Abstract
Clinical data suggest that optic neuropathy and retinal ganglion cell loss are the main cause of visual decline in patients with familial dysautonomia, but this has not previously been confirmed by pathological analyses. We studied retinas and optic nerves in 6 eyes from 3 affected patients obtained at autopsy. Analyses included routine neurohistology and immunohistochemistry for neurofilaments, cytochrome c oxidase (COX), and melanopsin-containing ganglion cells. We observed profound axon loss in the temporal portions of optic nerves with relative preservation in the nasal portions; this correlated with clinical and optical coherence tomography findings in 1 patient. Retinal ganglion cell layers were markedly reduced in the central retina, whereas melanopsin-containing ganglion cells were relatively spared. COX staining was reduced in the temporal portions of the optic nerve indicating reduced mitochondrial density. Axonal swelling with degenerating lysosomes and mitochondria were observed by electron microscopy. These findings support the concept that there is a specific optic neuropathy and retinopathy in patients with familial dysautonomia similar to that seen in other optic neuropathies with mitochondrial dysfunction. This raises the possibility that defective expression of the IkB kinase complex-associated protein (IKAP) resulting from mutations in IKBKAP affects mitochondrial function in the metabolism-dependent retinal parvocellular ganglion cells in this condition.
Keywords: Hereditary optic neuropathy, Histopathology, Familial dysautonomia, Optical coherence tomography, Retina, Riley-Day syndrome
INTRODUCTION
Visual impairment is a disabling feature of familial dysautonomia (Riley-Day syndrome, hereditary sensory and autonomic neuropathy type III), a rare, infantile-onset, autosomal recessive disease caused by a mutation in the IKBKAP gene that leads to defective expression of IkB kinase complex-associated protein (IKAP) (1, 2). The defective IKAP protein is strongly expressed in neuronal and retinal tissue and appears to be involved in cell migration and adhesion during embryogenesis, causing impaired development of sensory and afferent autonomic nerves (3).
Hallmarks of familial dysautonomia are impaired pain and temperature sensation, absent deep tendon reflexes, gait ataxia (4), chronic lung disease (5), and afferent baroreflex failure causing orthostatic hypotension and paroxysmal hypertension (6), all which contribute to morbidity and mortality (2, 7).
Neuropathological findings of the disease include brainstem, pontine, medullary, and spinal atrophy with neuronal depletion in dorsal root (sensory) and sympathetic ganglia, as well as reduced numbers of small myelinated and nonmyelinated (sensory) axons of peripheral nerves (8–12).
Previous studies from our group using high-definition optical coherence tomography (OCT) showed that 100% of patients with familial dysautonomia suffer from an optic neuropathy featured by reductions in the macular retinal ganglion cell layer and their axons in the retinal nerve fiber layer (RNFL), whereas more peripheral ganglion cells are relatively spared (13, 14). This optic neuropathy is universal in familial dysautonomia, in contrast to corneal opacities due to neurotrophic keratopathy and insufficient tear secretion. These were previously considered to be the main cause of visual loss but are present in less than 50% of patients (13, 14). In subjects with relatively normal corneas, visual decline becomes symptomatic around the second or third decade of life and can progress to legal blindness in older patients, although the retinal ganglion cell loss begins at earlier ages (13). However, to date, there has not been pathological confirmation of this.
We here describe the histopathological findings of eyes from patients with familial dysautonomia and correlate these with the clinical ophthalmological findings and the retinal structure abnormalities measured by OCT. To the best of our knowledge, this is the first reported histopathological study of the retina and the optic nerve of patients with familial dysautonomia.
MATERIALS AND METHODS
Patients
We studied autopsy specimens from 3 subjects (6 eyes) with genetically confirmed familial dysautonomia who died due to systemic complications. Clinical information was obtained from the medical charts of the New York University (NYU) Dysautonomia Center. In 2 patients, ocular histories were obtained from records submitted by their primary ophthalmologists. Patient #1 was examined at our center and a comprehensive neuro-ophthalmological evaluation was performed 10 weeks prior to death. Relatives gave full consent for autopsy in all cases. The New York University School of Medicine Institutional Review Board approved this study.
Optical Coherence Tomography
In Patient #1, OCT was performed to obtain retinal images and measure the RNFL and the ganglion cell complex (GCC) thicknesses (Cirrus 4000; Carl Zeiss, Dublin, CA) 10 weeks prior to death. Images were acquired in the seated position with the patient facing the OCT equipment. Both eyes were scanned 3 times with 2 standard Cirrus HD-OCT acquisition protocols: macular cube 512 × 128 and optic disc cube 200 × 200. The quality of the obtained images was assessed by evaluation of the signal strength (a value from 0 to 10 in arbitraries units), automatically provided by system. Only scans with signal strength above 6 units were accepted.
Ocular Pathology Studies
In all cases, the postmortem delay was <15 hours; both eyes were fixed immediately in 10% buffered formalin. The optic nerves were 20 mm in length and cut into cross sections, 2 mm thick and approximately 3 mm posterior to the globes. Orientation was established by razor nicks. The eyes were cut in the horizontal plane to section the macular and optic disc area (PO section). Paraffin-embedded 6-μm-thick tissue sections were stained with hematoxylin and eosin, periodic acid Schiff, and Luxol fast blue stains and Bielschowsky impregnation. The formalin fixed paraffin-bedded tissue sections were deparaffinized and rehydrated for immunohistochemistry. Immunohistochemical staining was performed using mouse monoclonal antineurofilament (Ventana Medical, Tucson, AZ), antimelanopsin, and anticytochrome c oxidase (COX) antibodies (Abcam, Cambridge, MA). COX is a component of the mitochondrial respiratory chain; therefore, COX immunostaining in normal subjects is usually more intense in the prelaminar and unmyelinated portion of the optic nerve, particularly in the temporal portion, where mitochondria abound in order to provide energy for nerve conduction. In contrast, the retro-laminar portion of the optic nerve is myelinated, allowing more efficient nerve conduction, thus requiring less energy and fewer mitochondria, leading to less intense COX immunostaining. The binding of these 3 antibodies (antineurofilament, antimelanopsin, and antiCOX) was visualized as brown pigment using a secondary antibody Avidin-Biotin Complex Kit (Vectastain Elite ABC Kit; Vector Laboratories, Burlingame, CA). Appropriate positive and negative controls were used.
In 1 patient (Patient #2), electron microscopy was also performed. The eye was first fixed in 10% buffered formalin and then transferred to 2.5% glutaraldehyde in 100 mM phosphate buffer at pH 7.0. The tissue in the area of the prelaminar and lamina cribrosa of the optic nerve head area was cut into 2 mm3 tissue blocks and postfixed in 1% osmium tetroxide in sodium cacodylate buffer for 3 hours at 20 °C. These were then en-block stained with 5% aqueous uranyl acetate, dehydrated in graded ethanol solutions, and embedded in resin (Epon-812). Thick sections (1 μm) were stained with toluidine blue. Thin sections were cut at 50–70 nm using an ultramicrotome (LKB8801), stained with uranyl acetate and lead citrate, and examined with a transmission electron microscope (Phillips EM 201).
RESULTS
Patients’ characteristics are summarized in the Table. Patient #1 was 61 years old, whereas Patients #2 and #3 were younger (19 and 11 years old, respectively). Patient #1 had worse visual acuity; Patient #3 was visually asymptomatic. Red-green color vision was reduced in 2 patients. On visual field testing, there were cecocentral scotomas in both eyes of Patient #2, and generalized sensitivity depression was seen in both eyes of Patient #1. Neurotrophic keratopathy, corneal hypoesthesia, and impaired tear production were found in all 3 patients. Corneal opacities due to recurrent abrasion and ulcers were present in 2 patients: mild in Patient #1 and moderate to severe in Patient #2, but were not dense enough to preclude fundus examination or OCT image acquisition. On fundus examination, all patients had optic nerve pallor that was more evident in the temporal portion.
TABLE.
Patients Clinical Features
| Age/Sex | Eye | Visual Acuity | Color Vision | Visual Fields Defects | Anterior Segment | Fundoscopic Exam | Optical Coherence Tomography | |
|---|---|---|---|---|---|---|---|---|
| Patient #1 | 61/M | Right | 20/200 | 2/16 | Ceco-central with generalized sensitivity depression | Corneal hypoesthesia and hypolacrima, mild corneal opacities | Diffuse optic nerve pallor, papillomacular RNFL loss | Global RNFL loss mainly affecting temporal axons, severe loss of macular GCC |
| Left | 20/200 | 1/16 | ||||||
| Patient #2 | 17/M | Right | 20/100 | 8/16 | Ceco-central scotoma | Corneal anesthesia and hypolacrima, moderate to severe corneal opacities due to recurrent abrasions | Temporal optic nerve pallor, papillomacular RNFL loss | N/A |
| Left | 20/80 | 6/16 | ||||||
| Patient #3 | 11/F | Right | 20/30 | N/A | N/A | Corneal hypoesthesia and hypolacrima, no corneal opacities | Temporal optic nerve pallor, papillomacular RNFL loss | N/A |
| Left | 20/25 |
M, male; F, female: N/A, not available; GCC, ganglion cell complex, RNFL: retinal nerve fiber layer.
OCT Findings
In Patient #1, in vivo OCT showed significant reductions of the global RNFL and GCC thicknesses especially in the temporal portion (corresponding to the maculopapular bundle), consistent with previous reports by our group in patients with familial dysautonomia (13). These OCT findings were later confirmed in retinal histopathological cross sections showed thinning of the ganglion cell layer (mostly midget/parvocellular, P-cells), and its axons in the RNFL (Fig. 1).
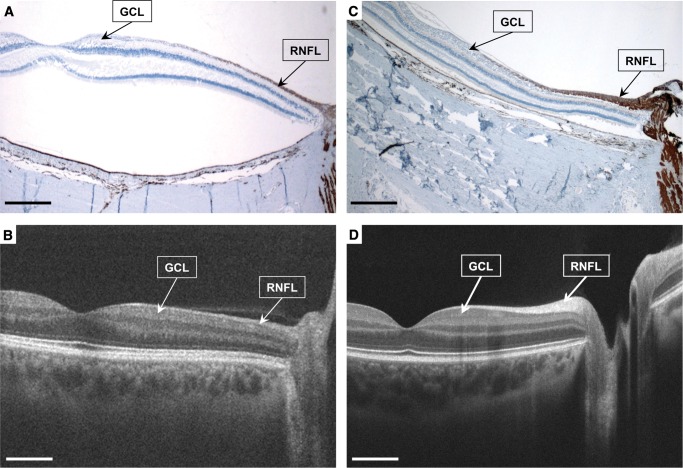
FIGURE 1.
Patient #1 with familial dysautonomia (A) and (B) and aged-matched normal control (C) and (D). Anti-neurofilament immunostained maculopapillary retinal cross-section (2x, (A) and (C)) and matching images from in vivo high definition-optical coherence tomography (B) and (D) show that in the familial dysautonomia patient eye there is marked thinning of the ganglion cell layer ([GCL], mostly midget/parvocellular, P-cells), and their the axons in the retinal nerve fiber layer (RNFL), which occurred primarily in the central retina in the distribution of the maculopapillary bundles (A), retinal detachment is a processing artifact). This was previously documented with the in vivo optical coherence tomography (B). Scale bars, 1 mm.
Histopathology
In Patient #1, cross sections of both optic nerves showed diffuse depletion of axon bundles of the entire optic nerve associated with thickened connective tissue septae in affected regions. Axonal depletion was more evident in the temporal portion of the optic nerve (corresponding to maculopapillary nerve fibers), better seen in neurofilament immunostained sections (Fig. 2E, F). In the central retina, the number of macular ganglion cells (mostly parvocellular ganglion cells) was reduced (stained blue, Fig. 3A). Melanopsin immunohistochemical staining demonstrated preservation of the intrinsic photosensitive melanopsin-containing ganglion cells (stained brown, Fig. 3A), despite significant macular ganglion cell loss. The peripheral retina was mostly devoid of ganglion cells. COX immunostaining showed accumulation of mitochondria in the prelaminar and amyelinic portion of the optic nerve axons, with less staining density in the temporal portion (indicating extensive retinal nerve fiber loss secondary to macular ganglion cell death). In contrast, the staining was normally preserved in the nasal portion.
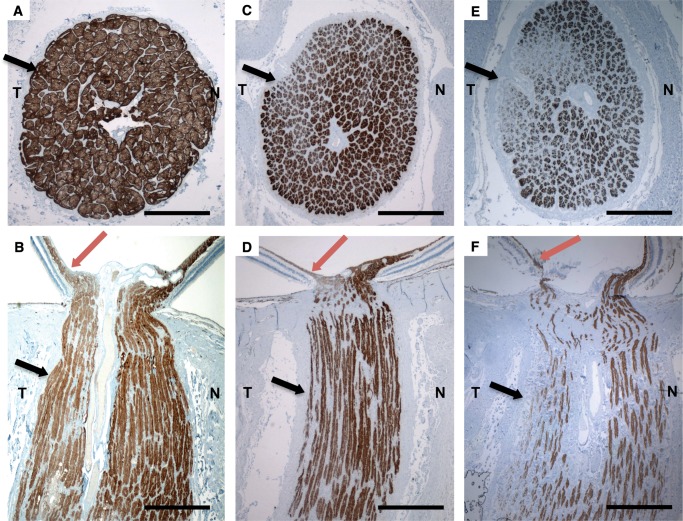
FIGURE 2.
(A–F) Anti-neurofilament-immunostained histopathologic cross and axial sections (2x) of the optic nerve of a 48-year-old normal control (A) and (B) and patients with familial dysautonomia (C–F). Sections from 11-year-old patient #3 (C) and (D) and 61-year-old patient #1 (E) and (F) show significant axonal loss in the optic nerve that is more evident in the temporal optic nerve portion ([T] black arrows), than in the nasal portion [N], as well as thinning in the maculopapillary nerve fiber layer (red arrows). Nerve loss is more prominent in the older patient (Patient #1, (E) and (F)). Scale bars: 1 mm.
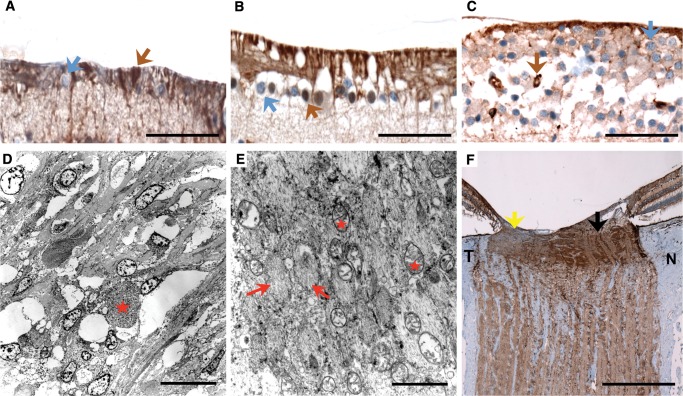
FIGURE 3.
Histopathological cross sections and electron microscopy of the retina and optic nerves of patients with familial dysautonomia. (A–C) Immunohistochemical staining for melanopsin in retinal ganglion cells (40x, scale bars are 50 μm) in Patient #1 (A), Patient #3 (B), and a normal control (C). Macular ganglion cells (stained blue, blue arrow) are markedly reduced in patients with familial dysautonomia (A) and (B) compared to the normal control (C), whereas melanopsin retinal ganglion cells (stained in brown, brown arrow) are preserved in the 3 subjects. Melanopsin retinal ganglion cells are more resistant to mitochondrial stress and are also preserved in Leber hereditary optical atrophy and autosomal dominant optic atrophy (17). (D) and (E) Electron microscopy examination of the prelaminar temporal portion of the optic nerve head in Patient #2 (scale bars: 2 μm). Nerve fibers are arranged in axonal bundles surrounded by astrocytes and a narrow connective tissue space containing capillaries. Low magnification of the optic nerve head shows cells with focal axonal swelling/degeneration (red star). Temporal portion of the optic nerve head pre-laminar region showing nerve bundles (red arrows) with atrophy and mitochondria (red stars). There is axonal swelling with degenerating organelles (lysosomes and mitochondria). Focal myelinated axons were identified beyond the lamina cribrosa area. (F) COX immunostaining (2x, scale bar: 0.5 mm) of the optic nerve of Patient #3 is less intense in the temporal portion ([T], yellow arrow), indicating extensive retinal nerve fiber loss secondary to macular ganglion cell death, compared to more normal staining in the nasal portion ([N], black arrow).
In Patient #2, electron microscopy the optic nerve head surface, nerve fiber layer, and prelaminar temporal region showed optic nerve fibers arranged in axonal bundles surrounded by astrocytes (or glial cells) and narrow connective tissue space containing capillaries. Astrocytes contained cytoplasmic intermediate filaments, and degenerating organelles. The mitochondria, from the fibers that were still viable, had a membrane, matrix, and internal ridges (cristae) and showed normal range and size. Focally, there were axonal swellings with degenerating organelles, presumably lysosomes and mitochondria. These findings are similar to those described in Leber hereditary optic neuropathy (15). The region of the lamina cribrosa showed normal connective tissue collagen fibers and elastic complexes admixed with nerve fibers and glial cells. Focal myelinated axons were identified beyond the lamina cribrosa (Fig. 3D, E).
Histopathological analysis in Patient #3 showed markedly reduced neurofilament-immunostained optic nerve axons particularly at the temporal portion (Fig. 2C, D), although this was less marked than in Patient #1 (Fig. 2E, F). Melanopsin immunostaining of the retina also showed preservation of the melanopsin-containing ganglion cells (stained brown, Fig. 3B) in spite of generalized reductions in the number of macular ganglion cells (stained blue, Fig. 3B). In the peripheral retina, the ganglion cell population was also mildly reduced although less affected than in the macula. COX immunostaining showed accumulation of mitochondria in the prelaminar and amyelinic portion of the optic nerve axons, although the staining was less intense in the temporal portion (indicating extensive retinal nerve fiber loss secondary to macular ganglion cell death), compared to normal staining in the nasal portion (Fig. 3F). This pattern has been described in other mitochondrial optic neuropathies (16).
In summary, histopathological analysis of ocular tissue of patients with familial dysautonomia showed thinning of the retinal ganglion cell layer with widespread axonal depletion, more evident in the temporal portion of the optic nerve (where axons of the ganglion cell layer are located). This ganglion cell layer thinning was due to depletion of macular ganglion cells (mostly parvocellular ganglion cells). In contrast, melanopsin-containing ganglion cells were preserved. Mitochondrial density was reduced in the temporal portion of the prelaminar area (further indicating retinal nerve fiber damage secondary to macular ganglion cell death). Mitochondrial abnormalities were confirmed with electron microscopy showing axonal swelling with degenerating lysosomes and mitochondria. These abnormalities appear to worsen with age. Overall, these findings are similar to those described in mitochondrial optic neuropathies such as Leber hereditary optic neuropathy and dominant optic atrophy (16, 17).
DISCUSSION
In this, the first histopathological postmortem study of ocular tissue in patients with familial dysautonomia, we confirmed our hypothesis that retinal macular ganglion cell loss and progressive optic nerve axonal degeneration are the primary cause of visual loss in these patients (13, 14). Our findings provide novel information on the mechanisms involved in the pathogenesis of visual loss in familial dysautonomia.
In the 3 patients in this study, clinical evidence of optic neuropathy included visual acuity loss, abnormal red-green color perception, central visual field defects and thinning of the maculopapillary RNFL, and macular GCC on OCT. In all of them, histopathological examination showed that the loss of ganglion cells (mostly midget/parvocellular, P-cells) occurred primarily in the central retina with loss of the small optic nerve axons in the in the distribution of the maculopapillary bundles. These findings correlate with our previous OCT observations and were demonstrated in Patient #1 (3, 4). An additional histopathological finding was the relative preservation of melanopsin-containing ganglion cells in the most affected regions of the retina, suggesting that less energy-dependent cells, which mainly project to the hypothalamus and are responsible for the extrageniculate visual afferents, are spared. This supports our hypothesis that more energy-demanding maculopapillary ganglion cells are preferentially damaged in familial dysautonomia.
Interestingly, a recent retinal histopathological study in a mouse model of familial dysautonomia (IKBKAP conditional knockout) showed significant retinal ganglion cells loss, particularly in the temporal portion, with preservation of melanopsin ganglion cells (18). These findings are remarkably similar as the ones we document here.
The optic neuropathy of familial dysautonomia closely resembles that seen in mitochondrial optic neuropathies such as Leber hereditary optic neuropathy (15, 19), autosomal dominant optic atrophy (20), and tobacco/alcoholic (21), Cuban epidemic (22), B12 deficiency, and drug-related optic neuropathies (23). Pathological studies in Leber hereditary optic neuropathy and dominant optic atrophy have shown relatively preservation of melanopsin-containing ganglion cells in spite of extensive loss of the predominantly small midget/parvocellular P-ganglion cells (17, 24). This is because melanopsin retinal ganglion cells are more resistant to mitochondrial stress and neurodegeneration (17). The optic neuropathy observed in familial dysautonomia particularly resembles that seen in dominant optic atrophy, in which the optic nerve damage is present since childhood, even in visually asymptomatic patients, which can be proven by OCT (25). In both familial dysautonomia and dominant optic atrophy, the genetic defect is located at the nuclear DNA (IKBKAP gene in 9q31 in familial dysautonomia; and OPA1 gene in 3q28 in dominant optic atrophy) (1, 25). While the OPA1 gene in involved in mitochondrial membrane stabilization, COX function, and mitochondrial respiratory chain metabolism (26), the role of the IKBKAP gene in mitochondrial metabolism remains unexplored. It is possible that the loss of functional IKAP protein (expressed by the IKBKAP gene) also affects mitochondrial function in familial dysautonomia (27, 28), although additional research is required to confirm this hypothesis.
In conclusion, familial dysautonomia is associated with a characteristic type of optic neuropathy resembling other disorders affecting mitochondrial protein production and function. The possibility that IKAP deficiency may impair the function of mitochondria of the retinal ganglion cells may potentially have therapeutic implications, although this remains to be ascertained in future studies. Additionally, the retinal ganglion cells are accessible by intravitreal injection and, therefore, more amenable to potential therapies. Also, monitoring optic nerve structure and function may be useful to assess disease progression in clinical trials of potential disease-modifying drugs for familial dysautonomia.
Financial support: This work was supported by the Dysautonomia Foundation Inc., the National Institutes of Health (U54-NS065736-01), and The Massachusetts Lions Clubs/Research to Prevent Blindness Challenge Grant.
Conflict of interest: The authors declare that they have no conflict of interests relevant to this paper.
REFERENCES
- 1.Slaugenhaupt SA, Blumenfeld A, Gill SP, et al. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet 2001;68:598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norcliffe-Kaufmann L, Slaugenhaupt SA, Kaufmann H.. Familial dysautonomia: History, genotype, phenotype and translational research. Prog Neurobiol 2016; pii: S0301-0082(16)30020-X. doi: 10.1016/j.pneurobio.2016.06.003 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Hunnicutt BJ, Chaverra M, George L, et al. IKAP/Elp1 is required in vivo for neurogenesis and neuronal survival, but not for neural crest migration. PloS One 2012;7:e32050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macefield VG, Norcliffe-Kaufmann L, Gutierrez J, et al. Can loss of muscle spindle afferents explain the ataxic gait in Riley-Day syndrome? Brain 2011;134:3198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maayan HC. Respiratory aspects of Riley-Day Syndrome: familial dysautonomia. Paed Resp Rev 2006;7 Suppl 1:S258–59 [DOI] [PubMed] [Google Scholar]
- 6.Norcliffe-Kaufmann L, Axelrod F, Kaufmann H.. Afferent baroreflex failure in familial dysautonomia. Neurology 2010;75:1904–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palma JA, Norcliffe-Kaufmann L, Fuente-Mora C, et al. Current treatments in familial dysautonomia. Exp Opin Pharmacother 2014;15:2653–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson J, Pytel BA, Grover-Johnson N, et al. Quantitative studies of dorsal root ganglia and neuropathologic observations on spinal cords in familial dysautonomia. J Neurol Sci 1978;35:77–92 [DOI] [PubMed] [Google Scholar]
- 9.Pearson J, Dancis J, Axelrod F, et al. The sural nerve in familial dysautonomia. J Neuropathol Exp Neurol 1975;34:413–24 [DOI] [PubMed] [Google Scholar]
- 10.Yatsu F, Zussman W.. Familial Dysautonomia (Riley-Day syndrome). Case report with postmortem findings of a aatient at age 31. Arch Neurol 1964;10:459–63 [DOI] [PubMed] [Google Scholar]
- 11.Cohen P, Solomon NH.. Familial dysautonomia; case report with autopsy. J Pediatr 1955;46:663–70 [DOI] [PubMed] [Google Scholar]
- 12.Brown WJ, Beauchemin JA, Linde LMA.. Neuropathological study of familial dysautonomia (Riley-Day Syndrome) in siblings. J Neurol Neurosurg, Psych 1964;27:131–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendoza-Santiesteban CE, Hedges TR III, Norcliffe-Kaufmann L, et al. Selective retinal ganglion cell loss in familial dysautonomia. J Neurol 2014;261:702–09 [DOI] [PubMed] [Google Scholar]
- 14.Mendoza-Santiesteban CE, Hedges TR 3rd, Norcliffe-Kaufmann L, et al. Clinical neuro-ophthalmic findings in familial dysautonomia. J Neuro-Ophthalmol 2012;32:23–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadun AA, Win PH, Ross-Cisneros FN, et al. Leber's hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc 2000;98:223–32;discussion 32-5 [PMC free article] [PubMed] [Google Scholar]
- 16.Yu-Wai-Man P, Griffiths PG, Chinnery PF.. Mitochondrial optic neuropathies—disease mechanisms and therapeutic strategies. Prog Ret Eye Res 2011;30:81–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Morgia C, Ross-Cisneros FN, Sadun AA, et al. Melanopsin retinal ganglion cells are resistant to neurodegeneration in mitochondrial optic neuropathies. Brain 2010;133:2426–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueki Y, Ramirez G, Salcedo E, et al. Loss of Ikbkap causes slow, progressive retinal degeneration in a mouse model of familial dysautonomia. eNeuro 2016;3:pii: ENEURO.0143-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barboni P, Carbonelli M, Savini G, et al. Natural history of Leber's hereditary optic neuropathy: Longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology 2010;117:623–27 [DOI] [PubMed] [Google Scholar]
- 20.Cohn AC, Toomes C, Hewitt AW, et al. The natural history of OPA1-related autosomal dominant optic atrophy. Br J Ophthalmol 2008;92:1333–336. [DOI] [PubMed] [Google Scholar]
- 21.Kesler A, Pianka P.. Toxic optic neuropathy. Curr Neurol Neurosci Rep 2003;3:410–14 [DOI] [PubMed] [Google Scholar]
- 22.Johns DR, Neufeld MJ, Hedges TR. 3rd. Mitochondrial DNA mutations in Cuban optic and peripheral neuropathy. J Neuro-Ophthalmol 1994;14:135–40 [PubMed] [Google Scholar]
- 23.Grzybowski A, Zulsdorff M, Wilhelm H, et al. Toxic optic neuropathies: An updated review. Acta Ophthalmol 2015;93:402–10 [DOI] [PubMed] [Google Scholar]
- 24.La Morgia C, Ross-Cisneros FN, Hannibal J, et al. Melanopsin-expressing retinal ganglion cells: implications for human diseases. Vision Res 2011;51:296–302 [DOI] [PubMed] [Google Scholar]
- 25.Yu-Wai-Man P, Griffiths PG, Burke A, et al. The prevalence and natural history of dominant optic atrophy due to OPA1 mutations. Ophthalmology 2010;117:1538–54646 e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadun AA, La Morgia C, Carelli V.. Mitochondrial optic neuropathies: Additional facts and concepts - response. Clin Exp Ophthalmol 2014;42:207–8 [DOI] [PubMed] [Google Scholar]
- 27.Lee G, Papapetrou EP, Kim H, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 2009;461:402–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palma JA, Roda R, Norcliffe-Kaufmann L, et al. Increased frequency of rhabdomyolysis in familial dysautonomia. Muscle Nerve 2015;52:887–90 [DOI] [PMC free article] [PubMed] [Google Scholar]