Abstract
Phosphatase and tensin homolog (PTEN) is a major negative regulator of the Akt/mammalian target of rapamycin (mTor) pathway. Mutations in PTEN have been found in a subset of individuals with autism and macrocephaly. Further, focal cortical dysplasia (FCD) has been observed in patients with PTEN mutations prompting us to examine the role of Pten in neuronal migration. The dentate gyrus of PtenFlox/Flox mice was injected with Cre- and non-Cre-expressing retroviral particles, which integrate into the dividing genome to birthdate cells. Control and Pten knockout (KO) cell position in the granule cell layer was quantified over time to reveal that Pten KO neurons exhibit an aberrant migratory phenotype beginning at 7.5 days-post retroviral injection (DPI). We then assessed whether rapamycin, a mTor inhibitor, could prevent or reverse aberrant migration of granule cells. The preventative group received daily intraperitoneal (IP) injections of rapamycin from 3–14 DPI, before discrepancies in cell position have been established, while the reversal group received rapamycin afterward, from 14–24 DPI. We found that rapamycin prevented and reversed somal hypertrophy. However, rapamycin prevented, but did not reverse aberrant migration in Pten KO cells. We also find that altered migration occurs through mTorC1 and not mTorC2 activity. Together, these findings suggest a temporal window by which rapamycin can treat aberrant migration, and may have implications for the use of rapamycin to treat PTEN-mutation associated disorders.
Introduction
Phosphatase and tensin homolog (Pten) is a protein/lipid phosphatase that directly antagonizes the actions of phosphatidylinositol-3-kinase (PI3K) through the dephosphorylation of phosphatidylinositol (3,4,5)-triphosphate (PIP3) to phosphatidylinositol (4,5)-biphosphate (PIP2), to negatively regulate the Akt/mammalian target of rapamycin (mTor) pathway (Chalhoub and Baker, 2009a; Endersby and Baker, 2008). Neuronal deletion of Pten results in over-activation of the PI3K/Akt/mTOR pathway, leading to neuronal hypertrophy, hyper-excitability, and enhanced excitatory connectivity (Kwon et. al., 2001, 2006; Luikart et. al., 2011; Williams et. al., 2015). Mutations in PTEN have been linked to disorders including Cowden Syndrome, Bannayan-Riley-Ruvalcaba Syndrome, and Autism Spectrum Disorder (Butler et. al., 2005; Buxbaum et. al., 2007; Eng, 2003). In rare cases, patients with Cowden Syndrome and Epilepsy present with focal cortical dysplasia (FCD; Cheung et. al., 2014; Child and Cascino, 2013; Elia et. al., 2012; Merks et. al., 2003).
Studies addressing the role of Pten in neuronal migration have varied. Previous studies in mice have shown that conditional loss of Pten in the cerebellum leads to neuronal dysplasia (Kwon et. al., 2001; Marino et. al., 2002) In addition, complete loss of Pten in embryonic brains leads to aberrant histoarchetecture (Groszer et. al., 2001). The rate of neuronal migration has been reported to increase along the rostral migratory stream (RMS) to the olfactory bulb in Pten heterozygous mice (Li et. al., 2002). However, migration is largely unaffected with loss of Pten in Nestin-CreERT2 mediated neuronal precursors, with only a subset of cells showing decreased migratory rates along the RMS (Zhu et. al., 2012). These discrepant findings could be due to differences in cell-intrinsic properties or alterations in cell-extrinsic cues, as many of these studies showed Pten loss in both neurons and glia. Thus, the nature of migrational abnormalities associated with PTEN mutations and underlying mechanisms are unclear.
Rapamycin, a mTor inhibitor, is currently in the testing stage as a potential therapeutic for patients with autism and Tuberous Sclerosis Complex (TSC) mutations (https://clinicaltrials.gov/ct2/show/NCT01289912?term=rapamycin+and+autism&rank=3). Studies in mice have shown that rapamycin treatment can dramatically reduce the frequency of seizure activity in mice containing mutations in mTOR, Tuberous Sclerosis Complex 1 (Tsc1), and Pten (Lim et. al., 2015; Zeng et al., 2008; Zhou et al., 2009; Ljunberg et al., 2009). Rapamycin treatment has been shown to rescue autism-like behavioral phenotypes in neuron specific enolase (Nse)-PtenFlox/+ mice (Zhou et al., 2009). Previous studies have also shown that treatment with rapamycin reduces somal hypertrophy in both neuron specific- and Nse-PtenFlox/+ mice (Ljungberg et. al., 2009; Zhou et al., 2009). To our knowledge, there have been no published studies examining the effect of rapamycin treatment on deficits of neuronal migration.
Dentate gyrus granule neurons are generated throughout life; they arise from type IIb precursor cells in the subgranular zone, migrate into the granule cell layer, and integrate into the existing hippocampal circuitry (Kempermann et. al., 2015). Retroviral injections into adult mice have been shown to specifically label the dividing transiently amplifying (type II) precursor cells. By three days post injection, greater than 80% of the retroviral labeled cells are post-mitotic differentiated neurons (van Praag et. al., 2002; Zhao et. al., 2006; Kempermann et. al., 2015). These neurons then migrate into the granule cell layer and extend dendrites into the molecular layer over the next two weeks (Kemperman et. al., 2003; Zhao et. al., 2006). This has been defined as migration, because the movement of the soma into the granule cell layer occurs in conjunction with the extension of dendritic processes into the molecular layer (Nadarajah and Parnavelas, 2002).
To examine the cell intrinsic properties of Pten loss on migration, we used a Cre-mediated retroviral strategy to knockout Pten in the dentate gyrus of neonatal Ptenflox/flox mice. In neonatal animals, the process through which newborn neurons integrate into the dentate gyrus is equivalent to but accelerated compared to adults (Zhao et. al., 2006, Overstreet-Wadiche et. al., 2006). Because retroviral particles only infect dividing cells, the number of days post-injection (DPI) defines the post-mitotic age of the neurons (Lentz et al., 2012). Thus we are able to define the precise temporal development of neuronal migratory deficits and hypertrophy in response to Pten deletion. We then leveraged this finding to assess whether the abnormal migratory and hypertrophic phenotypes can be prevented or reversed by treatment with rapamycin.
Materials and Methods
Animals
All procedures were approved by both the Dartmouth Institutional Animal Care and Use Committee, and the Association for Assessment and Accreditation of Laboratory Animal Care Review Board. We utilized Pten flox/flox (B6.129S4-Ptentm1Hwu/J) mice of either sex, which were backcrossed into the C57BL/6J background at least five generations.
Stereotaxic injections
Mice were anesthetized with isoflurane on postnatal day 7 (P7), and the hippocampal dentate gyrus was injected with replication incompetent retroviral particles based on the pRubi construct (Williams et. al., 2015). These particles contained either fluorescent proteins or fluorescent proteins and Cre recombinase linked to a T2A-motif (Donnelly et al., 2001). Retroviral production and methodology for viral injection are further described in Williams et al. (2015) and Luikart et al. (2011), respectively. Injected animals were transcardially perfused with 4% paraformaldehyde and 4% sucrose for immunohistochemical analysis at varying days-post injection (DPI) utilizing previously described methods (Luikart et. al., 2011; Fricano et. al., 2014).
Rapamycin-treatment
Virally injected mice received daily intraperitoneal (IP) injections of either rapamycin (10 mg/kg body weight; Cayman Chemical) or vehicle from either 3–14 DPI, referred to as the preventative group, or 14–24 DPI, referred to as the reversal group. Mice were weighed prior to rapamycin administration, occurring every 22–26 hours. The mice were perfused following final rapamycin injection, at 14 and 24 DPI for the prevention and reversal groups, respectively. Rapamycin was dissolved in ethanol and stored at −20°C as a stock at a concentration of 25 mg/mL. A 1 mg/mL working solution was made daily with 4% rapamycin or ethanol, 5% tween80 (Fisher Biosciences), 5% PEG400 (Sigma Aldrich), as previously described (Fricano et. al., 2014; Ljungberg et. al., 2009; Zeng et. al., 2008; Zhou et. al., 2009).
5-bromo-2’-deoxyuridine (BrDU) Treatment
Virally injected mice received one IP injection of BrDU (150mg/kg in saline; Sigma Aldrich) at 0, 1, or 2 DPI. The 0 DPI group received BrDU just before viral injection. The 1 and 2 DPI groups received a BrDU injection at the same time as the 0 DPI group, but delayed 24 or 48 hours. Mice were perfused between 7 and 12 DPI, and each BrDU time point is reflected at all DPIs examined.
Immunohistochemistry
Sections were cut at a 50 µm and stained using mouse anti-mCherry (1:5000, Clontech), chicken anti-GFP (1:3000, abcam), rabbit anti-Pten (1:50, Cell Signaling Technology), mouse G3G4 (anti-BrDU (1:200, DSHB)), rabbit anti-phospho-S6 (Ser235/236; 1:100, Cell Signaling Technology), rabbit anti-phospho-Akt (Ser473; 1:100, Cell Signaling Technology), and rabbit anti-phospho-GSK-3β (Ser9; 1:50, Cell Signaling Technology). The secondary antibody for GFP was Alexa488 anti-chicken, mCherry was Cy3 anti-mouse, and all others were Alexa647 anti-rabbit (1:200; all from Jackson immunoresearch). Antigen retrieval was performed on tissue stained with rabbit anti-Pten, and mouse anti-BrDU (Tang et. al., 2007). To do this, sections were permeabilized for 30 minutes in phosphate buffered saline with 4% triton, pH 7.4 (PBS-T). Sections were then placed in 1.5 mL eppendorf tubes containing 1 mL of sodium citrate buffer, pH 6.0, and placed on a heat block at 95°C for 10 minutes. Following that, sections were taken off the heat block and allowed to rest at room temperature in the eppendorf tubes for 10 minutes, then taken out of the tubes and allowed to rest in individual wells of a well plate for 10 minutes in PBS-T. Sections then underwent two quick rinses in PBS-T and placed in blocking solution for 1 hour at room temperature; blocking solution is comprised of 10% normal donkey serum in phosphate buffered saline (PBS).
Soma size and migration tissue stained for mouse anti-mCherry and chicken anti-GFP received primary and secondary antibody staining for 24 and 24 hours, respectively. Pten, BrDU, and normalized intensity tissue received primary and secondary antibody staining for 48 and 48 hours, respectively. To ensure that intense staining in the red channel did not affect intensity measurement in the far-red channel, tissue that was stained for either rabbit anti-p-Akt or rabbit anti-p-GSK-3β was not immunostained for mCherry or GFP, but relied on endogenous fluorescence of mCherry and GFP to identify KO and control neurons. Protocols for primary and secondary antibody application are further described in Luikart et. al. (2011) and Fricano et. al. (2014).
Microscopy and Image Analysis
Z-stacked images of dentate gyrus granule cell neurons were taken on a Zeiss LSM 510 laser-scanning confocal microscope. Images were acquired of both the supra- and infrapyramidal blades with a 40×/1.3µm oil emersion lens with 1× zoom, at 1024 × 1024, and a 2 µm z-step for the timepoints and rapamycin treatment groups (Fricano et. al., 2014). For BrDU and p-S6, images were acquired with a 20×/0.75µm plan-apochromat lens with 2× zoom, at 512 × 512, and a 2 µm z-step. For p-Akt (S473) and p-GSK3β (S9), images were acquired with a 63×_1.4NA_190WD lens with 0.7× zoom, at 512 × 512, and a 2 µm z-step.
Soma size was analyzed as previously described in Fricano et. al. (2014). Migration analysis was previously described by Perederiy et. al. (2013). Briefly, a line was drawn at the border of the granule cell layer (GLC) on the hilar border of the subgranular zone to delineate cell origin. Another line was drawn perpendicular from the hilar border to the center of the cell at its largest circumference. The center of the cell is unbiasedly determined by the centroid function of ImageJ for each circled soma. This line is considered distance migrated. A third line is drawn through the cell to characterize the local thickness of the GCL. The thickness of the GCL varies along the rostrocaudal and mediolateral axes; further, rapamycin treatment decreases the overall thickness of the GCL. Therefore, the distance from the hilar boarder is divided by GCL width, so that cell migration is expressed as a percentage of granule cell layer (GCL) thickness.
BrDU images were analyzed by first counting Pten KO and control neurons, then counting the number of BrDU-positive cells that were also infected with retrovirus. The proportion of cells that were BrDU-positive and retrovirally infected were compared at each time point (0, 1, 2 DPI BrDU IP). These results were further broken down into Pten KO and control cells that were also BrDU-positive.
Normalized p-S6, p-Akt (S473), p-GSK3β (S9) intensities were analyzed as previously described in Fricano et. al., (2014). Briefly, following soma size analysis, intensity values in the far red channel (p-S6, p-Akt, p-GSK3β) were normalized to that of the average control neuronal intensity in that image. This controls for variability in staining intensity across different tissues and treatments.
Statistics
Mice were assigned to rapamycin or vehicle groups, taking into account sex and initial body weight such that each group was evenly weighted by either factor. Overall animal weights were compared using a standard two-way ANOVA with Bonferroni’s post-hoc. Measurements of cell size and migration were obtained post-treatment and varied between animals, depending on viral infection level. Estimated mean±SEM was expressed using the average of all neurons obtained from unadjusted comparisons. Statistical comparisons for soma size and migration were made using a statistical model that utilizes a standard regression coefficient to examine the interaction effects of rapamycin, a between-mouse factor, and Pten loss, a within-mouse factor (Fricano et. al., 2014). In addition, this model clusters data points obtained from each animal, such that neurons from the same animal are not considered independent measures. Finally, this model was used to examine migration as a function of time. The predictor variables, migration and Pten status, were tested for a pointwise difference in diffusion curves over the specified DPI. P-values were obtained using a post-hoc analysis, which performs a pairwise comparison between all groups within an experiment. Selected p-values are presented in tables and figures to highlight the most relevant changes. These statistics are described further in Moen et al (2016).
Results
Pten loss in granule neurons of the hippocampus leads to aberrant migration
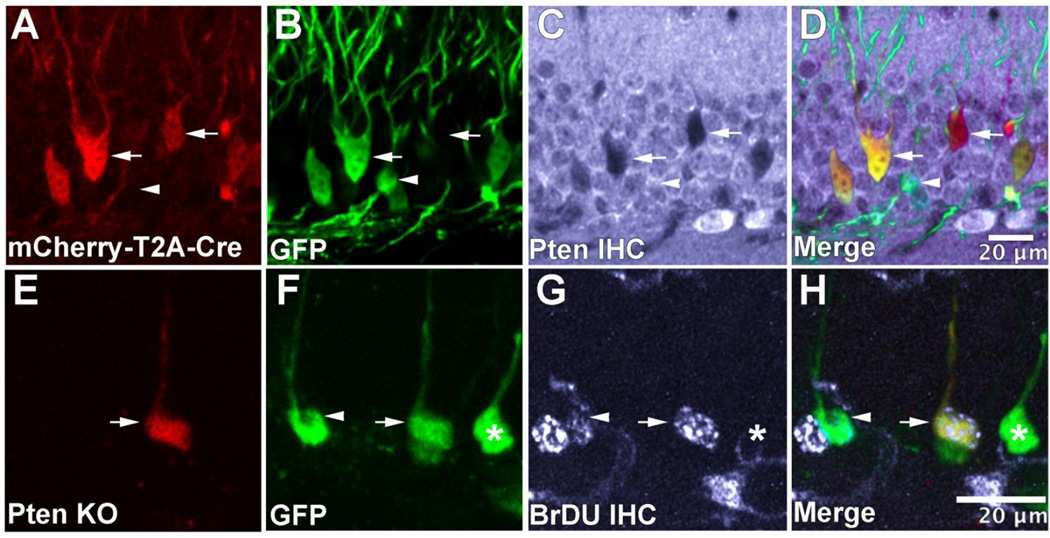
To examine the role of Pten in the development of dentate gyrus granule neurons a retrovirus expressing mCherry and Cre, along with a GFP-expressing control retrovirus, was co-injected into the dentate gyrus of Ptenflox/flox mice at P7. In doing so, Pten KO neurons fluoresce red, while wild-type controls fluoresce green. We confirmed that mCherry-T2A-Cre positive neurons were lacking Pten using immunohistochemical staining against Pten (Figure 1A–D). To determine whether Pten knockout alters mitosis or differentiation of dentate gyrus precursor cells we injected BrDU either just before viral injection, or 24 or 48-hours after viral injection and examined co-expression between 7 and 12 days post-injection (DPI; Figure 1E–H). We found that injecting BrDU just before viral injection resulted in 20.03±3.22% cells being co-labeled by BrDU and the retrovirus. By 24 and 48 hours post-injection, BrDU labeled 0.53±0.43% and 3.15±1.10% of retrovirus-infected cells, respectively. There was no difference in the proportion Pten KO and control cells co-labeled by BrDU at any time-point. These results indicate that greater than 83% of cells labeled by the retrovirus are post-mitotic within 24 hours post-injection. Previous research clearly indicates that Pten is important for mitosis and differentiation. However, in our system by the time Cre is expressed and Pten protein levels drop the majority of transiently amplifying precursor cells have already differentiated into neurons. Therefore we are unable to detect changes in differentiation into post-mitotic neurons due to Pten knockout.
Figure 1.
Retrovirus-mediated knockout of Pten in newborn neurons of the neonatal mouse dentate gyrus. Retroviral particles containing mCherry and Cre linked by a T2A-motif (A) was co-injected with a GFP-expressing control retrovirus into the dentate gyrus of PtenFlox/Flox mice on P7 (B). Pten loss was confirmed through immunohistochemical staining against Pten (C). The channels were merged to aid in the visualization of Cre-positive and Cre-negative neurons (D). Arrows denote Cre-positive cells that lack expression of Pten, while arrowheads denote Cre-negative cells. To examine a potential change in differentiation or mitosis following Pten loss, animals were injected with BrDU either just before viral injection, or 24 or 48 hours after. When BrDU is injected at the time of viral injection we find that ~ 20% of control (F; green) and Pten knockout (E; red) retrovirus labeled cells are co-labeled with BrDU (G; gray) There is no difference in the percentage of BrDU co-labeling between Pten KO and control neurons (H). By 24 and 48 hours post injection less than 4% of retrovirus labeled neurons were co-labeled with BrDU (not shown) indicating specificity of viral labeling for cells dividing on the day of viral injection.
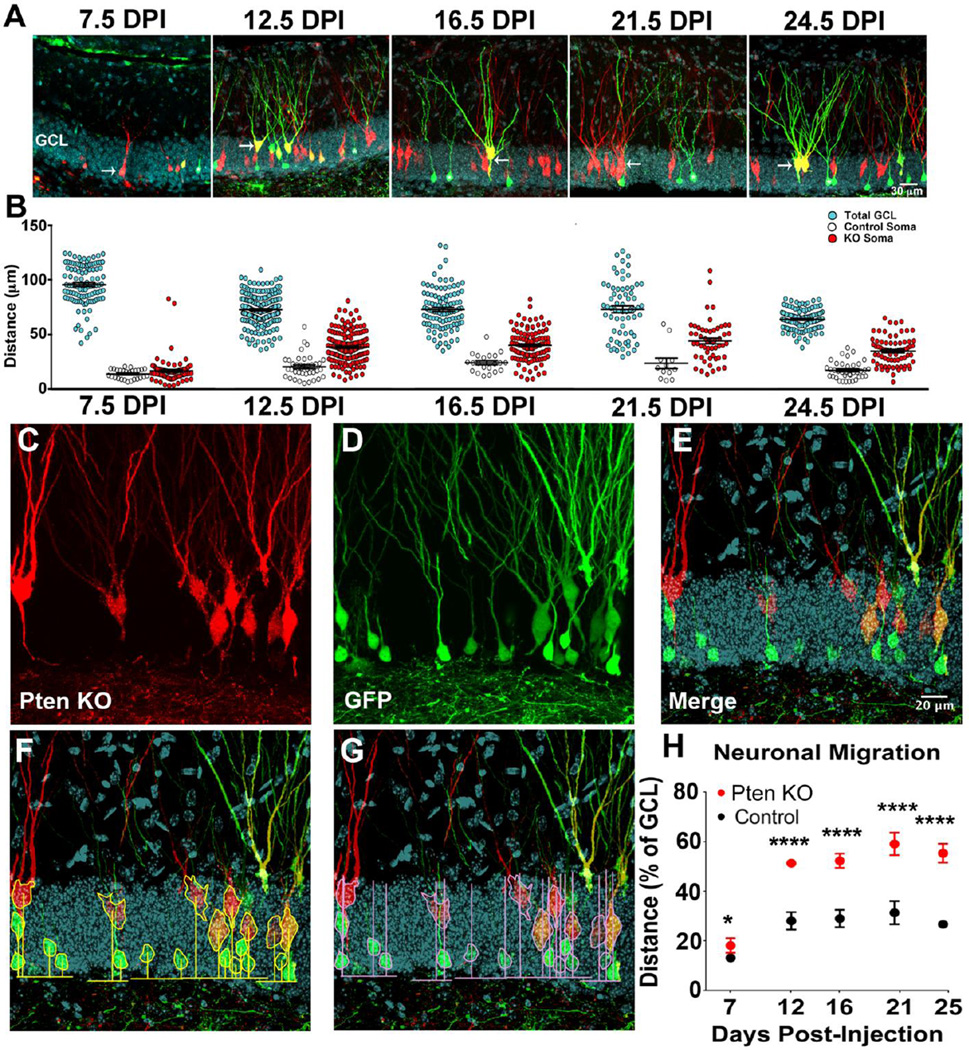
To examine how Pten affects granule neuron migration animals were sacrificed at 7.5, 12.5, 16.5, 21.5, and 24.5 DPI (Figure 2A). We found that the overall thickness of the dentate gyrus granule cell layer varied depending on the precise anatomical position (not shown) as well as the developmental time-point (Figure 2B). Despite this variability it was clear from quantification of the migratory distance that Pten knockout neurons were migrating farther into the GCL than control neurons (Figure 2B). To isolate the effect of Pten knockout on migration distance across time-points and anatomical positions we expressed migration as a percentage of the overall granule cell thickness for every individual cell (Figure 2C–G). Further, we measured control and Pten knockout neurons in every tissue section to ensure that gross anatomical position and developmental time-point were ballanced for the control and Pten knockout groups. Pten KO neurons migrated significantly farther from the hilus into the granule cell layer than did retrovirus labeled control neurons imaged in the same tissue section at 7.5 DPI, as well as every time point thereafter (Figure 2H; Table I.a). We have previously shown a similar time course for somal hypertrophy in Pten KO neurons beginning at 7.5 DPI through 24.5 DPI Williams et al. (2015).
Figure 2.
Pten knockout neurons migrate farther into the dentate gyrus granule cell layer. The dentate gyrus of PtenFlox/Flox mice was injected co-injected with a retrovirus expressing mCherry and Cre (red), to label and KO Pten, and one that expresses GFP only (green), to label controls. Mice were perfused at 7.5, 12.5, 16.5, 21.5, and 24.5 DPI to examine control vs Pten KO granule neurons. Arrows denote Pten KO neurons (red or yellow), asterisks denote control (only green). DAPI staining (turquoise) was used to visualize the granule cell layer (GCL). Newborn granule neurons migrate into the GCL in conjunction with the extension of dendrites through the molecular layer prior to 12.5 DPI (A). Scatter plots of the thickness of the GCL as measured at the same position of each control and Pten KO neuron indicates variability in GCL thickness across time and anatomical region (B; turquoise). Despite this variability, measurement of the raw distance from the hilus/GCL border into the GCL indicate that Pten KO neurons (B; red) migrate farther into the GCL compared to controls (B; white). To control for variability in GCL thickness the position Pten KO neurons (C; red) and control neurons (D; green) were expressed as a percentage of the thickness of the GCL as visualized by the DAPI stain (E; turquoise). Migration was quantified by measuring the distance to the center of the neurons from the hilar border (F) and expressed as a percentage of the thickness of the GCL at the position of each neuron measured (G). Pten KO neurons migrate farther from the hilus compared to controls beginning around 7.5 DPI (p<0.05), with a marked discrepancy in migration around 12.5 DPI (p<0.0001) and at every other time point following 12.5 DPI (p<0.0001; G). See Table I for quantitative values. (Statistical model described in methods; *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001)
Table I.
Statistics
| I.a | Migration | |||||
|---|---|---|---|---|---|---|
| DPI | 7.5 | 12.5 | 16.5 | 21.5 | 24.5 | |
| Mean±SEM Control (% of GCL) | 13.86±0.87 | 28.93±2.28 | 30.71±2.96 | 34.63±3.16 | 26.00±2.53 | |
| Mean±SEM KO (% of GCL) | 16.94±1.30 | 51.24±1.25 | 53.19±1.77 | 58.71±2. | 55.74±2.56 | |
| p-value | 0.013 | < 0.0001 | < 0.0001 | < 0.000 | < 0.0001 | |
| n, cells | 28, 83 | 41, 166 | 17, 87 | 13, 53 | 37, 72 | |
| n, animals | 3 | 3 | 3 | 3 | 3 | |
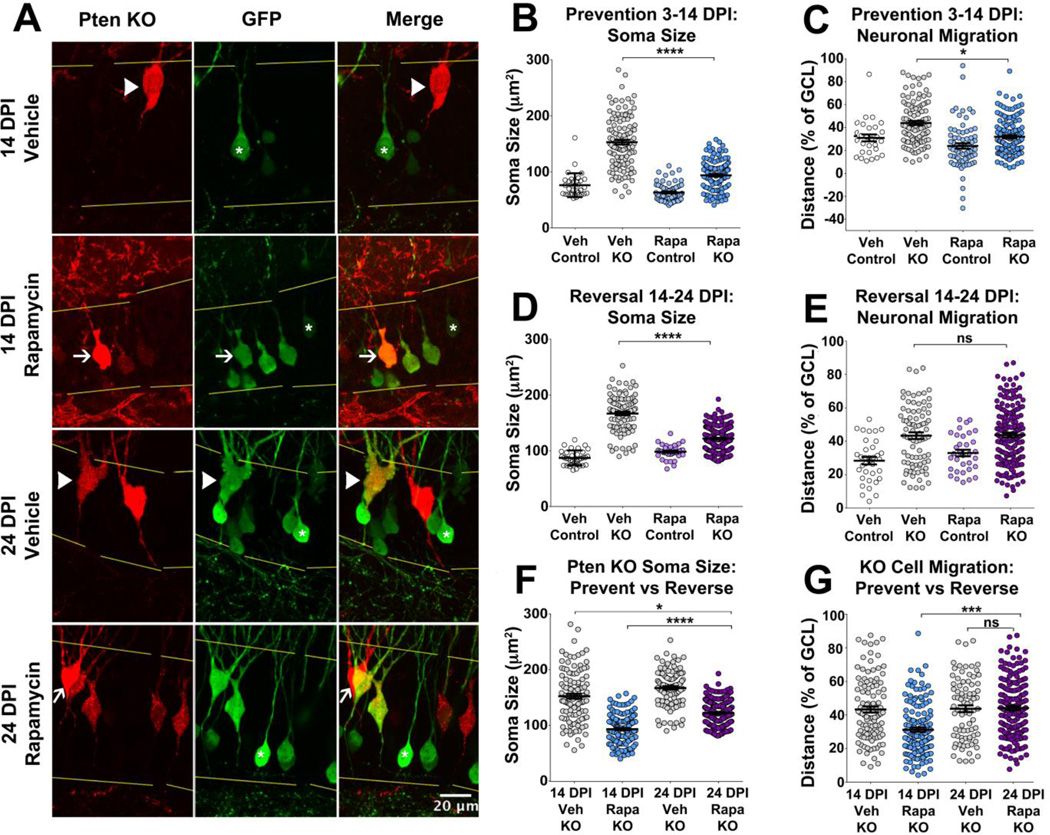
| I.b | Soma Size | Migration | ||||
|---|---|---|---|---|---|---|
| Mean±SEM (µm2) | p-value | n, cells (animals) | Mean±SEM (% of GCL) | p-value | n, cells (animals) | |
| 14 DPI Veh Control | 76.22±3.76 | < 0.0001 | 32 (3) | 30.23±3.01 | < 0.0001 | 28 (3) |
| 14 DPI Veh KO | 152.90±4.21 | 118 (3) | 43.24±1.81 | 105 (3) | ||
| 14 DPI Veh KO | 152.90±4.21 | < 0.0001 | 118 (3) | 30.23±3.01 | < 0.05 | 105 (3) |
| 14 DPI Rapa KO | 93.81±2.04 | 146 (4) | 31.22±1.32 | 139 (4) | ||
| 24 DPI Veh Control | 88.08±2.30 | < 0.0001 | 34 (3) | 28.73±2.20 | < 0.0001 | 34 (3) |
| 24 DPI Veh KO | 167.65±3.21 | 102 (3) | 42.77±1.90 | 93 (3) | ||
| 24 DPI Veh KO | 167.65±3.21 | < 0.0001 | 102 (3) | 42.77±1.90 | 0.946 | 93 (3) |
| 24 DPI Rapa KO | 122.8±1.43 | 220 (4) | 44.12±1.34 | 169 (4) | ||
| 14 DPI Veh KO | 152.90±4.21 | < 0.05 | 118 (3) | 30.23±3.01 | 0.999 | 105 (3) |
| 24 DPI Rapa KO | 122.8±1.43 | 220 (4) | 44.12±1.34 | 169 (4) | ||
| 14 DPI Rapa KO | 93.81±2.04 | < 0.0001 | 146 (4) | 31.22±1.34 | < 0.001 | 139 (4) |
| 24 DPI Rapa KO | 122.8±1.43 | 220 (4) | 44.12±1.34 | 169 (4) | ||
| I.c | Normalized Intensity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| p-S6 | p-Akt (S473) | P-GSK3β (S9) | |||||||
| Mean±SEM (%) | p-value | n, cells (animals) | Mean±SEM (%) | p-value | n, cells (animals) | Mean±SEM (%) | p-value | n, cells (animals) | |
| 14 DPI Veh Control | 1.000±0.04 | < 0.0001 | 30 (2) | 1.000±0.03 | < 0.0001 | 60 (3) | 1.000±0.03 | < 0.0001 | 45 (3) |
| 14 DPI Veh KO | 3.448±0.21 | 30 (2) | 2.756±0.20 | 64 (3) | 2.714±0.27 | 62 (3) | |||
| 14 DPI Veh KO | 3.448±0.21 | < 0.0001 | 30 (2) | 2.756±0.2 | 0.89 | 64 (3) | 2.714±0.27 | 0.029 | 62 (3) |
| 14 DPI Rapa KO | 1.110±0.018 | 40 (3) | 3.042±0.25 | 74 (4) | 1.897±0.09 | 73 (4) | |||
Rapamycin prevents and reverses somal hypertrophy, but it only prevents aberrant migration in Pten KO neurons
To investigate potential avenues for therapeutic intervention, we administered daily IP injections of rapamycin to animals either during neuronal migration or post-migration. The majority of the aberrant migration occurred between 7.5 and 12.5 DPI, with marked discrepancy in cell position established by 12.5 DPI (Figure 2H). Therefore, to test if rapamycin can prevent or reverse these phenotypes, the dentate gyrus of Ptenflox/flox mice was co-injected with a retrovirus expressing mCherry and Cre and a GFP-control retrovirus at P7. One group received rapamycin from 3–14 DPI to test whether treatment could prevent the migratory defect, while another received rapamycin from 14–24 DPI to test whether treatment could reverse the migratory defect (Figure 3A). In the prevention group, rapamycin treated animals weighed less than vehicle-treated controls by 12 DPI (Figure 3B). While the rapamycin treated animals tended to weigh less than the vehicle treated controls, this did not reach significance for the reversal group (Figure 3C).
Figure 3.
Rapamycin-treated animals have decreased weight gain. GFP only and mCherry with Cre retroviruses were co-injected into the dentate gyrus of Ptenflox/flox mice on postnatal day 7 (P7). Rapamycin was injected prior to the establishment of the migratory defect (prevention group) via daily IP injections from 3–14 DPI (P10–21), then perfused on 14 DPI. Rapamycin was also injected after the establishment of the migratory defect (reversal group) via daily IP injections from 14–24 DPI (P21–31), then perfused on 24 DPI (A). Mice were weighed daily and the graphical values represent mean+/−SEM over time to find that rapamycin-treated mice grew less than vehicle-treated controls in the prevention group, although the change in weight was not significant considering all time points, pairwise comparisons reveal a divergence in weight beginning at 12 DPI (treatment p=0.0627, 12 DPI p<0.01, 13 DPI p<0.0001, 14 DPI p<0.0001, B). Rapamycin-treated mice trended towards decreased weight gain in the reversal group but this was not significant (treatment p=0.3852, C). For both growth curves, the number in parenthesis next to rapamycin and vehicle indicate number of animals and the number next to the brackets represent the overall statistical significance of rapamycin when all time-points are considered (two-way ANOVA). Stars represent significance at individual time points after pairwise comparison (Bonferroni’s; *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001).
Pten KO somas from vehicle-treated animals were larger than vehicle-treated controls (Table I.b; Figure 4A, B; 14 DPI Veh Control vs 14 DPI Veh KO). We found that rapamycin treatment decreases the overall thickness of the GCL (prevention group rapamycin-treated GCL thickness: 70.37±0.62µm; vehicle: 102.52±0.87µm; p<0.0001; t-test). To control for the effect on the overall GCL thickness we continued to express migration distance for individual neurons as a percentage of the GCL width. Pten KO neurons from animals treated with vehicle migrated farther from the hilus than control neurons (Table I.b; Figure 4A, C; 14DPI Veh Control vs 14DPI Veh KO). Rapamycin treatment reduced the size of Pten KO neurons but not to levels of vehicle-treated controls (Veh control vs Rapa KO p=0.004; Table I.b; Figure 4A, B; 14 DPI Veh KO vs 14 DPI Rapa KO). Rapamycin also prevented Pten KO neurons from migrating as far as vehicle-treated Pten KO neurons (Table I.b; Figure 4A, C; 14 DPI Veh KO vs 14 DPI Rapa KO).
Figure 4.
Rapamycin can prevent and reverse somal hypertrophy, but can only prevent aberrant migration in Pten KO neurons. Representative images of prevention (3–14 DPI) and reversal (14–24 DPI) groups, arrowheads denote KO neurons from vehicle-treated animals, while arrows denote KO neurons from rapamycin-treated animals. Asterisks denote GFP-control cells, while yellow lines represent the GCL border (A). Quantification of soma size and migration in the prevention group reveals that KO cells from vehicle-treated animals were larger and migrated farther from the hilus compared to control cells and Pten KO cells from rapamycin-treated animals (p<0.0001, B; p<0.05, C). In the reversal group, KO cells from vehicle-treated animals were larger but showed no difference in migratory distance compared to KO cells from rapamycin-treated animals (p<0.0001, D; p=0.946, E). Comparisons of soma size and migration between prevention and reversal groups reveal rapamycin treatment could shrink KO cells, as KO cells from rapamycin-treated animals in the reversal group were smaller than KO cells from vehicle-treated animals in the prevention group (p<0.05, F), but it could not shrink KO cells to the size of KO somas from rapamycin-treated animals (p<0.0001, F). Rapamycin-treatment could prevent but not reverse aberrant migration, as KO neurons from rapamycin-treated animals in the reversal group migrate farther from the hilus compared to KO neurons from rapamycin-treated animals in the prevention group (p<0.001, G), but show no difference in migration compared to KO neurons from vehicle-treated animals in the reversal group (p=0.946, G). See Table I for quantitative results. (Statistical model described in methods; *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001)
In the reversal group (14–24 DPI) the somas of vehicle-treated Pten KO granule cells were larger and migrated farther from the hilus compared to controls (Table I.b; Figure 4A, D–E; 24 DPI Veh Control vs 24 DPI Veh KO). Rapamycin-treated Pten KO somas were smaller than vehicle-treated Pten KO neurons but not as small as vehicle-treated controls (Veh control vs Rapa KO p<0.001; Table I.b; Figure 4A, D; 24 DPI Veh KO vs 24 DPI Rapa KO). However, rapamycin treatment was unable to reverse the increased migration distance of Pten KO neurons (Table I.b; Figure 4A, E; 24 DPI Veh KO vs 24 DPI Rapa KO).
We next performed additional statistical analyses to compare the effects of rapamycin on Pten loss between the prevention and reversal groups. Rapamycin treatment reduced somal hypertrophy caused by Pten knockout in both the prevention and reversal groups (Table I.b; Figure 4F; 14 DPI Veh KO vs 14 DPI Rapa KO, 24 DPI Veh KO vs 24 DPI Rapa KO). However, rapamycin was only able to decrease migration in the prevention group (Table I.b; Figure 4G; 14 DPI Rapa KO vs 24 DPI Rapa KO). There was no difference in migration distance between rapamycin-treated Pten KO neurons and Vehicle-treated Pten KO neurons in the reversal group (Table I.b; Figure 4G; 24 DPI Veh KO vs 24 DPI Rapa KO). Taken together, rapamycin can prevent and reverse somal hypertrophy, but it can only prevent aberrant migration.
Aberrant migration in Pten KO neurons occurs through mTorC1 pathway activity, not mTorC2
To begin to elucidate the mechanism by which Pten loss leads to aberrant migration, we examined phosphorylation of proteins downstream of mTorC1 and mTorC2 in response to rapamycin treatment. Vehicle- and rapamycin-treated tissue from the preventative group was stained for p-S6 (S235,236), p-Akt (S473), and p-GSK3β (S9). Normalized p-S6 intensity, an indicator of mTorC1 activity, revealed that Pten KO neurons from vehicle-treated animals had a greater normalized intensity compared to both control neurons from vehicle-treated animals (Table I.c; Figure 5A, B; 14 DPI Veh Cont vs 14 DPI Veh KO), and KO neurons from rapamycin-treated animals (Table I.c; Figure 5A, B; 14 DPI Veh KO vs 14 DPI Rapa KO). This suggests that rapamycin is suppressing mTorC1 activity. To examine mTorC2 activity, we stained for p-Akt (S473) and found that Pten KO neurons from vehicle-treated animals also had a greater normalized intensity compared to control neurons from vehicle-treated animals (Table I.c; Figure 5C, D; 14 DPI Veh Cont vs 14 DPI Veh KO). There was, however, no difference in normalized p-Akt (S473) intensity between KO neurons after treatment with rapamycin (Table I.c; Figure 5C, D; 14 DPI Veh KO vs 14 DPI Rapa KO). This suggests that rapamycin is not affecting mTorC2 activity. Finally, normalized levels of p-GSK3β (S9) were examined as a marker of p-Akt activity. Pten KO neurons from vehicle-treated animals showed increased levels of p-GSK3β (S9) as compared to control neurons from vehicle-treated animals (Table I.c; Figure 5E, F; 14 DPI Veh Cont vs 14 DPI Veh KO). Rapamycin was unable to restore p-GSK3β in Pten KO neurons to the levels of control neurons (14 DPI Rapa Cont vs 14 DPI Rapa KO p<0.0001; mean±SEM: Rapa Cont 1±0.02, Rapa KO 1.897±0.09; n, cells: Rapa Cont 72, Rapa KO 73; n, animals: 4). However, Pten KO neurons from rapamycin-treated animals showed a modest but significant decrease in p-GSK3β (S9) intensity as compared to KO neurons from vehicle-treated animals (Table I.c; Figure 5E, F; 14 DPI Veh KO vs 14 DPI Rapa KO). This decrease was due to a reduction in the number of Pten KO cells that had very high levels (>5) of normalized p-GSK3β (Figure 6F). Overall, these results indicate that rapamycin was working specifically through mTorC1.
Figure 5.
Rapamycin effects aberrant migration through mTorC1 and not mTorC2. Tissue from vehicle- and rapamycin-treated animals in the prevention group was stained for p-S6 (Ser235/236), an indicator of mTorC1 activity (A), to reveal that Pten KO neurons from vehicle-treated animals have a greater normalized p-S6 intensity compared to control cells from vehicle-treated animals (B; p<0.0001), and KO cells from rapamycin-treated animals (B; p<0.0001). P-Akt (Ser473), an indicator of mTorC2 activity (C), revealed that Pten KO neurons from vehicle-treated animals have a greater normalized p-Akt intensity compared to control cells from vehicle-treated animals (D; p<0.0001), but show no significant difference in intensity as compared to KO cells from rapamycin-treated animals (D; p=0.89). Normalized intensity levels of phosphorylated-GSK3β (Ser9), an indicator of p-Akt activity (E), revealed that Pten KO neurons from vehicle-treated animals have a greater normalized p-GSK3β intensity compared to control cells from vehicle-treated animals (F; p<0.0001). However, KO cells from rapamycin-treated animals showed a modest decrease in p-GSK3β intensity as compared to KO cells from vehicle-treated animals (F; p=0.029; F). In A–C, arrowheads denote Pten KO neurons from vehicle-treated animals, arrows denote Pten KO neurons from rapamycin-treated animals, and asterisks denote control neurons See Table I for quantitative results. (Statistical model described in methods; *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001)
Discussion
In this study, we quantified the effects of Pten loss on migration by injecting Cre- and non-Cre-expressing retroviruses into the dentate gyrus of Ptenflox/flox mice. When examining Pten loss over a time, we found that Pten KO neurons show a marked discrepancy in cell position at 12.5 DPI and every time-point thereafter. We next showed that rapamycin, a mTor inhibitor, can prevent and reverse somal hypertrophy, but it can only prevent and not reverse aberrant migration in Pten KO cells. This suggests there is a temporal window in which rapamycin is effective in mitigating migration deficits. Finally, we showed that rapamycin-treatment reduced levels of p-S6 (S235,236) and p-GSKβ (S9) but not p-Akt (S473) in Pten KO neurons. This suggests that this migration deficit occurs in an mTorC1 dependent manner.
Examining the temporal regulation of Pten loss on migration in the GCL using a retroviral Cre-loxP strategy allows us to address the cell-intrinsic properties of the role of Pten on migration. We were able to show that over time, loss of Pten lead to mislamination of cells in the dentate gyrus. Previous studies have shown that loss of Pten leads to neuronal dysplasia in the cerebellum (Kwon et. al., 2001; Marino et. al., 2002). However, with regards to the effects of Pten loss on migration, some studies reported increased migratory speeds without deficits in lamination, while others observed normal to slowed migratory speeds with lamination deficits (Zhu et al., 2012; Li et. al., 2002). One complication of previous studies has been the inability to label a single homogeneous population of cells with a quantifiable migratory pattern. Here, we targeted a single neuronal type and followed its position over time to demonstrate that Pten loss alters cell-position in a cell-autonomous fashion.
Although rare, mutations in PTEN and FCD do occur (Cheung et. al., 2014; Child and Cascino, 2013; Elia et. al., 2012; Merks et al., 2003). Jansen et. al. (2015) identified patients with cortical malformations with mutations in PIK3CA (PI3K), AKT3 (Akt), and PTEN with downstream p-S6 activation. Further, activating mutations in MTOR have been found in several patients with cortical dysplasia (Lim et al., 2015). A cross between a conditional Pten knockout mouse presenting with neuronal dysplasia in the cerebellum with a conditional phosphate dependent kinase-1 (Pdk-1) knock-out mouse, which is downstream of Pten in the Akt/mTor pathway, rescued soma size but not migration (Chalhoub et. al., 2009b). They showed that the Pten- and Pdk-1- double knockout mice maintained p-Akt (S473) levels, indicating continued mTORC2 activation after Pdk-1 deletion.
Here, we see that rapamycin reduced mTorC1 activity, without effecting mTorC2, because p-S6 (S9) levels were reduced, while p-Akt (S473) levels remained unchanged in Pten KO neurons after rapamycin treatment. This suggests that mTorC2 activity is not altered by the presence of rapamycin, and thus is not mechanistically crucial to the aberrant migratory phenotype caused by loss of Pten. The decrease in levels of p-GSK3β (S9) in Pten knockout neurons treated with rapamycin suggests that p-Akt function is downregulated. This supports a model indicating that rapamycin decreases phosphorylation of Akt Thr308, possibly due to feedback through IRS-1 and Pdk1 activity. This decrease in p-GSK3β in Pten KO cells after rapamycin treatment was subtle and warrants additional investigation into the mechanisms through which rapamycin may feedback onto GSK3β activity. It is also important to consider that there may be cell type specific differences in the migratory deficits that have been identified in the rostral migratory stream, cerebellum, and now, the dentate gyrus.
In our study, rapamycin rescued both neuronal hypertrophy and migration when given preventatively, supporting studies indicating that mTORC1 function may critically regulate cell motility. One caveat to this interpretation is the issue of whether mTOR inhibition results in feedback onto elements generally considered upstream of mTorC1. Further studies should be done to elucidate the mechanism by which loss of Pten leads to aberrant neuronal migration; more specifically, regulation of IRS1, Pdk1, and cytoskeletal elements downstream of mTorC1 should be investigated in conjunction with Pten loss.
Clinical trials addressing the potential therapeutic benefits of mTor inhibitors in autism with PTEN or TSC mutations are planned or ongoing, respectively. However, preclinical models can be used to test potential outcomes of different treatment schemes. To address this, we administered rapamycin at a period before onset of somal hypertrophy and aberrant migration, and at a period after somal hypertrophy and aberrant migration has already been established. We found treatment with rapamycin could prevent and reverse somal hypertrophy, which is consistent with previous studies (Ljungberg et. al., 2009; Zhou et. al., 2009). We next found that rapamycin could prevent aberrant migration in Pten KO cells, but it could not reverse the mislamination of cells once established. This suggests that rapamycin may not be able to reverse all cellular phenotypes in patients by the time diagnosis is established. Because it is unknown how discrete cellular phenotypes can contribute to the emergent autism phenotype it remains to be determined whether the inability to reverse some pathologies will manifest as decreased treatment efficacy. However, our results indicate that a maximal treatment benefit may not be possible without genetic screening and preventative therapy.
Highlights.
Pten loss results in somal hypertrophy and aberrant neuronal migration in the dentate gyrus
Rapamycin, a mTor inhibitor, prevents and reverses somal hypertrophy in Pten knockout neurons
Rapamycin can only prevent and not reverse aberrant migration in Pten knockout neurons
Aberrant migration following loss of Pten occurs through mTorC1 and not mTorC2
Significance Statement.
Mutations in phosphatase and tensin homolog (PTEN) have been linked to a subset of individuals with autism and macrocephaly, as well as Cowden Syndrome and focal cortical dysplasia. Pten loss leads to neuronal hypertrophy, but the role of Pten in neuronal migration is unclear. Here we have shown that loss of Pten leads to aberrant migration, which can be prevented but not reversed by treatment with rapamycin, a mTor inhibitor. These results are important to consider as clinical trials are developed to examine rapamycin as a therapeutic for autism with PTEN mutations. Our findings show that some abnormalities cannot be reversed, and suggest the potential need for genetic screening and preventative treatment.
Acknowledgments
We would like to thank Dr. Alistair J. O’Malley for the code he wrote for our statistical analysis. This work was supported by NIH grant R01 MH097949, and by the Autism Speaks Pilot Grant #7359. This work was also supported by the Optical Cellular Imaging Shared Resource and the Norris Cotton Cancer Center at the Geisel School of Medicine at Dartmouth (5 P30 CA023108).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors declare no competing financial interest.
References
- Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, Eng C. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumor suppressor gene mutations. J Med Genet. 2005;42:318–321. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Cai G, Chaste P, Nygren G, Goldsmith J, Reichert J, Anckarsäter H, Rastam M, Smith CJ, Silverman JM, Hollander E, Leboyer M, Gillberg C, Verloes A, Betancur C. Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. Am J Med Genet. 2007;144B:484–491. doi: 10.1002/ajmg.b.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalhoub N, Baker SJ. Pten and the pi3-kinase pathway in cancer. Annu Rev Pathol. 2009a;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalhoub N, Zhu G, Zhu X, Baker SJ. Cell type specificity of pi3k signaling in pdk1- and pten-deficient brains. Genes & Devel. 2009b;23:1619–1624. doi: 10.1101/gad.1799609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KM, Lam CW, Chan YK, Siu WK, Yong L. Atypical focal cortical dysplasia in a patient with cowden syndrome. Hong Kong Med J. 2014;20(2):165–167. doi: 10.12809/hkmj133863. [DOI] [PubMed] [Google Scholar]
- Child ND, Cascino GD. Mystery case: cowden syndrome presenting with partial epilepsy related to focal cortical dysplasia. Neurology. 2013;81:e98–e99. doi: 10.1212/WNL.0b013e3182a55ef0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly ML, Hughes LE, Luke G, Mendoza H, tenDam E, Gani D, Ryan MD. The ‘cleavage’ activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring ‘2A-like’ sequences. J Gen Virol. 2001;82:1027–1041. doi: 10.1099/0022-1317-82-5-1027. [DOI] [PubMed] [Google Scholar]
- Elia M, Amato C, Bottitta M, Grillo L, Calabrese G, Esposito M, Carotenuto M. An atypical patient with cowden syndrome and pten gene mutation presenting with cortical malformation and focal epilepsy. Brain & Devel. 2012;34:873–876. doi: 10.1016/j.braindev.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Endersby R, Baker SJ. Pten signaling in brain: neuropathology and tumorigenesis. Oncogene. 2008;27:5416–5430. doi: 10.1038/onc.2008.239. [DOI] [PubMed] [Google Scholar]
- Eng C. Pten: one gene, many syndromes. Human Mutation. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- Fricano CJ, Despenza T, Jr, Frazel PW, Li M, O’Malley AJ, Westbrook GL, Luikart BW. Fatty acids increase neuronal hypertrophy of Pten knockdown neurons. Front Mol Neurosci. 2014;7:30. doi: 10.3389/fnmol.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- Jansen LA, Mirzaa GM, Ishak GE, O’Roak BJ, Hiatt JB, Roden WH, Gunter SA, Christian SL, Collins S, Adams C, Rivère, St-Onge J, Ojemann JG, Shendure J, Henver RF, Dobyns WB. Pi3k/akt pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain. 2015;138(6):1613–1628. doi: 10.1093/brain/awv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi, Gage FH. Early determination and long-term persistence of adult –generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015;7(a018812):1–14. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29:404–411. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz TB, Gray SJ, Samulski J. Viral vectors for gene delivery to the central nervous system. Neruobiol of Disease. 2012;48:179–188. doi: 10.1016/j.nbd.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liu F, Salmonsen RA, Turner TK, Litofsky NS, Di Cristofano A, Pandolfi PP, Jones SN, Recht LD, Ross AH. Pten in neural precursor cells: regulation of migration, apoptosis, and proliferation. Mol and Cell Neurosci. 2002;20:21–29. doi: 10.1006/mcne.2002.1115. [DOI] [PubMed] [Google Scholar]
- Lim JS, Kim W, Kang HC, Kim SH, Park AH, Park EK, Cho YW, Kim S, Kim HM, Kim JA, Kim J, Rhee H, Kang SG, Kim HD, Kim D, Kim DS, Lee JH. Brain somatic mutations in mtor cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med. 2015;21(4):395–400. doi: 10.1038/nm.3824. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Sunnen NC, Lugo JN, Anderson AE, D’Arcangelo G. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. DMM. 2009;2:389–398. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart BW, Schnell E, Washburn EK, Bensen AL, Tovar KR, Westbrook GL. Pten knockdown in vivo increases excitatory drive onto dentate granule cells. J Neurosci. 2011;31:4345–4354. doi: 10.1523/JNEUROSCI.0061-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S, Krimpenfort P, Leung C, van der Korput HAGM, Trapman J, Camenisch I, Berns A, Brandner S. Pten is essential for cell migration but not for fate determination and tumourigenesis in the cerebellum. Development. 2002;129:3513–3522. doi: 10.1242/dev.129.14.3513. [DOI] [PubMed] [Google Scholar]
- Merks JHM, de Vries LS, Zhou XP, Nikkels P, Barth PG, Eng C, Hennekam RCM. Pten hamartoma tumor syndrome: variability of an entity. J Med Genet. 2003;40(e111):1–4. doi: 10.1136/jmg.40.10.e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen EL, Fricano-Kugler CJ, Luikart BW, O’Malley AJ. Analyzing clustered data: why and how to account for multiple observations nested within a study participant? PLOSone. 2016;11(1):e0146721. doi: 10.1371/journal.pone.0146721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Parnavelas JG. Modes of neuronal migration in the developing cerebral cortex. Nature Reviews Neuroscience. 2002;3:423–432. doi: 10.1038/nrn845. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26:2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perederiy JV, Luikart BW, Washburn EK, Schnell E, Westbrook GL. Neural injury alters proliferation and integration of adult-generated neurons in the dentate gyrus. J Neurosci. 2013;33(11):4754–4767. doi: 10.1523/JNEUROSCI.4785-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Falls DL, Li X, Lane T, Luskin MB. Antigen-retrieval procedure for bromodeoxyuridine immunolabeling with concurrent labeling of nuclear dna and antigens damaged nby hcl pretreatment. J Neurosci. 2007;27(22):5837–5844. doi: 10.1523/JNEUROSCI.5048-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schnider AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, DeSpenza T, Jr, Li M, Gulledge AT, Luikart BW. Hyperactivity of newborn pten knockout neurons results from increased excitatory synaptic drive. J Neurosci. 2015;35(3):943–959. doi: 10.1523/JNEUROSCI.3144-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63(4):444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RGJR, Ming G, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mtorc1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific pten knock-out mice. J Neurosci. 2009;29(6):1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Chow LML, Bayazitov IT, Tong Y, Gilbertson RJ, Zakharenko SS, Solecki DJ, Baker SJ. Pten deletion causes mtorc1-dependent ectopic neuroblast differentiation without causing uniform migration defects. Development. 2012;139:3422–3431. doi: 10.1242/dev.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]