Abstract
Atopic dermatitis (AD) is an inflammatory skin condition accompanied by symptoms such as edema and hemorrhage. Kimchi is a traditional fermented Korean dish consisting of various probiotics. In this study, the therapeutic effect of Lactobacillus plantarum CJLP55 isolated from Kimchi was studied in AD-induced mice. Orally administered Lactobacillus strain, CJLP55, suppressed AD symptoms and high serum IgE levels. CJLP55 administration reduced the thickness of the epidermis, infiltration of mast cells and eosinophils into the skin lesion, enlargement of axillary lymph nodes, and increase in cell population in axillary lymph nodes. CJLP55 treatment decreased the production of type 2 cytokines, such as interleukin (IL)-4, IL-5, IL-10, IL-12, interferon (IFN)-γ, and IL-6,which were stimulated by house dust mite extracts, in the axillary lymph node cells. Orally administered CJLP55 exhibited a therapeutic effect on house dust mite-induced AD in NC/Nga mice after onset of the disease by altering immune cell activation. The Lactobacillus strain, CJLP55, isolated from Kimchi, suppressed AD. Our results suggest its possible use as a potential candidate for management of AD.
Keywords: Atopic, Dermatitis, Dermatophagoides farinae, Lactobacillus, Probiotics, Th2 cells
INTRODUCTION
Atopic dermatitis (AD) is a common inflammatory skin disease affecting 15~30% of the children and 2~10% of the adults [1]. Inappropriate immune response, especially excessive Th2 response, plays an important role in the pathogenesis of AD [2]. Although genetic background is known as an important risk factor for the disease, increased prevalence of the disease due to modernisation of lifestyle suggests that environmental factors also importantly act on the onset of the disease. The composition of the gut microbiota can be altered because of bacterial infections, antibiotic treatment, lifestyle changes, surgical intervention, and instant food consumption [3]. The altered microbiota can affect the immune system and induce misdirected immune responses, rather than immune tolerance, in people with modernised lifestyle. It contributes to increase and induce allergies such as AD and rhinitis [4].
Probiotics are generally defined as live microorganisms that are thought to confer health benefits when consumed [5]. Oral administration of probiotics is an attractive approach to modulate immune responses by altering the microbiota. Therefore, numerous studies have been conducted to evaluate the effect of probiotics on a variety of immune disorders [6,7]. In our previous study, we investigated the immunomodulatory effects of 26 strains belonging to Lactobacillus spp. isolated from Kimchi, a traditional fermented Korean dish among them, four strains were identified to be effective in maintaining homeostasis between Th1 and Th2 responses in vitro [8]. In order to elucidate whether the four selected strains affect the induction of AD, the symptoms and immune responses were studied in mice with AD induced by the topical application of house dust mite Dermatophagoides farinae body (Dfb) extract followed by administration of the strains [9]. Two of the four selected strains, CJLP55 and CJLP133, showed a definite effect on immune response modulation during the induction of AD. Although it is very meaningful to identify the lactic acid bacteria that have a preventive effect on AD, they do not show the effect on patients already suffering from AD. Based on the questions that arose from a prior study [9], in this study, we investigated whether the administration of CJLP55 can treat AD after its onset. To examine the therapeutic effect of CJLP55, AD was primarily induced for three weeks in NC/Nga mice; later, CJLP55 strain was administered in mice for eight weeks. We also examined the effect of Lactobacillus spp. on infiltration of effector cells into the skin lesion and immunological responses from the axillary lymph nodes.
METHODS
Animals
Six-week-old female mice were purchased from Central Lab Animal Incorporation (Seoul, Korea), randomised, and maintained under specific pathogen-free conditions under controlled temperature of 24±2℃ and light/dark cycle of 12 h/12 h. All experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the protocol was approved by the Chung-Ang University Institutional Animal Care and Use Committee of the Laboratory Animal Research Center (IACUC number: 14-0034).
Induction of AD
The dorsal portion of the mice were shaved using an electric clipper and hair removal cream. In order to disrupt the skin barrier, 150 µL of 4% sodium dodecyl sulfate was applied on the shaved surface 4 h before Dfb ointment application. Then, 100 mg Biostir AD (Biostir, Hiroshima, Japan), an ointment containing Dfb extract, was applied onto the precisely shaved dorsal surface. Non-induction mice were applied vaseline instead of Dfb extract. Topical application of the ointment was accomplished twice a week for 21 d (Fig. 1A). From day zero, the mice were administered dietary powder mixed with lyophilised Lactobacillus strains daily, and the ointment was applied once a week to maintain AD induced by the Dfb extract.
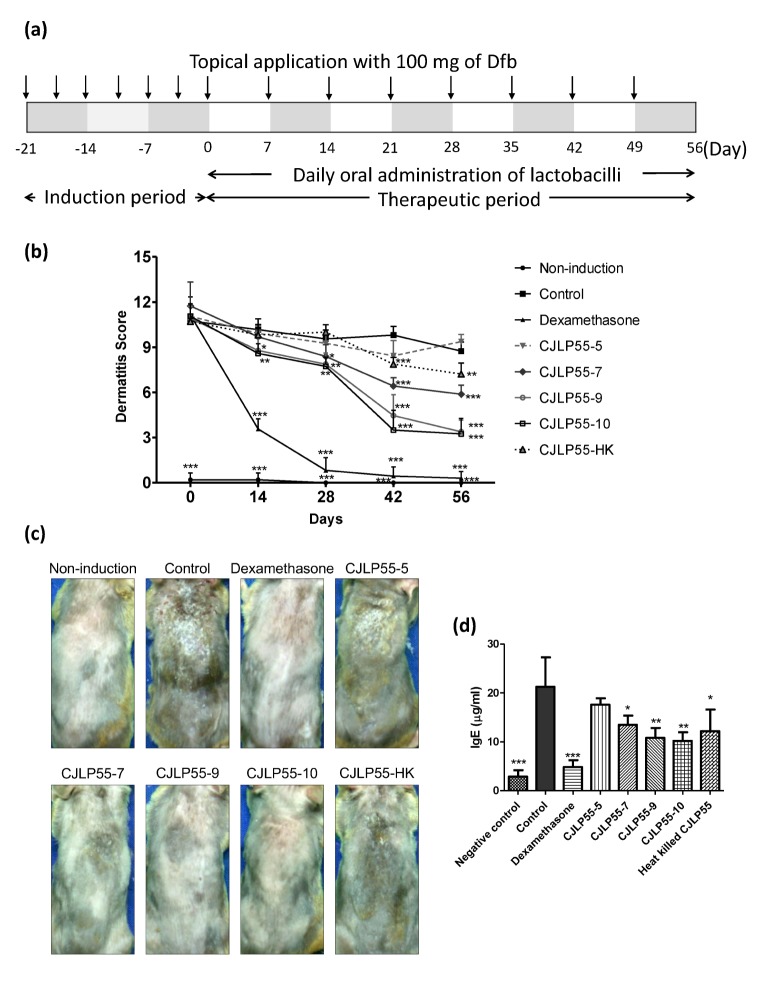
Fig. 1. Experimental procedure and effects of orally administered CJLP55 on atopic dermatitis induced by house dust mite extract.
(a) Experimental procedure for induction of atopic dermatitis and administration of lactobacilli was illustrated. Day zero is defined as the first day of administration of CJLP55 strain. (b) The dermatitis scores were evaluated by observing erythema/hemorrhage, scarring/dryness, excoriation/erosion, and edema. The score was noted once every two weeks until day 56. (c) The photographs were obtained in week eight before the mice were sacrificed. The photographs of NC/Nga mice with Dfb-induced dermatitis were obtained on day 55. (d) The level of total IgE in collected serum on day 28 was determined by ELISA. Data are shown as mean±SD of changes in the dermatitis score and total IgE level of five mice (n=5). *p<0.05; **p<0.01; ***p<0.001 compared with control.
Microorganisms and diets
Lactobacillus plantarum CJLP55 (KCTC 11401BP) was obtained from CJ Foods R&D Center, CJ CheilJedang Corporation (Suwon-si, Gyeonggi-do, Korea). Lactobacilli were incubated in MRS broth at 37℃ for 24 h and were lyophilised. A mouse was fed 5 g of dietary powder daily, which was uniformly mixed with 1×105, 1×107, 1×109, 1×1010 CFU of CJLP55, respectively. In the case of heat-killed CJLP55 group, mice were administered with 1×1010 CFU of heat-killed CJLP55 daily. Fresh feed was maintained by replacing the feed daily. Dexamethasone, positive control for treatment, was applied to the dorsal skin twice a week (50 µg/mouse).
Evaluation of dermatitis scores
The severity of dermatitis was evaluated on the 0, 14, 28, 42, and 56 d. The degree of four symptoms—erythema/hemorrhage, scarring/dryness, excoriation/erosion, and edema—was scored as, zero (none), one (mild), two (moderate), or three (severe). The dermatitis score was calculated as the sum of these four scores.
Measurement of IgE in serum
On day 28, blood specimens were collected and total immunoglobulin E (IgE) levels were measured by ELISA. Briefly, immunoplates were coated with the purified IgE antibody overnight, blocked for 1 h with 3% BSA in PBS, and then incubated with appropriately diluted serum samples overnight. The bound IgE was detected using biotinylated antibodies and streptavidin-alkaline phosphatase. Nitrophenyl phosphate was used as a substrate for color development.
Histological analysis
The dorsal skins were excised on d56, fixed in 10% phosphate-buffered formalin, and embedded in paraffin. The skin sections were cut at 4-µm thickness, and stained with hematoxylin and eosin (H&E) for the measurement of skin thickness. Toluidine blue and Congo red stains were used to analyse the number of mast cells and eosinophils, respectively. Cell infiltration was observed under a microscope at 400× magnification.
Evaluation of cell population in the axillary lymph nodes
For evaluation of total cell count, the axillary lymph nodes were homogenised, and the cells in suspension were stained with 0.4% trypan blue. For the determination of number of T cells, B cells, macrophages, and dendritic cells, the cell suspension was incubated with FITC-labeled anti-Thy1.2, anti-CD19, anti-F4/80, and anti-CD11c monoclonal antibodies, respectively, on ice for 30 min. The cell populations were investigated by using a BD Biosciences FACSCalibur using CellQuest Pro software.
Determination of cytokine production in the axillary lymph node cells
The cells purified from the axillary lymph nodes were cultured with 10 µgmL−1 Dfb extract for 48 h. The culture supernatants were collected for the measurement of cytokine levels. Interleukin (IL)-4, IL-5, IL-10, IL-12, interferon (IFN)-γ, and IL-6 levels were measured by ELISA as described above.
Statistical analysis
Statistical interpretation was conducted by Student's t-test. Results are expressed as mean±SD. p values<0.05 were considered significant and are indicated by asterisks in the figures.
RESULTS
Effect of orally administered CJLP55on AD symptoms inNC/Nga mice
To evaluate the therapeutic effect of CJLP55 on AD, AD was induced in mice by topical application of Dfb extract for 21 d prior to the administration of Lactobacillus spp. Mice with induced AD showed similar degree of symptoms. On day zero, mice were administered diets containing various concentrations of CJLP55, and the Dfb application was performed once a week to maintain AD. On day 14, mice treated with dexamethasone, a positive control used in the study, exhibited a significant recovery of the skin lesion (Fig. 1B and 1C). High doses of CJLP55, such as 109 and 1010 CFU/mouse, showed a little improvement in the dermatitis score, while low doses of CJLP55, such as 105 and 107 CFU/mouse, and heat-killed CJLP55-treated group showed no effect on the score. After day 28, symptoms of dermatitis almost disappeared in the dexamethasone-treated mice. All CJLP55-administered groups without CJLP55-5 group, displayed a great recovery in a dose dependent manner after day 42. On the final day of experiment, CJLP55-9 and CJLP55-10 administration reduced the dermatitis score to more than half of that of control, and the difference in the effect was not observed for 109 and 1010 CFU/mouse group. Administration of CJLP55-7 clearly diminished the manifestation of dermatitis, but showed only half of the effect than that of CJLP55-9 and CJLP55-10. CJLP55-HK also exhibited the effect by reducing the dermatitis score, although less effect than 107 live bacteria. The lowest concentration of CJLP55 did not notably lower the dermatitis score throughout the experimental period. Additionally, it is well known that serum IgE concentration is generally elevated in patients with AD. As shown in Fig. 1D, IgE level in the control group was significantly higher than that in mice in the non-induction group. CJLP55-administered mice showed lower serum IgE levels with the exception of mice administered with 105 CFU of CJLP55. Similarly to regulation of dermatitis symptom, CJLP55-9 and CJLP55-10 showed higher effect on IgE regulation, without a significant difference between the two groups. Heat-killed CJLP55 administration also downregulated IgE levels in serum. Thus, CJLP55 possibly may have a therapeutic effect that could alleviate the symptoms of AD depending on the concentration of the administered strain. Furthermore, heat-killed bacteria showed a therapeutic effect, although a weaker effect than living lactic acid bacteria.
CJLP55 administration decreased skin thickness induced by Dfb application
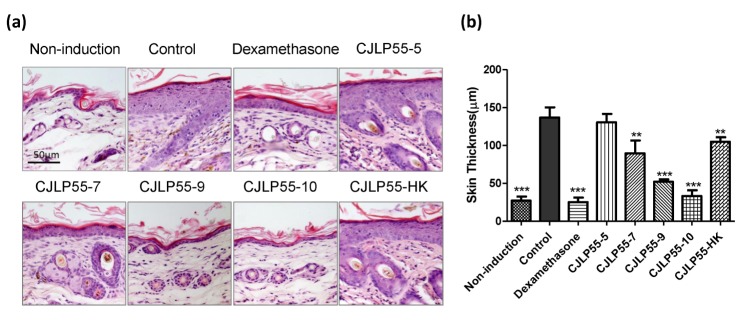
Lichenification is a symptom of chronic AD that is characterised by thickening and darkening of the skin. After the administration of lactic acid bacteria for 56 d, H&E staining was conducted on the dorsal skin lesion. The thickness of the dermis was measured to evaluate the progress of lichenification. The mice in the control group showed epidermal hyperplasia, but it was not observed in mice in the non-induction group (Fig. 2A and 2B). The epidermal layer was five times thicker in mice in the control group than in mice in the non-induction group. This indicates that lichenification clearly occurred in dermatitis induced by Dfb extract. Dexamethasone-treated mice showed recovery in the increased thickness of the skin, which was similar to mice without dermatitis. In contrast, administration of 107, 109, and 1010 CFU of CJLP55 exhibited a dose-dependent reduction in the skin thickness. Heat-killed CJLP55 also showed an effect on restoration of lichenification, but displayed only a similar degree of effect as shown by CJLP55-7. Collectively, administration of lyophilised CJLP55 prevents dermal hyperplasia, except at a low dose, and heat-killed strains exhibit the effect, which is weaker than that of the live strain.
Fig. 2. Effect of orally administered CJLP55 on the thickness of the dorsal skin.
(a) Histological analysis of the skin lesion after H&E staining. This is a representative image of five mice. (b) Epidermal thickness of the dorsal skin was measured. Data are shown as mean±SD of changes in the skin thickness of five mice (n=5). *p<0.05; **p<0.01; ***p<0.001 compared with control.
Local infiltration of mast cells and eosinophils after administration of CJLP55
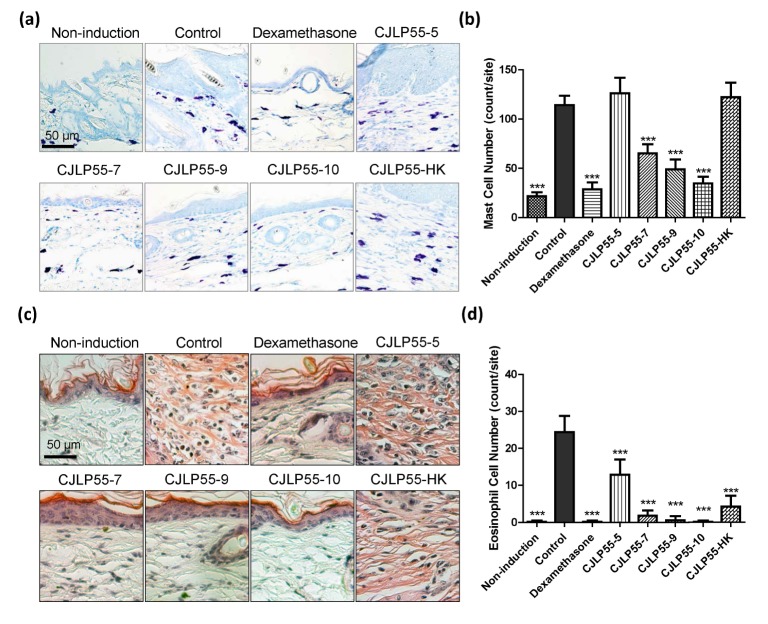
Infiltration of mast cells and eosinophils in the skin is involved in the induction of AD. Infiltrated mast cells in the skin lesion were visualised with toluidine blue and counted (Fig. 3A and 3B). Consistent with the results of dermatitis symptoms, these results showed that dexamethasone effectively regulated the mast cell infiltration during induction of AD, and CJLP55 suppressed the accumulation of the mast cells in the dorsal skin lesion in a dose dependent manner, excluding CJLP55-5. Unlike previous result of CJLP55-HK, heat-killed CJLP55 administration did not show any effect on infiltration of mast cells. Histological analysis by Congo red staining indicated that the number of eosinophils was significantly increased in the skin of AD-induced mice (Fig. 3C and 3D). The mice treated with Dexamethasone and all doses of CJLP55 blocked eosinophil infiltration in the skin lesion. Heat-killed CJLP55 also suppressed the eosinophil infiltration to 20% of that noted in mice in the control group. Thus, CJLP55 administration suppressed infiltration of mast cells and eosinophils, and the effect on the eosinophils was more distinct than that on the mast cells.
Fig. 3. Effects of orally administered CJLP55 on infiltration of mast cells and eosinophils in the inflammatory site.
The paraffin block of the dorsal skin was sectioned and stained with toluidine blue (a) and congo red (c) for mast cells and eosinophils, respectively. The number of mast cells (b) and eosinophils (d) were counted under a microscope. Data are shown as mean±SD of five mice. *p<0.05; **p<0.01; ***p<0.001 compared with control.
Population of the axillary lymph node cells regulated by orally administered CJLP55
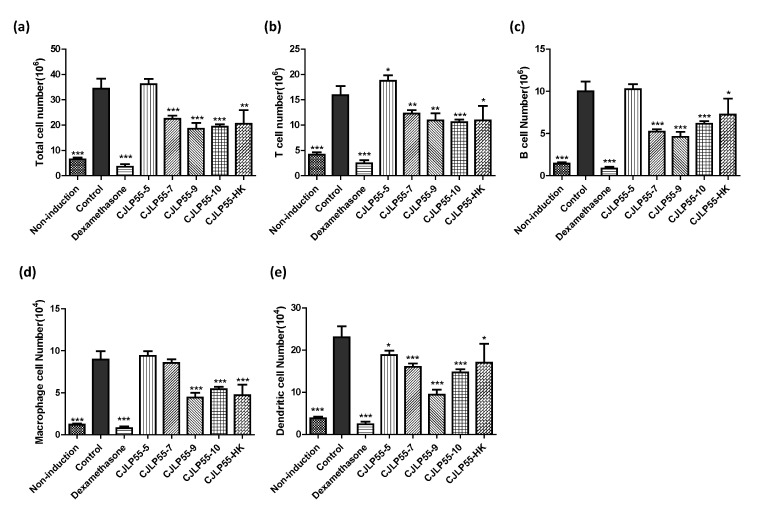
The axillary lymph nodes are a type of lymphoid organs present near the dorsal surface of the skin, and are involved in eliciting immune response in mice with AD. Enlargement of the axillary lymph nodes was observed in mice with AD, but not in non-induction mice. Therefore, we analysed the change in number of immune cells in the axillary lymph nodes to elucidate the alteration in lymphoid organ near the skin. Total cell number in the axillary lymph node of mice in the control group increased five-fold as compared to that in the axillary lymph node of mice in the non-induction group, which can be inferred from the change in the size of the lymph node (Fig. 4A). The number of T cells, B cells, macrophages, and dendritic cells was analysed along with the total number of cells in axillary lymph node. The mice with AD in the control group showed a significant increase in the number of immune cells as compared to mice without the disease in the non-induction group (Fig. 4B~4E). The number of total cells, T cells, B cells, macrophages, and dendritic cells were extremely low in dexamethasone-treated mice than in non-induction mice. Oral administration of 109 and 1010 CFU of CJLP55 lowered the number of cells analysed in these experiments. The lowest dose of CJLP55 did not show any effect on controlling the number of most cells increased by AD, and a slight decrease in the effect was observed in the case of dendritic cells. In contrast, administration of 107 CFU of CJLP55 also showed the regulatory effect on increased immune cells, except on macrophages. In mice administered with heat-killed CJLP55, the cell number modulating effect was noted to be similar to that of the high dose group. These results suggest that administration of CJLP55 lowered the number of immune cells increased due to dermatitis.
Fig. 4. Effects of orally administered CJLP55 on the axillary lymph nodes in NC/Nga mice.
(a) Total cell number was measured from cell suspensions of the axillary lymph nodes. The number of T cells (b), B cells (c), macrophages (d), and dendritic cells (e) was analysed by flow cytometry. Data are shown as mean±SD of five mice. *p<0.05; **p<0.01; ***p<0.001 compared with control.
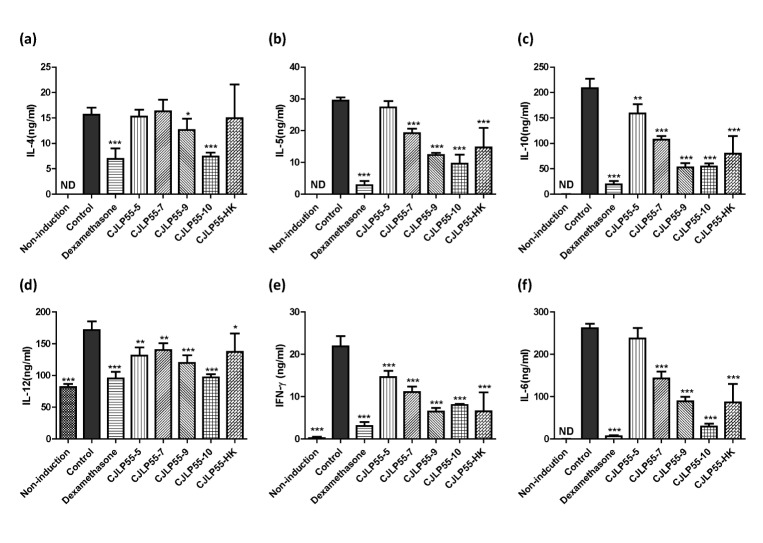
Administration of CJLP55 decreases the cytokine production in lymph node cells
To determine whether orally administered CJLP55 affects the production of various cytokines, cells isolated from the axillary lymph nodes were cultured with Dfb extract for 48 h, and then, the concentration of cytokines in the supernatant was analysed. Levels of Th2 cytokines, such as IL-4 and IL-5, produced by the lymph node cells were increased in AD-induced mice in control group without Lactobacillus administration (Fig. 5A and B). Administration of more than 109 CFU of CJLP55 decreased the production of the two Th2 cytokines in a dose dependent manner. However, 107 CFU CJLP55 and heat-killed strain exhibited the reducing effect on the production of IL-5 but not IL-4, and the lowest dose of CJLP55 did not show a regulatory effect on both cytokines. The elevated production of IL-10, IL-12, IFN-γ, and IL-6 in mice in the control group was lowered by administration of CJLP55 in a dose dependent manner. Heat-killed CJLP55 showed a regulatory effect on the production of these cytokines. These results suggest that CJLP55 strain decreased the production of various cytokines regardless of sub-population of the T cells.
Fig. 5. Effects of orally administered CJLP55 on the cytokine production in the axillary lymph node cells.
On day 56, axillary lymph node cells were isolated and cells were stimulated with 10 µgmL−1 of Dfb extract for 48 h. The concentration of IL-4 (a), IL-5 (b), IL-10 (c), IL-12 (d), IFN-γ (e), and IL-6 (f) was measured by ELISA. Data are shown as mean±SD of three independent experiments. *p<0.05; **p<0.01; ***p<0.001 compared with control.
DISCUSSION
Modulating allergic disease using probiotic is a promising strategy and it has been reported in many studies [10]. In particular, among various allergic disease, studies about the role of the probiotic in atopic dermatitis have been briskly conducted. Although various types of organisms belong to the probiotic, lactobacilli and bifidobacteria which are well known to be effective in atopic dermatitis [11,12]. In our previous study, we described the prophylactic effect of lactobacilli CJLP55 that is able to suppress the induction of AD in NC/Nga mice [9]. Other lactobacilli were also tested for their suppressive effect in the development of AD [13,14,15]. The experiments in this study were accomplished similar to those in our previous study. These previous studies focused on the preventive effect of lactic acid bacteria on AD because the induction of AD was performed after the administration of Lactobacillus spp. Therefore, we wanted to study if CJLP55 has a therapeutic effect on AD induced by Dfb extract along with its preventive effect. AD was preferentially induced for 21 d and CJLP55 was administered in mice for the next 56 d. On day zero, the mice in the control group exhibited a similar dermatitis score as the mice in the other group without non-induction group. After the administration of the lactic acid bacteria, the dermatitis score reduced in a dose dependent manner. The dermatitis scores were evaluated by observing erythema/hemorrhage, scarring/dryness, excoriation/erosion, and edema as defined by Matsuda et al. [16]. Therefore, it was observed that the administration of CJLP55 effectively regulated various clinical symptoms associated with AD. IgE dysregulation is correlated with severity of AD and is affected by abnormality of the skin barrier, a key factor in AD [16,17]. IgE level was determined to assess the degree of AD because it is observed in many other studies with a model similar to that in our study [18,19,20]. In this study, significant increase in serum IgE was observed in the mice with AD in the control group. Orally administered live and heat-killed CJLP55 decreased the serum IgE levels. It suggests a possibility that CJLP55 is able to modulate the immune activation of mast cells and eosinophils, because these cells have the surface receptor for IgE to induce their activation and secretion of inflammatory mediators. In the present study, the skin thickness was used as an indicator of lichenification, frequently found in chronic AD. The dermal thickness in CJLP55-administered mice was lowered in a dose dependent manner as compared to that in mice in the control group. It indicates that CJLP55 administration improves the skin condition decreasing discomfort and deformation of the skin. The infiltration of various immune cells, such as mast cells and eosinophils, into the skin lesion is a characteristic feature of AD [21,22]. In the skin of mice with AD, mast cell infiltration was significantly increased and the administration of CJLP55 over 107 CFU/mouse reduced the infiltration. Mast cells play a central role in AD through degranulation of inflammatory mediators by binding IgE onto the cells. Decreased infiltration of mast cells by CJLP55 administration is expected to have a role in reducing inflammation of the skin. The infiltration of eosinophils was effectively regulated by orally administered CJLP55, more than the regulation of mast cells. Although tissue eosinophilia is not a key factor in AD, it is believed to mediate the pathogenetic late-phase immune reaction involved in the destruction of the inflamed tissue [23]. Eosinophils also play an important role in inducing allergic reaction through the production of cytokines, such as IL-4 and IL-13. These two cytokines act as regulators of T and B cells [24]. Based on the role of eosinophils, their regulation by CJLP55 may affect the late-phase reactions and the function of T and B cells. We also analysed the cell population in the axillary lymph node. In mice with AD, the axillary lymph nodes were enlarged and the total cell number was significantly increased. The number of T and B cells, involve in adaptive immune responses, were also increased. Dendritic cells and macrophages, participating in antigen presentation, were also augmented in the axillary lymph node by induction of AD. Immune cell accumulation by excessive immune activation was adequately regulated after high dose of CJLP55 was administered in mice. This result suggests that Lactobacillus spp. isolated from Kimchi have the ability to regulate immune responses. To confirm the immunological responses in the local tissue, the cells from axillary lymph nodes were isolated from the experimental mice on day 56 and cultured with Dfb extracts to determine the alteration in cytokine production by lymphocytes. High dose of CJLP55 inhibited the production of Th2 cytokines, such as IL-4 and IL-5, expectedly. Various cell types produce IL-10. IL-10 is generally known to regulate immune responses efficiently, but it also plays an important role in Th2 response to antigen and in eosinophil infiltration in the skin [25]. The production of IL-10 was significantly increased in AD-induced mice, and CJLP55 alleviated the production in a dose dependent manner. It is well known that Th1 and Th2 differentiation are mutually controlled by IL-12 and IFN-γ, respectively, which are representative Th1 cytokines [26]. However, the levels of IL-12 and IFN-γ are increased in chronic AD [27]. Although immune reaction of Th2 cells is mainly responsible for the development and progress of AD, Th1 cells are also involved in chronic states of the disease. In our experimental setting, the production of cytokines induced by Dfb stimulation was analysed. Dfb-sensitised lymphocytes showed a more strong response to the antigen than the non-sensitised lymphocytes did, and the production of IL-12 and IFN-γ was significantly increased in mice in the control group. Orally administered CJLP55 regulated the level of these two cytokines and it indicates that Lactobacillus spp. regulated lymphocyte activation induced by antigen stimulation. The association of IL-6 to AD has not been fully known until now. Increased IL-6 production and the difference of IL-6 genotype was observed in patients with AD [28,29], but most studies involving AD do not confirm the effect on IL-6, maybe because of its unexplained mechanisms. In this study, IL-6 production from the lymph node cells was increased in mice in the control group, and CJLP55 suppresses it in a dose dependent manner. This result suggests the need to further study about the role and regulation of IL-6 in AD.
Heat-killed CJLP55-administered mice were maintained as an experimental group in this study because some studies reported that non-viable microbes also exhibit the health benefits of probiotics [30]. In our study, 1010 CFU of live CJLP55 were heat-killed and administered in mice. The mice in this experimental group showed an improvement in AD, which was similar to the effect of 107 CFU of live lactobacilli-administered group. These results indicate that heat-killed CJLP55 also has a therapeutic effect due to its component, but it causes a reduced effect as compared to that of live microbes. Other previous studies have reported that dead lactic acid bacteria have immunomodulatory effects similar to this study. In these studies, it is anticipated that the components of the cell wall affect the innate immune cells and thus exhibit immunomodulatory effects. However, studies that elucidate the specific component of lactic acid bacteria have not been done at present. It should be studied further for obtaining a clear understanding of Lactobacillus spp. and its application in human diseases.
In conclusion, orally administered viable and heat-killed CJLP55 suppressed the skin inflammation and AD-like skin lesions, and altered the immune cell populations and activation. These results suggest that CJLP55 may have a therapeutic potential in the treatment of AD.
ACKNOWLEDGEMENTS
This research was supported by the Chung-Ang University Excellent Student Scholarship and the CJ CheilJedang Corporation.
Footnotes
Author contributions: S.J.K. and D.K.L. managed overall mice-related experiments. Y.W.J. and K.H.H. performed the cell-based assay experiments. E.S.P. performed histological analysis. B.S.M., B.J.K. and H.Y.A. performed preparation of lactic acid bacteria. K.W.H. S.Y.P. and U.D.S. supervised and coordinated the study. H.K.E. wrote the manuscript and managed whole experiments.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogg G. Role of T cells in the pathogenesis of atopic dermatitis. Clin Exp Allergy. 2009;39:310–316. doi: 10.1111/j.1365-2222.2008.03146.x. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, Marchesi JR, Collado MC. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050. doi: 10.3402/mehd.v26.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West CE, Jenmalm MC, Prescott SL. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin Exp Allergy. 2015;45:43–53. doi: 10.1111/cea.12332. [DOI] [PubMed] [Google Scholar]
- 5.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B6, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 6.Schiavi E, Barletta B, Butteroni C, Corinti S, Boirivant M, Di Felice G. Oral therapeutic administration of a probiotic mixture suppresses established Th2 responses and systemic anaphylaxis in a murine model of food allergy. Allergy. 2011;66:499–508. doi: 10.1111/j.1398-9995.2010.02501.x. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzaki T, Takagi A, Ikemura H, Matsuguchi T, Yokokura T. Intestinal microflora: probiotics and autoimmunity. J Nutr. 2007;137(3 Suppl 2):798S–802S. doi: 10.1093/jn/137.3.798S. [DOI] [PubMed] [Google Scholar]
- 8.Won TJ, Kim B, Song DS, Lim YT, Oh ES, Lee DI, Park ES, Min H, Park SY, Hwang KW. Modulation of Th1/Th2 balance by Lactobacillus strains isolated from Kimchi via stimulation of macrophage cell line J774A.1 in vitro. J Food Sci. 2011;76:H55–H61. doi: 10.1111/j.1750-3841.2010.02031.x. [DOI] [PubMed] [Google Scholar]
- 9.Won TJ, Kim B, Lim YT, Song DS, Park SY, Park ES, Lee DI, Hwang KW. Oral administration of Lactobacillus strains from Kimchi inhibits atopic dermatitis in NC/Nga mice. J Appl Microbiol. 2011;110:1195–1202. doi: 10.1111/j.1365-2672.2011.04981.x. [DOI] [PubMed] [Google Scholar]
- 10.Yang G, Liu ZQ, Yang PC. Treatment of allergic rhinitis with probiotics: an alternative approach. N Am J Med Sci. 2013;5:465–468. doi: 10.4103/1947-2714.117299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matricardi PM, Bjorksten B, Bonini S, Bousquet J, Djukanovic R, Dreborg S, Gereda J, Malling HJ, Popov T, Raz E, Renz H, Wold A EAACI Task Force 7. Microbial products in allergy prevention and therapy. Allergy. 2003;58:461–471. doi: 10.1034/j.1398-9995.2003.00175.x. [DOI] [PubMed] [Google Scholar]
- 12.Prescott SL, Björkstén B. Probiotics for the prevention or treatment of allergic diseases. J Allergy Clin Immunol. 2007;120:255–262. doi: 10.1016/j.jaci.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Segawa S, Hayashi A, Nakakita Y, Kaneda H, Watari J, Yasui H. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates the development of dermatitis and inhibits immunoglobulin E production in atopic dermatitis model NC/Nga mice. Biol Pharm Bull. 2008;31:884–889. doi: 10.1248/bpb.31.884. [DOI] [PubMed] [Google Scholar]
- 14.Tobita K, Yanaka H, Otani H. Heat-treated Lactobacillus crispatus KT strains reduce allergic symptoms in mice. J Agric Food Chem. 2009;57:5586–5590. doi: 10.1021/jf900703q. [DOI] [PubMed] [Google Scholar]
- 15.Sunada Y, Nakamura S, Kamei C. Effect of Lactobacillus acidophilus strain L-55 on the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol. 2008;8:1761–1766. doi: 10.1016/j.intimp.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, Matsumoto M, Ushio H, Saito S, Askenase PW, Ra C. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 1997;9:461–466. doi: 10.1093/intimm/9.3.461. [DOI] [PubMed] [Google Scholar]
- 17.Liu FT, Goodarzi H, Chen HY. IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:298–310. doi: 10.1007/s12016-011-8252-4. [DOI] [PubMed] [Google Scholar]
- 18.Lim HS, Ha H, Lee MY, Jin SE, Jeong SJ, Jeon WY, Shin NR, Sok DE, Shin HK. Saussurea lappa alleviates inflammatory chemokine production in HaCaT cells and house dust mite-induced atopic-like dermatitis in Nc/Nga mice. Food Chem Toxicol. 2014;63:212–220. doi: 10.1016/j.fct.2013.10.050. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka H, Maki N, Yoshida S, Arai M, Wang J, Oikawa Y, Ikeda T, Hirota N, Nakagawa H, Ishii A. A mouse model of the atopic eczema/dermatitis syndrome by repeated application of a crude extract of house-dust mite Dermatophagoides farinae. Allergy. 2003;58:139–145. doi: 10.1034/j.1398-9995.2003.23790.x. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Haruna T, Yasui K, Takahashi H, Iduhara M, Takaki S, Deguchi M, Arimura A. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergol Int. 2007;56:139–148. doi: 10.2332/allergolint.O-06-458. [DOI] [PubMed] [Google Scholar]
- 21.Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, Nakanishi K, Yamanishi K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staumont-Sallé D, Fleury S, Lazzari A, Molendi-Coste O, Hornez N, Lavogiez C, Kanda A, Wartelle J, Fries A, Pennino D, Mionnet C, Prawitt J, Bouchaert E, Delaporte E, Glaichenhaus N, Staels B, Julia V, Dombrowicz D. CX3CL1 (fractalkine) and its receptor CX3CR1 regulate atopic dermatitis by controlling effector T cell retention in inflamed skin. J Exp Med. 2014;211:1185–1196. doi: 10.1084/jem.20121350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapp A. The role of eosinophils in the pathogenesis of atopic dermatitis--eosinophil granule proteins as markers of disease activity. Allergy. 1993;48:1–5. doi: 10.1111/j.1398-9995.1993.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 24.Simon D, Braathen LR, Simon HU. Eosinophils and atopic dermatitis. Allergy. 2004;59:561–570. doi: 10.1111/j.1398-9995.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 25.Laouini D, Alenius H, Bryce P, Oettgen H, Tsitsikov E, Geha RS. IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J Clin Invest. 2003;112:1058–1066. doi: 10.1172/JCI18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fishman MA, Perelson AS. Th1/Th2 differentiation and cross-regulation. Bull Math Biol. 1999;61:403–436. doi: 10.1006/bulm.1998.0074. [DOI] [PubMed] [Google Scholar]
- 27.Grewe M, Bruijnzeel-Koomen CA, Schöpf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, Krutmann J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998;19:359–361. doi: 10.1016/s0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]
- 28.Toshitani A, Ansel JC, Chan SC, Li SH, Hanifin JM. Increased interleukin 6 production by T cells derived from patients with atopic dermatitis. J Invest Dermatol. 1993;100:299–304. doi: 10.1111/1523-1747.ep12469875. [DOI] [PubMed] [Google Scholar]
- 29.Gharagozlou M, Farhadi E, Khaledi M, Behniafard N, Sotoudeh S, Salari R, Darabi B, Fathi SM, Mahmoudi M, Aghamohammadi A, Amirzargar AA, Rezaei N. Association between the interleukin 6 genotype at position -174 and atopic dermatitis. J Investig Allergol Clin Immunol. 2013;23:89–93. [PubMed] [Google Scholar]
- 30.Kataria J, Li N, Wynn JL, Neu J. Probiotic microbes: do they need to be alive to be beneficial? Nutr Rev. 2009;67:546–550. doi: 10.1111/j.1753-4887.2009.00226.x. [DOI] [PubMed] [Google Scholar]