Abstract
Background:
Dendritic arbor simplification and dendritic spine loss in the hippocampus, a limbic structure implicated in mood disorders, are assumed to contribute to symptoms of depression. These morphological changes imply modifications in dendritic cytoskeleton. Rho GTPases are regulators of actin dynamics through their effector Rho kinase. We have reported that chronic stress promotes depressive-like behaviors in rats along with dendritic spine loss in apical dendrites of hippocampal pyramidal neurons, changes associated with Rho kinase activation. The present study proposes that the Rho kinase inhibitor Fasudil may prevent the stress-induced behavior and dendritic spine loss.
Methods:
Adult male Sprague-Dawley rats were injected with saline or Fasudil (i.p., 10 mg/kg) starting 4 days prior to and maintained during the restraint stress procedure (2.5 h/d for 14 days). Nonstressed control animals were injected with saline or Fasudil for 18 days. At 24 hours after treatment, forced swimming test, Golgi-staining, and immuno-western blot were performed.
Results:
Fasudil prevented stress-induced immobility observed in the forced swimming test. On the other hand, Fasudil-treated control animals showed behavioral patterns similar to those of saline-treated controls. Furthermore, we observed that stress induced an increase in the phosphorylation of MYPT1 in the hippocampus, an exclusive target of Rho kinase. This change was accompanied by dendritic spine loss of apical dendrites of pyramidal hippocampal neurons. Interestingly, increased pMYPT1 levels and spine loss were both prevented by Fasudil administration.
Conclusion:
Our findings suggest that Fasudil may prevent the development of abnormal behavior and spine loss induced by chronic stress by blocking Rho kinase activity.
Keywords: behavior, dendritic spines, antidepressant, ROCK inhibitor Fasudil, stress
Significance Statement
This study examined the effects of a ROCK inhibitor, fasudil, on depressive-like behavior and neuronal changes associated with exposure to chronic restraint stress in a rodent model of depression. We observed that our stress model induced depressive-like behaviors in rats, which was prevented by Fasudil treatment. Furthermore, we observed that chronic stress decreased spine density in CA1 pyramidal neurons, modification that was related to an enhancement in phospho-MYPT1 levels, a known ROCK effector. These changes were prevented by Fasudil treatment, a ROCK inhibitor.
Introduction
Neuroplasticity, the ability of the brain to change and adapt in response to experience and fluctuating environment, involves several mechanisms, ranging from synaptic remodeling to functional modification of synapses and neural circuitries. Repeated exposure to unpredictable and uncontrollable stressors may result in brain modifications that decrease the capacity to appropriately respond to subsequent stressors, thus increasing the risk for developing mental disorders, including depression (McEwen and Gianaros, 2010). Furthermore, several studies have shown that functional modifications of the hippocampus, amygdala, prefrontal cortex, and other structures are responsible for depression symptoms (Drevets et al., 2008; Price and Drevets, 2010). The hippocampus, a stress-sensitive limbic structure, is crucial for episodic and spatial memory; these functions are impaired in depressive disorder (Austin et al., 2001; Drevets et al., 2008; Price and Drevets, 2010). Human brain imaging and preclinical studies in rodents have revealed that stress and depression are associated with reduced hippocampal volume, neuronal atrophy, and dendritic spine loss (McEwen and Seeman, 1999; Vyas et al., 2002; Fernandez-Guasti et al., 2012; Castaneda et al., 2015). In addition, the hippocampus participates in the intersection between cognition and emotion and plays a crucial in the pathophysiology of mood disorders (Campbell and Macqueen, 2004; Femenía et al., 2012). Similar to other studies (Bondi et al., 2008), we previously reported that chronic stress in rodents produces anhedonia, impairs associative learning, and increases immobility in the forced swimming test (FST) (Bravo et al., 2009; Ulloa et al., 2010; Castaneda et al., 2015). Notably, these behavioral modifications are accompanied by dendritic atrophy and spine density reduction of pyramidal neurons in the hippocampus and prefrontal cortex (McEwen and Seeman, 1999; Vyas et al., 2002; Fernandez-Guasti et al., 2012; Castaneda et al., 2015). Overall, this evidence suggests that the mechanisms involved in excitatory synapse formation and maintenance can be altered in stress-related mood disorders, contributing to the phenotypic alterations observed in depressive disorder.
The formation and elimination of dendritic spines are mechanisms by which neuronal connections can be shaped (Chklovskii et al., 2004). The growth of dendrites, filopodia, and dendritic spines occurs by protrusive forces of actin polymerization (Luo, 2002). Thus, it is plausible that the morphological alterations observed under chronic stress are produced by changes in signal transduction pathways that target the reorganization of the actin cytoskeleton. Members of the Rho-GTPase family regulate actin cytoskeleton dynamics and modulate axonal growth, dendritic arborization, and spine growth during development and adulthood (Luo, 2000; Nakayama et al., 2000; Tashiro and Yuste, 2004; Govek et al., 2005; Elia et al., 2006). For instance, activated RacGTP-ases stimulate the formation of thin spines (Nakayama et al., 2000) and through the activation of p21-activated kinase 1 trigger the activation of LIM-kinase, which phosphorylates and inhibits cofilin, a potent actin-depolymerizing molecule (Calabrese et al., 2006). In contrast, RhoA-GTPases promote neuronal dendritic arbor simplification and reduce spine length and number (Nakayama et al., 2000; Nakayama and Luo, 2000). RhoA signaling is mediated by Rho serine/threonine kinase (ROCK) isoforms 1 and 2, the latter being highly expressed in the brain and muscle tissue (Hashimoto et al., 1999). ROCK regulates cytoskeleton dynamics by phosphorylation of the myosin light chain (MLC) at Ser19, probably facilitating acto-myosin interaction (Hirose et al., 1998), which may produce fast neuronal remodeling, such as retraction of dendrites. Furthermore, ROCK can phosphorylate and inactivate the myosin phosphatase-targeting subunit 1 (MYPT1) of MLC phosphatase and may indirectly increase MLC phosphorylation state, favoring acto-myosin interaction (Amano et al., 1996).
Studies have shown high immunoreactivity of ROCK2 in several brain areas, including pyramidal neurons of the hippocampus (Hashimoto et al., 1999), suggesting a particular role of ROCK in these structures of the adult brain. Although ROCK2-deficient mice display normal brain anatomy, electrophysiological studies in hippocampal slices evidenced an impairment of both basal synaptic transmission and long-term potentiation (Zhou et al., 2009). These electrophysiological alterations were consistent with modifications in dendritic spine length and morphology, accompanied by an increase in activated cofilin (unphosphorylated form). Nonetheless, this study did not explore whether the deletion of ROCK2 modifies the behavior of animals (Zhou et al., 2009).
Considering that ROCK has several downstream effectors and that some of them are related to the regulation of cytoskeleton dynamics, studies have evaluated the effect of different ROCK inhibitors on brain function. For example, in vascular smooth cells, Fasudil increases cerebral blood flow after stroke by reducing vasoconstriction (Shin et al., 2007). Furthermore, systemic administration of hydroxyfasudil, an active metabolite of Fasudil, improves the cognitive deficits in aged rats and also improves learning and memory in a model of sporadic Alzheimer’s disease (Hou et al., 2012). According to the relevance of the Rho-ROCK pathway in neuronal morphology in vitro and the effect of ROCK inhibitors on cognition, learning, and memory, it is particularly important to address whether RhoA signaling is being altered under some pathological conditions, such as depressive disorders. Recently, in an animal model of depression induced by chronic restraint stress, we observed an increase in hippocampal phospho-MYPT1 levels, suggesting that ROCK2 is activated by this experimental paradigm (Castaneda et al., 2015). Interestingly, the rise in phospho-MYPT1 levels was associated with the spine loss observed in secondary dendrites of CA1 pyramidal neurons of the hippocampus (Castaneda et al., 2015). Moreover, it has been demonstrated that bilateral infralimbic administration of Y-27632, a specific ROCK inhibitor, promotes antidepressant-like effects in naive rats that are similar to those produced by the antidepressant fluoxetine (Inan et al., 2015).
Considering that stress increases immobility in the FST, an effect that is accompanied by a dendrite spine loss in CA1 pyramidal neurons and along with an increase in hippocampal phospho-MYPT1 levels, indicative of RhoA-ROCK pathway activation (Castaneda et al., 2015), we hypothesized that pharmacological inhibition of ROCK with Fasudil prevents both the increase in immobility in FST and dendrite spine loss in the hippocampus of rats under chronic restraint stress paradigm, an animal model of depression.
Materials and Methods
Animals
The Sprague-Dawley rats used in these experiments were obtained from the Faculty of Chemical and Pharmaceutical Sciences, Universidad de Chile. Efforts were made to minimize the number of animals and their suffering. The rats were handled according to guidelines outlined and approved by the Ethical Committee of the Faculty of Chemical and Pharmaceutical Sciences, Universidad de Chile and the Science and Technology National Commission (CONICYT), in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication, 8th Edition, 2011).
The adult male Sprague-Dawley rats (250–280 g) were housed in groups of 4/cage in a temperature (22–23ºC) and humidity (55–65%) controlled room with a standard light: dark cycle (12 hours:12 hours). Food (standard rat chow) and water were freely provided, except when restraint stress was applied. The rats were handled once per day for 7 days prior to initiating the experimental procedures. The handling procedure consisted of picking up the rat by its body, weighing it, and finally returning it to its home cage.
Restraint Stress and Pharmacological Treatment
An animal model of chronic restraint stress was used to evaluate changes in ROCK activity and the effect of the ROCK inhibitor Fasudil on behavior. A dose of 10 mg/kg i.p. was selected based on the observation that i.p. administration of 5 to 15 mg/kg of Fasudil was previously reported to induce neuroprotection in the CNS (Wu et al., 2012; Song et al., 2013; Wei et al., 2014). The rats were randomly assigned to weight-matched groups that received one of the following treatments prior to the restraint protocol: (1) unstressed control animals injected every day for 18 days with saline (0.9% NaCl) (CONTROL, n=16) or with 10 mg/kg Fasudil (LC Laboratories, Woburn, MA) (FASUDIL, n = 13), and (2) stressed animals injected for 18 days with saline (STRESS, n = 16) or 10 mg/kg Fasudil (STRESS-FASUDIL, n=15). All injections were carried out i.p. 15 minutes prior to the stress protocol. To examine the preventive action of Fasudil on chronic stress effects, the stress procedure was initiated 4 days after the first drug or saline was administered. In this study, we used restraint stress as previously described, which consisted of placing the rats in Plexiglas tubes (25 x 8 cm) that were wide enough to allow comfortable breathing but restricting the movement of the animals for 2.5 h/d for 14 consecutive days. Every stress session was performed between 9:00 am and 12:00 pm to avoid any effects due to changes in circadian rhythms. After the procedure, the animals were returned to their respective cages. After vehicle or Fasudil administration, unstressed animals were maintained in their home cages and left in another room. Twenty-four hours after the last treatment, animals were evaluated in a behavioral test (described below) or were killed to obtain either brain tissue to conduct morphological analyses, or the hippocampus for protein level determinations by immuno-western blot.
FST
The behavioral test consisted of blinded observations, which was carried out 24 hours after the last restraint procedure, in a quiet room. This test was performed as previously described (Lucki, 1997). A transparent Plexiglas cylinder (50 cm high x 20 cm wide) was filled up to a depth of 30 cm with water at 24ºC. Four hours after the last stress session and Fasudil treatment on day 14, rats were trained for 15 minutes by placing them in the water-filled cylinder. On day 15 (24 hours after cessation of stress and Fasudil treatment), the rats were subjected to 5 minutes of forced swimming and escape behaviors (climbing and swimming) that were registered by trained observers who were blind to the treatments. Climbing was defined as upward-directed movements of the forepaws along the side of the swim chamber, while swimming consisted of movements throughout the swim chamber. Duration of immobility was defined as the time the animal was not actively involved in escape responses (i.e., total time of the test minus the time the animal spent climbing or swimming).
Golgi Staining and Evaluation of Dendritic Spine Density
After decapitation, one brain hemisphere was used for morphological studies and in the other, the hippocampus was dissected and rapidly frozen in liquid nitrogen and stored at -80°C. The FD Rapid GolgiStain kit (FD Neuro Technologies, Baltimore, MD) was used as recently described (Castaneda et al., 2015). Secondary apical dendrites of pyramidal neurons from the CA1 region were selected for spine analyses (bregma -2.3 to -4.3) (Paxinos and Watson, 1982). The criteria for the selection of Golgi-impregnated neurons for morphological analyses were recently described (Castaneda et al., 2015). Confocal z-stacks of identified intersections were captured on a Zeiss LSM 700 confocal laser scanning microscope. Each designated segment was located in the microscope field, and confocal stacks of 15 to 30 digital images, separated by a z-step of 0.5 to 1 μm, were captured using a Plan-Apochromat 40 × 1.4 NA Zeiss oil-immersion objective. Protrusions in direct connection with the dendritic shaft, irrespective of their morphological characteristics, were considered as spines. A mushroom spine type was identified when its head diameter exceeded 0.6 μm; the remaining spines (filopodia, stubby and other protrusions) were classified as “non-mushroom.” Spines were counted in segments of 8 µm, starting from the origin of the branch, along a distance of 80 µm of the secondary dendrite. The number of spines at a given distance from the origin of the branch were then averaged using all of the neurons from the same animal (at least 6 cells/animal), and these data were pooled with the mean of the other animals belonging to the same experimental group. The total number of spines corresponded to the sum of spines along the dendritic length of 80 µm.
Immunoblot Analysis
Whole hippocampus was homogenized in a glass-glass homogenizer in the presence of 50 mM Tris (pH 7.4), 150 mM NaCl, 0.5 mM EGTA, 0.5 mM EDTA, 0.5 mM DTT, 0.125 mM Na3VO4, 0.2 mM PMSF, 2 µg/mL leupeptin, 2 μg/mL aprotinin, 2 mM NaF, 0.25 mM Na2P2O7, and 1% Triton X-100 and then sonicated on ice for 5 minutes. After centrifuging lysates at 17860 x g for 30 minutes, the supernatant was collected and a sample was saved for protein determination using the bicinchoninic method (Pierce BCA Protein Assay Kit ThermoFisher Scientific). The remaining supernatant was boiled immediately in sample loading buffer. A total of 25 to 50 μg of each protein extract was resolved on 12% SDS-polyacrylamide gels and then blotted onto 0.2 μm nitrocellulose (for detection of total and phosphorylated form of LIMK and MYPT1) or 0.2 μm PVDF membranes (for determination of total and phosphorylated form of cofilin). After blocking, membranes were incubated overnight with the appropriate primary antibodies diluted in blocking solution and then, with their corresponding secondary antibody (Table 1), as we described previously (Castaneda et al., 2015). All membranes were then incubated with enhanced chemiluminescent substrate (Perkin Elmer Life Sciences, Boston, MA) and detected by a chemiluminescence imager (Syngene). Band intensities were determined and analyzed using the UN SCAN IT software. The levels of β-actin were used to verify equivalent protein loading. For detection of total MYPT1 and LIMK, blots were stripped in Ponceau S solution for 1 hour, then incubated with the appropriate dilution of the antibodies (Table 1). For detection of total cofilin, blots were stripped with ReBlotPlus Mild Antibody Stripping Solution during 10 minutes and were then incubated overnight with an appropriate dilution of anti-cofilin (Table 1). After rinses, the procedure continued as described above.
Table 1.
Western-Blot Conditions and Antibodies
| Antibody | Host, Isotype | Source/Catalog No. | Blocking Solution | Primary Antibody | Secondary Antibody |
|---|---|---|---|---|---|
| β-Actin | Mouse | Sigma-Aldrich/A5316 | 3% nonfat milk-TBS 0.1% Tween-20 | 1:10000; 1 h | 1:10000; 2 h |
| pThr508-LIMK | Rabbit | Sigma-Aldrich/SAB 4504460 | 3% nonfat milk-TBS 0.1% Tween-20 | 1:500; overnight | 1:10000; 2 h |
| LIMK 1 | Rabbit | Sigma-Aldrich/L2290 | 1% nonfat milk-TBS 0.1% Tween-20 | 1:4000; overnight | 1:10000; 2 h |
| pSer3-cofilin | Rabbit | Cell Signaling/77G2 | 5% BSA-TBS 0.1% Tween-20 | 1:1000; overnight | 1:10000; 2 h |
| cofilin | Rabbit | Cytoskeleton/ACFL02 | 3% nonfat milk-TBS 0.1% Tween-20 | 1:8000; overnight | 1:10000; 2 h |
| pThr853-MYPT1 | Rabbit | Cell Signaling/4563 | 5% nonfat milk-TBS 0.1% Tween-20 | 1:500; overnight | 1:10000; 2 h |
| MYPT1 | Rabbit | Cell Signaling/2634 | 5% nonfat milk-TBS 0.1% Tween-20 | 1:250; overnight | 1:10000; 2 h |
Statistical Analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA). Data are expressed as mean ± SEM and were analyzed by 2-way ANOVA followed by Tukey’s post-hoc test.
Results
Effect of Stress and Fasudil Treatment on Body Weight Gain
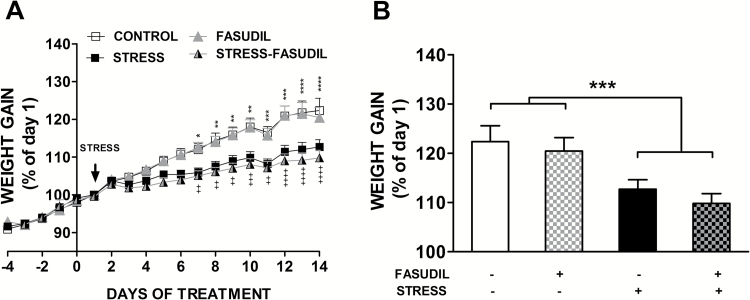
Because basic studies have shown that stress affects physiological parameters related to hypothalamic-pituitary-adrenal activation, including energy mobilization mediated by glucocorticoid action, we tested whether mild restraint and Fasudil led to changes in body weight gain. We determined the percentage of body weight gain for each day either prior (days -4 to 0) or during the stress procedure (day 1 to day 14) compared with the initial weight at the beginning of the stress procedure (day 1), expressed as daily weight x 100/start weight at day 1. We observed no difference in weight gain prior to the beginning of the stress procedure, in which rats were injected with saline (CONTROL) or Fasudil (FASUDIL) (Figure 1A). Nonetheless, 2-way ANOVA analysis performed with the daily variation of weight gain during the 14 days of stress and Fasudil administration showed that treatment (or experimental groups) (F3,56 = 7.45, P < .0003), time (days) (F13,56 = 132.2, P < 0.0001), and the interaction between these factors (F39,56 = 5.54 P < .0001) had a significant effect on body weight gain. Moreover, Tukey’s post-hoc analysis indicated that stressed rats injected either with saline solution (STRESS) or Fasudil (STRESS-FASUDIL) showed a reduction in weight gain observable after 7 days of stress (Figure 1A). Additionally, 2-way ANOVA analysis of both factors at the end point of treatment showed that stress (F1,56 = 15.93, P = 0.0002), but not Fasudil treatment (F1,56 = 0.8979, P = 0.3474), had a significant effect on body weight gain (Figure 1B). The interaction between both factors was not significant (F1,56 = 0.03771, P = .8467). Moreover, no significant change in adrenal weight was observed by restraint stress (CONTROL, 28.9 ± 1.3 mg vs STRESS, 26.23 ± 2.2 mg) and Fasudil, compared with control (24.1±1.4 mg) and stressed rats (24.0±1.3 mg). Thus, the restraint stress, but not Fasudil, produced a reduction in weight gain, but this was not related to changes in adrenal weight, suggesting that our stress model is mild in terms of HPA activation.
Figure 1.
Effect of chronic restraint on body weight gain. (A) The graph represents mean ± SEM of the change in body weight as a percentage of the initial weight for control animals that were left untreated (CONTROL, n=16) or treated with Fasudil (FASUDIL, n=13), untreated animals subjected to chronic restraint stress (STRESS, n=16) and Fasudil-treated animals subjected to chronic restraint (STRESS-FASUDIL, n=15). Two-way ANOVA followed by Tukey’s post-hoc analysis. CONTROL vs STRESS *P <.05, **P<.01, ***P<.001, ****P<.0001. FASUDIL vs STRESS-FASUDIL ++P<.01, +++P < .001, ++++P < .0001. (B) Variation of body weight gain was evaluated at the endpoint of treatments. Two-way ANOVA, ***P<.001.
Effects of Fasudil on Activity in the FST
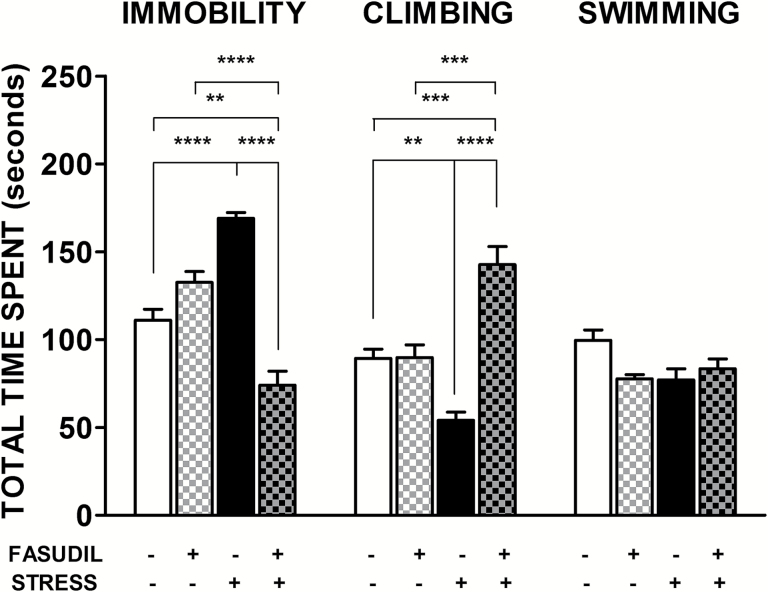
The antidepressant effect of Fasudil was examined in the FST, which is a highly reliable behavioral assay for the detection of antidepressant activity exerted by pharmacological agents (Cryan et al., 2002). As shown in Figure 2, control animals spent approximately 60% of the time in active response, that is, swimming and climbing. The 2-way ANOVA analysis showed a significant main effect of Fasudil treatment (F1,21=36.59, P<.0001) but not for stress (F1,21=0.004, P=.95) on spent time in immobility. Nonetheless, the interaction between these factors was significant (F1,21=91.75, P<.0001). Tukey post-hoc analysis showed that stressed animals increased their time in immobility (P<.0001), which was prevented by Fasudil (P<.0001), suggesting an antidepressant-like effect of Fasudil. In addition, the 2-way ANOVA analysis on spent time in climbing showed a significant main effect of Fasudil treatment (F1,21 = 40.11, P<.0001) but not of stress (F1,21 = 1.559, P = .226). We found a significant interaction between stress and Fasudil treatment (F1,21 = 39.19, P<.0001), evidencing that Fasudil treatment in stressed animals induces an increment in the spent time in climbing, indicating that this response is higher than control rats (P<.001). In the case of the swimming behavior, we observed that neither stress (F1,21 = 1.45, P=.1578) nor Fasudil treatment (F1,21 = 1.850, P=.1882) showed a significant effect.
Figure 2.
Effects of Fasudil administration on immobility and active response duration in the forced swimming test (FST). Bar graph indicates mean ± SEM of total time spent in immobility and climbing behaviors. Two-way ANOVA followed by Tukey’s post-hoc analysis indicated that Fasudil-treated animals (FASUDIL, n= 5) showed similar durations of immobility and active behaviors as animals injected with saline (CONTROL, n = 7). Stressed animals (STRESS, n = 7) spent more time in an immobile posture and significantly reduced climbing behavior. Chronic treatment with Fasudil (STRESS-FASUDIL, n=6) prevented the stress-induced immobility by increasing active behaviors, mainly in the form of climbing. **P < .01; ***P < .001; ****P < .0001.
Effects of Stress and Fasudil on Dendritic Spine Number of CA1 Pyramidal Cells
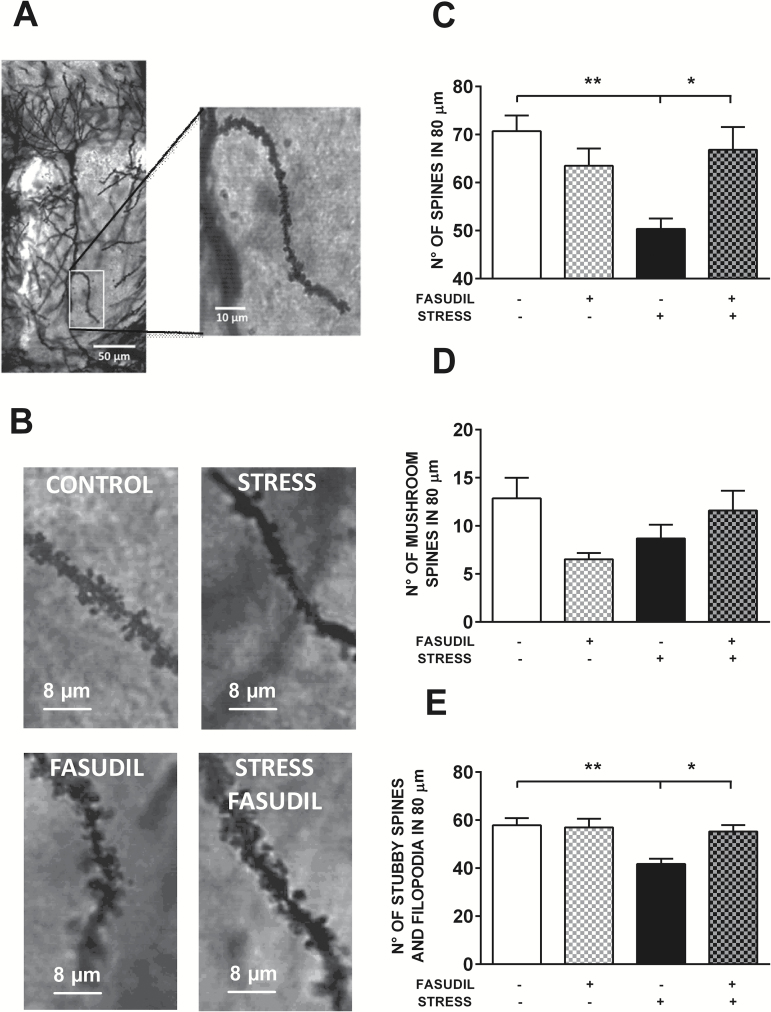
We analyzed the effect of stress and Fasudil on dendritic spine number of secondary apical dendrites from pyramidal neurons of CA1. We evaluated whether treatments modified the number of spines regardless of their morphological features. Figure 3A shows an example of a CA1 pyramidal neuron, and the magnification shows the segment of a secondary dendrite used to count the number of spines along 80 µm. Figure 3B shows the magnification of a segment of a secondary dendrite, where the effect of treatments on the density of protrusions along the dendrite can be observed. When the total number of spines counted in 80 µm was analyzed by the 2-way ANOVA test, we observed a significant main effect of stress (F1,15 = 5.666, P<.03) but not of Fasudil treatment (F1,15 = 1.67, P<.3); nonetheless a significant interaction was detected (F1,15 = 10.99, P<.005). Post-hoc analysis revealed that stress induced a reduction in spine density (P<.01) and that Fasudil prevented the stress-induced reduction in spine density (Figure 3C). We segregated the spines according to their morphology to visualize a differential effect of treatments. We observed that neither stress (F1,15 = 0.06797, P=.7979) nor Fasudil treatment (F1,15 = 0.9393, P = .3478) induced variation in the number of mushroom spines (Figure 3D). Nonetheless, we found a significant effect of stress (F1,15=9.750, P<.007), Fasudil treatment (F1,15 = 4.863, P=.0435), and interaction between these factors (F1,15=6.324, P=.0238) on the number of nonmushroom spines in 80 µm (Figure 3E). Furthermore, Fasudil administration prevented the stress-induced reduction in spines (Figure 3C), mainly in nonmushroom spines (Figure 3E).
Figure 3.
Effects of stress and Fasudil treatment on spine number along apical dendrites of pyramidal neurons in the CA1 hippocampal region. (A) The photograph shows a representative isolated Golgi-stained pyramidal neuron of the CA1 hippocampal region of control animals, and a magnification of a secondary dendritic branch in the stratum radiatum area, which was used to count spines. (B) Photographs show the qualitative effects of stress and Fasudil treatment on spine density from a secondary dendrite. (C) Effect of treatments on total spine number in a dendritic segment of 80 µm. Two-way ANOVA followed by Tukey’s post-hoc analysis revealed that stress induced a reduction in spine density and that Fasudil prevented the stress-induced reduction in spine density. (D) Effect of treatments on mushroom spine number in a dendritic segment of 80 µm. (E) Effect of treatments on nonmushroom spine number in a dendritic segment of 80 µm. Two-way ANOVA followed by Tukey’s post-hoc analysis indicated that stress induced a reduction in spine density and that Fasudil prevented the stress-induced reduction in spine density. Values are the mean ± SEM (CONTROL, n = 5; FASUDIL, n = 4; STRESS, n = 5; STRESS-FASUDIL, n = 5). *P < .05; **P < .01.
Effect of Stress and Fasudil on Phosphorylation Levels of MYPT1, LIMK, and Cofilin in Hippocampus Extract
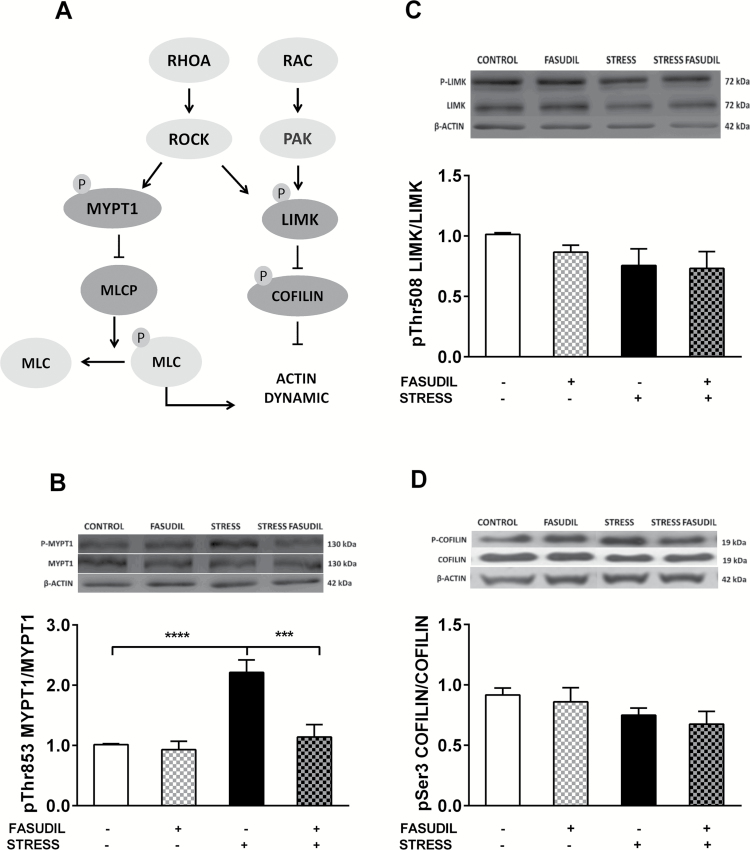
To evaluate whether chronic stress and Fasudil modulate ROCK activity, we evaluated the phosphorylation state of myosin phosphatase targeting protein 1 (MYPT1, also known as Myosin Binding Subunit) in its Thr853 residue, an exclusive target of ROCK. We observed a significant main effect of stress (F1,19=21.19, P=.0002), Fasudil treatment (F1,19 =13.74, P=.0015), and strong interaction between factors (F1,19=10.49, P=.0043) on the levels of p-MYPT1, relative to MYPT1 (Figure 4B). Post-hoc analysis showed that stress induced an increase of 50% in p-MYPT1 levels (P <.0001), variation that was prevented by Fasudil (P<.001) in the hippocampus of stressed animals, an effect probably related to ROCK inhibition.
Figure 4.
Effect of stress and Fasudil on the phosphorylation state of proteins related to actin dynamics, myosin phosphatase targeting subunit 1 (MYPT1), LIMK, and cofilin. (A) Pathways related to RAC and RhoA activation and their downstream effectors. (B) phospho-MYPT1 expressed as a ratio of phospho-MYPT1 relative to MYPT1. Two-way ANOVA followed by Tukey’s post-hoc analysis indicated an increase in p-MYPT1 levels that was prevented by Fasudil. (CONTROL n = 7; FASUDIL n = 5; STRESS n = 6 and STRESS-FASUDIL n = 5). ***P < .001, ****P < .0001 (C) phospho-LIMK expressed as a ratio of phospho-LIMK relative to LIMK. (CONTROL n = 5; FASUDIL n = 4; STRESS n = 5 and STRESS-FASUDIL n = 4). (D) phospho-cofilin expressed as a ratio of phospho-cofilin relative to cofilin (CONTROL n=6; FASUDIL n=5; STRESS n = 6 and STRESS-FASUDIL n = 5). β-Actin was used as the loading control. Values are the mean ± SEM.
We also evaluated changes in the phosphorylation state of LIMK, which is a common effector of Rho and RAC GTPases in vitro. We observed that neither stress (F1,14 = 0.955, P = .345) nor Fasudil treatment (F1,14=1.028, P=.328) influenced p-LIMK levels, relative to total LIMK (Figure 4C). Accordingly, there were no significant effect of stress (F1,18 = 3.09, P = .095) and Fasudil (F1,18 = 0.87, P = .361) on p-cofilin levels relative to total cofilin (Figure 4D).
Discussion
Alteration in neuronal morphology related to dendritic arbor and spines are hallmarks of several psychiatric disorders, including major depressive disorders (Licznerski and Duman, 2013). Stress exposure may contribute to the development of depression (Kendler et al., 1999), and several studies support the idea that stress-induced brain atrophy might be responsible, in part, for the phenotypic characteristics of depressive disorders (Pittenger and Duman, 2008). The observed morphological changes include dendritic tree simplification accompanied by a reduction in the number of dendritic spines (Lin and Koleske, 2010). We have recently described that dendritic spine loss in the hippocampus of chronically stressed rats is related to an increase in phospho-MYPT1, a well-known downstream target of ROCK, suggesting that stress promotes the activation of the RhoA-ROCK pathway and therefore induces changes in cytoskeleton dynamics (Castaneda et al., 2015). Our main findings in the present study performed in rats can be summarized as follows: Fasudil, a ROCK inhibitor, prevents the stress-induced immobility observed in the FST, spine loss in CA1 neurons of the hippocampus, and increase in pMYPT1 levels.
By using a chronic restraint stress paradigm in rats, we have previously reported anhedonic behavior accompanied by a significant reduction in escape-directed behavior (Bravo et al., 2009; Castaneda et al., 2015). In the present study, we tested the effect of Fasudil in the FST, a test widely used to assess antidepressant activity of pharmacological agents (Porsolt et al., 1979; Cryan et al., 2005). It has been described that all antidepressant drugs reduce behavioral immobility. However, those antidepressants that increase serotonergic neurotransmission predominantly increase swimming behavior, whereas those that increase catecholaminergic neurotransmission increase climbing behavior (Cryan et al., 2005). We demonstrated that Fasudil treatment reduced stress-induced immobility through an increase in active responses, mainly in climbing behavior, to a level that was even higher than in control rats. Notably, the effect of Fasudil in stressed animals resembles the action of the antidepressant DMI (Bravo et al., 2009), which blocks the norepinephrine transporter. However, in our model, we do not know whether chronically administered Fasudil modifies noradrenergic neurotransmission. Furthermore, a recent study demonstrated that acute bilateral microinjection of the ROCK inhibitor Y-27632 (0.25 μg) into the infralimbic cortex of naïve rats increased active behavior during the FST (swimming and climbing) (Inan et al., 2015), similarly to drugs with dual effects on norepinephrine and serotonin transporters that extend the duration of both active behaviors (Detke et al., 1995, 1997). Thus, it seems plausible that drugs that do not share the same pharmacological profile, that is, antidepressant molecules and ROCK inhibitors, may converge into a common intracellular pathway that underlies the action of antidepressants. The specificity of the FST for screening antidepressant-like agents has been widely questioned (Petit-Demouliere et al., 2005), because a large variety of nonantidepressant drugs have been shown to increase the locomotion that can be erroneously interpreted with an antidepressant-like effect (for review, see Yin et al., 2016). For this reason, some studies that evaluate the effects of antidepressant drugs in FST also evaluate potential hyperactivity in the open field arena. Although we did not use parallel test to exclude drug-induced locomotor false positives, there is a report indicating that Fasudil did not change locomotor activity in the open field test (Yoshimi et al., 2010). Additionally, we have observed that the total arm visits in elevated plus-maze was similar in control and stressed animals with or without Fasudil administration, suggesting that treatments did not alter locomotor activity (data not shown).
On the other hand, studies have used FST to visualize the “depressogenic effect” of various types of stress; however, their effects seem to be dependent on the stress paradigm used (for review, see Bogdanova et al., 2013). Some authors have proposed that the immobility observed during the test session of the FST indicates that the animal “learned” a strategy to save energy during the FST training session. Thus, in this scenario, the increase in the time spent in immobility does not seem to be related to “behavioral despair” or depressive-like symptoms (for review, see de Kloet and Molendijk, 2016). Considering that in our stress model Fasudil increases the time spent in climbing in the FST, the increment in this active response may be indicative of changes in the activity of some neural circuits related to this behavior promoted by the ROCK inhibitor. In the context of depressive behavior, motivation has been evaluated by the exposure of rodents to an inescapable stressor, such as the tail-suspension test or FST, and quantifying the proportion of time spent performing escape-related behavior (struggling) relative to time spent immobile. Recently, and by using an optogenetic approach, acute and selective inhibition of ventral tegmental area (VTA) dopaminergic neurons was shown to promote several depression-like behaviors, such as anhedonia (sucrose consumption) and reduced struggling behavior in the tail-suspension test (Tye et al., 2013). Interestingly, this study also showed that the phasic activation of VTA dopaminergic neurons reverts the reduction in escape behavior (struggling) induced by chronic mild stress (Tye et al., 2013). Furthermore, other optogenetic approaches revealed that a selective population of neurons in medial prefrontal cortex (PFC) is implicated in the active response to behavioral challenges (Warden et al., 2012). More recently, optogenetic approaches have also shown that activation of the ventral-hippocampus-medial PFC pathway is required for the sustained action of ketamine antidepressant action, observed as an increase in climbing in the FST (Carreno et al., 2016). Although a similar mechanism probably operates in our stress model, the suggested Fasudil antidepressant action in the FST must be interpreted with caution, and further studies are required to prove its action on specific circuits that are sensitive to stress, such as those related to motivated behavior.
Structural neuronal changes within the hippocampus and the prefrontal cortex are increasingly recognized as key to the pathophysiology of depression (Pittenger and Duman, 2008). A reduction in spine densities in CA1 neurons has been associated with depression-like behaviors in several animal models of depression induced by restraint stress or by chronic exposure to light at night, suggesting an altered glutamatergic excitatory neurotransmission in the hippocampus (Bedrosian et al., 2012; Fernandez-Guasti et al., 2012; Castaneda et al., 2015; Huang et al., 2015). Some studies have reported that antidepressant drugs with different primary mechanisms of action such as imipramine and fluoxetine (Bessa et al., 2009) revert both behavioral deficits and spine loss in CA3 (Bessa et al., 2009) caused by stress. Accordingly, we next analyzed the potential contribution of Fasudil on dendritic spine density that may explain its stress preventive effect on depression-like behaviors. Thus, in the present study, we found that stress-induced spine loss (nonmushroom spines) in secondary dendrites of CA1 pyramidal neurons was prevented by Fasudil treatment, suggesting that ROCK inhibition and perhaps other kinases can modify spine density. Recently, it was shown that primary hippocampal neurons acutely exposed to Y-27632, a ROCK inhibitor, specifically increased the number of filopodia and thin spines (Swanger et al., 2015). Hence, this evidence suggests that ROCK inhibition promotes variation in the density of spines, mainly the immature forms.
The effect of Fasudil may be related to modulation of the cytoskeleton in vivo that favors spine stability, an effect that correlates well with the observed antidepressant-like action of this drug in stressed animals. In accordance with this, ROCK directly phosphorylates MLC, at least in vitro (Amano et al., 1996), and probably favors actomyosin interaction and contraction. In addition, some studies have indicated that ROCK activates the LIMK-cofilin pathway (Maekawa et al., 1999), but we found that phosphorylation levels of this kinase are insensitive to stress and Fasudil treatment. Moreover, ROCK2 activity may indirectly increase the level of phospho-MLC by phosphorylating the Thr853 residue of the MLC phosphatase regulator MYPT1 (Hartshorne et al., 1998; Somlyo and Somlyo, 2000; Somlyo et al., 2000), resulting in a decrease in MLC phosphatase activity (Kimura et al., 1996). Recently, we reported a rise in phospho-MYPT1 levels in the hippocampus of stressed animals and considering that this protein is an exclusive target of ROCK (Grassie et al., 2011), we suggested that chronic stress activates ROCK (Castaneda et al., 2015). The present study showed that both factors, increased phospho-MYPT1 levels and reduced spine density triggered by chronic stress, are prevented by Fasudil, suggesting that this drug may mediate those effects by inhibiting ROCK activity.
Overall, this evidence suggests that the disruption of the Rho-ROCK pathway and/or inhibition of other kinases by Fasudil seem to exert antidepressant-like actions, probably by preventing spine loss in some areas (e.g., hippocampus). However, further studies are necessary to confirm whether Fasudil acts on similar substrates in different brain areas. We should also consider that Fasudil, through the inhibition of ROCK, may affect several transduction pathways. In vivo reports have shown that i.p. administration of Fasudil in a dose similar to the present study (10 mg/kg) implicated ROCK in the negative regulation of PTEN activity and the enhancement of AKT activity, a neuroprotective transduction pathway (Wu et al., 2012). Moreover, many new effects of Fasudil have been described, particularly in the CNS. It has been shown that systemic administration of Fasudil protects against ischemia (15 mg/kg) (Wei et al., 2014) and attenuates neuronal apoptosis and proinflammatory cytokine production (5–10 mg/kg) (Song et al., 2013) in animals models of neurodegeneration. Interestingly, diverse evidences have situated neuroinflammation and proinflammatory cytokines as principal players in depressive disorder pathogenesis and recurrence (Slavich and Irwin, 2014). Thus, it remains to be elucidated whether Fasudil, through its antidepressant-like actions and preventive effect on dendritic spine loss, are related to ROCK inhibition or to other kinases sensitive to this drug and/or are associated with modulatory effects on glial and inflammatory cells in the CNS. However, considering that systemic administration of Fasudil may act as a potent vasodilator, it will be important to consider this action in vivo, which may mediate not only effects on peripheral organs but also cause protective effects in the brain.
Conclusion
The present study suggests that Fasudil could prevent both increase in immobility in the FST and spine loss induced by chronic restraint stress in the rat hippocampus. Although further experiments are necessary to reveal the molecular mechanisms underlying the Fasudil-induced improvement in activity level in the FST and spine loss prevention, the present results can be considered as an initial step by providing support for the hypothesis that ROCK inhibition initiates a cascade of events that can prevent stress-induced behavior and spine loss. Finally, our findings offer new perspectives on pharmacological intervention in depressive disorders.
Statement of Interest
None.
Acknowledgments
We are grateful to Constanza Fuentealba for technical assistance. This work was supported by the National Commission for Scientific and Technological Research of Chile (FONDECYT 1120528 to J.L.F., FONDEQUIP EQM120114 to J.L.F., and 21120711 to G.G.) and Fondo Central de Investigación, Universidad de Chile (ENL025/16 to J.L.F.).
References
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271:20246–20249. [DOI] [PubMed] [Google Scholar]
- Austin MP, Mitchell P, Goodwin GM. (2001) Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry 178:200–206. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Weil ZM, Nelson RJ. (2012) Chronic citalopram treatment ameliorates depressive behavior associated with light at night. Behav Neurosci 126:654–658. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. (2009) The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14:764–773, 739. [DOI] [PubMed] [Google Scholar]
- Bogdanova OV, Kanekar S, D’Anci KE, Renshaw PF (2013) Factors influencing behavior in the forced swim test. Physiol Behav 118:227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. (2008) Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33:320–331. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Diaz-Veliz G, Mora S, Ulloa JL, Berthoud VM, Morales P, Arancibia S, Fiedler JL (2009) Desipramine prevents stress-induced changes in depressive-like behavior and hippocampal markers of neuroprotection. Behav Pharmacol 20:273–285. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Wilson MS, Halpain S (2006) Development and regulation of dendritic spine synapses. Physiology (Bethesda) 21:38–47. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. (2004) The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, Lodge DJ (2016) Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol Psychiatry 21:1298–1308. [DOI] [PubMed] [Google Scholar]
- Castaneda P, Munoz M, Garcia-Rojo G, Ulloa JL, Bravo JA, Marquez R, Garcia-Perez MA, Arancibia D, Araneda K, Rojas PS, Mondaca-Ruff D, Diaz-Veliz G, Mora S, Aliaga E, Fiedler JL (2015) Association of N-cadherin levels and downstream effectors of Rho GTPases with dendritic spine loss induced by chronic stress in rat hippocampal neurons. J Neurosci Res 93:Spc1. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. (2005) Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29:547–569. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. (2004) Cortical rewiring and information storage. Nature 431:782–788. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Molendijk ML (2016) Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural Plast 2016:6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 121:66–72. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Johnson J, Lucki I. (1997) Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol 5:107–112. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213:93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia LP, Yamamoto M, Zang K, Reichardt LF. (2006) p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins. Neuron 51:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femenía T, Gómez-Galán M, Lindskog M, Magara S (2012) Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain Res 1476:58–70. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Fiedler JL, Herrera L, Handa RJ (2012) Sex, stress, and mood disorders: at the intersection of adrenal and gonadal hormones. Horm Metab Res 44:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Van Aelst L (2005) The role of the Rho GTPases in neuronal development. Genes Dev 19:1–49. [DOI] [PubMed] [Google Scholar]
- Grassie ME, Moffat LD, Walsh MP, MacDonald JA (2011) The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1delta. Arch Biochem Biophys 510:147–159. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ, Ito M, Erdodi F. (1998) Myosin light chain phosphatase: subunit composition, interactions and regulation. J Muscle Res Cell Motil 19:325–341. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Nakamura Y, Kosako H, Amano M, Kaibuchi K, Inagaki M, Takeda M. (1999) Distribution of Rho-kinase in the bovine brain. Biochem Biophys Res Commun 263:575–579. [DOI] [PubMed] [Google Scholar]
- Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar WH, Matsumura F, Maekawa M, Bito H, Narumiya S. (1998) Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol 141:1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Zhou L, Yang QD, Du XP, Li M, Yuan M, Zhou ZW. (2012) Changes in hippocampal synapses and learning-memory abilities in a streptozotocin-treated rat model and intervention by using fasudil hydrochloride. Neuroscience 200:120–129. [DOI] [PubMed] [Google Scholar]
- Huang P, Li C, Fu T, Zhao D, Yi Z, Lu Q, Guo L, Xu X. (2015) Flupirtine attenuates chronic restraint stress-induced cognitive deficits and hippocampal apoptosis in male mice. Behav Brain Res 288:1–10. [DOI] [PubMed] [Google Scholar]
- Inan SY, Soner BC, Sahin AS. (2015) Infralimbic cortex Rho-kinase inhibition causes antidepressant-like activity in rats. Prog Neuropsychopharmacol Biol Psychiatry 57:36–43. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. (1999) Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. (1996) Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273:245–248. [DOI] [PubMed] [Google Scholar]
- Licznerski P, Duman RS. (2013) Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience 251:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Koleske AJ. (2010) Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci 33:349–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. (1997) The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol 8:523–532. [DOI] [PubMed] [Google Scholar]
- Luo L. (2000) Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci 1:173–180. [DOI] [PubMed] [Google Scholar]
- Luo L. (2002) Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol 18:601–635. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. (1999) Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285:895–898. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ (2010) Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci 1186:190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Seeman T (1999) Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci 896:30–47. [DOI] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L. (2000) Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci 20:5329–5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama AY, Luo L. (2000) Intracellular signaling pathways that regulate dendritic spine morphogenesis. Hippocampus 10:582–586. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CW. 1982. The rat brain in stereotaxic coordinates. Waltham, MA: Academic Press. [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M (2005) Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 177:245–255. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33:88–109. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Blavet N, Deniel M, Jalfre M. (1979) Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. Eur J Pharmacol 57:201–210. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. (2010) Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HK, Salomone S, Potts EM, Lee SW, Millican E, Noma K, Huang PL, Boas DA, Liao JK, Moskowitz MA, Ayata C. (2007) Rho–kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J Cereb Blood Flow Metab 27:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. (2014) From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 140:774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AV, Bradshaw D, Ramos S, Murphy C, Myers CE, Somlyo AP. (2000) Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochem Biophys Res Commun 269:652–659. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV (2000) Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522Pt 2:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Chen X, Wang LY, Gao W, Zhu MJ. (2013) Rho kinase inhibitor fasudil protects against beta-amyloid-induced hippocampal neurodegeneration in rats. CNS Neurosci Ther 19:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanger SA, Mattheyses AL, Gentry EG, Herskowitz JH. (2015) ROCK1 and ROCK2 inhibition alters dendritic spine morphology in hippocampal neurons. Cell Logist 5:e1133266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Yuste R. (2004) Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci 26:429–440. [DOI] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. (2013) Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa JL, Castaneda P, Berrios C, Diaz-Veliz G, Mora S, Bravo JA, Araneda K, Menares C, Morales P, Fiedler JL (2010) Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol Biochem Behav 97:213–221. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S (2002) Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22:6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. (2012) A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XE, Zhang FY, Wang K, Zhang QX, Rong LQ. (2014) Fasudil hydrochloride protects neurons in rat hippocampal CA1 region through inhibiting GluR6-MLK3-JNKs signal pathway. Cell Biochem Biophys 70:415–421. [DOI] [PubMed] [Google Scholar]
- Wu J, Li J, Hu H, Liu P, Fang Y, Wu D. (2012) Rho-kinase inhibitor, fasudil, prevents neuronal apoptosis via the Akt activation and PTEN inactivation in the ischemic penumbra of rat brain. Cell Mol Neurobiol 32:1187–1197. [DOI] [PubMed] [Google Scholar]
- Yoshimi E, Kumakura F, Hatori C, Hamachi E, Iwashita A, Ishii N, Terasawa T, Shimizu Y, Takeshita N. (2010) Antinociceptive effects of AS1892802, a novel Rho kinase inhibitor, in rat models of inflammatory and noninflammatory arthritis. J Pharmacol Exp Ther 334:955–963. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Meng Y, Asrar S, Todorovski Z, Jia Z. (2009) A critical role of Rho-kinase ROCK2 in the regulation of spine and synaptic function. Neuropharmacology 56:81–89. [DOI] [PubMed] [Google Scholar]