Abstract
Background:
Although dopamine has been suggested to play a role in mediating social behaviors of individual animals, it is not clear whether such dopamine signaling contributes to attributes of social groups such as social hierarchy.
Methods:
In this study, the effects of the pharmacological manipulation of dopamine D1 receptor function on the social hierarchy and behavior of group-housed mice and macaques were investigated using a battery of behavioral tests.
Results:
D1 receptor blockade facilitated social dominance in mice at the middle, but not high or low, social rank in the groups without altering social preference among mates. In contrast, the administration of a D1 receptor antagonist in a macaque did not affect social dominance of the drug-treated animal; however, relative social dominance relationships between the drug-treated and nontreated subjects were altered indirectly through alterations of social affiliative relationships within the social group.
Conclusions:
These results suggest that dopamine D1 receptor signaling may be involved in social hierarchy and social relationships within a group, which may differ between rodents and primates.
Keywords: monoamine, social affiliation, cognitive function, evolution, psychiatric disorder
Significance Statement
This study shows that the neurotransmitter dopamine plays important roles in attributes of social groups such as social hierarchy through one of the dopamine receptors, D1 receptor. In particular, we found that low D1 receptor function, although it has been known to cause detrimental effects such as cognitive dysfunction, could still yield beneficial changes such as facilitation of social dominance in animals living in social groups. Moreover, such effects were different between rodents and primates. Although the effects were more substantial in rodents, they were less clear in primates, which appeared to be due to contributions of other social factors, such as social affiliation, in determining social hierarchy. These results raise a possibility that low D1 receptor function may be under the balance between benefits and disadvantages in social group environments.
Introduction
Humans and many animals organize into social groups and follow a social hierarchy (Chase et al., 2002). In a hierarchical social group, social class determines the behavioral fitness, such as health and reproduction, of subjects in the group (Sapolsky, 2005; Majolo et al., 2012). Accumulating evidence suggests that neural transmission of monoamines, such as dopamine (DA), is involved in social hierarchy, although how monoamine signaling plays roles in social hierarchy construction and maintenance has largely remained elusive. For instance, in nonhuman primates, striatal DA D2 receptor availability is correlated with social class (Nader et al., 2012). Consistent with this, human functional imaging studies have reported that striatal D2 availability is correlated with social dominance (Cervenka et al., 2010) and social status (Martinez et al., 2010). In addition, D2 function also mediates social dominance in rodents (Jupp et al., 2016). The roles of D1 function in social hierarchy remain less clear. However, a recent human positron emission tomography study has also reported that subjects with low D1 receptor availability in the limbic-striatal regions exhibit higher aggression and socially dominant personalities (Plaven-Sigray et al., 2014), suggesting that D1 and D2 receptors may have opposite functions for determining social hierarchy.
Several macaque species (de Waal, 1996) and rodents (Lund, 1975) construct a linear hierarchy in their societies. Behavioral factors, such as aggression and impulsivity, which are behavioral traits associated with psychiatric disorders (Robbins et al., 2012), play roles in determining the social class of an animal. Since social hierarchy is an emerging property consequence of competition among subjects in a group, more highly aggressive and impulsive nonhuman primates and mice may gain higher social status (Machida et al., 1981; Morgan et al., 2000; Fairbanks et al., 2004; Koski et al., 2015). DA D1 receptor signaling is important for cognitive and affective functions (Seamans and Yang, 2004). In contrast, a mouse strain with low D1 expression exhibits higher aggression than another strain with high D1 expression (Couppis et al., 2008). Moreover, blockade of the D1 receptor also induces impulsivity (van Gaalen et al., 2006). Thus, although low D1 receptor signaling causes cognitive dysfunctions, low D1 signaling is also expected to augment impulsivity and aggression, which may assist in attaining higher social dominance, and consequently higher social class, in a social hierarchy.
In this study, we examined the effects of the pharmacological manipulation to inhibit D1 receptor function in socially housed mice and macaques, two different species, both of which exhibit rigorous linear social hierarchies in their social groups. We have hypothesized that administration of the D1 receptor antagonist augmented social dominance of the drug-treated animals in both rodent and primate social groups.
Methods
Subjects
Mice and nonhuman primates were used in this study. All experiments were conducted in accordance with the Guidelines for Proper Conduct of Animal Experiments by the Science Council of Japan and were approved by the Kyoto University Primate Research Institute animal ethics committee. A total of 116 adult male CD1 mice were used for the experiments. Four mice per cage were grouped and housed together in a cage. To extend the rodent findings to nonhuman primates, which are thought to have more complex social relationships with hierarchy and that are closely related to humans, we also conducted the experiments using a social group consisting of 6 Japanese macaques (Macaca fuscata). These macaques were 3 years old each, and the group consisted of 4 males and 2 females (supplementary Table 1). Further detail on the animals is provided in the supplementary Materials.
Social Rank Test in Mice
To determine social hierarchy of mice housed in a group, the tube rank test, which is similar to that used in other studies (Wang et al., 2011), was conducted. In this test, an apparatus consisting of a transparent plexiglass tube connected to transparent boxes at each end was used. A pair of mice from the same group were taken and simultaneously placed with one mouse into each of the boxes on either side of the tube. When mice from each side reached the middle of the tube, the partition wall was removed. The mouse that caused the other to retreat was designated as the “winner,” and the mouse that was retreated out of the tube was designated as the “loser”; they were scored with +1 or 0, respectively. Tournaments of all possible combinations of matches by pairs of mice in each group (e.g., in a group of 4 mice denoted as “A-D”, combinations are 6 pairs of A-B, A-C, A-D, B-C, B-D, C-D) were conducted once per day, 5 times (5 trials). Social dominance was then quantified by David’s score (DS) (David, 1987). DS is defined as
The proportion of wins by individual i in his interactions with another individual j, (Pij) is the number of times that i defeats j, (αij) divided by the total number of interactions between i and j (nij), i.e. Pij=αij/nij. The proportion of losses by i in interactions with j, Pji=1-Pij...DS for each member, i, of a group is calculated with the formula: DS=w+w2-l-l2, where w represents the sum of i’s Pij values, w2 represents the summed w values (weighted by the appropriate Pij values) of those individuals with which i interacted, l represents the sum of i’s Pji values and l2 represents the summed l values (weighted by the appropriate Pji values) of those individuals with which i interacted... (Gammell et al., 2003).
An example of DS calculations is shown in supplementary Table 2.
Administration of the DA D1 receptor antagonist SCH23390 (SCH) at the dose of 0.1 mg/kg was given to mice i.p. As a control, an equivalent volume of saline (SAL) was administered. Twenty-five groups of 4 mice each (100 mice total) were tested. The 20 groups were divided into 4 sets of drug administration conditions. In one set of 5 groups, the drug was administered to first-rank mice in each group; in another set of 5 groups, the drug was administered to second-rank mice in each group, and so on. In these 20 groups, the drug-administered mice were returned to the groups, and housed together immediately after drug administration. In an additional 5 groups in which the second-rank mice received drug administration, the drug-administered mice were isolated from the groups for 6 hours after drug administration, by which the drug effects were expected to be largely decreased, and then returned to the groups. After training, the tube rank test for the baseline (BASE) condition was conducted to determine the social rank of each mouse in each group (supplemental Figure 1a). Then, SAL was given once per day for 5 days in mice at each rank in their home cages and was followed by the tube rank test for 5 days. After confirming no change in social rank with SAL administration, SCH was given once per day for 5 days in the mouse that had received SAL beforehand. After 5 days of repeated SCH administration, the tube rank test was conducted again for another 5 days. No SAL or SCH was administered during the tube rank test. Further detail on the method is provided in the supplementary Materials.
Social Behavior Test in Mice
Although social affiliations have been suggested to underlie determination of social class in a hierarchy in primates (Raleigh and McGuire, 1991; Higley et al., 1996), it has remained elusive in rodents. Thus, alterations of social hierarchy by drug administration may be consequences of behavioral changes, such as enhancement of impulsivity and aggression, but also alterations of social affiliative bonds. To investigate this issue, we further examined the effects of SCH administration on motivation to socially interact with mates in mice with the 3-chamber social preference test modified from that used in other studies (Moy et al., 2004; Pearson et al., 2010). In this test, subject mice were placed in the middle chamber, which was connected to 2 other chambers on each side. The mice were allowed to freely enter into these chambers through the openings on the walls. Either (1) normal adult mice of the same gender with the test-subject mice and that had no previous contact with the test-subject mice, or (2) cage mates that were housed together with the test-subject mice were placed in a metal mesh cage that was positioned in the center of one of the sides of the chamber. On the other side of the chamber, an identical mesh cage without mice was placed. The amount of time that the test-subject mice spent on each side of the chamber was measured for 10 minutes. Animals that spent more time in the side of the chamber with the trapped mice were considered to be more strongly motivated to interact with mates.
A total of 56 mice were used in this test, of which 16 mice were paired with unfamiliar trapped mice (having no previous interaction), and 20 pairs of one higher and one lower ranked mice in the same home cages (which were also used for the tube rank test) were subjected to the test with SAL and SCH administration. Further detail on the method is provided in the supplementary Materials.
Other Behavioral Tests in Mice
D1 receptor signaling plays critical roles in cognitive functions, such as working memory (Zahrt et al., 1997; Seamans et al., 1998), behavioral inhibition (Rodgers et al., 1994; van Gaalen et al., 2006), and behavioral flexibility (Ragozzino, 2002). We investigated the effects of SCH administration on the associations between social rank and these nonsocial cognitive factors to address how alterations in them may contribute to social hierarchical changes using food foraging and elevated plus maze tests.
The random foraging task, which is similar to that used in other studies (Bond et al., 1981; Floresco et al., 1997; Jung et al., 1998), was conducted to examine working memory and behavioral flexibility. In this task, the radial 8-arm maze was used. Mice were placed in the central arena of a maze that was connected to 8 arms. At the beginning of the test, a piece of a cereal was baited at the end of each arm. Mice were allowed to freely explore the maze for 5 minutes or until consuming all baited cereals, whichever happened first. The number of times that mice reentered arms that they had already visited was recorded. Repeated entries into the same arms were divided into 2 categories. One category was re-entries into the same arm at least 2 nonconsecutive times (NC entrance). This pattern of entries into the same arms was measured as a reflection of working memory deficit. The other category was reentries into the same arm consecutively (C entrance). This pattern of entries into the same arm was measured as a reflection of behavioral flexibility deficit. SCH and SAL were given to animals approximately 10 minutes before starting tests.
The elevated plus maze task was conducted to examine inhibition of inappropriate behaviors. Mice were placed in the central crossing area of the arms and were allowed to freely enter into the arms for 5 minutes. Studies, including ours (Griebel et al., 1997; Ueno et al., 2002; Lee and Goto, 2011), have shown that the elevated plus maze can be used to examine behavioral inhibition, because entering into the opened arms is considered to be an inappropriate behavior and thereby should be inhibited. Accordingly, we measured the number of times that mice entered into the opened and enclosed arms. SCH and SAL were given to animals approximately 10 minutes before starting tests. Further detail on the method is provided in the supplementary Materials.
Behavioral Observations and Recordings in Nonhuman Primates
Behavioral observations were conducted with the focal animal sampling method. Observations and recordings were conducted for 15 min/d per monkey at the frequency of 3 or 4 d/week for 4 weeks (14 days of sampling) for the BASE observations, followed by another 4 weeks of observations after chronic SAL administration and then 4 additional weeks of observations after chronic SCH administration (supplementary Figure 1b). For later analysis of video recordings, 15-minute recordings were segmented into 10 seconds, and the presence or absence of specific behaviors in each segment was counted. Locomotion was separately quantified by subtracting the amount of time that animals were motionless from the whole 15-minute recording period. We measured “individual behavior” (goal-directed action, stereotypy, agonistic display, scanning, locomotion, unusual) of the drug-treated subject, and “social behavior” (affiliation, aggression, social inhibition, escape, mounting) of each animal in the group. Items of behavioral assessments and their detailed criteria are described in Table 1. Further detail on the method is provided in the supplementary Materials.
Table 1.
Items of behavioral assessment used in primates.
| Individual behavior | Definition |
|---|---|
| Goal-directed action | Any behavioral action that an observer can predict a target that a subject is approaching a target at least 1 sec before reaching the target. These actions include approaching to (1) a drinking fountain for drinking water, (2) other subjects or objects in the cage, and (3) a specific area within the cage (e.g., the area where a subject can see outside of the cage through the window). |
| Stereotypy | (1) Repetitive circular running at the same orbit in the cage for more than 2 rounds or (2) Repeated licking of an iron bar at constant speed for more than 2 rounds |
| Agonistic display | Banging (hard striking with making loud noises) any object in the cage (e.g. an iron bar), floor, or wall of the cage more than twice repeatedly. |
| Scanning | Sustained gazing at others for longer than 1 sec |
| Unusual | Behavior that is not observed in a normal condition, e.g., falling asleep or ataxia. |
| Locomotion | The amount of time a subject is moving, which is calculated by subtraction of stillness time from the whole recording time |
| Social behavior | Definition |
| Affiliation | (1) Proximate sitting (sitting with the body touching another animal, or less than a 30-cm distance between subjects for longer than 1 sec), (2) Grooming (for longer than 1 sec), or (3) Playing (biting, hitting, grabbing without facial expressions associated with aggression). Although biting, hitting, and grabbing are also actions associated with aggression, these actions exhibited in the context of playing behavior are clearly distinct, i.e., with a subject making these actions much slower and softer than when the actions are made in the context of aggression. |
| Aggression | Either (1) biting, (2) hitting, (3) grabbing, or (4) threatening with an open-mouth facial expression by an attacker and a simultaneous bared-teeth display or scream by an attack-recipient |
| Social inhibition | Stopping on-going (planed) actions due to interference by others |
| Escape | Changing locomotive orbit to avoid others |
| Mounting | Mounting on the back of others |
Social Rank Determination in Nonhuman Primates
A typically employed index that determines social rank is the direction of aggressive attacks, which are usually made from higher to lower social rank subjects (Alexander and Bowers, 1969). Thus, social rank was estimated by counting the number of aggressive attacks from one monkey to other cage mates. DS was also calculated with the number of aggressive attacks from an initiator to a receiver.
Another index for social rank is food incentive priority (Richards, 1974). Thus, we also conducted a food priority test to further determine social rank. In this test, food presentation (a portion of a sweet potato) was given at roughly equal distance from all subjects, and the order of obtaining a food was recorded. After one subject accessed and consumed a food, the next sweet potato portion was presented immediately. This process was repeated until the last subject obtained a piece of a sweet potato. However, to emphasize the priority for accessing a food, the portioned size of the food was consecutively reduced to be approximately two-thirds smaller than the formerly presented food portion. Ten trials at the frequency of one trial per day were conducted in each of the control and drug administration conditions. Quantification of social rank was attempted by scoring (food priority score or FPS) for the orders of food acquiring from 6 to 1 points with 6 points for the first access. Further detail on the method is provided in the supplementary Materials.
Drug Administration in Nonhuman Primates
SCH was administered in the subject that was at second rank in the social group, based on the observation that the increase of social rank with SCH administration was the largest in second-rank subjects in mice. The drug was delivered by subcutaneous implantation of an ALZET osmotic pump, with which drug delivery was expected to persist for up to 4 weeks (Hill et al., 2013). The concentration of SCH was adjusted to be approximately 0.1 mg/kg/d. As a control treatment, an equivalent volume of 0.9% saline in an osmotic pump was also implanted for 1 month before the drug was administered. Further detail on the method is provided in the supplementary Materials.
Data Analysis
Drug effects on social hierarchy and affiliations were analyzed with ANOVA with repeated measures. In addition, drug effects on the linearity and steepness of the hierarchy were analyzed with linear regression analysis, with the linearity expressed as Pearson product-moment correlation coefficients, and the steepness as slopes of linear regressions. Concurrence of social affiliations was calculated by absolute difference of normalized affiliative contacts for each observation between specific bonds (denoted as “similarity index”). Lower index values indicated higher similarity between the contacts, with an index score=0 the similarity identical. Further detail on the data analysis is provided in the supplementary Materials.
Results
Social Hierarchy of Mice
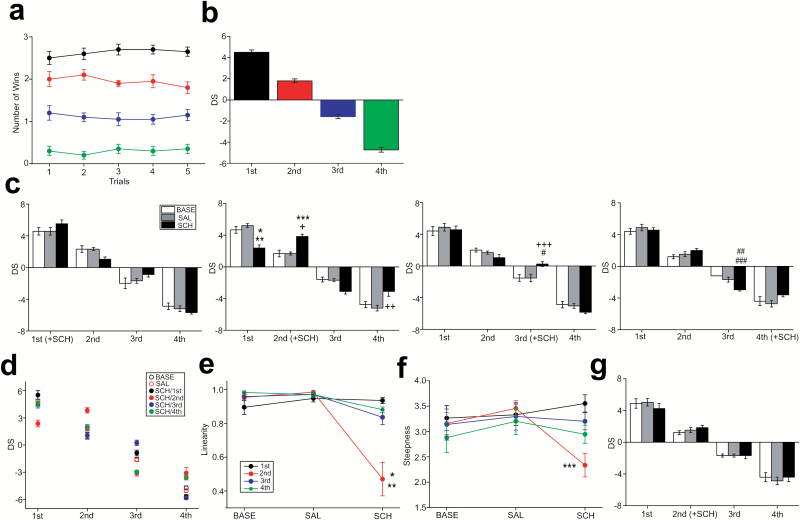
A stable, linear social hierarchy was established in all groups under the BASE condition (Figure 1a-b). Three-way repeated-measures ANOVA with the condition (i.e., drug treatment in different rank of mice; n=20 groups in total, n=5 groups for SAL/SCH administration at each rank) and rank (i.e., original rank of each mouse in a group) as independent variables and the treatment (BASE vs SAL vs SCH) as repeated measures revealed the significant effects of SCH administration on DS in the interactions of treatment x rank and treatment x rank x condition (F3,64=0.00, P=1.00 in condition; F3,64=869.6, P<.001 in rank; F9,64=3.73, P<.001 in condition x rank; F2,128=0.00, P=1.00 in treatment; F6,128=0.00, P=1.00 in treatment x condition; F6,128=2.48, P=.026 in treatment x rank; F18,128=11.24, P<.001 in treatment x condition x rank). Posthoc Tukey analysis has revealed that in a set of 5 groups in which SCH was administered to the first-rank mice, DS was not significantly altered at any rank (Figure 1c). In another set of the 5 groups in which SCH was administered to the second-rank mice, DS was significantly increased in the drug-treated mice (P<.001 in BASE vs SCH, P<.001 in SAL vs SCH) (Figure 1c). SCH administration of the second-rank mice also decreased DS of the first-rank mice (P<.001 in BASE vs SCH, P<.001 in SAL vs SCH; Figure 1c) and increased DS of the fourth-rank mice (P=.001 in SAL vs SCH; BASE vs SCH was marginally significant with P=.075; Figure 1c) within the same groups. In the groups in which the third-rank mice received SCH administration, DS was significantly increased only in the drug-treated mice (P=.033 in BASE vs SCH, P=.033 in SAL vs SCH) (Figure 1c). In a set of the groups in which SCH was administered to the fourth-rank mice, DS was not altered in the drug-treated mice, but a significant decrease in DS was observed in the third-rank mice (P<.001 in BASE vs SCH, P=.033 in SAL vs SCH).
Figure 1.
Alterations of social hierarchy in mouse groups by the D1 antagonist. (a) A graph showing a stable, linear hierarchy in all tested groups at the baseline (BASE) condition expressed as a number of wins over 5 trials of the tube rank test. Black, red, blue, and green colors indicate mice at first, second, third, and fourth rank, respectively, in each group. Error bars in the graphs indicate SEM. (b) A graph showing David’s score (DS) of mice at each rank in the BASE condition. (c) Graphs showing alterations of DS with SCH23390 (SCH) administration in mice at each rank (graphs from the left to the right showing SCH administration of first to fourth rank). *P<.001 vs BASE, **P<.001 vs SAL, ***P<.001 vs BASE, †P<.001 vs SAL, ††P=.001 vs SAL, †††P=.033 vs BASE, #P=.033 vs SAL, ##P<.001 vs BASE, ###P=.033 vs SAL. (d) A graph showing DS of mice at each rank that illustrates the linearity and steepness of the hierarchy in the BASE, saline (SAL), and SCH conditions. (e-f) Graphs showing the linearity (e) and steepness (f) of the hierarchy. *P<.001, **P<.001, ***P=.003. (g) A graph showing DS with SCH administration into the second-rank subject, but the drug-administered mice were isolated for 6 hours before returning to the groups.
Further analysis revealed that SCH administration in mice at the second rank but not other ranks significantly decreased the linearity (F3,16=8.61, P=.001 in rank; F2,32=33.8, P<.001 in treatment; F6,32=13.4, P<.001 in interaction; P<.001 in BASE vs SCH at second rank, P<.001 in SAL vs SCH at second rank; Figure 1d-e) and steepness (F3,16=1.05, P=.398 in rank; F2,32=3.41, P=.045 in treatment; F6,32=3.29, P=.012 in interaction; P=.003 in SAL vs SCH at second rank; Figure 1d-f) of the hierarchy, which may be associated with the SCH effects on DS most pronounced in the second-rank mice.
To further elucidate an insight on how social hierarchy was changed with D1 antagonist administration, the effects of drug administration was examined in the second-rank mice, but the drug-administered subjects were isolated, although this manipulation also became the confounding factor of repeated acute social isolation, until the drug effects were waned before returning to the groups. DS of the drug-administered mice in this condition was unchanged (Figure 1g).
These results suggest that pharmacological manipulation to inhibit D1 receptor signaling may facilitate social dominance in the drug-administered mice at the middle, but not in the highest or lowest social rank in their social groups. Such alterations in the drug-administered mice may simultaneously alter the social status of other mates within the same social group. Moreover, such social dominance alterations may also involve altered social interactions between the drug-administered mice and other mates in the groups immediately after drug administration.
Behavioral Factors Associated with Social Hierarchical Changes in Mice
Social Behavior
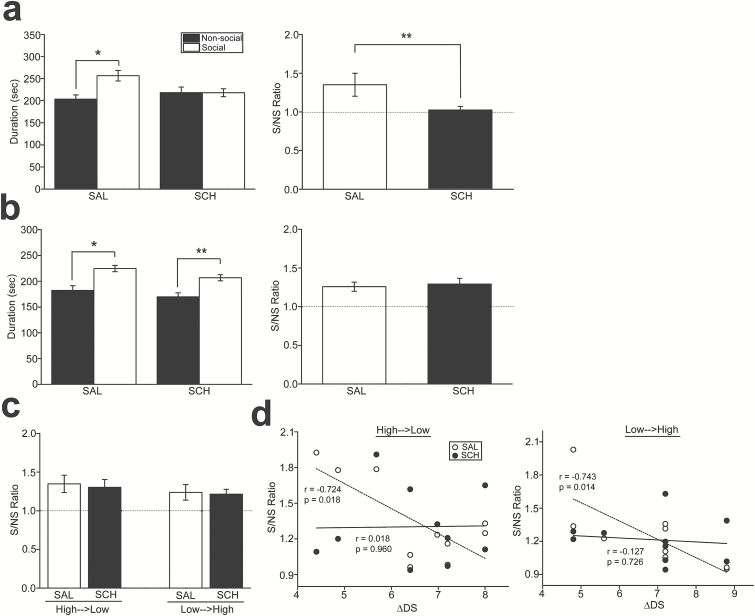
First, we investigated the interactions between test-subject mice and unfamiliar trapped mice that were not from the home cage of the test subject mice (n=16). Test subject mice that received SAL but not with SCH administration stayed longer in the area where there were trapped mice than in the area where mates were absent (F1,30=7.12, P=.012 in area; F1,30=1.00, P=.325 in treatment; F1,30=5.14, P=.031 in interaction; P=.004 in social vs nonsocial area under SAL condition) (Figure 2a). Accordingly, social preference, which was expressed as the ratio of time in the social to the non-social area was significantly decreased with SCH administration (paired t-test, t15=2.16, P=.047; Figure 2a).
Figure 2.
Alterations of social preference in mice by the D1 antagonist. (a) Graphs showing duration of time spent in the social and nonsocial areas (left) and the ratio of the time spent in the social to nonsocial areas (S/NS ratio; right) when the trapped mates were unfamiliar ones to the test-subject mice. *P=.004, **P=.047. (b) Graphs similar to a, but the trapped mates were chosen from the groups in which the test-subject mice were housed together. *P=.006, **P=.048. (c) A graph showing that social rank relationships between the test-subject mice and trapped mates are considered in analysis. High→Low indicates the condition in which the social rank of the test-subject mice is higher than that in the trapped mates and vice versa for Low→High. (d) Graphs showing the linear regression analyses between the social preference ratio and the difference of the David’s score (DS) between the test-subject mice and the trapped mates (∆DS; subtraction of DS in lower-ranked mice from that of higher-ranked mice) with saline (SAL; open circles and dashed lines) and SCH23390 (SCH; black circles and solid lines) administration. In the graph on the right (showing Low→High), one of white circles (S/NS ratio of approximately 2.0) appears to be an outlier. Linear regression analysis excluding this outlier still yields a significant correlation (r=−0.709, P=.032).
We then examined the interactions between test-subject mice and familiar trapped mice selected from the home cages of the test subject mice (n=20). In this condition, test-subject mice that received both SAL and SCH administration stayed longer in the area with other mice than in the area with no mates (F1,38=19.3, P<.001 in area; F1,38=0.111, P=.741 in treatment; F1,38=0.803, P=.376 in interaction; P=.006 and P=.048 in the social vs nonsocial area with SAL and SCH administration, respectively; Figure 2b), and consequently, social preference was not different between the SAL and SCH conditions (t19=0.383, P=.406; Figure 2b). Further analysis was conducted in which social dominance of the test-subject and trapped mice was considered, that is, a pair of high and low dominance ranked test-subject and trapped mice, respectively (n=10) and vice versa (n=10). SCH administration did not alter longer time spent in the social area compared with the nonsocial area in either low→high (t9=0.189, P=.854; Figure 2c) or high→low pairs (t9=0.331, P=.748; Figure 2c). Social preference was discovered to be higher when social dominance between the test-subject mice and trapped mice was closer in the SAL condition (r=−0.724, P=.018 in high→low rank; r=−0.743, P=.014 in low→high rank; Figure 2d). In contrast, a correlation was not observed in the SCH condition (Figure 2d). However, statistical analyses for comparison of the correlation coefficients between the SAL and SCH conditions did not reveal any significant difference (F2,16=1.75, P=.206 in high→low; F2,16=2.12, P=.153 in low→high).
These results suggest that the roles of D1 receptor signaling on social behaviors in rodents may depend on the social contexts, such as familiarity and social status of interacting mates. Since D1 receptor antagonist administration did not affect social preference with mates that were housed together in the same groups, the SCH effects to alter social dominance in mice may not involve social affiliative changes.
Impulsivity and Cognitive Functions
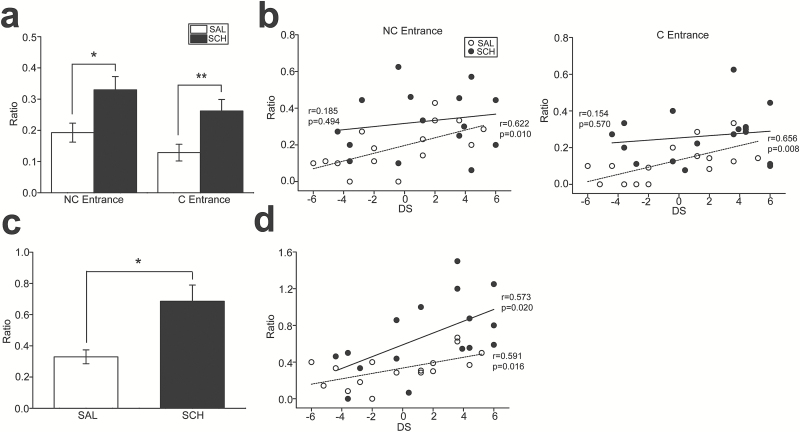
The random foraging test using the radial 8-arm maze was conducted to examine the effects of SCH administration on working memory (NC entrance) and behavioral flexibility (C entrance) and their relations to social dominance. SCH administration increased the number of both NC (t15=3.06, P=.008; Figure 3a) and C (t15=2.44, P=.028; Figure 3a) entries compared with those with SAL administration. Significant positive correlations were found between DS and the number of both NC (r=0.622, P=.010; Figure 3b) and C (r=0.656, P=.008; Figure 3b) entries in the SAL condition, which indicates that higher-ranked mice tended to perform worse in the test. In contrast, no such correlation was observed with SCH administration (Figure 3b). Statistical analyses for comparison of the correlation coefficients between the SAL and SCH conditions revealed significant (F2,28=3.79, P=.035) and marginally significant (F2,28=2.79, P=.079) differences in the number of C and NC entries, respectively.
Figure 3.
Associations between cognitive alterations and social hierarchy, and their modulations by the D1 antagonist in mice. (a) A graph showing the ratio of nonconsecutive (NC entrance) and consecutive (C entrance) reentries into the previously visited arms relative to the total number of arm entries in the random foraging task. *P=.008, **P=.028. (b) Graphs showing the correlations between DS and the number of NC (left) and C (right) entries with saline (SAL) and SCH23390 (SCH) administration. The dashed and solid lines indicate the linear regression analyses for SAL (open circles) and SCH (black circles) administration, respectively. (c) A graph showing the ratio of the number of opened-arm entries relative to the number of enclosed-arm entries in the elevated plus maze task. *P=.004. (d) A graph showing the correlations between David’s score (DS) and the number of opened-arm entries with SAL and SCH administration. The dashed and solid lines indicate the linear regression analyses for SAL and SCH administration, respectively.
The elevated plus maze test was also conducted to examine behavioral inhibition as measured by the number of inappropriate actions, that is, entering into the opened-arms of the maze, during the test. The number of opened-arm entries was significantly higher with SCH than with SAL administration (t15=3.38, P=.004) (Figure 3c). Moreover, statistically significant correlations were observed between the number of opened-arm entries and DS in the SAL (r=0.591, P=.016; Figure 3d) and SCH (r=0.573, P=.020; Figure 3d) conditions, which indicates that higher social rank mice exhibit more impulsive behavior.
These results suggest that impairments of behavioral inhibition resulting in higher impulsivity may be involved in promotion of social rank with SCH administration, whereas deficits in other cognitive functions may not have significant impacts on determining social hierarchy in rodents.
Social Hierarchy of Nonhuman Primates
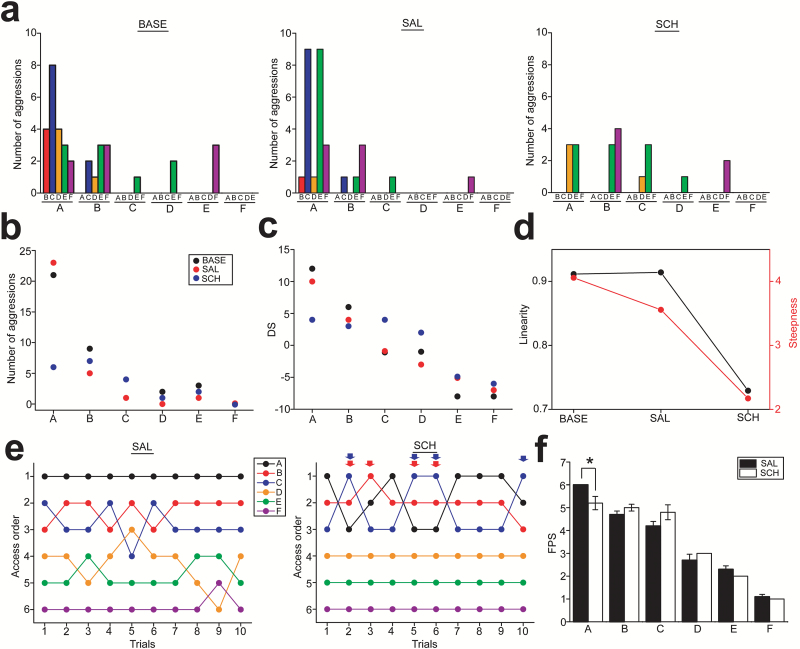
Social rank was determined by aggression between subjects and the food priority test. The highest-ranked subject (denoted as “A” in Figure 4a) exhibited aggressions over all other members in the group. The second-ranked subject (denoted as “B” in Figure 4a) exhibited aggressions to others except the highest ranked subject. Aggressions were observed only from higher to lower ranked subjects (Figure 4a). The social rank, which was determined by aggressions, was further confirmed with the food priority test in which the order of food access in each subject was recorded and quantified by the FPS (Figure 4e). Social rank was consistent independent of whether it was determined by aggressions or the food priority test (Figure 4a,e).
Figure 4.
Alterations of social hierarchy in the nonhuman primate group with D1 antagonist administration into the second-rank subject. (a) Histograms showing the number of aggressions that a subject made against each of other group members during the observation periods in the baseline (BASE), saline (SAL), and SCH23390 (SCH) conditions. The letters A to F at the bottom of the X-axis indicate the initiators, and the smaller letters above them indicate the receivers. (b) A graph showing the total number of aggressions that each subject made to all other group members in the BASE (black circles), SAL (red circles), and SCH (blue circles) conditions. (c) A graph showing the David’s score (DS) of the subjects at each rank in the BASE, SAL, and SCH conditions. (d) A graph showing the linearity and steepness of the hierarchy in group. (e) Diagrams showing the order of food access in each subject in the food priority test under the SAL (left) and SCH (right) conditions. The orders of food access and trials are shown on the y- and x-axes, respectively. The red and blue arrows indicate the events when the drug-treated (second rank; red circles) or third rank subject accessed food prior to the first-rank subject in the SCH condition. (f) A graph showing the average of the food priority score (FPS) over 10 trials in each subject under the SAL and SCH conditions. *P=.043.
Aggressions from the first to the second (i.e., the drug-treated) and third-rank subjects as well as from the second to the third rank subjects were observed in the BASE and SAL conditions, but these were diminished when SCH administration was given to the second-rank subject (Figure 4a-b). The DS that was calculated with aggressions illustrated a linear hierarchical relationship in the group under the BASE and SAL conditions (Figure 4c-d). SCH administration resulted in the reduction of the linearity and steepness of the hierarchy (Figure 4c-d). In the food priority test, the second- and third-rank subjects were able to obtain foods before the first-rank subject 4 times out of 10 trials for each of them in the SCH condition, which had not been observed in the SAL condition (Fisher’s exact test, χ2=5, df=1, P=.025 in SAL vs SCH; Figure 4e). The FPS of the first-rank subject was significantly decreased (Wilcoxon matched pairs test, Z=2.023, P=.043 in SAL vs SCH; Figure 4f) but was unaffected in others, including the drug-administered (second rank) subject.
These results suggest that SCH administration in the second-rank subject may not affect social dominance of the drug-administered subject but attenuates dominance of the highest-ranked subject over others in the group, and this may thereby promote stronger competitions among higher-ranked subjects.
Individual Behavior of the Nonhuman Primate with Drug Administration
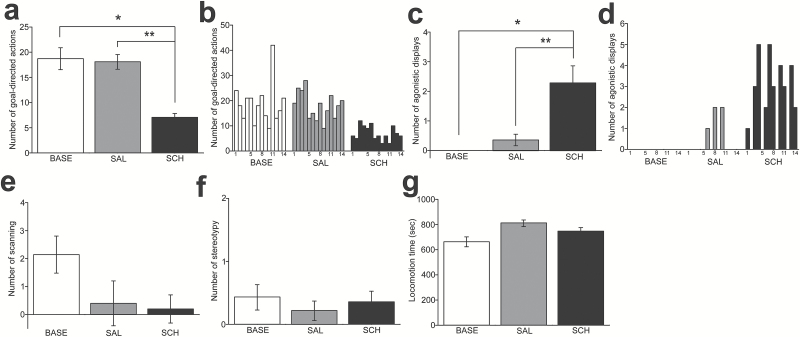
The effects of SCH administration were confirmed with several behavioral alterations in the drug-administered subject. SCH administration caused a significant decrease of goal-directed actions (F2, 12 =24.76, P<.001; posthoc Tukey test, P<.001 in BASE vs SCH, P=.002 in SAL vs SCH; Figure 5a) and an increase in the number of agonistic displays (F2, 12 =9.795, P=.003; P=.001 in BASE vs SCH, P=.005 in SAL vs SCH; Figure 5c), which indicates heightened impulsive and aggressive behaviors, respectively, in the drug-treated subject. These alterations persisted for approximately 1 month of the observation period (Figure 5b,d). None of the stereotypy, scanning, or locomotion was altered by SCH administration (Figure 5e-g).
Figure 5.
Behavioral alterations by the D1 antagonist in the nonhuman primate. (a) A graph showing the number of goal-directed actions per observation day in the drug-treated subject under the baseline (BASE), saline (SAL), and SCH23390 (SCH) conditions. *P<.001, **P=.002. (b) A histogram illustrating the changes in number of goal-directed actions on each observation day across the BASE, SAL, and SCH conditions. (c-d) Graphs similar to a and b, but showing agonistic display. *P=.001, **P=.005. (e-g) Graphs showing that neither scanning (e), stereotypy (f), nor locomotion (g) was altered by SCH administration.
Social Behaviors of Nonhuman Primates
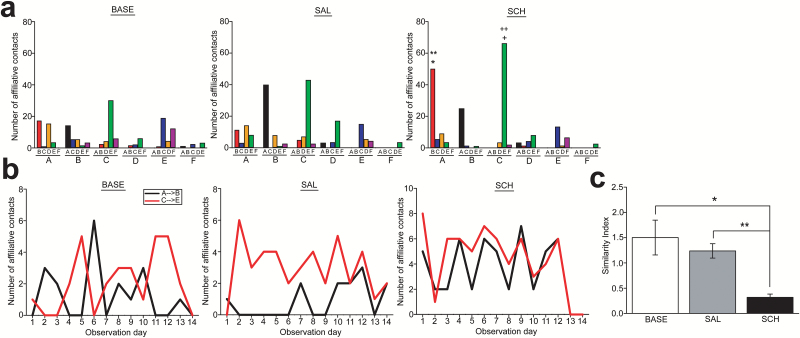
Social affiliations (i.e., proximate sitting, grooming, and playing; see Table 1 for the details of the criteria on these behaviors) between subjects were also altered by SCH administration into the second-rank subject. Because affiliative contacts occurred at relatively variable frequencies even between the BASE and SAL conditions (Figure 6a), the drug effects were determined when affiliations in the SCH condition were significantly different compared with both SAL and BASE conditions. The alterations matching with this criterion were the affiliative contacts from the first- to second-rank subjects (F2,12 =10.16, P=.003; P=.005 in BASE vs SCH, P=.001 in SAL vs SCH) and from the third- to fifth-rank subjects (F2,12 =8.771, P=.004; P=.02 in BASE vs SCH, P=.042 in SAL vs SCH) (Figure 6a). Although these affiliative bonds between first- and second- and between third- and fifth-rank subjects were independent in the BASE and SAL conditions, concurrences between them had emerged in the SCH condition (Figure 6b), which was evidenced by a significantly smaller similarity index score in the SCH condition than in the BASE and SAL conditions (1-way ANOVA, F2,39 =8.088, P=.001; P=.001 in BASE vs SCH, P=.014 in SAL vs SCH) (Figure 6c). SCH administration in the second-rank subject did not alter other social behaviors (supplementary Figure 2a-c).
Figure 6.
Alterations of social affiliations in the nonhuman primate group with D1 antagonist administration into the second-rank subject. (a) Histograms showing the number of affiliations that a subject made against each of other group members in the baseline (BASE), saline (SAL), and SCH23390 (SCH) conditions. The letters A to F at the bottom and the letters above them indicate the initiators and receivers, respectively. *P=.005, vs A→B in BASE; **P=.001, vs A→B in SAL; †P=.02, vs C→E in BASE; ††P=.042, vs C→E in SAL. (b) Graphs showing the affiliations from the subjects A to B (black line) and from the subjects C to E (red line) on each observation day in the BASE (left), SAL (middle), and SCH (right) conditions. (c) A graph showing the similarity index for the affiliative contacts between A→B and C→E. *P=.001, **P=.014.
These results suggest that SCH administration in one member of a group may cause complex affiliative social relationship alterations not only between the drug-treated subject and other mates, but also between subjects that are not directly related to the drug-treated subject (supplementary Figure 3a-c).
Discussion
In this study, by comparing mice and macaque social groups, we examined whether the mechanisms underlying social hierarchy were mutual across the 2 species. We found that D1 antagonist administration facilitated social dominance in the drug-treated mice. In contrast, in nonhuman primates, although D1 antagonist administration into the second-rank macaque in the social group did not alter social dominance of the drug-treated subject, this manipulation resulted in greater competitiveness between the drug-treated subject and other higher ranked (i.e., dominant) subjects, along with the alterations of social affiliations that consequently worked beneficially for the drug-treated subject. Thus, the findings suggest that D1 antagonist administration induces beneficial effects in both mice and macaques living in social groups, although the underlying processes may differ between species. Our study found that there was a loss of social dominance in the first-rank subject and a gain of social dominance in the third-rank subject after drug administration in the second-rank subject, which could be associated with altered social affiliation relationships. This is consistent with the studies suggesting that affiliative abilities are important in the attainment of dominance for primate species (Raleigh and McGuire, 1991; Higley et al., 1996). Such social relationship alterations with SCH administration into the second-rank macaque within the group did not cause a major devastating effect in each individual macaque in the group, as no significant changes in the amount of chronic stress hormones in the drug-treated subject and other mates were observed (supplementary Methods; supplementary Results; supplementary Figure 4a-b, for stress hormone assays in macaques). In contrast, the role of affiliative ability in social dominance was less clear in the mouse social groups. We also found that social preference between subjects housed in the same group was not altered by D1 receptor antagonist, suggesting that social affiliations may be less important in determination of a social hierarchy in rodents than in primates. These findings suggest that rodents and primates may have different levels of social complexity (social tactics including various aspects of interactions, such as affiliation and dominance, among subjects in a group).
Social hierarchy of animals and humans has been shown to be related to various factors such as kin relationship, age, and body size/weight (Koski et al., 2015). However, these factors had little effect in our study, because the animals used in this study had no kin relationships and were age matched. Moreover, social group dynamics in nonhuman primates were also independent of the gender of subjects, as we did not observe gender-specific social status and affiliative relationships in the group consisting of male and female macaques.
In mice, the SCH effects were not uniform in all drug-treated animals but were dependent on social rank in the groups. Facilitations of social dominance were observed in the second- and third-rank mice, but not in the first- and fourth-rank mice. One of possible explanations for no increase of social dominance in the first-rank mice may be the ceiling effect. In contrast, absence of increase in social dominance by SCH administration in the fourth-rank mice may involve other factors whose effects to place the mice into the lowest rank are substantially larger than the effects yielded by SCH administration.
Several studies have investigated the impacts of pharmacological manipulation of DA transmission on social behaviors of nonhuman primates housed in social groups. Amphetamine administration in such group-housed nonhuman primates decreases social interactions with other mates in the drug-treated subjects, which is reversed by the D1 antagonist (Ellenbroek et al., 1989; Melega et al., 2008). Consistent with these previous studies, we found no apparent decrease in social interactions of the drug-treated Japanese macaque with other group members following D1 receptor antagonist administration. Nonetheless, these findings contradict the study showing that D1 receptor stimulation facilitates social interactions in rodents (Gunaydin et al., 2014). Importantly, we found that the effects of D1 antagonist administration on social preference in mice was different when the drug-treated subjects interacted with familiar vs unfamiliar mates. Moreover, the difference of social dominance between the drug-treated subjects and other mates also affected social preference. Thus, social contexts, such as familiarity and social rank, play important roles on DA-dependent regulation of social behaviors. In this regard, also note that administration of a low dose of phencyclidine, which is used as an animal model of schizophrenia, causes deteriorative effects, such as “decreased” social interactions in individually housed rodents and nonhuman primates (Sams-Dodd, 1999; Mao et al., 2008). In contrast, the same drug treatment has been found to actually “increase” social affiliations with other mates without altering aggressions in socially housed nonhuman primates (Linn et al., 1999, 2007).
D1 receptors are distributed in several brain areas, including the prefrontal cortex (PFC), limbic structures, and the basal ganglia nuclei (Grace et al., 1998). This study did not unveil which brain regions and neural mechanisms were involved in the alterations of social hierarchy. In rodents, glutamatergic transmission in the PFC has been shown to be associated with social hierarchy (Wang et al., 2011), suggesting that the PFC is one of the promising targets. However, in nonhuman primates, the anterior and dorsal parts of the PFC gray matters have been demonstrated to correlate to not only social class of each subject in a group but also social group size (Noonan et al., 2014). In contrast, the volumes of the subcortical structures where D1 receptors are expressed, such as the striatum, amygdala, and hypothalamus, are associated sorely with social status of each subject in a group (Noonan et al., 2014). Collectively, DA-dependent information processing in the PFC and its striatal and limbic pathways may be the most promising neural substrates that determine social group dynamics.
In addition to DA, serotonin (5-HT) is another neurotransmitter involved in social dominance and social affiliations, and thereby, 5-HT and DA would work together to orchestrate social group dynamics. Administration of selective 5-HT reuptake inhibitor has been shown to attenuate dominant and submissive behaviors in the dominant and subordinate rank, respectively, of primates (Shively et al., 2014) and other animals (Larson and Summers, 2001). In contrast, selective 5-HT reuptake inhibitor administration has also been demonstrated to facilitate social affiliations in humans (Knutson et al., 1998). Collectively, increasing 5-HT transmission may result in social groups of less clear hierarchy and stronger social affiliative bonds.
In the social contexts of nonhuman primates and rodents and possibly other animals that organize into social hierarchy, behavioral changes caused by D1 receptor inhibition, which causes cognitive dysfunction, cannot simply be interpreted as “deficits.” Thus, a counter-balance could be established in D1 receptor signaling for cognitive deficits and facilitation of social dominance. Lower social ranked animals may perform better at foraging to facilitate reproductive fitness in a social group. This may also be associated with the facet of cognitive functions playing crucial roles in food foraging, and this may be relaxed for animals in social groups where resources can be shared compared with those in solitary environments. Finally, the evolutionary origins of psychiatric disorders that may involve D1 receptor alterations, such as schizophrenia (Okubo et al., 1997; Abi-Dargham et al., 2012), may be explained by such balancing selection mechanisms.
In conclusion, our study suggests that D1 receptor functions play significant roles in social group dynamics, such as social hierarchy. In rodents, low D1 functions facilitate social dominance in subjects housed in social groups. In contrast, D1 facilitation of social dominance is less clear in nonhuman primate social groups, which may be because social hierarchy determination in nonhuman primates may involve more complex social interaction dynamics than those in rodents.
Statement of Interest
None.
Supplementary Material
Acknowledgments
We thank Drs. Mary Ann Raghanty and Michael A. Huffman for comments and editing of the manuscript, and Ms. Tsukasa Obora and the staff of the Center for Human Evolution Modeling Research at Kyoto University Primate Research Institute for technical assistance.
This work was supported by Kyoto University Young Investigator Step-up and Core-stage Research Grants, Chubei Ito Foundation Research Grant, Sumitomo Foundation Research Grant, Inamori Foundation Research Grant, Institute of Seizon & Life Sciences Research Grant, JSPS Grant-in-Aid for challenging Exploratory Research #26640044 (Y.G.), JSPS Research Fellowship for Young Scientists #15J01210 (Y.Y.), and National Research Foundation of Korea #NRF-2014R1A1A3052796 (Y.A.L.).
References
- Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban N, Narendran R, Hwang DR, Laruelle M, Slifstein M (2012) Increased prefrontal cortical D(1) receptors in drug naive patients with schizophrenia: a PET study with [(1)(1)C]NNC112. J Psychopharmacol 26:794–805. [DOI] [PubMed] [Google Scholar]
- Alexander BK, Bowers JM. (1969) Social organization of a troop of Japanese monkeys in a two-acre enclosure. Folia Primatol 10:230–242. [DOI] [PubMed] [Google Scholar]
- Bond AB, Cook RG, Lamb MR. (1981) Spatial memory and the performance of rats and pigeons in the radial-arm maze. Anim Learn Behav 9:575–580. [Google Scholar]
- Cervenka S, Gustavsson JP, Halldin C, Farde L. (2010) Association between striatal and extrastriatal dopamine D2-receptor binding and social desirability. NeuroImage 50:323–328. [DOI] [PubMed] [Google Scholar]
- Chase ID, Tovey C, Spangler-Martin D, Manfredonia M (2002) Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proc Natl Acad Sci U S A 99:5744–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couppis MH, Kennedy CH, Stanwood GD. (2008) Differences in aggressive behavior and in the mesocorticolimbic DA system between A/J and BALB/cJ mice. Synapse 62:715–724. [DOI] [PubMed] [Google Scholar]
- David HA (1987) Ranking from unbalanced paired-comparison data. Biometrika 74. [Google Scholar]
- de Waal FB (1996) Macaque social culture: development and perpetuation of affiliative networks. J Comp Psychol 110:147–154. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Willemen AP, Cools AR (1989) Are antagonists of dopamine D1 receptors drugs that attenuate both positive and negative symptoms of schizophrenia? A pilot study in Java monkeys. Neuropsychopharmacology 2:191–199. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Jorgensen MJ, Huff A, Blau K, Hung YY, Mann JJ. (2004) Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. Am J Primatol 64:1–17. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. (1997) Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci 17:1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammell MP, Vries Hd, Jennings DJ, Carlin CM, Hayden TJ (2003) David’s score: a more appropriate domminance ranking method than Clutton-Brock et al.’s index. Anim Behav 66:601–605. [Google Scholar]
- Grace AA, Gerfen CR, Aston-Jones G (1998) Catecholamines in the central nervous system. Overview. Adv Pharmacol 42:655–670. [DOI] [PubMed] [Google Scholar]
- Griebel G, Rodgers RJ, Perrault G, Sanger DJ. (1997) Risk assessment behaviour: evaluation of utility in the study of 5-HT-related drugs in the rat elevated plus-maze test. Pharmacol Biochem Behav 57:817–827. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K. (2014) Natural neural projection dynamics underlying social behavior. Cell 157:1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, King ST, Hasert MF, Champoux M, Suomi SJ, Linnoila M (1996) Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology 14:67–76. [DOI] [PubMed] [Google Scholar]
- Hill A, Geissler S, Meyring M, Hecht S, Weigandt M, Mader K. (2013) In vitro-in vivo evaluation of nanosuspension release from subcutaneously implantable osmotic pumps. Int J Pharmaceut 451:57–66. [DOI] [PubMed] [Google Scholar]
- Jung MW, Qin Y, McNaughton BL, Barnes CA (1998) Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex 8:437–450. [DOI] [PubMed] [Google Scholar]
- Jupp B, Murray JE, Jordan ER, Xia J, Fluharty M, Shrestha S, Robbins TW, Dalley JW (2016) Social dominance in rats: effects on cocaine self-administration, novelty reactivity and dopamine receptor binding and content in the striatum. Psychopharmacology (Berl) 233:579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Wolkowitz OM, Cole SW, Chan T, Moore EA, Johnson RC, Terpstra J, Turner RA, Reus VI. (1998) Selective alteration of personality and social behavior by serotonergic intervention. Am J Psychiatry 155:373–379. [DOI] [PubMed] [Google Scholar]
- Koski JE, Xie H, Olson IR (2015) Understanding social hierarchies: the neural and psychological foundations of status perception. Soc Neurosci:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ET, Summers CH. (2001) Serotonin reverses dominant social status. Behav Brain Res 121:95–102. [DOI] [PubMed] [Google Scholar]
- Lee YA, Goto Y. (2011) Neurodevelopmental disruption of cortico-striatal function caused by degeneration of habenula neurons. PloS One 6:e19450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn GS, O’Keeffe RT, Schroeder CE, Lifshitz K, Javitt DC (1999) Behavioral effects of chronic phencyclidine in monkeys. Neuroreport 10:2789–2793. [DOI] [PubMed] [Google Scholar]
- Linn GS, O’Keeffe RT, Lifshitz K, Schroeder C, Javitt DC (2007) Behavioral effects of orally administered glycine in socially housed monkeys chronically treated with phencyclidine. Psychopharmacology (Berl) 192:27–38. [DOI] [PubMed] [Google Scholar]
- Lund M. (1975) Social mechanisms and social structure in rats and mice. Ecol Bull 19:255–260. [Google Scholar]
- Machida T, Yonezawa Y, Noumura T. (1981) Age-associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Horm Behav 15:238–245. [DOI] [PubMed] [Google Scholar]
- Majolo B, Lehmann J, de Bortoli Vizioli A, Schino G (2012) Fitness-related benefits of dominance in primates. Am J Phys Anthropol 147:652–660. [DOI] [PubMed] [Google Scholar]
- Mao CV, Hori E, Maior RS, Ono T, Nishijo H. (2008) A primate model of schizophrenia using chronic PCP treatment. Rev Neurosci 19:83–89. [DOI] [PubMed] [Google Scholar]
- Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, Broft A, Van Heertum R, Kleber HD (2010) Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry 67:275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Jorgensen MJ, Lacan G, Way BM, Pham J, Morton G, Cho AK, Fairbanks LA. (2008) Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. Neuropsychopharmacology 33:1441–1452. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. (2000) Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am J Primatol 52:115–131. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. (2004) Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Gene Brain Behav 3:287–302. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Nader SH, Morgan D (2012) Nonhuman primate models of social behavior and cocaine abuse. Psychopharmacology (Berl) 224:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Sallet J, Mars RB, Neubert FX, O’Reilly JX, Andersson JL, Mitchell AS, Bell AH, Miller KL, Rushworth MF (2014) A neural circuit covarying with social hierarchy in macaques. PLoS Biol 12:e1001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. (1997) Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature 385:634–636. [DOI] [PubMed] [Google Scholar]
- Pearson BL, Defensor EB, Blanchard DC, Blanchard RJ. (2010) C57BL/6J mice fail to exhibit preference for social novelty in the three-chamber apparatus. Behav Brain Res 213: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaven-Sigray P, Gustavsson P, Farde L, Borg J, Stenkrona P, Nyberg L, Backman L, Cervenka S (2014) Dopamine D1 receptor availability is related to social behavior: A positron emission tomography study. NeuroImage 102:590–595. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. (2002) The effects of dopamine D1 receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn Mem 9:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MJ, McGuire MT (1991) Bidirectional relationships between tryptophan and social behavior in vervet monkeys. Adv Exp Med Biol 294:289–298. [DOI] [PubMed] [Google Scholar]
- Richards SM. (1974) The concept of dominance and methods of assessment. Anim Behav 22:914–930. [Google Scholar]
- Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD (2012) Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trend Cogn Sci 16:81–91. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Nikulina EM, Cole JC. (1994) Dopamine D1 and D2 receptor ligands modulate the behaviour of mice in the elevated plus-maze. Pharmacol Biochem Behav 49:985–995. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F (1999) Phencyclidine in the social interaction test: an animal model of schizophrenia with face and predictive validity. Rev Neurosci 10:59–90. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. (2005) The influence of social hierarchy on primate health. Science 308:648–652. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. (1998) D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci 18:1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. (2004) The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74:1–58. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Higley JD, Willard SL (2014) Sertraline effects on cerebrospinal fluid monoamines and species-typical socioemotional behavior of female cynomolgus monkeys. Psychopharmacology (Berl) 231:1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno KI, Togashi H, Mori K, Matsumoto M, Ohashi S, Hoshino A, Fujita T, Saito H, Minami M, Yoshioka M. (2002) Behavioural and pharmacological relevance of stroke-prone spontaneously hypertensive rats as an animal model of a developmental disorder. Behav Pharmacol 13:1–13. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ (2006) Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry 60:66–73. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. (2011) Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science 334:693–697. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. (1997) Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17:8528–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.