3.9–28.0) days with deltoid vs gluteal administration, respectively. Median time to steady-state Cmax after the fifth aripiprazole once-monthly 400-mg injection was 3.95 days.
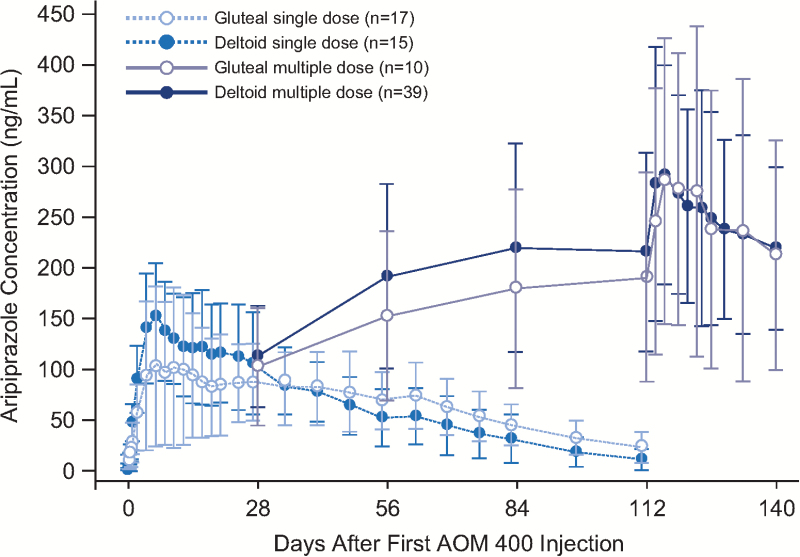
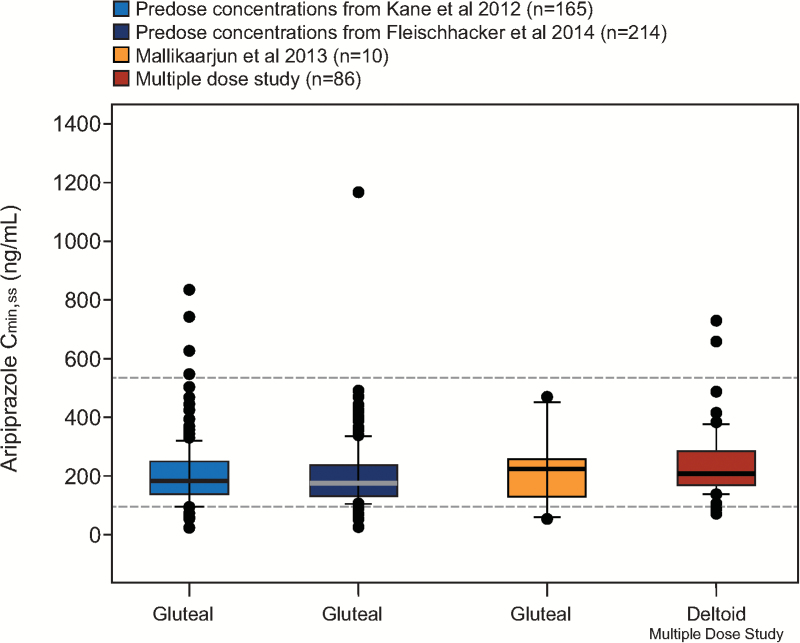
Based on visual inspection of predose concentrations from the multiple-dose study and historical data from a phase 1 study in patients with schizophrenia, aripiprazole steady state was reached by the fourth injection in the deltoid muscle (Mallikaarjun et al., 2013) (Figure 1). After the fifth injection, values for aripiprazole Cmin,ss were comparable between injection site groups, regardless of first injection site (mean [SD], 351 [161] and 299 [80.6] ng/mL for deltoid/deltoid and gluteal/deltoid groups, respectively) and were also comparable with historical data for gluteal administration from earlier studies in patients with schizophrenia (Figure 2).
Figure 1.
Mean (SD) aripiprazole concentration-time profiles after deltoid or gluteal injection of aripiprazole once-monthly 400 as single- or multiple-dose administrations. Because data are from multiple trials, only time points that were common in all trials are displayed. In multiple-dose studies (injections given every 28 d), samples at days 28, 56, 84, and 112 are predosing concentrations (trough levels), and more frequent sampling was done at steady state from days 112 to 140. Historical gluteal multiple-dose data are from an open-label, parallel-arm, multiple-dose, 24-week study (Mallikaarjun et al., 2013).
Figure 2.
Aripiprazole Cmin,ss after monthly injections of aripiprazole 400 mg at the deltoid site and comparison with historical data from the gluteal site. Steady-state concentration data for the deltoid site are from the multiple-dose study following the fifth injection, with gluteal/deltoid and deltoid/deltoid administration groups combined. Historical data are from patients with schizophrenia in a phase 3 randomized, placebo-controlled, 52-week study (Kane et al., 2012); a phase 3, randomized, active-controlled 38-week study (Fleischhacker et al., 2014); and a phase 1, open-label, parallel-arm, multiple-dose, 24-week study (Mallikaarjun et al., 2013). Boxes span the 25th and 75th percentiles with a thick horizontal bar at the median. Error bars denote the 10th and 90th percentiles. Dashed horizontal lines represent the therapeutic window (i.e., the median simulated Cmin,ss for oral aripiprazole 10 mg/d [94.0 ng/mL] and the 75th percentile of the simulated Cmax,ss for oral aripiprazole 30 mg/d [534 ng/mL]) based on population pharmacokinetic (PK) simulations of once-daily oral aripiprazole (Raoufinia et al., 2015). Points represent individual outliers below the 10th and above the 90th percentiles. Cmax,ss=maximum plasma concentration at steady state; Cmin,ss=minimum plasma concentration at steady state.
Safety and Tolerability
Treatment-Emergent Adverse Events
There were no deaths reported and no treatment-emergent AEs (TEAEs) related to suicidal ideation in the single- or multiple-dose studies. During the single-dose study, there were no serious TEAEs. In the multiple-dose study, 4 of 138 patients (2.9%) experienced a serious TEAE (1 patient with psychotic disorder and 1 with schizophrenia in the gluteal/deltoid group; 1 patient with schizophrenia and 1 with atrial fibrillation in the deltoid/deltoid administration site group). In the single-dose study, TEAEs for ≥10% of patients overall occurred with similar frequency between the 2 administration site groups, including injection site pain (supplementary Table 2). The majority of TEAEs were transient and mild in severity. In the deltoid administration site group, 1 patient (5.9%) had a meniscus lesion (right knee) that was considered severe and unrelated to study treatment. In the multiple-dose study, the most frequently reported TEAEs overall are shown in supplementary Table 3; the majority were mild or moderate in severity. The incidence of TEAEs was comparable between administration site groups overall and after the first injection. A clinically relevant increase in weight (≥7% increase from baseline) was reported in 21.5% of patients at the last visit, with overall mean (SD) increase in weight over the duration of the study period of 2.4 (5.6) kg and change in BMI of 0.8 (1.9) kg/m2 (n=135).
There were no clinically relevant mean changes from baseline in serum chemistry, prolactin, urinalysis test results, vital signs, or electrocardiogram parameters during aripiprazole once-monthly 400-mg treatment in either study. An AE of increase in blood glucose and triglycerides was reported in 1 patient in the single-dose study deltoid group. In the multiple-dose study, increased blood glucose was reported in 1 patient in the deltoid/deltoid group, and hypercholesterolemia in 1 patient in the gluteal/deltoid group. There were no clinically relevant changes in hematology in the single-dose study, but anemia was reported for 1 patient in the multiple-dose study. Changes from baseline on EPS scales were minimal and comparable between deltoid and gluteal administration groups in the single-dose (supplementary Table 2) and multiple-dose (supplementary Table 3) studies; last observation carried forward and observed cases analyses were consistent. There were no adverse findings in the C-SSRS in the single-dose trial. In the multiple-dose trial, based on the C-SSRS, suicidality, suicidal ideation, and emergence of suicidal ideation occurred in 2 patients; no patients completed suicide or reported emergence of suicidal behavior.
Injection Site Assessments
In both studies, pain at injection site was the most commonly reported TEAE (Table 3). In the single-dose study, 1 patient in the gluteal administration group (5.6%) experienced severe injection site pain; all other injection site TEAEs were mild in severity. In both studies, investigator-assessed pain, redness, swelling, and induration at the injection site was rated as absent or mild in both administration site groups at all time points (Table 4). Likewise, patient-rated pain scores based on a visual analog scale (100 mm) 1 hour postdose were low in both studies (Table 4).
Table 3.
Injection Site Treatment-Emergent Adverse Events
| Single-Dose Study | Multiple-Dose Study | ||
|---|---|---|---|
| Injection Site Adverse Events, n (%) | Deltoid (n = 17) | Gluteal (n = 18) | Total (n = 138) |
| Pain | 6 (35.3) | 5 (27.8) | 38 (27.5) |
| Rash | 1 (5.9) | 0 | 0 |
| Erythema | 0 | 0 | 1 (0.7) |
| Induration | 0 | 0 | 1 (0.7) |
| Swelling | 0 | 0 | 1 (0.7) |
Table 4.
Injection Site Assessments
| Single-Dose Study | Multiple-Dose Study | ||
|---|---|---|---|
| Deltoid (n = 17) | Gluteal (n = 18) | Total (n = 138) |
|
| Investigator-rated injection site reactions, n (%) of patientsa | |||
| Pain, 1 h after first injection | 4 (23.5) | 1 (5.6) | 12 (8.7) |
| Pain, last visit/injectionb | 2 (11.8) | 0 | 9 (6.5) |
| Swelling, 1 h after first injection | 0 | 0 | 2 (1.4) |
| Swelling, last visit/injectionb | 0 | 0 | 0 |
| Redness, 1 h after first injection | 0 | 0 | 1 (0.7) |
| Redness, last visit/injectionb | 0 | 0 | 0 |
| Induration, 1 h after first injection | 0 | 1 (5.6) | 2 (1.4) |
| Induration, last visit/injectionb | 0 | 0 | 0 |
| Patient-reported pain (VAS scale score),a,c mean (SD) | |||
| 1 h after first injection | 3.1 (7.3) | 1.7 (2.7) | - |
| Deltoid/deltoid (n = 71) | - | - | 2.3 (4.1) |
| Gluteal/deltoid (n = 67) | - | - | 1.8 (4.7) |
| Last visit/5th injectionb | 2.2 (7.2) | 0.5 (1.3) | 1.2 (3.4) |
Abbreviation: VAS, visual analog scale.
a Investigator or patient ratings for the first injection.
b Last visit for the single-dose study; fifth injection (1 h postdose) for the multiple-dose study (n = 100).
c Assessed using a VAS ranging from 0 mm (no pain) to 100 mm (unbearable pain).
Rates of injection site pain reported as a TEAE were slightly numerically higher with deltoid than gluteal injections but decreased with subsequent injections. After the first injection in the multiple-dose study, 18 of 71 patients (25.4%, deltoid/deltoid) and 15 of 67 (22.4%, gluteal/deltoid) had injection site pain as a TEAE, and this number decreased to 4 of 122 patients (3.3%), 0 of 109 (0.0%), 0 of 106 (0.0%), and 3 of 100 (3.0%) after the second, third, fourth, and fifth injections, respectively.
Other Assessments
In the multiple-dose study, patients remained stable during the trial based on standard measures of psychiatric symptoms and disease severity (Positive and Negative Syndrome Scale and CGI-S and CGI-I scales; data not shown).
Discussion
Single-dose administration of aripiprazole once-monthly 400 mg in the deltoid muscle resulted in similar aripiprazole exposure (based on AUCs) and slightly higher Cmax (31%) and shorter tmax (7.1 days vs 24.1 days) compared with its gluteal administration. The observed differences in aripiprazole Cmax and tmax after deltoid and gluteal administration of aripiprazole once-monthly 400 mg are due to the higher absorption rate of aripiprazole once-monthly 400 mg after deltoid administration because of the smaller mass and higher perfusion of the deltoid muscle. Other LAIs show similar patterns in Cmax and tmax when administered in the deltoid muscle vs the gluteal muscle (Yin et al., 2015). Aripiprazole concentration-time profiles after multiple doses of aripiprazole once-monthly 400 mg in the deltoid muscle were comparable with the historical data after multiple gluteal administrations. In addition, no differences in the multiple-dose PK data were observed when patients received the first dose of aripiprazole once-monthly 400 mg in the deltoid site vs the gluteal site. Although higher Cmax and shorter tmax were observed after deltoid administration of aripiprazole once-monthly 400 mg in the single-dose study, no apparent differences vs gluteal administration with respect to Cmax were observed in the multiple-dose setting during the 28 days after the first administration. Results for dehydro-aripiprazole followed a similar pattern. The differences in Cmax and tmax between gluteal and deltoid injections observed in the single-dose study but not in the multiple dose study are likely due to the different sampling schedules after the first injection in the 2 studies. The multiple-dose deltoid study was prospectively designed to randomly initiate aripiprazole once-monthly 400 mg in the deltoid or gluteal site, and no appreciable differences in the PK or safety data after the first and subsequent doses of aripiprazole once-monthly 400 mg were observed. Likewise, multiple-dose PK results for the active, major metabolite, dehydro-aripiprazole, showed a similar pattern to that of the parent drug. Dehydro-aripiprazole has affinity for dopamine D2 receptors comparable to that of aripiprazole (Otsuka Pharmaceutical Europe Ltd., 2016a; Otsuka Pharmaceutical Co., Ltd., 2016b) and is present at plasma concentrations 30% to 50% of aripiprazole concentrations (Kirschbaum et al., 2008; Hiemke et al., 2011; Otsuka Pharmaceutical Europe Ltd., 2016a; Otsuka Pharmaceutical Co., Ltd., 2016b). Nevertheless, the extent of the contribution of dehydro-aripiprazole to the clinical effect of aripiprazole is unknown. Thus, it is concluded that treatment of aripiprazole once-monthly 400 mg can be initiated in either the deltoid or gluteal muscle. Furthermore, given the similar safety and PK profiles of aripiprazole once-monthly 400 mg administered to the deltoid vs historical data of its gluteal administration (Kane et al., 2012; Mallikaarjun et al., 2013; Fleischhacker et al., 2014), the safety and efficacy of aripiprazole once-monthly 400-mg deltoid administration are expected to be comparable with its gluteal administration. Comparable safety and efficacy with deltoid and gluteal injections can likewise be expected based on the observed mean Cmin,ss and Cmax,ss, both of which were within the established therapeutic drug monitoring reference range (150–500 ng/mL) (Hiemke et al., 2011).
Previously, the Cmax attained by multiple gluteal administrations of aripiprazole once-monthly 400 mg was reported to approximate steady-state plasma levels achieved after oral dosing of 20 to 30 mg/d, and Cmin to approximate steady-state plasma levels achieved after oral dosing of 15 to 20 mg/d (Mallikaarjun et al., 2004; Sparshatt et al., 2010). Based on the Cmin and Cmax concentrations achieved after multiple deltoid administrations of aripiprazole once-monthly 400 mg, one can conclude that deltoid administration of aripiprazole once-monthly 400 mg also attains the aforementioned steady-state plasma levels achieved after oral aripiprazole dosing. Furthermore, examination of the range of aripiprazole Cmin concentrations, as displayed in Figure 2, shows that aripiprazole plasma concentrations for most patients (>95%) are maintained >94.0 ng/mL with multiple-dose administration of aripiprazole once-monthly 400 mg in the deltoid or gluteal site. The maintenance of efficacy of aripiprazole once-monthly 400 mg has previously been reported (Kane et al., 2012; Fleischhacker et al., 2014) with the rate of impending relapse of 10.0% and 7.1% over 52 or 38 weeks, respectively. The performance of aripiprazole once-monthly 400 mg, as evidenced by its long-term clinical data, can be at least partly attributed to its robust maintenance of Cmin aripiprazole concentrations similar to those observed after daily administration of 15 to 20 mg of oral aripiprazole.
Deltoid administration of paliperidone palmitate, a once-monthly LAI atypical antipsychotic, also showed potential differences in plasma concentrations and shorter t1/2 compared with gluteal administration (Yin et al., 2015). Both the US and EU prescribing information recommend initiating paliperidone palmitate treatment at the deltoid site, with deltoid or gluteal maintenance injections thereafter (Janssen Pharmaceuticals, Inc., 2015a; Janssen Pharmaceuticals N.V., 2015b). The data reported here demonstrate that mean aripiprazole concentration over time is similar with deltoid and gluteal administration after single or multiple injections, and there is no requirement in the US or EU prescribing information to preferentially initiate aripiprazole once-monthly 400 mg with deltoid vs gluteal administration (Otsuka Pharmaceutical Europe Ltd., 2016a; Otsuka Pharmaceutical Co., Ltd., 2016b).
In both aripiprazole once-monthly 400-mg deltoid administration studies, the majority of TEAEs were mild or moderate in severity. The incidence of TEAEs was comparable between administration site groups overall and after the first injection. Furthermore, investigators assessed injection site pain as absent or mild, and mean patient-rated pain scores 1-hour postdose were low in both studies. Injection site pain reported as a TEAE following the first injection was slightly more common with deltoid than gluteal injections (25.4% vs 22.4%, respectively) but decreased to 3.0% after the fifth injection, which was into the deltoid muscle.
The multiple-dose study did not include a separate arm in which all injections were administered at the gluteal site. However, the rich historical data for multiple gluteal site injections of aripiprazole once-monthly 400 mg in patients with schizophrenia in phase 1 and 3 trials allowed us to use an alternative approach to compare deltoid vs gluteal administration site PK in which all patients were exposed to deltoid injections in the current multiple-dose study. Administration of aripiprazole once-monthly 400 mg to the deltoid site resulted in comparable aripiprazole concentrations relative to the gluteal site. For the duration of the single-dose study, patients continued their current oral antipsychotic. During the multiple-dose study, patients continued their current oral antipsychotic medication 14 days following the first injection. It is unclear to what extent these concurrent therapies may have contributed to the observed efficacy or safety outcomes in either study. One patient in the single-dose study and 4 in the multiple-dose study were determined to be CYP2D6 poor metabolizers and found to have higher plasma concentrations of aripiprazole. The PK data for these patients are not presented separately here due the small sample size, which precludes meaningful analysis. No new safety signals emerged in either study, and patients in the multiple-dose study remained stable based on standard clinical assessments. The comparable safety, tolerability, and PK profiles support the deltoid muscle as an alternative injection site for administration of aripiprazole once-monthly 400 mg in patients with schizophrenia.
Statement of Interest
Ross A. Baker, Na Jin, Patricia Bricmont, Jennifer Repella, Robert D. McQuade, Arash Raoufinia, and Timothy Peters-Strickland are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. Peter Hertel, Frank Larsen, and Anna-Greta Nylander are employees of H. Lundbeck A/S. Anna Eramo is an employee of Lundbeck LLC.
Supplementary Material
Acknowledgments
Editorial support for development of this manuscript was provided by Amy Roth Shaberman at C4 MedSolutions, LLC (Yardley, PA), a CHC Group company, and funded by Otsuka Pharmaceutical Development & Commercialization, Inc, and H. Lundbeck A/S.
This work was supported by H. Lundbeck A/S and Otsuka Pharmaceutical Development & Commercialization, Inc.
References
- Barnes TR. (1989) A rating scale for drug-induced akathisia. Br J Psychiatry 154:672–676. [DOI] [PubMed] [Google Scholar]
- Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB. (2002) Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 302:381–389. [DOI] [PubMed] [Google Scholar]
- Caseiro O, Perez-Iglesias R, Mata I, Martinez-Garcia O, Pelayo-Teran JM, Tabares-Seisdedos R, Ortiz-Garcia de la Foz V, Vazquez-Barquero JL, Crespo-Facorro B. (2012) Predicting relapse after a first episode of non-affective psychosis: a three-year follow-up study. J Psychiatr Res 46:1099–1105. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Sanchez R, Perry PP, Jin N, Peters-Strickland T, Johnson BR, Baker RA, Eramo A, McQuade RD, Carson WH, Walling D, Kane JM. (2014) Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry 205:135–144. [DOI] [PubMed] [Google Scholar]
- Guy W. (1976a) Abnormal Involuntary Movement Scale (AIMS). ECDEU Assessment Manual for Psychopharmacology, pp 534–537. Rockville, MD: US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs. [Google Scholar]
- Guy W. (1976b) ECDEU Assessment manual for psychopharmacology. Rockville, MD: US Department of Health Services. [Google Scholar]
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Moller HJ, World Federation of Societies of Biological Psychiatry Task Force on Treatment Guidelines for Schizophrenia (2013) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry 14:2–44. [DOI] [PubMed] [Google Scholar]
- Heres S, Frobose T, Hamann J, Leucht S, Maino K, Reichhart T, Stiegler M, Kissling W. (2012) Patients’ acceptance of the deltoid application of risperidone long-acting injection. Eur Neuropsychopharmacol 22:897–901. [DOI] [PubMed] [Google Scholar]
- Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, Fric M, Gerlach M, Greiner C, Grunder G, Haen E, Havemann-Reinecke U, Jaquenoud Sirot E, Kirchherr H, Laux G, Lutz UC, Messer T, Muller MJ, Pfuhlmann B, Rambeck B, et al. (2011) AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry 44:195–235. [DOI] [PubMed] [Google Scholar]
- Hough D, Lindenmayer JP, Gopal S, Melkote R, Lim P, Herben V, Yuen E, Eerdekens M. (2009) Safety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 33:1022–1031. [DOI] [PubMed] [Google Scholar]
- Janssen Pharmaceuticals, Inc. (2015a) InvegaR SustennaR (paliperidone palmitate). Full prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc. [Google Scholar]
- Janssen Pharmaceutica N.V. (2015b) XeplionR (paliperidone palmitate). Full prescribing information. Beerse, Belgium: Janssen Pharmaceutica N.V. [Google Scholar]
- Jusko WJ. (1992) Guidelines for collection and analysis of pharmacokinetic data. Applied pharmacokinetics: principles of therapeutic drug monitoring (third edition) (pp. 1–43). Vancouver, WA: Applied Therapeutics, Inc. [Google Scholar]
- Kane J, Sanchez R, Perry P, Jin N, Johnson B, Forbes R, McQuade R, Carson W, Fleischhacker W. (2012) Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 73:617–624. [DOI] [PubMed] [Google Scholar]
- Kane JM, Zhao C, Johnson BR, Baker RA, Eramo A, McQuade RD, Duca AR, Sanchez R, Peters-Strickland T. (2015) Hospitalization rates in patients switched from oral anti-psychotics to aripiprazole once-monthly: final efficacy analysis. J Med Econ 18:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. (1987) The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Kirschbaum KM, Muller MJ, Malevani J, Mobascher A, Burchardt C, Piel M, Hiemke C. (2008) Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J Biol Psychiatry 9:212–218. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. (2013) Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry 74:957–965. [DOI] [PubMed] [Google Scholar]
- Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB, Schizophrenia Patient Outcomes Research Team (2010) The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull 36:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MR, Soumerai SB, Ross-Degnan D, Adams AS. (2008) A longitudinal study of medication nonadherence and hospitalization risk in schizophrenia. J Clin Psychiatry 69:47–53. [DOI] [PubMed] [Google Scholar]
- Mallikaarjun S, Kane JM, Bricmont P, McQuade R, Carson W, Sanchez R, Forbes RA, Fleischhacker WW. (2013) Pharmacokinetics, tolerability and safety of aripiprazole once-monthly in adult schizophrenia: an open-label, parallel-arm, multiple-dose study. Schizophr Res 150:281–288. [DOI] [PubMed] [Google Scholar]
- Mallikaarjun S, Salazar DE, Bramer SL. (2004) Pharmacokinetics, tolerability, and safety of aripiprazole following multiple oral dosing in normal healthy volunteers. J Clin Pharmacol 44:179–187. [DOI] [PubMed] [Google Scholar]
- Otsuka Pharmaceutical Europe Ltd. (2016a) Abilify MaintenaR EU (aripiprazole). Full prescribing information. Wexham, UK: Otsuka Pharmaceutical Europe Ltd. [Google Scholar]
- Otsuka Pharmaceutical Co., Ltd. (2016b) Abilify MaintenaR US (aripiprazole). Full prescribing information. Tokyo: Otsuka Pharmaceutical Co., Ltd. [Google Scholar]
- Posner K, Brent D, Lucas C, Gould M, Stanley B, Brown G, Fisher P, Zelazny J, Burke A, Oquendo M, Mann J. Columbia Suicide Severity Rating Scale. Available at: http://cssrs.columbia.edu/docs/C-SSRS_1_14_09_Baseline.pdf Accessed April 19, 2016. [Google Scholar]
- Raoufinia A, Baker RA, Eramo A, Nylander AG, Landsbeg W, Kostic D, Larsen F. (2015) Initiation of aripiprazole once-monthly in patients with schizophrenia. Curr Med Res Opin 31:583–592. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW. (1970) A rating scale for extrapyramidal side effects. Acta Psychiatr Scand 212:11–19. [DOI] [PubMed] [Google Scholar]
- Sparshatt A, Taylor D, Patel MX, Kapur S. (2010) A systematic review of aripiprazole--dose, plasma concentration, receptor occupancy, and response: implications for therapeutic drug monitoring. J Clin Psychiatry 71:1447–1456. [DOI] [PubMed] [Google Scholar]
- Taylor D, Olofinjana O. (2014) Long-acting paliperidone palmitate: interim results of an observational study of its effect on hospitalization. Int Clin Psychopharmacol 29:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Collier AC, Barr AM, Honer WG, Procyshyn RM. (2015) Paliperidone palmitate long-acting injectable given intramuscularly in the deltoid versus the gluteal muscle: are they therapeutically equivalent? J Clin Psychopharmacol 35:447–449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.