Abstract
Background:
Homocysteine, a risk factor for Alzheimer’s disease, induces cognitive dysfunction. Reactive aldehydes play an important role in cognitive dysfunction. Aldehyde-dehydrogenase 2 detoxifies reactive aldehydes. Hydrogen sulfide, a novel neuromodulator, has neuroprotective effects and regulates learning and memory. Our previous work confirmed that the disturbance of hydrogen sulfide synthesis is invovled in homocysteine-induced defects in learning and memory. Therefore, the present work was to explore whether hydrogen sulfide ameliorates homocysteine-generated cognitive dysfunction and to investigate whether its underlying mechanism is related to attenuating accumulation of reactive aldehydes by upregulation of aldehyde-dehydrogenase 2.
Methods:
The cognitive function of rats was assessed by the Morris water maze test and the novel object recognition test. The levels of malondialdehyde, 4-hydroxynonenal, and glutathione as well as the activity of aldehyde-dehydrogenase 2 were determined by enzyme linked immunosorbent assay; the expression of aldehyde-dehydrogenase 2 was detected by western blot.
Results:
The behavior experiments, Morris water maze test and novel objects recognition test, showed that homocysteine induced deficiency in learning and memory in rats, and this deficiency was reversed by treatment of NaHS (a donor of hydrogen sulfide). We demonstrated that NaHS inhibited homocysteine-induced increases in generations of MDA and 4-HNE in the hippocampus of rats and that hydrogen sulfide reversed homocysteine-induced decreases in the level of glutathione as well as the activity and expression of aldehyde-dehydrogenase 2 in the hippocampus of rats.
Conclusion:
Hydrogen sulfide ameliorates homocysteine-induced impairment in cognitive function by decreasing accumulation of reactive aldehydes as a result of upregulations of glutathione and aldehyde-dehydrogenase 2.
Keywords: ALDH2, cognitive dysfunction, GSH, homocysteine, hydrogen sulfide, reactive aldehydes
Significance Statement
Homocysteine (Hcy), an independent risk factor for Alzheimer’s disease (AD), induces the deficit in learning and memory. Thus, finding a new strategy to antagonize Hcy-induced cognitive dysfunction has important values in the prevention and treatment of AD. This study is to explore whether hydrogen sulfide (H2S), the third gasotransmitter, improves the Hcy-generated cognitive dysfunction and the underlying mechanisms. In the present study, we found that H2S ameliorated Hcy-induced cognitive dysfunction. We also demonstrated that H2S inhibited reactive aldehydes accumulation, preserved glutathione homeostasis, and upregulated aldehyde-dehydrogenase 2 activity and expression in the hippocampus of Hcy-exposed rats. Our present findings identify that H2S is a potential target for therapeutic intervention in Hcy-induced cognitive impairment and provide a new strategy in the prevention and treatment of Hcy-induced neurodegenerative diseases.
Introduction
Homocysteine (Hcy), a sulfur-containing amino acid derived from the metabolism of methionine (Prudova et al., 2006; Parkhitko et al., 2016), is an independent risk factor for Alzheimer’s disease (AD) (Seshadri et al., 2002; Dwyer et al., 2004; Van Dam and Van Gool, 2009; Miwa et al., 2015; Hu et al., 2016a). Hcy markedly enhances the vulnerability of neuronal cells to excitotoxicity and elicits neuronal cell death in a variety of neuronal types (Hankey and Eikelboom, 1999; Kim et al., 2007). Furthermore, it was reported that treatment of rats with Hcy induces deficits in learning and memory function (Zhang et al., 2009; Li et al., 2014b; Agrawal et al., 2015; Miyazaki et al., 2015), suggesting that Hcy has harmful effects on cognitive function. Thus, finding a new strategy to antagonize Hcy-induced cognitive dysfunction has important value in the prevention and treatment of AD.
Hydrogen sulfide (H2S), the third endogenous gasotransmitter, is an important neuromodulator and neuroprotectant (Kimura, 2002; Hu et al., 2011; Zhou and Tang, 2011; Kimura et al., 2012). It has been reported that H2S facilitates the formation of hippocampal LTP (Abe and Kimura, 1996) and attenuates lipopolysaccharide- or beta-amyloid-induced spatial learning and memory impairment (Gong et al., 2011a, 2011b). A recent study suggested that H2S ameliorates Hcy-induced AD-like pathology, blood-brain barrier disruption (Kamat et al., 2016). The most recent in vitro and in vivo studies by our group have demonstrated that inhibition of endogenous H2S generation is associated with Hcy-induced neurotoxicity (Tang et al., 2011), while H2S antagonizes Hcy-induced neurotoxicity in PC12 cells (Tang et al., 2010), and that decrease in endogenous H2S generation contributes to Hcy-induced deficit in learning and memory of rats (Li et al., 2014b). Therefore, we speculated that H2S can ameliorate Hcy-induced cognitive dysfunction.
“Aldehyde load” has become a new therapeutic target in neurodegenerative disease (Wood, 2006; Wood et al., 2006, 2007). Reactive aldehydes are the product of lipid peroxidation, including malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) (Aldini et al., 2007; Mali et al., 2016). MDA and 4-HNE are the most important products of peroxidation of cellular membrane lipids. The accumulation of reactive aldehydes such as MDA and 4-HNE has been observed in the brain tissues in neurodegenerative disorders such as AD and Parkinson’s disease (Lovell et al., 1997; Montine et al., 1997; Sayre et al., 1997; Ishrat et al., 2009). It has been demonstrated that the accumulation of reactive aldehydes not only lead to neuronal death (Mark et al., 1997) but also result in a decline in cognitive function (Zarkovic, 2003). Therefore, this work investigated whether H2S-exerted melioration in Hcy-induced cognitive impairment involves the reduction of reactive aldehydes in hippocampus of Hcy-exposed rats. Aldehyde dehydrogenase 2 (ALDH2) is the principal enzyme involved in detoxifying aldehydes, including MDA, 4-HNE, and other aldehydes, by converting them to less toxic acid products (Ohta et al., 2004; Marchitti et al., 2007; Chen et al., 2014; Doorn et al., 2014). It has been reported that the deficiency of ALDH2 in neuronal cells exhibited increased vulnerability to 4-HNE (D’Souza et al., 2015), while overexpression of ALDH2 protects neuronal survival against 4-HNE (Bai and Mei, 2011; Ma et al., 2011; Lee et al., 2012). Thus, we suggest that clearance of reactive aldehydes via upregulation of ALDH2 may be a new opportunity for intervention in neurodegenerative diseases. Therefore, we evaluated whether H2S upregulates the activity and expression of ALDH2 in the hippocampus of Hcy-exposed rats.
In the present work, we demonstrated that H2S ameliorated the cognitive dysfunction, inhibited hippocampal reactive aldehydes generation, and upregulated hippocampal glutathione (GSH) as well as ALDH2 activity and expression in Hcy-exposed rats. Our findings therefore suggest that H2S has the therapeutic potential to intervent Hcy-induced cognitive impairment.
Materials and Methods
Reagents
Sodium hydrosulfide (NaHS, a donor of H2S) and Hcy were purchased from Sigma Chemical. Anti-ALDH2 antibody was purchased from Abcam Technology. ALDH2 activity assay kit was obtained from Abcam. MDA enzyme linked immunosorbent assay (ELISA) kit was purchased from Uscn Life Science. 4-HNE and GSH ELISA kits were obtained from Bio-Swamp Life Science.
Animals
Adult male Sprague-Dawley rats (260–300 g) were used for all experiments. Animals were purchased from the SJA Lab Animal Center of Changsha. They were housed individually in a temperature- and humidity-controlled room under standard 12-h-light/-dark cycle and given access to standard laboratory food and water. Before the experiments, all rats were allowed to adapt to their new environment for 7 days. The experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Use and Protection Committee of University of South China. All efforts were made to minimize animal suffering.
Drugs and Treatments
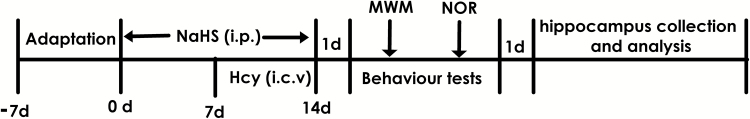
A total of 1.68 mg or 5.6 mg of NaHS was dissolved in 1 mL of phosphate-buffered saline (PBS) to equal concentrations of NaHS 30 or 100 μmol/mL, respectively. Hcy was diluted in sterilized artificial cerebrospinal fluid/50% DMSO and intracerebroventricularly injected (i.c.v.) at a dose of 0.6 μmol. After adaptation for 7 days, the rats were pretreated with 30 or 100 μmol/mL NaHS (1 mL/kg/d, i.p.) for 7 days and then cotreated with Hcy (0.6 μmol/d, i.c.v.) for 7 days. Behavioral tests were performed 24 hours after the last injection. One day after the behavioral tests, animals were killed, and the hippocampus region tissues of the brain were rapidly removed on the ice-cold artificial cerebrospinal fluid and stored at -80°C for analysis (Figure 1).
Figure 1.
Schematic diagram of the experimental schedule. MWM, Morris water maze test; NOR, novel objects recognition test.
Intracerebroventricular Injection
Animals were anesthetized with sodium pentobarbital (45 mg/kg, i.p.) and placed in a stereotaxic frame for operation. The area around the incision was trimmed. PBS or Hcy (0.6 μmol) in 4 μL was injected into the unilateral ventricle using the following coordinates: AP, -1.4; ML, 1.8; DV, -3.0 (in millimeters with respect to bregma and dura; tooth bar at 3.3 mm), with an injection rate of 0.4 μL/min using a 10-μL Hamilton syringe. To ensure that the entire injection had been delivered, the injection needle should be slowly pulled out halfway and kept in position for an additional 2 minutes before being removed. Rats were kept in a warm environment until they recovered from the anesthesia.
Morris Water Maze (MWM) Test
Before the MWM test, the rats of all groups were kept in a controlled room temperature (20°C -24°C) and humidity (60–80%) for 3 days to adapt to the environment and then submitted to the water maze test. The water maze was mainly composed of a large circular pool(180 cm diameter, 60 cm high) with water at 23 ± 2°C and a hidden platform (12.5 cm diameter, 38 cm high) in the pool where they can escape. The pool was divided into 4 quadrants, and the escape platform was placed at a fixed position in the center of one quadrant as the target quadrant, which was hidden 2 cm below the water surface. The walls of the water maze room were fixed with several landmarks as the distal spatial extra-maze cues for the rats to find the platform. The procedure of the MWM test was recorded by an MT-200 Morris Image Motion System (Chengdu Technology and Market Corp). It was carried out 24 hours after the cessation of Hcy treatment and this test was run 8 consecutive days.
Acquisition Trial
The acquisition trial involves training rats to find the hidden platform at a fixed location in space over a series of trials. It uses 7 training days (days 1–7) and 4 trials per day with a 20-minute intertrial interval. The starting position, which was randomly chosen across the 4 trials each day, was equally spaced along the circumference of the pool. The order of the quadrants that rats were placed into was changed each day such that they were never exposed to a sequence of trials that they had had before. Each rat was allowed to swim for a maximum duration of 120 s in each trial to find the platform. After finding and climbing onto the platform, the rat was allowed to remain there for 20 seconds. If an animal did not find the platform within 120 seconds, it was guided to the platform and remained there for 20 seconds before being returned to its home cage. Rats were kept dry and warm between trials.
Probe Trial
After finishing the place navigation task, the rats were subjected to the probe trial (day 8) to assess spatial memory. The platform was removed from the pool and animals were placed into the pool from another quadrant, which was opposite to the target quadrant. The rats were allowed to swim freely for 120 seconds in the water maze. The time that an animal spent in the target quadrant and the number of time that the same animal crossed the former platform area were recorded for measuring spatial memory maintenance.
Visible Platform Test
After the probe test, each rat proceeded to the visible platform test to rule out the possible deficits (as visual, motor, and motivation skills) in sensorimotor processes. The water surface was 2 cm beneath the height of the platform. The escape platform was moved to a novel quadrant in the pool at a fixed location for the next 4 trials. The latency to reach the platform and the average speed were recorded. After finishing the last trial, rats were gently dried and kept warm before returned to their home cages.
Novel Objects Recognition Test
Novel object recognition test was performed to assess hippocampus-dependent cognitive function (D’Agostino et al., 2012; Tang et al., 2013). Before testing, each rat was individually habituated to the test box (50.0×50.0×60) for 5 minutes once per day without objects. During the training session, 2 different types of objects used in the current study were 10-cm diameter × 7-cm high red crystal balls and 10-cm white plastic objects. On the day of testing, each rat was allowed to freely explore the 2 identical objects secured to the floor in opposite corners of the test box for 5 minutes (familiarization phase), and the time rats spent exploring each object was recorded with stopwatches. After a 60-minute retention interval, each rat was returned to the test box with one identical and one novel object. Each rat was again allowed to explore the objects for 5 minutes (testing phase), and the time rats spent exploring each object was recorded. Training trials were video-taped and the location of the novel object was varied in random order within groups. Exploratory behavior was defined as sniffing, touching, and direct attention to the object. The nose within 1 cm of the object was considered as exploratory behavior. The rats climbing on or chewing the object was not considered as exploratory behavior. The discrimination index (= (novel object−familiar object) / (novel object + familiar object)) was used to measure the cognitive function of rats.
Tissue Sampling
The brain tissues (hippocampus) of the rats were harvested 24 hours after behavioral tests. Animals were given a lethal dose of sodium pentobarbital. The brain was immediately removed. Hippocampus and cortical tissue of the brain were dissected and rapidly frozen in liquid nitrogen. All samples were stored at -80°C until further processing.
The Bicinchoninic Acid (BCA) Assay for Protein Quantitation
Total protein quantification was assessed by BCA assay according to the manufacturer’s instructions. Bovine serum albumin (BSA) standard solution was diluted to a concentration range of 0–] to 2 μg/μL with PBS. Already diluted BSA standard solution and protein samples, which was diluted 10 times with PBS, were added to 96-well plates (25 μL/well), respectively. Two parallel wells were made for each sample. Subsequently, 200 μL BCA working solution (BCA reagent A and BCA reagent B by 50:1) was added to each well and incubated at 37°C for 30 minutes. After cooling to room temperature, the absorbance at 562 nm was determined using a microplate reader. The BSA standard curve is used to calculate the relative protein concentration of each sample.
Biochemical Analysis of MDA, 4-HNE, and GSH
The contents of MDA, 4-HNE, and GSH in hippocampus tissue homogenate were measured using MDA, 4-HNE, and GSH ELISA kit, respectively. Briefly, hippocampus tissues were lysed and centrifuged for 10 minutes at 5000 g. The supernatants were collected and total protein concentrations were quantified using the BCA protein assay kit. Protein sample (10 μg/mL, 50 μL) was added to the 96-well protein binding plate and incubated at 37°C for 2 hours. After washing 2 times with wash buffer, 100 μL of assay diluent per well was added and incubated for 2 hours on an orbital shaker. Plates were then washed completely with thorough aspiration between each wash, 50 μL of the diluted anti-MDA, anti-4-HNE, or anti-GSHantibody was added to all wells, and incubated for 1 hour. After washing 3 times with wash buffer, 50 μL of the diluted secondary antibody-HRP conjugate was added to each well and incubated for 1 hour on an orbital shaker. Then 50 μL of substrate solution was add to each well and incubated for 15 minutes at room temperature on an orbital shaker. Finally, 50 μL of stop solution was added to terminate the reaction, and the absorbance of each well was recorded using a microplate reader at 450 nm.
ELISA for ALDH2 Activity
The ALDH2 activity in hippocampus tissue homogenate was measured using ALDH2 Activity Assay Kit. Hippocampus tissues were lysed and centrifuged at 5000 g for 10 minutes at 4°C. After being quantified using BCA protein assay kit, the equal proteins were performed in a 96-well plate that was coated with an antibody specific for ALDH2. In a nutshell, 100 μL of each diluted sample was added to the 96-well protein binding plate and incubated for 3 hours at room temperature with gentle shaking and then washed 2 times with wash buffer. Next, 200 μL of activity solution (1×Coupler, 25 mM acetaldehyde, 1 mM NAD+, 1×Reagent Dye) was added to all wells. The absorbance at 450 nm of each well was read on a microplate reader. The concentration of ALDH2 in the samples was then determined by comparing the O.D. of the samples to the standard curve.
Western-Blot Analysis for ALDH2 Expression
The hippocampus tissues were homogenized in lysis buffer (150 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 7.5, 1% Triton X-100, 1 mmol/L phenyl methyl sulfonyl fluoride, 1 mmol/L Na3VO4, leupeptin, and EDTA) and incubated on ice for 30 minutes. After the samples were centrifuged at 5000 g for 10 minutes at 4°C, the supernatant was collected and the protein concentration quantified using a BCA Protein Assay kit (Beyotime). After the supernatant of sample was heated at 100°C for 5 minutes, an equivalent amount of protein extract for each sample was run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Subsequently, the protein was transferred to a PVDF membrane and blocked with TBST (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.1% Tween-20) containing 5% BSA (Sigma) for 2 hours at room temperature, and the membranes were incubated with the primary ALDH2 antibodies (Cambridge) diluted 1:1000 and antibody anti-β-actin (Epitomics), which was diluted 1:2000 overnight at 4°C. After washing with TBST buffer 3 times, the membranes were incubated in anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:5000) for 2 hours. Then the membranes were washed with TBST buffer again and developed with an enhanced chemiluminescence system (ECL) followed by apposition of the membranes with autoradiographic films (Kodak). The integrated optical density for the protein band was calculated by Image-J software.
Statistical Analysis
Data are displayed as the mean ± SEM. The significance of inter-group differences was evaluated by 1-way ANOVA. Differences were considered significant at P < .05.
Results
H2S Improves Spatial Learning and Memory of Hcy-Treated Rats in MWM
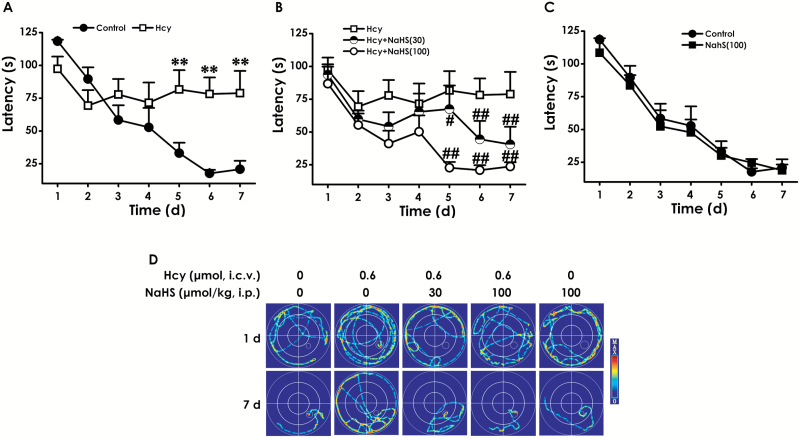
To investigate the protective role of H2S in cognitive dysfunction of Hcy-treated rats, after pretreatment with NaHS (the donor of H2S, 30 or 100 μmol/kg/d, i.p.) for 7 days and cotreatment with Hcy (0.6 μmol/d, i.c.v.) for 7 days, rats were subjected to the MWM task to test spatial learning and memory. The latency traveled to find the platform in the acquisition phase is shown in Figure 2A-C. All 5 groups over the 7 training days exhibited a decrease in the escape latency (Figure 2A-C). Hcy-treated alone rats exhibited significant higher escape latency on days 5, 6, and 7 during training trials compared with control group rats (Figure 2A), implying a significant impairment of spatial learning in Hcy-exposed rats, and this impairment occurred from training day 5 onward. However, treatment with NaHS (30 or 100 μmol/kg/d, i.p.) significantly decreased the escape latency of Hcy-treated alone rats from training day 5 onward (Figure 2B). Figure 2D shows the representative swimming tracks of rats searching for the underwater platform on the 1st and 7th training days. On the 1st training day, there was no difference of the distance in searching for the hidden platform among the 5 groups. On the 7th training day, Hcy-treated alone rats exhibited a significant increase in the distance swam compared with the control group; however, the rats cotreated with NaHS (30 or 100 μmol/kg/d, i.p.) and Hcy showed a significant decrease in the distance swam compared with the Hcy-treated alone group.
Figure 2.
Effects of hydrogen sulfide (H2S) on homocysteine (Hcy)-induced impairment in learning function of rats in navigation testing of the Morris water maze (MWM). After pretreatment with NaHS (30 or 100 μmol/kg/d, i.p.) for 7 days and then cotreatent with Hcy (0.6 μmol/d, i.c.v.) for 7 days, rats were tested in the MWM task. The latency traveled to find the platform during 7 days in the acquisition phase was recorded in the navigation training (A-C). The swimming tracks of rats searching for the underwater platform at the 1st and 7th training days (D). Values represented as mean ± SEM (n=8). **P < .01, vs control; ##P < .01, vs Hcy-treated alone group.
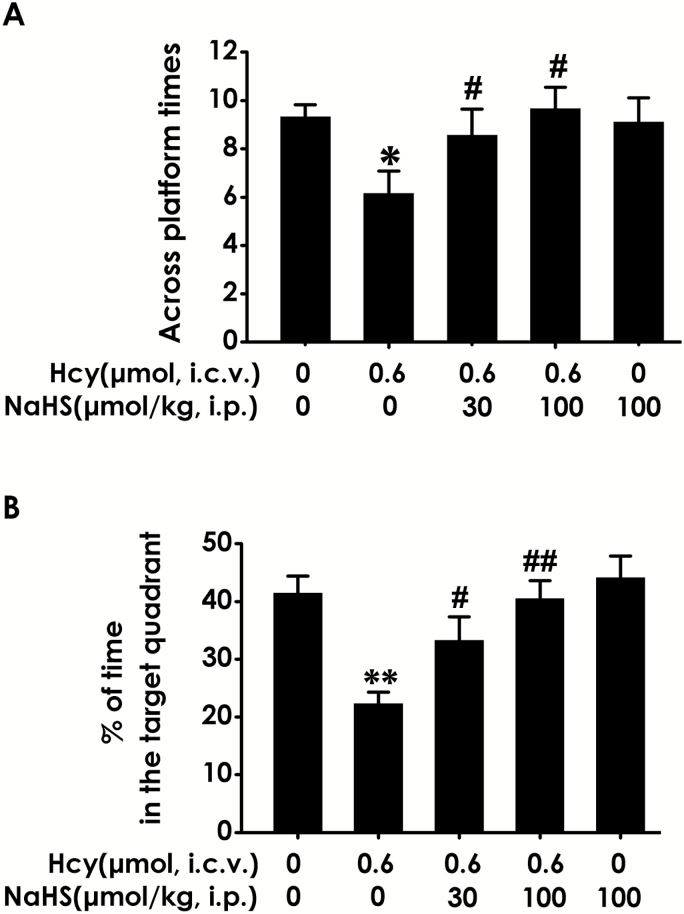
In the probe trial, the platform was removed, and the rats were placed into the quadrant opposite to the target quadrant and allowed to swim freely for 120 seconds. Hcy-treated alone rats showed impaired memory, as evident by their significant decreases in the number of times crossing the target quadrant (Figure 3A) and the time spend in the target quadrant (Figure 3B). However, NaHS (30 or 100 μmol/kg/d, i.p.) significantly increased the time that Hcy-treated alone rats spend in the target quadrant (Figure 3A) and the number of times that Hcy-treated alone rats crossed the target quadrant (Figure 3B). Taken together, these data suggest that H2S reverses the impairment in spatial learning and memory induced by Hcy.
Figure 3.
Effects of hydrogen sulfide (H2S) on homocysteine (Hcy)-induced impairment in memory function of rats in probe trial testing of Morris water maze (MWM). After finishing the navigation task, the platform was removed and the rats were submitted to the probe trail testing. The number of times that the animal crossed the platform area (A) and the percentage of time spent in the target quadrant (B) was analyzed. Values represented as mean ± SEM (n=8), *P < .05, **P < .01, vs control; #P < .05, ##P < .01, vs Hcy-treated alone group.
Ruling out the Effects of the Changes of Vision and Motor Ability on Learning and Memory in Rats
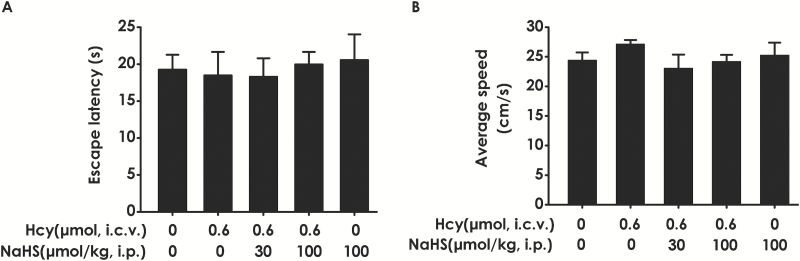
To exclude the possibility that the results are due to changes of vision and motor ability in the rats, we examined the escape latency and average swimming speed of rats by performing a visible platform test after finishing the transfer probe trials. There was no statistical difference in the escape latencies (Figure 4A) among all groups in the visible platform test, and there was no significant difference in swimming speed (Figure 4B), which indicated that the alterations of all parameters in the hidden platform tests and probe trials are not due to changes in visual or motor abilities of rats.
Figure 4.
Effects of hydrogen sulfide (H2S) and homocysteine (Hcy) on the motor function and vision of rats. After finishing the probe test, the rats were submitted to the visible platform test, the latency to reach the platform (A), and the average swimming speeds in probe trials (B) were recorded. Values represented as mean ± SEM, n=8.
H2S Enhances the Cognitive Function of Hcy-Exposed Rats in Novel Object Recognition Test
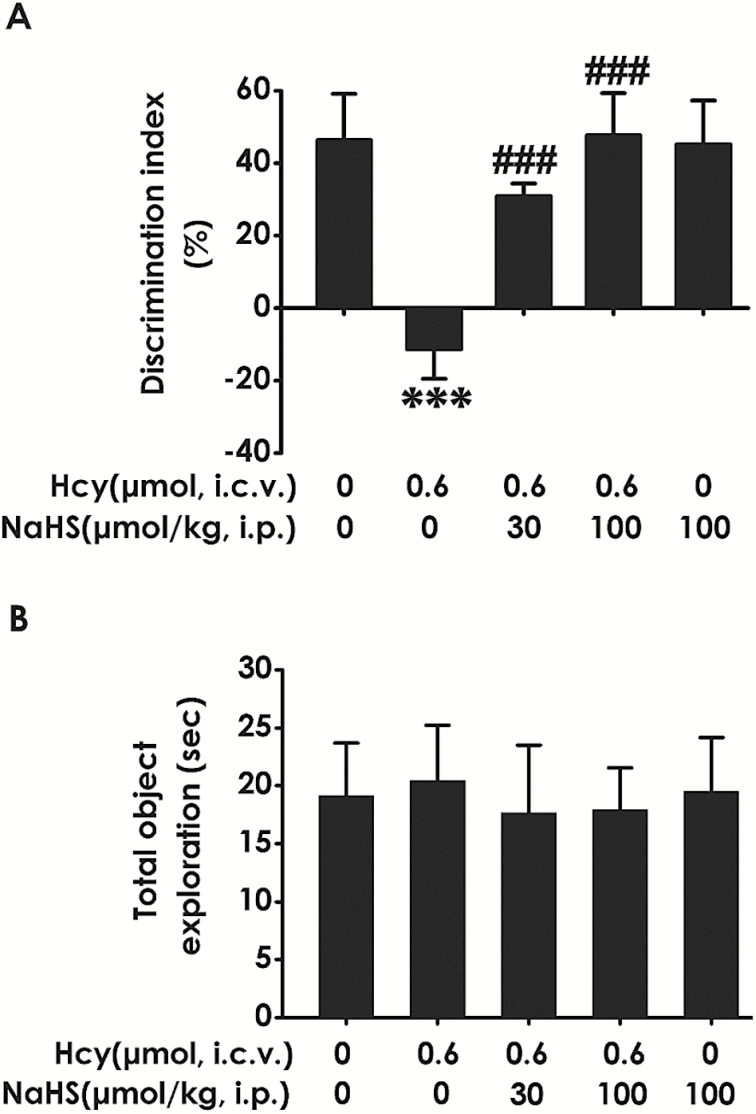
To further investigate whether H2S ameliorates the cognitive dysfunction of Hcy-exposed rats, we also examined the cognitive function of rats using the novel object recognition test. As shown in Figure 5A, the discrimination index in Hcy-exposed alone rats was significantly decreased compared with control; however, treatment with NaHS (30 or 100 μmol/kg/d, i.p.) significantly increased the discrimination index of Hcy-exposed alone rats. In addition, there was no significant difference of the total exploration time among the 5 groups (Fig. 5B). These data suggested that H2S prevents the decline in cognitive function triggered by the exposure of Hcy.
Figure 5.
Effects of hydrogen sulfide (H2S) on homocysteine (Hcy)-induced impairment in the function of cognition of rats determined by novel object discrimination test. After the Morris water maze (MWM) test, the rats were submitted to the novel object recognition test. The discrimination index (A) and the total exploring time of rats in each group (B) were recorded. Values represented as mean ± SEM (n=8), ***P < .001, vs control; ###P < .001, vs Hcy-treated alone group.
H2S Inhibits Hcy-Upregulated Levels of the Reactive Aldehydes in the Hippocampus of Rats
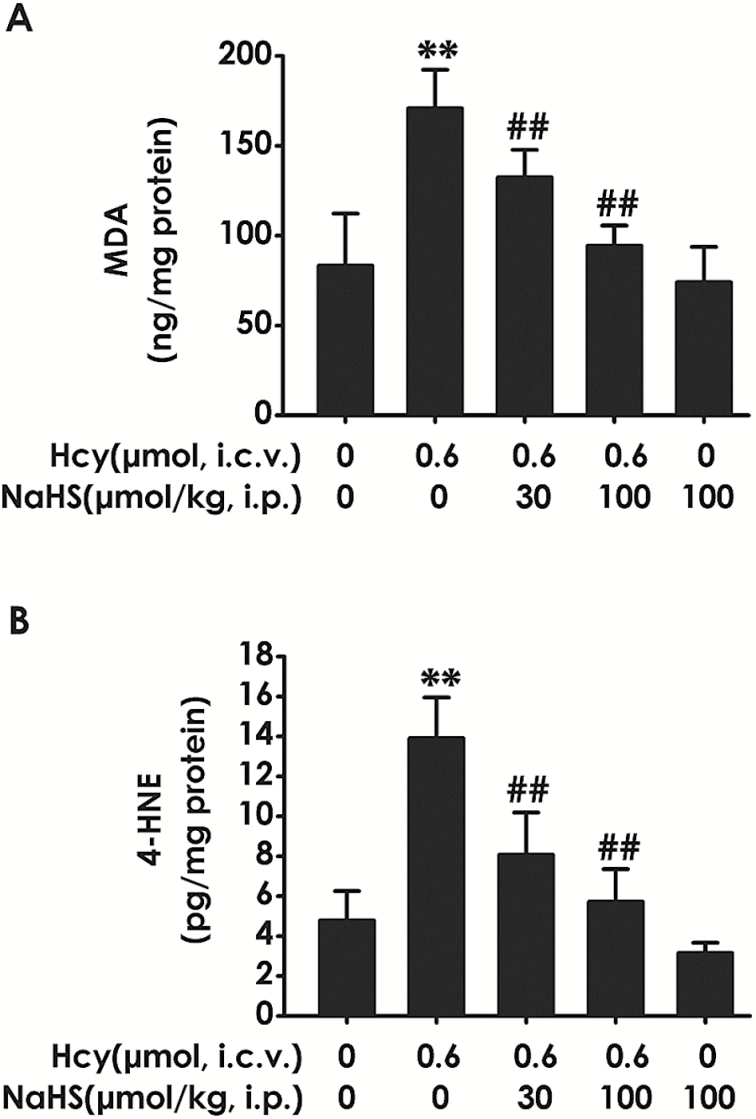
To explore the effect of H2S on Hcy-induced reactive aldehydes in the hippocampus of rats, we measured the levels of MDA and 4-HNE in the hippocampus of rats. As shown in Figure 6, the elevated levels of hippocampal MDA (Figure 6A) and 4-HNE (Figure 6B) by 7-d treatment with Hcy (0.6 μmol, i.c.v.) were markedly reduced by treatment with NaHS (30or 100 µmol/kg/d, i.p.). NaHS (100 µmol/kg) alone did not affect the levels of MDA (Figure 6A) and 4-HNE (Figure 6B) in control rats. These data indicated that H2S inhibits Hcy-triggered accumulation of hippocampal reactive aldehydes.
Figure 6.
Effects of hydrogen sulfide (H2S) on homocysteine (Hcy) upregulated the levels of reactive aldehydes in the hippocampus of rats. After finishing the behavioral tests, the hippocampus of rats was homogenized and the levels of reactive aldehydes were detected by measuring the contents of malondialdehyde (MDA) (A) and 4-hydroxynonenal (4-HNE) (B) using an ELISA kit. Values represented as mean±SEM (n = 3). **P < .01, vs control; ##P < .01, vs Hcy-treated alone group.
H2S Increased the Level of GSH in the Hippocampus of Rats Exposed to Hcy
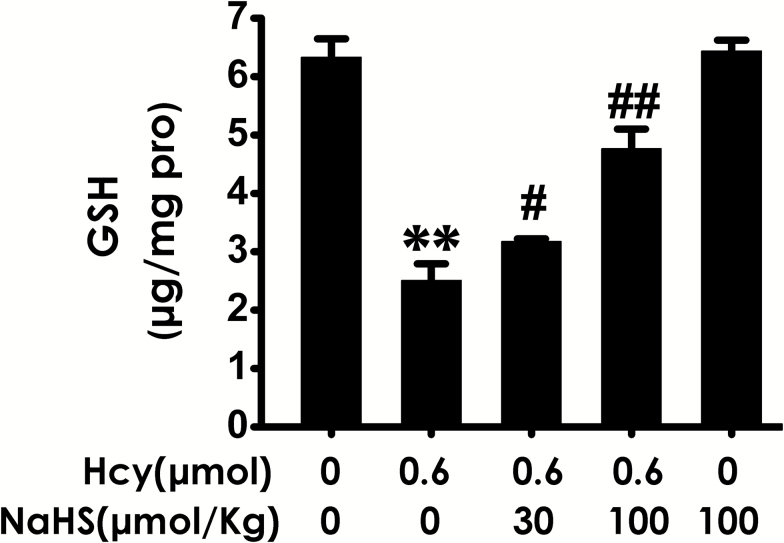
We also investigated the change in the level of GSH in the hippocampus. Hcy (0.6 μmol, i.c.v.) caused a decrease in the level of GSH in the hippocampus of rats, while treatment of NaHS (30or 100 µmol/kg/d, i.p.) markedly increased the level of GSH in the hippocampus of Hcy-exposed rats (Figure 7), which indicated that H2S inhibits the decrease in the level of hippocampal GSH induced by Hcy.
Figure 7.
Effects of hydrogen sulfide (H2S) on homocysteine (Hcy) decreased the level of glutathione (GSH) in the hippocampus of rats. After finishing the behavioral tests, the hippocampus of rats was homogenized and the level of GSH in hippocampus was determined using an ELISA kit. Values represented as mean ± SEM (n = 3). **P < .01, vs control; #P < .05, ##P < .01, vs Hcy-treated alone group.
H2S Up-Regulates the Activity and Expression of ALDH2 in the Hippocampus of Rats Exposed to Hcy
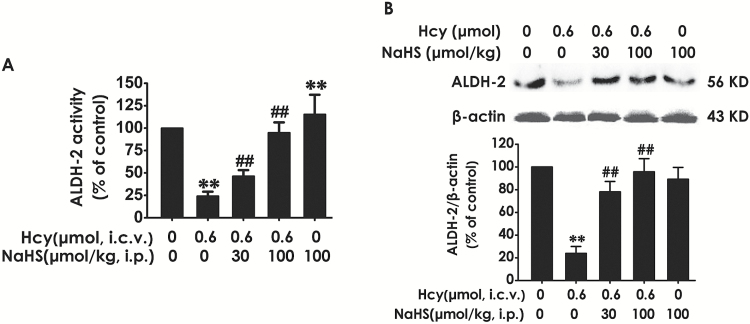
It has been certified that ALDH2 has the highest catalytic efficiency for oxidation of reactive aldehydes (Yoval-Sanchez and Rodriguez-Zavala, 2012). To explore whether the inhibitory role of H2S in Hcy-induced accumulation in hippocampal reactive aldehydes is associated with upregulation of hippocampal ALDH2, we detected the activity and expression of ALDH2 in the hippocampus of Hcy-exposed rats. Hcy (0.6 μmol, i.c.v.) caused decreases in the activity (Figure 8A) and expression (Figure 8B) of ALDH2 in the hippocampus of rats. However, treatment of NaHS (30or 100 µmol/kg/d, i.p.) markedly increased the activity (Figure 8A) and expression (Figure 8B) of ALDH2 in the hippocampus of Hcy-exposed rats, which indicated the upregulatory role of H2S in the activity and expression of hippocampal ADLH2.
Figure 8.
Effects of hydrogen sulfide (H2S) on homocysteine (Hcy) downregulated the activity and expression of aldehyde-dehydrogenase 2 (ALDH2) in the hippocampus of rats. After finishing the behavioral tests, the hippocampus of rats was homogenized. The activity of ALDH was determined by ALDH2 Activity Assay Kit (A) and the expression of ALDH2 was detected by western blot using an anti-ALDH2 antibody (B). In all blots, β-actin was used as a loading control. Values represented as mean ± SEM (n = 3). **P < .01, vs control; ##P < .01, vs Hcy-treated alone group.
Discussion
H2S plays an important role in the regulation of learning and memory (Nagpure and Bian, 2015). The aim of this study was to investigate the protective effects of H2S against Hcy-induced cognitive dysfunction and its mechanism. Our study showed that (1) H2S ameliorated the cognitive dysfunction of rats exposed to Hcy; (2) H2S inhibited Hcy-induced increase in generation of reactive aldehydes in the hippocampus of rats; and (3) H2S reversed Hcy-induced downregulation in the level of GSH as well as the activity and expression of ALDH2 in the hippocampus of rats. These data suggest that H2S ameliorates Hcy-induced impairment in cognitive function by decreasing accumulation of reactive aldehydes, which is involved in upregulation of hippocampal ALDH2.
A moderate elevation of plasma Hcy is a potential risk factor for AD (Seshadri et al., 2002; Dwyer et al., 2004; Van Dam and Van Gool, 2009; Li et al., 2014a; Miwa et al., 2015; Hu et al., 2016a), and Hcy induces a deficit in learning and memory function (Zhang et al., 2009; Li et al., 2014b; Agrawal et al., 2015; Miyazaki et al., 2015). Hcy increases reactive oxygen species (ROS) and stimulates neurotoxicity (Ho et al., 2001; White et al., 2001; Ataie et al., 2012; Sharma et al., 2015). H2S, a gaseous signaling molecule, scavenges ROS and protects neurons against oxidative stress (Kimura and Kimura, 2004; Whiteman et al., 2004, 2005; Tang et al., 2008; Sakamoto et al., 2014). Furthermore, H2S plays an important role in regulation of learning and memory (Nagpure and Bian, 2015). Therefore, elucidating the beneficial role of H2S in Hcy-induced deficit in learning and memory function has an important scientific research value. To investigate the potential treatment effect of H2S on Hcy-induced deficit in learning and memory, rats were pretreated with NaHS for 7 days and cotreated with Hcy for 7 days, and the functions of learning and memory of rats were tested with the MWM test and the novel object recognition test. The MWM is one of the most frequently used laboratory tools in behavioral neuroscience as a device to investigate spatial learning and memory in laboratory rats (D’Hooge and De Deyn, 2001; Li et al., 2014b). In MWM, the spatial learning of rats is investigated by the hidden-platform acquisition test, and spatial memory is evaluated through the probe trial test. In MWM test, we showed that treatment with NaHS decreases the escape latency in hidden-platform acquisition training and increases the crossing platform times and the percentage of time elapsed in the target quadrant in the probe trail in the Hcy-exposed rats, which indicated that H2S reverses the impairment in spatial learning and memory induced by Hcy. The novel object recognition test is based on the differential exploration of familiar and new objects (He et al., 2013), and it is used to study short-term declarative memory and attention. In the novel object recognition test, we showed that the discrimination index in Hcy-treated alone rats was significantly increased by treatment with NaHS, which also indicates the harmful role of Hcy in learning and memory can be reversed by H2S, a toxic gas whose toxicity is associated with concentration. In the present work, NaHS (30 or 100 μmol/kg/d, i.p.) did not lead to a deficit in learning and memory function. Taken together, our present work demonstrates that H2S prevents Hcy-induced cognitive impairment in rats.
It is known that reactive aldehydes (4-HNE and MDA) generated from lipids peroxides. These aldehydes can form adducts with lipids, proteins, and DNA, leading to their inactivation and damage of living cells (Esterbauer et al., 1991; Hill and Bhatnagar, 2009; Berg et al., 2011; He et al., 2012). It has been shown that the accumulation of aldehydes induces neuronal death and causes synaptic dysfunction (Pedersen et al., 1999) and that reactive aldehydes are increased in brain tissue in neurodegenerative disorders such as AD, amyotrophic lateral sclerosis, and Parkinson’s Disease (Zarkovic, 2003; Chiu et al., 2015). It has been reported that Hcy induces learning and memory deficit (Zhang et al., 2009; Li et al., 2014b; Agrawal et al., 2015; Miyazaki et al., 2015). Other studies have demonstrated that Hcy perturbs the methionine cycle (Alternative Medicine ReviewMiller, 2003), increases ROS, and induces oxidative damage to brain tissue (Obeid and Herrmann, 2006), which could be the cause of cognitive decline. Our present work showed that Hcy caused a significant increase in the levels of MDA and 4-HNE in the hippocampus of rats. Therefore, we suggested that accumulation of MDA and 4-HNE in the hippocampus plays a vital role in Hcy-induced cognitive dysfunction. It has been demonstrated that H2S scavenges ROS and protects neurons against oxidative stress (Kimura and Kimura, 2004; Whiteman et al., 2004, 2005; Tang et al., 2008). We now demonstrated that NaHS markedly reduced the elevated levels of MDA and 4-HNE in Hcy-exposed rats. This finding indicated that the role of H2S in the clearance of reactive aldehydes is implicated in its protective action against Hcy-induced cognitive function impairment. GSH is an important antioxidant in the brain. The potential HNE conjugations with GSH damages GSH homeostasis. In the present work, we found the level of GSH was decreased in the hippocampus of Hcy-exposed rats, while H2S reversed the Hcy-induced deficiency in GSH homeostasis. Thus, we envision that restoring GSH homeostasis plays an important role in H2S-exerted clearance of hippocampal reactive aldehydes and amelioration in cognitive function of Hcy-exposed rats.
ALDH2 is a key enzyme that metabolizes acetaldehyde to acetic acid and also detoxifies ROS-generated aldehyde adducts (Lagranha et al., 2010). In brain, reactive aldehydes are mainly detoxified by ALDHs (Conklin et al., 2007; Ellis, 2007). ALDH2 has the highest catalytic efficiency for oxidation of 4-HNE and MDA (Yoval-Sanchez and Rodriguez-Zavala, 2012). ALDH2 functions as a protector against oxidative stress (Gao et al., 2016; Hu et al., 2016b). Many researchers reported that the levels of MDA and 4-HNE were found to increase in the brain of AD patients (Khan et al., 2012; Zhou et al., 2013) and that a sufficient amount of active ALDH2 is needed to detoxify the increased reactive aldehydes that occur in neurodegenerative diseases (Ohsawa et al., 2008). Numerous studies have demonstrated that deficiency of ALDH2 in neuronal cells exhibited increased vulnerability to 4-HNE (D’Souza et al., 2015). Meanwhile, overexpression of ALDH2 rescues 4-HNE-induced ischemic damage (Bai and Mei, 2011; Ma et al., 2011; Lee et al., 2012) and decrease the levels of MDA and 4-HNE (He et al., 2012). Our results that H2S can clear reactive aldehydes led us to explore whether H2S upregulates the activity and expression of ALDH2 in the hippocampus of Hcy-exposed rats. Our present work showed that Hcy caused decreases in the activity and expression of ALDH2 in the hippocampus of rats, while NaSH markedly increased the activity and expression of ALDH2 in the hippocampus of Hcy-exposed rats, which indicated that H2S has the upregulatory role in hippocampal ALDH2. Taken together, our data suggested that the inhibitory role of H2S in Hcy-induced cognitive dysfunction may be associated with the upregulation of hippocampal ALDH2, which leads to clearance of reactive aldehydes (4-HNE and MDA) in hippocampus of rats.
In summary, our study elucidates the protective action of H2S against Hcy-induced impairment in cognitive function, which results from the clearance of hippocampal reactive aldehydes involving maintenance of hippocampal GSH homeostasis and upregulation of hippocampal ALDH2. Our findings signify that H2S is effective in providing protection against Hcy-induced cognitive dysfunction. To enhance the potential impact and validity of the conclusion, we will in depth explore the underlying mechanisms of the protective action of H2S in Hcy-generated cognitive dysfunction and investigate a clinically relevant model in our future work.
Statement of Interest
None potential conflict of interest.
Acknowledgments
The authors thank Fan Xiao and Yin Chen for technical assistance. We thank the Institute of Pharmacology, School of Pharmaceutical Science, University of South China for providing access to the autoradiographic films to complete western blot.
This study was supported by National Natural Science Foundation of China (81471310, 81400881), Zhengxiang Scholar Program of University of South China (2014-004), and the construct program of the key discipline in Hunan province.
References
- Abe K, Kimura H. (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Ilango K, Singh PK, Karmakar D, Singh GP, Kumari R, Dubey GP. (2015) Age dependent levels of plasma homocysteine and cognitive performance. Behav Brain Res 283:139–144. [DOI] [PubMed] [Google Scholar]
- Aldini G, Dalle-Donne I, Facino RM, Milzani A, Carini M. (2007) Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med Res Rev 27:817–868. [DOI] [PubMed] [Google Scholar]
- Alternative Medicine ReviewMiller AL (2003) The methionine-homocysteine cycle and its effects on cognitive diseases. Altern Med Rev 8:7–19. [PubMed] [Google Scholar]
- Ataie A, Ataee R, Shadifar M, Shahabi S, Aghajanpour SM, Hosseinpour Y. (2012) Interaction of memantine with homocysteine on the apoptosis in the rat hippocampus cells. Int J Mol Cell Med 1:145–152. [PMC free article] [PubMed] [Google Scholar]
- Bai J, Mei Y. (2011) Overexpression of aldehyde dehydrogenase-2 attenuates neurotoxicity induced by 4-hydroxynonenal in cultured primary hippocampal neurons. Neurotox Res 19:412–422. [DOI] [PubMed] [Google Scholar]
- Berg RM, Moller K, Bailey DM. (2011) Neuro-oxidative-nitrosative stress in sepsis. J Cereb Blood Flow Metab 31:1532–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. (2014) Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev 94:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CC, Yeh TH, Lai SC, Wu-Chou YH, Chen CH, Mochly-Rosen D, Huang YC, Chen YJ, Chen CL, Chang YM, Wang HL, Lu CS. (2015) Neuroprotective effects of aldehyde dehydrogenase 2 activation in rotenone-induced cellular and animal models of parkinsonism. Exp Neurol 263:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D, Prough R, Bhatanagar A. (2007) Aldehyde metabolism in the cardiovascular system. Mol Biosyst 3:136–150. [DOI] [PubMed] [Google Scholar]
- D’Agostino G, Russo R, Avagliano C, Cristiano C, Meli R, Calignano A. (2012) Palmitoylethanolamide protects against the amyloid-beta25-35-induced learning and memory impairment in mice, an experimental model of Alzheimer disease. Neuropsychopharmacology 37:1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36:60–90. [DOI] [PubMed] [Google Scholar]
- D’Souza Y, Elharram A, Soon-Shiong R, Andrew RD, Bennett BM. (2015) Characterization of Aldh2 (-/-) mice as an age-related model of cognitive impairment and Alzheimer’s disease. Mol Brain 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorn JA, Florang VR, Schamp JH, Vanle BC. (2014) Aldehyde dehydrogenase inhibition generates a reactive dopamine metabolite autotoxic to dopamine neurons. Parkinsonism Relat Disord 20Suppl 1:S73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer BE, Raina AK, Perry G, Smith MA. (2004) Homocysteine and Alzheimer’s disease: a modifiable risk? Free Radic Biol Med 36:1471–1475. [DOI] [PubMed] [Google Scholar]
- Ellis EM. (2007) Reactive carbonyls and oxidative stress: potential for therapeutic intervention. Pharmacol Ther 115:13–24. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhu S, Xu Y, Li Y, Zhou S, Liu X. (2016) [Mechanisms for inhibitory effect of ALDH2 on doxorubicin-induced cytotoxicity in C2C12 myogenic cell line]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 41:264–271. [DOI] [PubMed] [Google Scholar]
- Gong QH, Pan LL, Liu XH, Wang Q, Huang H, Zhu YZ. (2011. a) S-propargyl-cysteine (ZYZ-802), a sulphur-containing amino acid, attenuates beta-amyloid-induced cognitive deficits and pro-inflammatory response: involvement of ERK1/2 and NF-kappaB pathway in rats. Amino Acids 40:601–610. [DOI] [PubMed] [Google Scholar]
- Gong QH, Wang Q, Pan LL, Liu XH, Xin H, Zhu YZ. (2011. b) S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: involvement of TNF signaling and NF-kappaB pathway in rats. Brain Behav Immun 25:110–119. [DOI] [PubMed] [Google Scholar]
- Hankey GJ, Eikelboom JW. (1999) Homocysteine and vascular disease. Lancet 354:407–413. [DOI] [PubMed] [Google Scholar]
- He L, Liu B, Dai Z, Zhang HF, Zhang YS, Luo XJ, Ma QL, Peng J. (2012) Alpha lipoic acid protects heart against myocardial ischemia-reperfusion injury through a mechanism involving aldehyde dehydrogenase 2 activation. Eur J Pharmacol 678:32–38. [DOI] [PubMed] [Google Scholar]
- He P, Ouyang X, Zhou S, Yin W, Tang C, Laudon M, Tian S. (2013) A novel melatonin agonist Neu-P11 facilitates memory performance and improves cognitive impairment in a rat model of Alzheimer’ disease. Horm Behav 64:1–7. [DOI] [PubMed] [Google Scholar]
- Hill BG, Bhatnagar A. (2009) Beyond reactive oxygen species: aldehydes as arbitrators of alarm and adaptation. Circ Res 105:1044–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PI, Collins SC, Dhitavat S, Ortiz D, Ashline D, Rogers E, Shea TB. (2001) Homocysteine potentiates beta-amyloid neurotoxicity: role of oxidative stress. J Neurochem 78:249–253. [DOI] [PubMed] [Google Scholar]
- Hu LF, Lu M, Hon Wong PT, Bian JS. (2011) Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal 15:405–419. [DOI] [PubMed] [Google Scholar]
- Hu Q, Teng W, Li J, Hao F, Wang N. (2016. a) Homocysteine and Alzheimer’s Disease: evidence for a causal link from mendelian randomization. J Alzheimers Dis 52:747–756. [DOI] [PubMed] [Google Scholar]
- Hu XY, Fang Q, Ma D, Jiang L, Yang Y, Sun J, Yang C, Wang JS. (2016. b) Aldehyde dehydrogenase 2 protects human umbilical vein endothelial cells against oxidative damage and increases endothelial nitric oxide production to reverse nitroglycerin tolerance. Genet Mol Res 15. [DOI] [PubMed] [Google Scholar]
- Ishrat T, Hoda MN, Khan MB, Yousuf S, Ahmad M, Khan MM, Ahmad A, Islam F. (2009) Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer’s type (SDAT). Eur Neuropsychopharmacol 19:636–647. [DOI] [PubMed] [Google Scholar]
- Kamat PK, Kyles P, Kalani A, Tyagi N. (2016) Hydrogen sulfide ameliorates homocysteine-induced Alzheimer’s disease-like pathology, blood-brain barrier disruption, and synaptic disorder. Mol Neurobiol 53:2451–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MB, Khan MM, Khan A, Ahmed ME, Ishrat T, Tabassum R, Vaibhav K, Ahmad A, Islam F. (2012) Naringenin ameliorates Alzheimer’s disease (AD)-type neurodegeneration with cognitive impairment (AD-TNDCI) caused by the intracerebroventricular-streptozotocin in rat model. Neurochem Int 61:1081–1093. [DOI] [PubMed] [Google Scholar]
- Kim JH, Cho SY, Lee JH, Jeong SM, Yoon IS, Lee BH, Lee JH, Pyo MK, Lee SM, Chung JM, Kim S, Rhim H, Oh JW, Nah SY. (2007) Neuroprotective effects of ginsenoside Rg3 against homocysteine-induced excitotoxicity in rat hippocampus. Brain Res 1136:190–199. [DOI] [PubMed] [Google Scholar]
- Kimura H. (2002) Hydrogen sulfide as a neuromodulator. Mol Neurobiol 26:13–19. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kimura H. (2004) Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18:1165–1167. [DOI] [PubMed] [Google Scholar]
- Kimura H, Shibuya N, Kimura Y. (2012) Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid Redox Signal 17:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. (2010) Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res 106:1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Wong HY, Chai YY, Shi CW, Amino N, Kikuchi S, Huang SH. (2012) Lipid peroxidation dysregulation in ischemic stroke: plasma 4-HNE as a potential biomarker? Biochem Biophys Res Commun 425:842–847. [DOI] [PubMed] [Google Scholar]
- Li JG, Chu J, Barrero C, Merali S, Pratico D. (2014. a) Homocysteine exacerbates beta-amyloid pathology, tau pathology, and cognitive deficit in a mouse model of Alzheimer disease with plaques and tangles. Ann Neurol 75:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MH, Tang JP, Zhang P, Li X, Wang CY, Wei HJ, Yang XF, Zou W, Tang XQ. (2014. b) Disturbance of endogenous hydrogen sulfide generation and endoplasmic reticulum stress in hippocampus are involved in homocysteine-induced defect in learning and memory of rats. Behav Brain Res 262:35–41. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Ehmann WD, Mattson MP, Markesbery WR. (1997) Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer’s disease. Neurobiol Aging 18:457–461. [DOI] [PubMed] [Google Scholar]
- Ma H, Guo R, Yu L, Zhang Y, Ren J. (2011) Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J 32:1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali VR, Deshpande M, Pan G, Thandavarayan RA, Palaniyandi SS. (2016) Impaired ALDH2 activity decreases the mitochondrial respiration in H9C2 cardiomyocytes. Cell Signal 28:1–6. [DOI] [PubMed] [Google Scholar]
- Marchitti SA, Deitrich RA, Vasiliou V. (2007) Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacol Rev 59:125–150. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. (1997) Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci 17:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Tanaka M, Okazaki S, Yagita Y, Sakaguchi M, Mochizuki H, Kitagawa K. (2015) Increased Total Homocysteine Levels Predict the Risk of Incident Dementia Independent of Cerebral Small-Vessel Diseases and Vascular Risk Factors. J Alzheimers Dis 49:503–513. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Sugimoto Y, Fujita A, Kanouchi H. (2015) Ethanol extract of Brazilian propolis ameliorates cognitive dysfunction and suppressed protein aggregations caused by hyperhomocysteinemia. Biosci Biotechnol Biochem 79:1884–1889. [DOI] [PubMed] [Google Scholar]
- Montine KS, Olson SJ, Amarnath V, Whetsell WO, Jr., Graham DG, Montine TJ. (1997) Immunohistochemical detection of 4-hydroxy-2-nonenal adducts in Alzheimer’s disease is associated with inheritance of APOE4. Am J Pathol 150:437–443. [PMC free article] [PubMed] [Google Scholar]
- Nagpure BV, Bian JS. (2015) Brain, learning, and memory: role of H2S in neurodegenerative diseases. Handb Exp Pharmacol 230:193–215. [DOI] [PubMed] [Google Scholar]
- Obeid R, Herrmann W. (2006) Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett 580:2994–3005. [DOI] [PubMed] [Google Scholar]
- Ohsawa I, Nishimaki K, Murakami Y, Suzuki Y, Ishikawa M, Ohta S. (2008) Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity. J Neurosci 28:6239–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S, Ohsawa I, Kamino K, Ando F, Shimokata H. (2004) Mitochondrial ALDH2 deficiency as an oxidative stress. Ann N Y Acad Sci 1011:36–44. [DOI] [PubMed] [Google Scholar]
- Parkhitko AA, Binari R, Zhang N, Asara JM, Demontis F, Perrimon N. (2016) Tissue-specific down-regulation of S-adenosyl-homocysteine via suppression of dAhcyL1/dAhcyL2 extends health span and life span in Drosophila. Genes Dev 30:1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen WA, Cashman NR, Mattson MP. (1999) The lipid peroxidation product 4-hydroxynonenal impairs glutamate and glucose transport and choline acetyltransferase activity in NSC-19 motor neuron cells. Exp Neurol 155:1–10. [DOI] [PubMed] [Google Scholar]
- Prudova A, Bauman Z, Braun A, Vitvitsky V, Lu SC, Banerjee R. (2006) S-adenosylmethionine stabilizes cystathionine beta-synthase and modulates redox capacity. Proc Natl Acad Sci U S A 103:6489–6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Suzuki Y, Kurauchi Y, Mori A, Nakahara T, Ishii K. (2014) Hydrogen sulfide attenuates NMDA-induced neuronal injury via its anti-oxidative activity in the rat retina. Exp Eye Res 120:90–96. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. (1997) 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem 68:2092–2097. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. (2002) Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 346:476–483. [DOI] [PubMed] [Google Scholar]
- Sharma GS, Kumar T, Dar TA, Singh LR. (2015) Protein N-homocysteinylation: From cellular toxicity to neurodegeneration. Biochim Biophys Acta 1850:2239–2245. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Yang CT, Chen J, Yin WL, Tian SW, Hu B, Feng JQ, Li YJ. (2008) Effect of hydrogen sulphide on beta-amyloid-induced damage in PC12 cells. Clin Exp Pharmacol Physiol 35:180–186. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Shen XT, Huang YE, Chen RQ, Ren YK, Fang HR, Zhuang YY, Wang CY. (2011) Inhibition of endogenous hydrogen sulfide generation is associated with homocysteine-induced neurotoxicity: role of ERK1/2 activation. J Mol Neurosci 45:60–67. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Shen XT, Huang YE, Ren YK, Chen RQ, Hu B, He JQ, Yin WL, Xu JH, Jiang ZS. (2010) Hydrogen sulfide antagonizes homocysteine-induced neurotoxicity in PC12 cells. Neurosci Res 68:241–249. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Zhuang YY, Zhang P, Fang HR, Zhou CF, Gu HF, Zhang H, Wang CY. (2013) Formaldehyde impairs learning and memory involving the disturbance of hydrogen sulfide generation in the hippocampus of rats. J Mol Neurosci 49:140–149. [DOI] [PubMed] [Google Scholar]
- Van Dam F, Van Gool WA. (2009) Hyperhomocysteinemia and Alzheimer’s disease: a systematic review. Arch Gerontol Geriatr 48:425–430. [DOI] [PubMed] [Google Scholar]
- White AR, Huang X, Jobling MF, Barrow CJ, Beyreuther K, Masters CL, Bush AI, Cappai R. (2001) Homocysteine potentiates copper- and amyloid beta peptide-mediated toxicity in primary neuronal cultures: possible risk factors in the Alzheimer’s-type neurodegenerative pathways. J Neurochem 76:1509–1520. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. (2004) The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem 90:765–768. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Cheung NS, Zhu YZ, Chu SH, Siau JL, Wong BS, Armstrong JS, Moore PK. (2005) Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun 326:794–798. [DOI] [PubMed] [Google Scholar]
- Wood PL. (2006) Neurodegeneration and aldehyde load: from concept to therapeutics. J Psychiatry Neurosci 31:296–297. [PMC free article] [PubMed] [Google Scholar]
- Wood PL, Khan MA, Kulow SR, Mahmood SA, Moskal JR. (2006) Neurotoxicity of reactive aldehydes: the concept of “aldehyde load” as demonstrated by neuroprotection with hydroxylamines. Brain Res 1095:190–199. [DOI] [PubMed] [Google Scholar]
- Wood PL, Khan MA, Moskal JR. (2007) The concept of “aldehyde load” in neurodegenerative mechanisms: cytotoxicity of the polyamine degradation products hydrogen peroxide, acrolein, 3-aminopropanal, 3-acetamidopropanal and 4-aminobutanal in a retinal ganglion cell line. Brain Res 1145:150–156. [DOI] [PubMed] [Google Scholar]
- Yoval-Sanchez B, Rodriguez-Zavala JS. (2012) Differences in susceptibility to inactivation of human aldehyde dehydrogenases by lipid peroxidation byproducts. Chem Res Toxicol 25:722–729. [DOI] [PubMed] [Google Scholar]
- Zarkovic K. (2003) 4-hydroxynonenal and neurodegenerative diseases. Mol Aspects Med 24:293–303. [DOI] [PubMed] [Google Scholar]
- Zhang CE, Wei W, Liu YH, Peng JH, Tian Q, Liu GP, Zhang Y, Wang JZ. (2009) Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. Am J Pathol 174:1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CF, Tang XQ. (2011) Hydrogen sulfide and nervous system regulation. Chin Med J (Engl) 124:3576–3582. [PubMed] [Google Scholar]
- Zhou S, Yu G, Chi L, Zhu J, Zhang W, Zhang Y, Zhang L. (2013) Neuroprotective effects of edaravone on cognitive deficit, oxidative stress and tau hyperphosphorylation induced by intracerebroventricular streptozotocin in rats. Neurotoxicology 38:136–145. [DOI] [PubMed] [Google Scholar]