Abstract
Prostaglandin D2 (PGD2) may act against myocardial ischemia-reperfusion (I/R) injury and play an anti-inflammatory role in the heart. Although the effect of PGD2 in regulation of ANP secretion of the atrium was reported, the mechanisms involved are not clearly identified. The aim of the present study was to investigate whether PGD2 can regulate ANP secretion in the isolated perfused beating rat atrium, and its underlying mechanisms. PGD2 (0.1 to 10 µM) significantly increased atrial ANP secretion concomitantly with positive inotropy in a dose-dependent manner. Effects of PGD2 on atrial ANP secretion and mechanical dynamics were abolished by AH-6809 (1.0 µM) and AL-8810 (1.0 µM), PGD2 and prostaglandin F2α (PGF2α) receptor antagonists, respectively. Moreover, PGD2 clearly upregulated atrial peroxisome proliferator-activated receptor gamma (PPARγ) and the PGD2 metabolite 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2, 0.1 µM) dramatically increased atrial ANP secretion. Increased ANP secretions induced by PGD2 and 15d-PGJ2 were completely blocked by the PPARγ antagonist GW9662 (0.1 µM). PD98059 (10.0 µM) and LY294002 (1.0 µM), antagonists of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) signaling, respectively, significantly attenuated the increase of atrial ANP secretion by PGD2. These results indicated that PGD2 stimulated atrial ANP secretion and promoted positive inotropy by activating PPARγ in beating rat atria. MAPK/ERK and PI3K/Akt signaling pathways were each partially involved in regulating PGD2-induced atrial ANP secretion.
Keywords: Atrial natriuretic peptide, Mitogen-activated protein kinases, Peroxisome proliferator-activated receptor γ, Phosphatidylinositol-3-kinase, Prostaglandin D2
INTRODUCTION
Changes in the myocardial fatty acid composition of lipids were correlated with cardiovascular disease [1] and elevated phospholipase A2 (PLA2) activity caused accumulation of unesterified arachidonic acid (AA) in the heart [2,3]. Cardiac production of prostaglandin D2 (PGD2), derived from AA by the action of cyclooxygenase (COX), was demonstrated in the ischemic myocardium [4]. PGD2 may play both pro- and anti-inflammatory roles in different biological systems [5] through two distinct G-protein coupled receptors, the D-type prostanoid receptor (DP1) and the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2, also named DP2). Pronounced staining for both DP1 and DP2 proteins was observed in murine cardiomyocytes [4].
PGD2-derived metabolites were shown to significantly affect the cardiovascular system [6]. It was reported that 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2), the final dehydration product of PGD2, acted as a potent endogenous ligand for the nuclear peroxisome proliferator-activated receptor gamma (PPARγ) [7]. Nevertheless, recent findings suggested that 15d-PGJ2 also exerted a variety of cellular responses via PPARγ-independent mechanisms, for example, activation of mitogen-activated protein kinases (MAPKs) [8,9] and modulation of phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) signaling [10,11].
In addition, it was reported that PGD2 significantly stimulated atrial natriuretic peptide (ANP) secretion and caused a positive inotropic effect, primarily through the prostaglandin F2α receptor (FP) [12]. However, the mechanism by which PGD2 can stimulate atrial ANP secretion is not well known. Our study, therefore, aimed to investigate the mechanism of PGD2 induced regulation of atrial ANP secretion, using isolated perfused beating rat atria.
METHODS
Preparation of perfused beating rat atria
Sprague-Dawley rats of both sexes, weighing 250~300 g each, were used. The rats were decapitated and the isolated perfused beating left atria were prepared as previously described [12]. Once each perfused atrium was prepared, transmural electrical field stimulation with a luminal electrode was started at 1.5 Hz (0.3 ms, 30~40 V) and the atrium was perfused with HEPES buffer solution using a peristaltic pump (1.0 mL/min) to allow atrial pacing. The HEPES buffer contained (in µM) 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 10.0 glucose and 10.0 HEPES (pH 7.4 with NaOH), as well as 0.1% bovine serum albumin.
Experimental protocols
Each atrium was perfused for 60 min to stabilize the parameters of ANP secretion and mechanical dynamics. The perfusates were collected at 2-min intervals at 4℃ to assay for ANP levels.
The control period (12 min, as an experimental cycle) was followed by infusion of PGD2 (1.0 µM) or 15d-PGJ2 (0.1 µM) for 36 min, to determine changes in atrial ANP secretion in the perfusates and mechanical dynamics. Immediately after perfusion the atrial tissue was frozen and stored at −80℃ for subsequent western blotting.
To investigate the action mechanisms of PGD2-induced ANP secretion, a series of experiments was performed. After the control period, one pre-treatment cycle (12 min) was followed by 36-min of infusion of pre-treatment agent plus PGD2. The pre-treatment agents used in our study were: (1) AH-6809 (1.0 µM) and AL-8810 (1.0 µM), antagonists of PGD2 and PGF2α receptors, respectively; (2) GW 9662 (0.1 µM), an antagonist of PPARγ, and (3) PD98059 (10.0 µM) and LY294002 (1.0 µM), antagonists of MAPK/ERK and PI3K/Akt, respectively.
Measurement of ANP and atrial pulse pressure
The levels of ANP in the perfusates were measured by specific radioimmunoassay using an ANP assay kit (North Institute of Biological Technology, Beijing, China). Intra-assay and inter-assay coefficients of variation were less than 10 and 15%, respectively. The sensitivity of the assay is <0.05 ng/ml. All samples were assayed in the same run and at least in duplicate. The amounts of secreted ANP were expressed as ng/min/g of wet atrial tissue.
Intra-atrial pressure was recorded using a Physiograph (RM6240BD, Chengdu, China) via a pressure transducer (Statham P23Db, Oxnard, CA, USA) and pulse pressure was obtained from difference between systolic and diastolic pressure. The values of pulse pressure were expressed as cm-H2O.
Western blot analysis
Proteins from left atrial tissues were analyzed by western blotting. The atrial tissues were homogenized in radioimmunoprecipitation assay lysis buffer (Solarbio Institute of Biotechnology, Shanghai, China) and protein concentrations were determined using the Bradford Protein Assay Kit (BMG LABTECH, Offenburg, Germany). Solubilized protein was denatured in Lane Maker Loading Buffer (Cwbio, Beijing, China), separated by SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis on 10% or 8% gels and transferred to polyvinylidene difluoride filter membranes (Beyotime Institute of Biotechnology, Shanghai, China). Membranes were blocked with 5% nonfat dry milk in phosphate buffered saline (PBS) at room temperature. After 2 h, the membranes were incubated with rabbit phospho-PPARγ (S112) polyclonal antibody (1:1000; Elabscience, Wuhan, China) or with a rabbit polyclonal antibody to β-actin (1:1000; ComWin Biotech, Beijing, China) at 4℃ overnight. Membranes were incubated with secondary antibodies (1:1000; ZSGB-BIO, Beijing, China) for 2 h at room temperature. After extensive washing with phosphate buffered solution (PBST), bands were visualized with the ECL Plus western blotting detection system (ECL Western Blot Kit; CWBIO, Taizhou, China) and then quantified using Image J software (USA National Institute of Health, Bethesda, USA).
Statistical analysis
Significant differences between values were assessed by one-way ANOVA followed by Dunnett's multiple comparison test. An unpaired t-test was also applied. Statistical significance was defined as p<0.05. All data are presented as means±SEM.
RESULTS
Effects of PGD2 on atrial ANP secretion and pulse pressure
PGD2 significantly increased atrial ANP secretion, concomitantly with increased pulse pressure (both p<0.05 vs. control period; Fig. 1A and B). Different doses of PGD2 (0.1, 1.0 and 10.0 µM) clearly increased ANP secretion and atrial pulse pressure (all p<0.05 vs. control group; Fig. 2A and B). These results indicated that PGD2, in a dose-dependent manner, promoted atrial ANP secretion and a positive inotropic effect in isolated perfused beating rat atria.
Fig. 1. Prostaglandin D2 (PGD2, 1.0 µM) significantly increased ANP secretion (A) and mechanical dynamics (B) in isolated perfused beating rat atria.
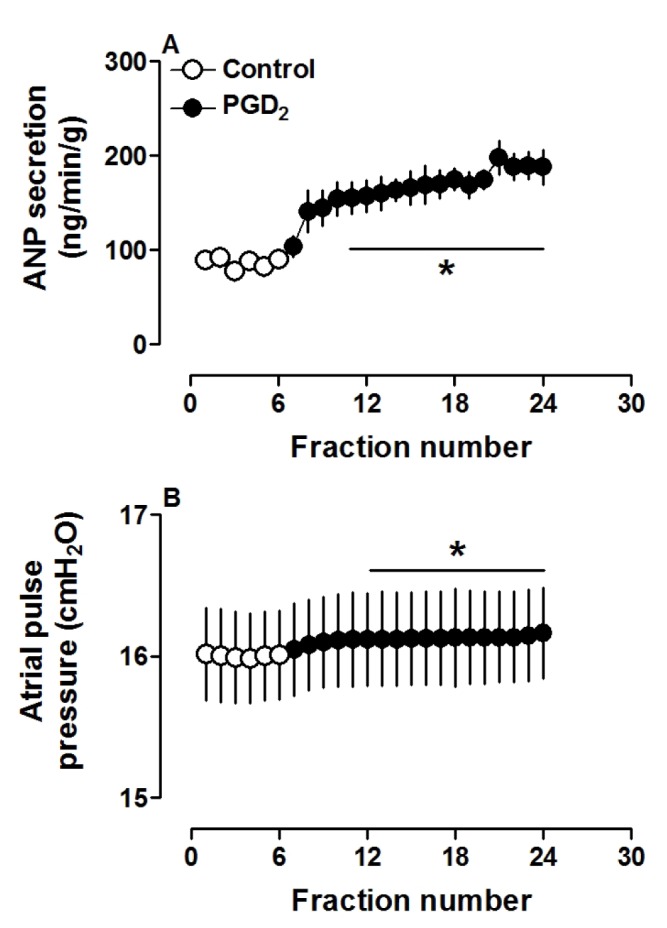
Data were expressed as mean±SEM, n=6. *p<0.05 vs. control period.
Fig. 2. PGD2 promotes secretion of ANP (A) and mechanical dynamics (B) by dose-dependent manner in isolated beating rat atria.
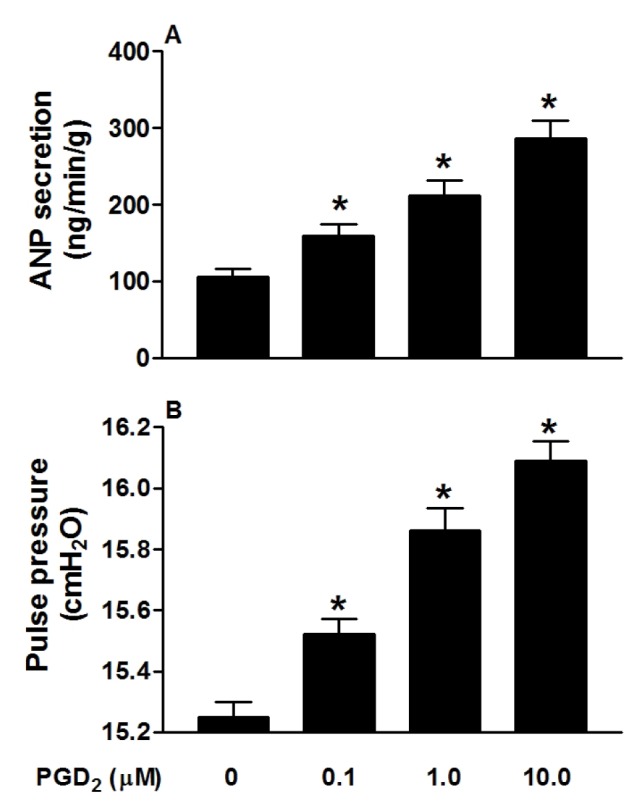
Data were expressed as mean±SEM, n=6 for each group. *p<0.05 vs. control (vehicle) group.
Effects of PGD2 and PGF2α receptor antagonists on PGD2-induced ANP secretion and mechanical dynamics
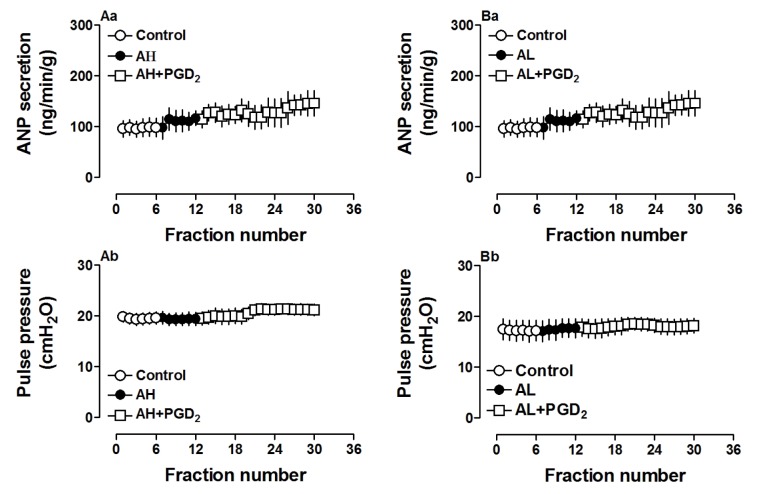
To investigate the role of receptors involved in PGD2-induced atrial ANP secretion and positive inotropy, antagonists of PGD2 and PGF2α receptors were utilized. As shown in Fig. 3, pretreatment with AH-6809 (1.0 µM), an antagonist of PGD2 receptor, completely blocked PGD2-induced atrial secretion of ANP (p>0.05 vs. control period, Fig. 3Aa) and positive inotropy (p>0.05 vs. control period, Fig. 3Ab). Similarly, an antagonist of PGF2α receptor, AL-8810 (1.0 µM), also completely abolished effects of PGD2 on atrial ANP secretion and mechanical dynamics (p>0.05 vs. control period, Fig. 3, Ba and Bb). The data demonstrated that PGD2 DP as well as PGF2α FP receptor was involved in PGD2-induced changes in atrial dynamics and ANP secretion.
Fig. 3. Effects of PGD2 and PGF2α receptor antagonists on PGD2-increased atrial ANP secretion and mechanical dynamics in isolated beating rat atria.
AH6809 (AH, 1.0 µM), an antagonist of PGD2 receptor; AL8810 (AL, 1.0 µM), an antagonist of PGF2α receptor. Data were expressed as mean±SEM, n=6 for each group.
Effect of 15d-PGJ2 on atrial ANP secretion and dynamics
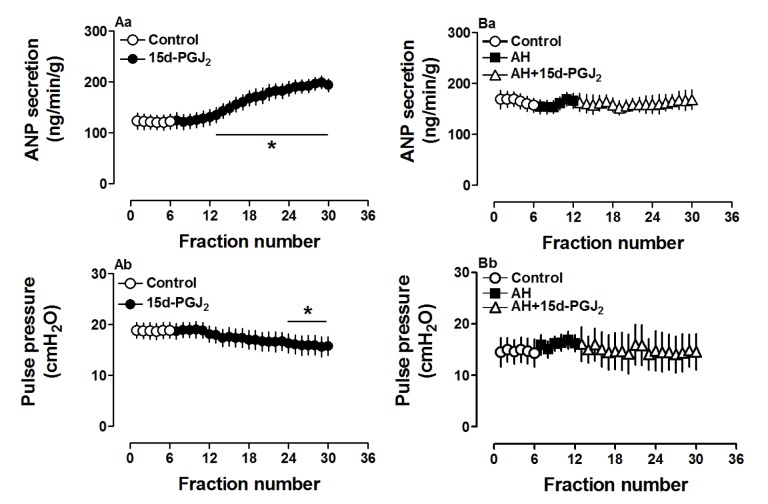
To define the role of 15d-PGJ2, a PGD2 metabolite, on atrial ANP secretion and mechanical dynamics, another series of experiments was performed on isolated beating rat atria. As shown in Fig. 4, 15d-PGJ2 (0.1 µM) dramatically increased atrial secretion of ANP (p<0.05 vs. control period, Fig. 4Aa), mimicking the effects of PGD2 on ANP secretion. However, 15d-PGJ2 slightly but significantly decreased atrial pulse pressure (p<0.05 vs. control period, Fig. 4Ab), thus having a different effect on mechanical dynamics than PGD2 in beating rat atria. The changes induced by 15d-PGJ2 on ANP secretion and mechanical dynamics were blocked by AH6809 (Fig. 4Ba and Bb). The results suggested that 15d-PGJ2 was involved in PGD2-induced atrial ANP secretion with difference in its effects on atrial dynamics.
Fig. 4. Effect of AH6809 (AH, 1.0 µM) on 15d-PGJ2 (0.1 µM)-induced atrial ANP and dynamics in isolated beating rat atria.
Data were expressed as mean±SEM, n=6 for each group. *p<0.05 vs. control period.
Effect of PPARγ signaling on PGD2- and 15d-PGJ2-induced ANP secretion and dynamics
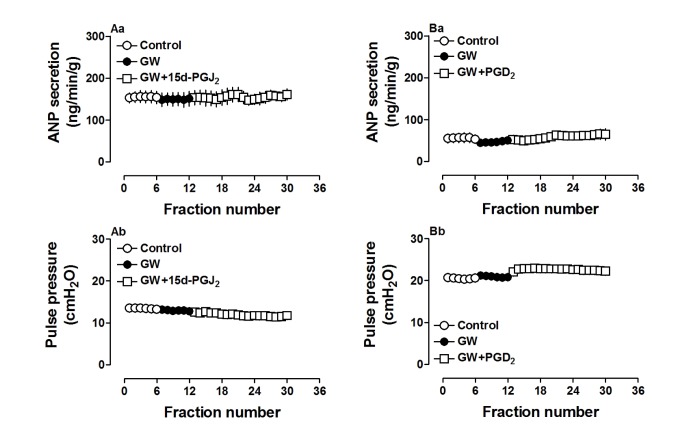
Because 15d-PGJ2 is a potent ligand for PPARγ, we used a PPARγ antagonist to investigate the action mechanism of ANP secretion stimulated by both 15d-PGJ2 and PGD2. GW 9662 (0.1 µM), a PPARγ antagonist, completely blocked the effects of 15d-PGJ2 on ANP secretion and its decreased atrial dynamics (p>0.05 vs. control period, Fig. 5Aa and Ab). GW 9662 also abolished effects of PGD2 on atrial ANP secretion and dynamics (p>0.05 vs. control period, Fig. 5Ba and Bb). There were no significant changes in ANP secretion and atrial dynamics induced by GW 9662 alone. Furthermore, PGD2 significantly upregulated PPARγ expression (p<0.05 vs. control group, Fig. 6) and this was abolished by GW9662 (p<0.05 vs. PGD2 group, Fig. 6). The data indicated that PGD2 induced atrial ANP secretion via its metabolite 15d-PGJ2 and activated PPARγ signaling in isolated perfused beating rat atria.
Fig. 5. GW 9662 (GW, 0.1 µM), an antagonist of PPARγ, inhibited the atrial secretion of ANP (Aa, Ba) and dynamics (Ab, Bb) induced by 15d-PGJ2 and PGD2 in isolated beating rat atria.
Data were expressed as mean±SEM, n=6 for each group.
Fig. 6. Effect of PGD2 on PPARγ expression in isolated beating rat atria.
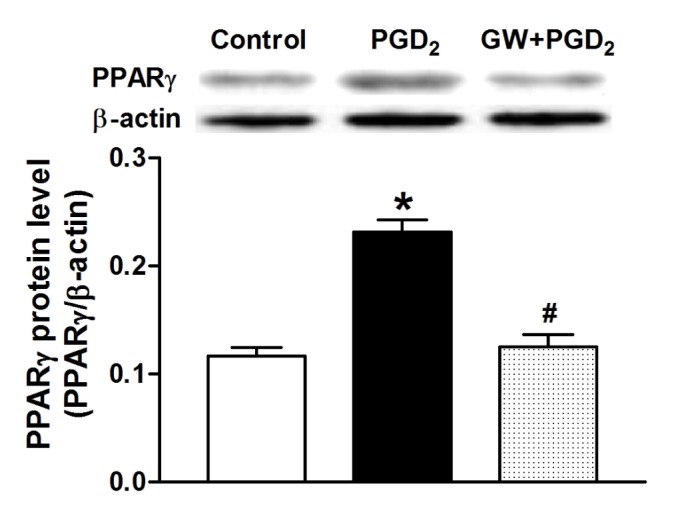
Data are means±SEM, n=5 for each group. GW, GW9662 (0.1 µM). *p<0.05 vs. control group; #p<0.05 vs. PGD2 group.
Effect of MAPK/ERK and PI3K/Akt on PGD2-induced atrial ANP secretion and dynamics
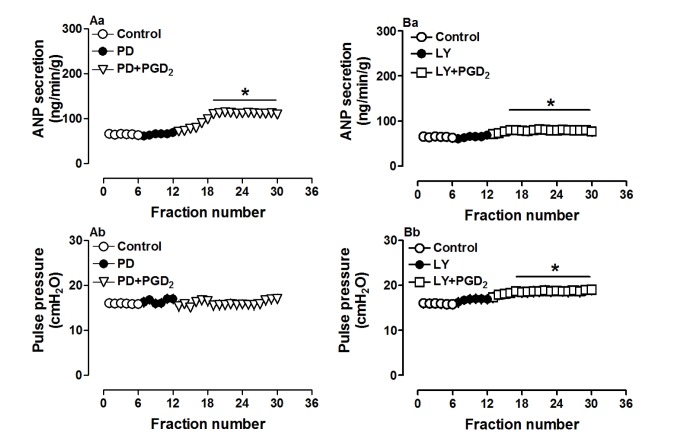
Because the PGD2 metabolite 15d-PGJ2 may activate signaling by MAPK as well as PI3K/Akt, antagonists of both pathways were used in another series of experiments. Pretreatment with PD98059 (10.0 µM), an antagonist of MAPK/extracellular signal-regulated kinase (ERK), clearly attenuated PGD2-induced atrial ANP secretion though ANP levels were still higher than those during the control period (p<0.05 vs. control period, Fig. 7Aa). PGD2-induced positive inotropy was also blocked by PD98059 (p>0.05 vs. control period, Fig. 7Ab). In addition, the PI3K/Akt antagonist LY294002 (1.0 µM) mimicked the effects of PD98059 on PGD2-induced atrial ANP secretion (p<0.05 vs. control period, Fig. 7Ba) but did not affect PGD2-induced positive inotropy (p<0.05 vs. control period, Fig. 7Bb). These results suggested that MAPK/ERK and PI3K/Akt signaling pathways were partially involved in PGD2-induced atrial ANP secretion.
Fig. 7. Effects of PD98059 (PD, 10.0 µM, A) and LY294002 (LY, 1.0 µM, B) on PGD2-induced atrial secretion of ANP and dynamics in isolated beating rat atria.
Data were expressed as mean±SEM, n=6 for each group. *p<0.05 vs. control period.
DISCUSSION
Our study showed that PGD2 significantly increased ANP secretion concomitantly with an increase of mechanical dynamics in isolated perfused beating left rat atria, which was completely abolished by a PPARγ antagonist. These results indicated that PPARγ signaling was essential for the changes in atrial ANP secretion and dynamics induced by PGD2. Our findings further suggested that both MAPK/ERK and PI3K/Akt signaling pathways were partially involved in the effects of PGD2.
A previous study showed that PGD2 significantly increased ANP release and atrial pulse pressure in isolated beating rat atria, which was blocked by an inhibition of PGF2α receptor [12]. Our findings agreed with this finding. The increased atrial ANP secretion and dynamics were completely blocked by an antagonist of the PGF2α receptor. These findings were consistent with a previous report that PGD2-induced ANP release was significantly attenuated by an inhibitor of the PGF2α receptor [12]. Further, we found that PGD2- and 15d-PGJ2-induced atrial ANP secretion and positive or negative inotropy were also blocked by an antagonist of the PGD2 receptor, in contrast to the previous report [12]. In cardiac myocytes, both DP1 and DP2 subtypes of PGD2 receptors are expressed [4,13]. Therefore, the present finding showing an accentuation of ANP secretion by PGD2 or 15d-PGJ2 and its blockade by an antagonist of PGD2 receptor is understandable. It is of interest that 15d-PGJ2 decreased mechanical dynamics. Previously, it was shown that DP2 subtype of the PGD2 receptors binds 15d-PGJ2 and results in a decrease in cAMP levels [14]. Although the exact reason of the difference between previous report [12] and present data is not clear at present, the wall stretch of the atrium during in vitro perfusion may, at least in part, be related. As shown in Fig. 1 of the present experiment and Fig. 1 of the previous report [12], the levels of atrial pulse pressure and atrial beating rate are different. As shown in the present study, higher pulse pressure and beating rate may result in a more distended atrial stretch and atrial workload, which is one of major factors in the regulation of ANP release from the atrium [15], and thus may elicit different responses to PGD2 and its receptor signaling. Further experiments are needed to more clearly define the question.
Several studies reported that PGD2 was converted to 15d-PGJ2, protecting the heart against ischemia-reperfusion (I/R) injury and suppressing inflammation by activating PPARγ [16,17]. In our study, we observed that PGD2 significantly increased atrial PPARγ expression and that this effect was abolished by pretreatment with the PPARγ antagonist GW9662. Moreover, GW9662 completely blocked the effects of both PGD2 and its metabolite 15d-PGJ2 on ANP secretion and atrial dynamics in beating rat atria. These results demonstrated that both the PGD2 and its metabolite 15d-PGJ2 promoted atrial ANP secretion by activating PPARγ signaling. In addition, our findings showed that an inhibitor of MAPK/ERK PD98059 attenuated PGD2-induced increase of ANP secretion with blockade of the positive inotropy. An inhibitor of PI3K/Akt LY294002 also clearly attenuated the PGD2-induced ANP secretion, but without significant effect on positive inotropy. These results suggested that the MAPK/ERK and PI3K/Akt signaling pathways were partially involved in PGD2-stimulated ANP secretion and that MAPK/ERK was also involved in PGD2-induced positive inotropy. The present data agree with our previous findings that MAPK/ERK and PI3K/Akt signaling pathways were important regulators for ANP secretion under hypoxic or normoxic conditions [18,19]. ANP was reported to be effective against myocardial ischemia-reperfusion injury and to have anti-inflammatory effects [20,21,22,23,24]. Therefore, the stimulation by PGD2 of atrial ANP secretion suggests its potential effectiveness against ischemia-reperfusion injury and inflammation in beating rat atria.
ACKNOWLEDGEMENTS
We thank Shan-ji Jin and Su-xiang Li for expert technical assistance. This work was supported by the National Natural Science Foundation of China (No. 81360061; 81660089).
Footnotes
Author contributions: Z.Y. and L.X. performed atrial perfused experiments. L.L.P and H.L. performed WB analysis. L.X. and Z.B. performed ANP measurement. W.C.Z. and C.X. designed experiments and wrote the manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Kalra BS, Roy V. Efficacy of metabolic modulators in ischemic heart disease: an overview. J Clin Pharmacol. 2012;52:292–305. doi: 10.1177/0091270010396042. [DOI] [PubMed] [Google Scholar]
- 2.Ryu SK, Mallat Z, Benessiano J, Tedgui A, Olsson AG, Bao W, Schwartz GG, Tsimikas S Myocardial Ischemia Reduction With Aggressive Cholesterol Lowering (MIRACL) Trial Investigators. Phospholipase A2 enzymes, high-dose atorvastatin, and prediction of ischemic events after acute coronary syndromes. Circulation. 2012;125:757–766. doi: 10.1161/CIRCULATIONAHA.111.063487. [DOI] [PubMed] [Google Scholar]
- 3.Gross GJ, Falck JR, Gross ER, Isbell M, Moore J, Nithipatikom K. Cytochrome P450 and arachidonic acid metabolites: role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res. 2005;68:18–25. doi: 10.1016/j.cardiores.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Qiu H, Liu JY, Wei D, Li N, Yamoah EN, Hammock BD, Chiamvimonvat N. Cardiac-generated prostanoids mediate cardiac myocyte apoptosis after myocardial ischaemia. Cardiovasc Res. 2012;95:336–345. doi: 10.1093/cvr/cvs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taba Y, Sasaguri T, Miyagi M, Abumiya T, Miwa Y, Ikeda T, Mitsumata M. Fluid shear stress induces lipocalin-type prostaglandin D(2) synthase expression in vascular endothelial cells. Circ Res. 2000;86:967–973. doi: 10.1161/01.res.86.9.967. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Young HA. PPAR and immune system--what do we know? Int Immunopharmacol. 2002;2:1029–1044. doi: 10.1016/s1567-5769(02)00057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim HJ, Lee KS, Lee S, Park JH, Choi HE, Go SH, Kwak HJ, Park HY. 15d-PGJ2 stimulates HO-1 expression through p38 MAP kinase and Nrf-2 pathway in rat vascular smooth muscle cells. Toxicol Appl Pharmacol. 2007;223:20–27. doi: 10.1016/j.taap.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Lee SJ, Kim MS, Park JY, Woo JS, Kim YK. 15-Deoxy-delta 12,14-prostaglandin J2 induces apoptosis via JNK-mediated mitochondrial pathway in osteoblastic cells. Toxicology. 2008;248:121–129. doi: 10.1016/j.tox.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Jung WK, Park IS, Park SJ, Yea SS, Choi YH, Oh S, Park SG, Choi IW. The 15-deoxy-Delta12,14-prostaglandin J2 inhibits LPS-stimulated AKT and NF-kappaB activation and suppresses interleukin-6 in osteoblast-like cells MC3T3E-1. Life Sci. 2009;85:46–53. doi: 10.1016/j.lfs.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Giri S, Rattan R, Singh AK, Singh I. The 15-deoxy-delta12,14-prostaglandin J2 inhibits the inflammatory response in primary rat astrocytes via down-regulating multiple steps in phosphatidylinositol 3-kinase-Akt-NF-kappaB-p300 pathway independent of peroxisome proliferator-activated receptor gamma. J Immunol. 2004;173:5196–5208. doi: 10.4049/jimmunol.173.8.5196. [DOI] [PubMed] [Google Scholar]
- 12.Bai G, Gao S, Shah A, Yuan K, Park WH, Kim SH. Regulation of ANP secretion from isolated atria by prostaglandins and cyclooxygenase-2. Peptides. 2009;30:1720–1728. doi: 10.1016/j.peptides.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Koyani CN, Windischhofer W, Rossmann C, Jin G, Kickmaier S, Heinzel FR, Groschner K, Alavian-Ghavanini A, Sattler W, Malle E. 15-deoxy-Δ12,14-PGJ2 promotes inflammation and apoptosis in cardiomyocytes via the DP2/MAPK/TNFα axis. Int J Cardiol. 2014;173:472–480. doi: 10.1016/j.ijcard.2014.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawyer N, Cauchon E, Chateauneuf A, Cruz RP, Nicholson DW, Metters KM, O'Neill GP, Gervais FG. Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br J Pharmacol. 2002;137:1163–1172. doi: 10.1038/sj.bjp.0704973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho KW, Lee SJ, Wen JF, Kim SH, Seul KH, Lee HS. Mechanical control of extracellular space in rabbit atria: an intimate modulator of the translocation of extracellular fluid and released atrial natriuretic peptide. Exp Physiol. 2002;87:185–194. doi: 10.1113/eph8702302. [DOI] [PubMed] [Google Scholar]
- 16.Katsumata Y, Shinmura K, Sugiura Y, Tohyama S, Matsuhashi T, Ito H, Yan X, Ito K, Yuasa S, Ieda M, Urade Y, Suematsu M, Fukuda K, Sano M. Endogenous prostaglandin D2 and its metabolites protect the heart against ischemia-reperfusion injury by activating Nrf2. Hypertension. 2014;63:80–87. doi: 10.1161/HYPERTENSIONAHA.113.01639. [DOI] [PubMed] [Google Scholar]
- 17.Kim KH, Sadikot RT, Xiao L, Christman JW, Freeman ML, Chan JY, Oh YK, Blackwell TS, Joo M. Nrf2 is essential for the expression of lipocalin-prostaglandin D synthase induced by prostaglandin D2. Free Radic Biol Med. 2013;65:1134–1142. doi: 10.1016/j.freeradbiomed.2013.08.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang QL, Cui BR, Li HY, Li P, Hong L, Liu LP, Ding DZ, Cui X. MAPK and PI3K pathways regulate hypoxia-induced atrial natriuretic peptide secretion by controlling HIF-1 alpha expression in beating rabbit atria. Biochem Biophys Res Commun. 2013;438:507–512. doi: 10.1016/j.bbrc.2013.07.106. [DOI] [PubMed] [Google Scholar]
- 19.Liu LP, Hong L, Yu L, Li HY, Ding DZ, Jin SJ, Cui X. Ouabain stimulates atrial natriuretic peptide secretion via the endothelin-1/ET(B) receptor-mediated pathway in beating rabbit atria. Life Sci. 2012;90:793–798. doi: 10.1016/j.lfs.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Mitaka C, Si MK, Tulafu M, Yu Q, Uchida T, Abe S, Kitagawa M, Ikeda S, Eishi Y, Tomita M. Effects of atrial natriuretic peptide on inter-organ crosstalk among the kidney, lung, and heart in a rat model of renal ischemia-reperfusion injury. Intensive Care Med Exp. 2014;2:28. doi: 10.1186/s40635-014-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong L, Xi J, Zhang Y, Tian W, Xu J, Cui X, Xu Z. Atrial natriuretic peptide prevents the mitochondrial permeability transition pore opening by inactivating glycogen synthase kinase 3β via PKG and PI3K in cardiac H9c2 cells. Eur J Pharmacol. 2012;695:13–19. doi: 10.1016/j.ejphar.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 22.Kiemer AK, Hartung T, Vollmar AM. cGMP-mediated inhibition of TNF-alpha production by the atrial natriuretic peptide in murine macrophages. J Immunol. 2000;165:175–181. doi: 10.4049/jimmunol.165.1.175. [DOI] [PubMed] [Google Scholar]
- 23.Kiemer AK, Weber NC, Fürst R, Bildner N, Kulhanek-Heinze S, Vollmar AM. Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-alpha-induced actin polymerization and endothelial permeability. Circ Res. 2002;90:874–881. doi: 10.1161/01.res.0000017068.58856.f3. [DOI] [PubMed] [Google Scholar]
- 24.Ladetzki-Baehs K, Keller M, Kiemer AK, Koch E, Zahler S, Wendel A, Vollmar AM. Atrial natriuretic peptide, a regulator of nuclear factor-kappaB activation in vivo. Endocrinology. 2007;148:332–336. doi: 10.1210/en.2006-0935. [DOI] [PubMed] [Google Scholar]