Abstract
Background:
Stress is a risk factor for depression and anxiety disorders, disrupting neuronal processes leading to exaggerated fear and compromised coping behaviors. Current antidepressants are only partially effective. Vortioxetine, a novel multimodal antidepressant, is a serotonin transporter inhibitor; 5-HT3, 5-HT7, and 5-HT1D receptor antagonist; 5-HT1B partial agonist; and 5-HT1A agonist. We have shown that chronic dietary vortioxetine administration reversed stress-induced deficits in cognitive flexibility. In the present studies, we investigated the generality of vortioxetine’s effects on other stress-related behavioral changes after different types of chronic stress.
Methods:
In experiment 1, rats were fear-conditioned by pairing a tone with footshock, then exposed to chronic plus acute prolonged stress. In experiment 2, rats were exposed to chronic unpredictable stress. In both experiments, beginning on day 4 of chronic stress, vortioxetine was given in the diet (24 mg/kg/d). In experiment 1, effects of vortioxetine were tested on stress-induced changes in retention and extinction of cue-conditioned fear, and in experiment 2, on coping behavior on the shock probe defensive burying test after chronic stress.
Results:
Chronic stress exaggerated the expression of conditioned fear memory. Vortioxetine restored fear memory to control levels and rendered extinction in stressed rats comparable with that in controls. In experiment 2, chronic unpredictable stress caused a shift from active to passive coping behavior, and vortioxetine restored active coping.
Conclusions:
Vortioxetine reduced exaggerated expression of conditioned fear and restored adaptive coping behavior following 2 different types of chronic stress, adding to the evidence of its therapeutic potential in the management of depression and anxiety disorders.
Keywords: coping, depression, fear memory, stress, vortioxetine
Significance Statement
Vortioxetine is a novel multimodal antidepressant. We have shown previously that vortioxetine improves stress-induced deficits in cognitive function mediated in the prefrontal cortex of rats that model cognitive components of depression and anxiety disorders in humans. In the present study, we expand the generality of these observations. The results show that chronic vortioxetine administration also reduces the exaggerated expression of fear memory and the shift from active to maladaptive passive coping behavior induced by 2 different chronic stress treatments. This provides further evidence of the therapeutic potential of vortioxetine in the management of depression and anxiety disorders.
Introduction
Chronic stress is a major risk factor for the development of depression and anxiety disorders (Kendler et al., 1999; Harris, 2001; Heim and Nemeroff, 2001; Caspi et al., 2003). In preclinical studies, we have shown different types of chronic stress to induce anxiety-like behaviors and deficits in cognition, executive function, and maladaptive coping behaviors that resemble symptoms of anxiety, cognitive disturbance, and avoidance behaviors that are prevalent in stress-related psychiatric disorders (Bondi et al., 2008; Lapiz-Bluhm and Morilak, 2010; Roth et al., 2012). Such symptoms can be particularly resistant to treatment (Hasselbalch et al., 2012). Current pharmacological approaches for the treatment of depression, including serotonin-specific reuptake inhibitors (SSRIs), are only partially effective, with an unacceptably high incidence of residual symptoms, relapse, and treatment resistance (Fava, 2006; Jaeger et al., 2006). SSRIs are also used for treatment of a spectrum of anxiety disorders, including panic disorder, posttraumatic stress disorder (PTSD), generalized anxiety disorder, and obsessive-compulsive disorder and others (see Charney et al., 1990), with varying degrees of efficacy.
Vortioxetine is a novel antidepressant with multimodal action approved by the Food and Drug Administration for the treatment of major depressive disorder (Sanchez et al., 2015). In addition to blocking the 5-HT transporter, vortioxetine is also an antagonist at 5-HT3A, 5-HT7, and 5-HT1D receptors; a partial agonist at 5-HT1B receptors; and a full agonist at 5-HT1A receptors (Bang-Anderson et al., 2011; Westrich et al., 2012). Preclinical and clinical studies have demonstrated antidepressant properties of vortioxetine (Alvarez et al., 2012; Katona et al., 2012; Mørk et al., 2012). We have previously shown that chronic dietary administration of vortioxetine reversed a deficit in reversal learning induced by exposing rats to chronic intermittent cold stress, suggesting that vortioxetine has beneficial effects in alleviating stress-induced cognitive impairments associated with dysregulation of the prefrontal cortex (Wallace et al., 2014). In the present study, we further tested the effects of vortioxetine on other types of stress-induced behavioral alterations relevant to depression and anxiety disorders, including the exaggerated expression of conditioned fear, and a shift from active to passive coping behavior resembling symptoms related to avoidance. In addition, we investigated the generality of the beneficial effects of vortioxetine, administered chronically in the diet, on behavioral alterations produced by 2 different types of chronic stress. The first was a combined Chronic plus Acute Prolonged Stress (CAPS) treatment (Roth et al., 2012), after which we measured the effects of vortioxetine on the stress-induced exaggeration of cue-conditioned fear and its extinction. In the second experiment, we measured the effects of vortioxetine treatment on the shift from active to maladaptive passive coping behavior induced on the shock probe defensive burying test after Chronic Unpredictable Stress (CUS) (Bondi et al., 2008). Portions of this work have been presented in abstract form (Evans et al., 2015).
Methods
Animals
A total of 82 adult male Sprague-Dawley rats (Harlan), 250 to 300 g were used in these experiments. Animals were group housed, 3 per cage, upon arrival and transferred to individual cages before beginning experiments. Food and water were available ad libitum. The animals were housed on a 12-hour-light/-dark cycle (lights on at 7:00 am), and experiments were conducted during the light phase of the cycle. All procedures were in accordance with National Institute of Health guidelines and approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center, San Antonio, Texas.
Chronic Dietary Vortioxetine Treatment
As described previously (Wallace et al., 2014), beginning on day 4 of CUS, CAPS, or the corresponding control treatments and continuing through behavioral testing, vortioxetine was administered in the diet prepared to contain 0.33 g/kg chow (Research Diets) corresponding to a dose of approximately 24 mg/kg/d based on estimated average daily food intake of 7.5 g/100 g body weight. This has been shown to achieve clinically relevant brain target occupancy levels (Wallace et al., 2014). Control diet was the standard rat chow (Purina no. 5001) used as the base for preparation of the drug chow, also provided by Research Diets, Inc. Rats were weighed 3 times per week during the period of drug treatment, and mean body weight gain during this period was calculated and compared to ensure equivalent food intake between treatment groups. Dietary treatment with vortioxetine was initiated during the chronic stress period, because preliminary experiments showed that the CUS treatment could not be extended to 4 weeks. This is what would be required to test a paradigm in which drug treatment was initiated after behavioral deficits had first been established by the full course of chronic stress, which would then have to be maintained for the duration of chronic drug treatment. An unacceptably high proportion of animals failed to perform on behavioral testing after this duration of chronic stress.
Experiment 1: Effects of Chronic Vortioxetine Treatment on Exaggerated Expression of Conditioned Fear Memory and Extinction after CAPS
Fifty-eight rats were randomly assigned to 4 treatment groups defined by stress (CAPS or control) and dietary treatment (vortioxetine or control). Figure 1A shows the time line for experiment 1.
Figure 1.
Animals exposed to chronic plus acute prolonged stress treatment exhibited an exaggeration of fear memory, and chronic treatment with vortioxetine in the diet normalized fear memory in stressed rats to a level comparable with that in unstressed controls. (A) Timeline for experiment 1. Rats were habituated to the chambers, fear conditioned, and assigned to groups prior to beginning the 15-day Chronic plus Acute Prolonged Stress (CAPS) stress treatment. Vortioxetine or control diets were given beginning on day 4 of CAPS treatment. Rats were tested for fear memory and extinction on the third day after the end of CAPS treatment. (B) As expected, because rats were assigned to groups after fear conditioning, there were no differences in freezing behavior during fear conditioning. (C) Chronically stressed rats that received control diet showed an exaggerated fear memory, measured as a significant increase in freezing during tone 1 of the extinction session (**P < .01 compared with unstressed rats receiving control diet). Chronic dietary vortioxetine treatment restored freezing during tone 1 to a level comparable to that in unstressed control rats (++P < .01 compared with stressed rats receiving control diet). (D) Consistent with the enhanced fear memory seen during tone 1, CAPS stress increased freezing only in response to the first 4 tones presented during extinction (*P < .05, CAPS control diet compared with unstressed-control diet). There were no other significant effects of either stress or drug on extinction, and the final level of freezing achieved at the end of extinction training was comparable in all groups. All data presented as mean ± SEM; n = 12–14 rats/group.
CAPS
CAPS was conducted as previously described (Green et al., 2011; Roth et al., 2012). CAPS consisted of 14 days of chronic intermittent cold stress followed on day 15 by a single 1-hour series of 3 acute stressors. For the chronic cold stress, rats were transported in their home cages into a cold room, (4°C) for 6 h/d, then returned to housing. This was repeated for 14 days. The acute prolonged stress on day 15 consisted of 20-minute social defeat, immediately followed by 30 minutes of immobilization, then a 10-minute swim stress. For social defeat, Long-Evans retired male breeders were pair-housed with ovariectomized females (Charles River) in large cages (63 x 63 x 40 cm) in a separate room. The female was removed from the resident cage, and the test rat placed in the cage with the resident Long-Evans male rat. Typically within approximately 20 seconds, the resident would attack and defeat the smaller “intruder” Sprague-Dawley test rat. Once defeat occurred, defined by the test rat assuming a supine submissive posture and the resident showing a dominant posture for at least 4 seconds, the test rat was placed under a wire mesh cage for 20 minutes, protecting it from further physical contact but allowing continued sensory exposure to the dominant rat. Immobilization involved taping the torso and limbs gently but securely in a prone position on a flat platform, allowing no movement for 30 minutes. For swim stress, the rat was placed in a cylindrical tank (30-cm diameter × 60-cm height) filled to a depth of 30 cm with water at approximately 23°C. Control rats were handled for 30 seconds daily.
Fear Conditioning and Extinction
Fear conditioning and extinction were performed as previously described with minor modification (Green et al., 2011). Two days before beginning CAPS or control treatment, rats were habituated to 2 contexts for 15 minutes each. Context A was a square chamber with metal walls and a metal grid floor. Context B was a round chamber with smooth vinyl floor and walls. Twenty-four hours after habituation, the rats received cued fear conditioning in context A. After 5-minute acclimation in the chamber, they experienced 4 pairings of a tone (10 kHz, 75 dB, 20 seconds) coterminating with a shock (0.8 mA, 0.5 seconds, average inter-trial interval = 120 seconds). After fear conditioning, the rats were then assigned to treatment groups, such that the average amount of freezing exhibited during conditioning was similar across groups. Twenty-four hours later, CAPS or control treatment began. Treatment with vortioxetine or control diet began on day 4 of CAPS. On the 3rd day after the end of CAPS treatment, fear extinction was conducted in context B to avoid contextual freezing. They were exposed to 16 trials of the tone alone, with an average inter-trial interval of 2 minutes. Behavior was recorded, and freezing during each tone was measured using FreezeFrame and FreezeView software (Coulbourn Instruments ACT-100).
Experiment 2: Effects of Chronic Vortioxetine Treatment on the CUS-Induced Shift from Active to Passive Coping Behavior on the Shock Probe Defensive Burying Test
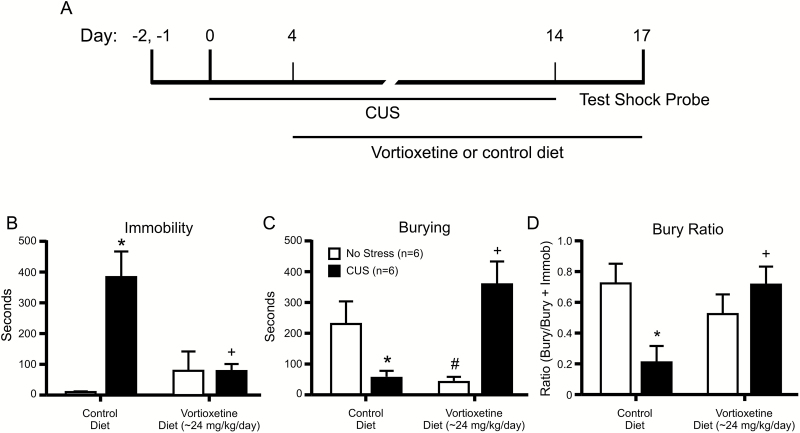
Twenty-four rats were randomly assigned to 4 treatment groups defined by stress (CUS or control) and dietary treatment (vortioxetine or control). Figure 2A shows the time line for experiment 2.
Figure 2.
Animals exposed to chronic unpredictable (CUS) stress exhibited a shift from active to passive coping behavior on the shock probe defensive burying test, and chronic treatment with vortioxetine normalized coping behavior in stressed rats. (A) Time line for experiment 2. Rats were assigned to groups prior to beginning the 14-day CUS treatment. Vortioxetine or control diets were given beginning on day 4 of CUS treatment. Rats were tested in the shock probe defensive burying test on the 3rd day after the end of CAPS treatment. (B) CUS increased immobility in rats receiving control diet (*P<.05 compared with unstressed controls), and vortioxetine in the diet reduced immobility of CUS-treated rats to a level comparable with that in controls (+P<.05 compared with CUS-control diet). (C) CUS reduced burying behavior in rats receiving control diet (*P<.05 compared with unstressed controls), and vortioxetine in the diet restored burying behavior of CUS-treated rats to a level comparable with that in controls (+P<.05 compared with CUS-control diet). In this experiment, vortioxetine alone reduced burying in unstressed rats (#P<.05 compared with unstressed-control diet). (D) To assess active coping behavior as a proportion of total coping behavior (active + passive), the bury ratio was analyzed. CUS decreased the bury ratio in rats receiving control diet, indicating a shift from active to passive coping behavior (*P<.05 compared with unstressed controls), and vortioxetine in the diet restored the bury ratio of CUS-treated rats to be comparable with that in unstressed control rats (+P<.05 compared with CUS-control diet). All data presented as mean ± SEM; n = 6 rats/group.
CUS
CUS was conducted as described previously (Bondi et al., 2008; Jett et al., 2015). Briefly, a different acute stressor was applied once daily for 14 consecutive days, as outlined in Table 1. After each stress, rats were placed in their home cages with fresh bedding in a separate room for 1 hour post-stress recovery and then returned to housing. Unstressed control rats were handled briefly each day. Vortioxetine or control diet was given beginning on day 4 of CUS or control treatment, continuing through behavioral testing. After the conclusion of CUS treatment on day 14, rats were left undisturbed in the housing room for 2 days and tested on day 17.
Table 1.
CUS Procedure
| Day 1 | Restraint (30 min) |
| Day 2 | Shaking (1 h) |
| Day 3 | Social defeat (45 min) |
| Day 4 | Warm swim (15 min) (Begin vortioxetine or control diet) |
| Day 5 | 24-h wet bedding |
| Day 6 | Cold swim (10 min) |
| Day 7 | Shaking (1 h) |
| Day 8 | Mild footshock (15 min) |
| Day 9 | Restraint (30 min) |
| Day 10 | Warm swim (15 min) |
| Day 11 | Mild footshock (15 min) |
| Day 12 | Tail pinch (10 min) |
| Day 13 | 24-h wet bedding |
| Day 14 | Mild footshock (15 min) |
Shock Probe Defensive Burying Test
On the 3rd day following the end of CUS or control treatment, a rat was placed into a modified cage containing 5 cm of clean bedding. A shock probe protruding 6 cm into one end of the cage was set to deliver 2 mA of current when touched. After the rat made contact with the probe and received a shock, the current was shut off. Behavior was recorded to video for offline scoring of the amount of time spent immobile and the amount of time spent engaged in actively burying the probe during the 15-minute test period following probe contact. Burying was defined as digging, plowing, pushing, or flicking bedding toward the probe. Immobility was defined as a lack of movement other than breathing or slight scanning movements of the head without moving the legs or torso. The measure of active coping relative to passive coping was the bury ratio, calculated as (bury time)/(bury time + immobility time).
Statistical Analysis
The investigator conducting the behavioral test was blind to the experimental treatment of the rat being tested. In both experiments, mean body weight gain during the period of drug treatment was compared between drug treatment groups using Student’s t test. In experiment 1, fear memory, measured by freezing on the first tone in the extinction test, was analyzed using 2-way ANOVA (stress x drug). Extinction of conditioned fear, indicated by the decrease in freezing across all tones in the extinction test, was analyzed by 3-way ANOVA (stress x drug x tone, with repeated measures over tone). For the shock-probe defensive burying data, bury time, immobility time, and bury ratio were analyzed by 2-way ANOVA. Pairwise comparisons to detect specific group differences were performed using Newman-Keuls test. In all analyses, significance was determined at P < .05.
Results
Experiment 1: Effects of Chronic Vortioxetine Treatment on Exaggerated Expression of Conditioned Fear Memory and Extinction after CAPS
All rats were fear-conditioned prior to group assignments. Thus, as expected, there were no differences in fear conditioning between groups prior to beginning chronic stress or drug treatment (all P>.23) (Figure 1B). Rats receiving both diets showed equivalent weight gain during the 2-week drug treatment period (control diet: 31.6 ± 0.9g, vortioxetine diet: 32.2 ± 0.8g; t(30) = 0.46, P=.65). Analysis of fear memory on tone 1, tested after chronic stress and drug treatment, indicated significant main effects of stress (F(1,48) = 11.26, P<.01) and drug (F(1,48) = 7.15, P<.01). Pairwise comparisons showed that CAPS treatment significantly elevated freezing on tone 1 in rats receiving control diet, and vortioxetine reduced freezing on tone 1 back to control levels (Figure 1C). Vortioxetine alone did not alter the expression of fear memory in nonstressed animals.
In the analysis of extinction (Figure 1D), there was the expected main effect of tone (F(15,720) = 17.25, P<.001), reflecting the decrease in freezing across tones. There was a significant stress x drug interaction (F(1,48) = 12.68, P<.001) and a stress x tone interaction (F(15,720) = 2.49, P<.01). There was no main effect of stress (F(1,48) = 2.69, P=.11) or drug (F(1,48) = 0.63, P=.43), and there were not significant interactions of tone x drug or tone x drug x stress (both P>.31). Pairwise comparisons revealed that the stress interactions were driven entirely by the exaggerated fear memory described above, as the only significant differences between groups were in the elevation in freezing during the first 4 tones in CAPS treated rats given control diet compared with all other groups (P<.05), consistent with the effects on tone 1.
Experiment 2: Effects of Chronic Vortioxetine Treatment on the CUS-Induced Shift from Active to Passive Coping Behavior on the Shock Probe Defensive Burying Test
Rats receiving both diets showed equivalent weight gain during the 2-week treatment period (control diet: 37.3 ± 0.8g, vortioxetine diet: 37.5 ± 0.7g; t(30) = 0.17, P=.87). CUS increased immobility, decreased burying, and reduced the bury ratio, and these effects were reversed by chronic dietary treatment with vortioxetine. For immobility, there were significant main effects of stress (F(1,20) = 11.60, P<.003), drug (F(1,20) =4.594, P<.05), and a stress x drug interaction (F(1,20) = 11.89, P<.003). Pairwise comparisons indicate that CUS increased immobility compared with controls (P<.001), and vortioxetine reversed this effect (P<.003) (Figure 2B). For burying time, there were no significant main effects, but there was a stress x drug interaction (F(1,20) = 17.84, P<.001). Stress decreased burying time, and vortioxetine restored burying to control levels (Figure 2C). As we have seen previously for other therapeutic interventions (e.g., see Jett et al., 2015), in this experiment vortioxetine alone also reduced burying but had no effect on immobility. In the analysis of bury ratio as a measure of active coping relative to passive coping, there was a main effect of stress (F(1,20) = 11.59, P<.03) and a stress x drug interaction (F(1,20) = 38.17, P<.01). Pairwise comparisons revealed that stressed rats given control diet showed a decrease in the bury ratio, reflecting a shift from active to a more passive behavioral coping strategy (P<.05). Chronic administration of vortioxetine in the diet reversed this effect (P<.01) (Figure 2D).
Discussion
In this study, we examined the effects of chronic dietary administration of vortioxetine on measures of exaggerated fear memory and maladaptive coping behavior following different types of chronic stress treatment. In the first experiment, in replication of our previous study (Roth et al., 2012), CAPS, administered after fear conditioning, enhanced the expression of cue-conditioned fear memory on a recall test after the end of chronic stress. Vortioxetine normalized the amount of freezing exhibited upon reexposure to the conditioned cue, without affecting the expression of conditioned fear memory in non-stressed animals. Enhanced fear memory and exaggerated response to fearful stimuli are common symptoms of mood and anxiety disorders, in particular PTSD. For instance, subjects with PTSD exhibited larger startle response magnitude relative to control subjects (Jovanovic et al., 2009). The amygdala is hyperresponsive in PTSD, which may account for exaggerated fear responses and persistence of traumatic memories (Shin et al., 2004). Amygdala activation is increased in PTSD patients in response to trauma-related imagery (Shin et al., 2004), fear conditioning (Bremner, 2005), and fearful facial expressions (Rauch et al., 2000). Further, SSRI treatment decreased the amygdala response to fearful facial expressions and normalized the amygdala response to aversive stimuli (Harmer et al., 2006b). The results of the present preclinical study suggest that vortioxetine may also be effective in reducing such symptoms.
Likewise, in response to subsequent tones administered during extinction training in the same test, chronic stress enhanced freezing during the first 4 tones, consistent with exaggerated fear memory. However, by the end of the session, all groups had achieved the same level of extinction. Thus, because extinction per se was apparently not compromised by chronic stress in this experiment, we can make no conclusions regarding the potential effects of vortioxetine on changes in the extinction of conditioned fear that may be associated with mood and anxiety disorders.
In the second experiment, also in replication of a previous study (Jett et al., 2015), CUS induced a shift from an active to a passive coping strategy in the shock probe defensive burying test. Chronic vortioxetine treatment normalized coping behavior in chronically stressed animals, restoring the proportional amount of active coping relative to total coping behavior (i.e., the bury ratio) to that seen in unstressed control rats. Our laboratory and others have shown active coping to be more adaptive in this test. Specifically, whereas active coping reduced stress hormone levels during the period of continued exposure to the probe, the passive immobility response was associated with elevated stress hormones (Bondi et al., 2007). Clinical studies have also shown that active coping, as opposed to avoidant coping, is associated with reduced stress and improved long-term mental and physical health outcomes (LeDoux and Gorman, 2001; Charney, 2004; Olff et al., 2005). Thus, vortioxetine may be effective clinically in reducing avoidance behavior and facilitate active coping. As we have also reported previously with other therapeutic interventions (e.g., see Jett et al., 2015), vortioxetine alone reduced burying behavior in unstressed rats, which may be interpreted as an anxiolytic response, as there was no increase in immobility. These findings are in agreement with a previous report that vortioxetine dose-dependently reduced anxiety in the marble burying test in mice (Bisulco et al., 2009). A similar effect has also been observed in previous studies in which SSRI treatment selectively decreased burying behavior (Degroot and Nomikos, 2005). The lateral septum is critical to determining coping behavior on the shock probe defensive burying test (Treit et al., 1993). Further, changes in serotonergic neurotransmission in the lateral septum have been shown to be involved in the behavioral effects of stress (Price et al., 2002) and respond to behaviorally effective doses of SSRIs (Contreras et al., 2001). Thus, this brain region is likely to be involved in the behavioral effects of CUS on the shock probe defensive burying test and is also a potential site of action for the beneficial effects of vortioxetine on this test.
The beneficial effects of vortioxetine on the shock probe defensive burying test, that is, restoring active coping behavior in rats that had been compromised by chronic stress, are consistent with the effects of SSRIs in reducing immobility and increasing active swimming behavior in the forced swim test, another test of active vs passive coping responses (Cryan et al., 2005). Further, chronic SSRI treatment also reduced immobility and increased swimming in rats compromised by chronic stress (Lapmanee et al., 2013). Vortioxetine has also been shown to have dose-dependent antidepressant-like effects in the forced swim test, decreasing immobility and increasing active responding (Mørk et al., 2013).
It is likely that the beneficial effects of vortioxetine on fear memory and anxiety-related behaviors were mediated by a combination of SERT inhibition, elevating extracellular 5-HT levels, together with direct activity at specific 5-HT receptors. Clinically effective doses in human (5–20 mg/d) generate approximately 50% to 80% SERT occupancy; primarily the SERT and 5-HT3 receptor are occupied at 5 mg, while at 20 mg, other targets are also occupied at functionally relevant levels (Sanchez et al., 2015). In a previous study using a dietary vortioxetine treatment comparable with that used in the present experiments, we reported approximately 85% occupancy of the SERT in the rat brain and approximately 50% to 60% occupancy of the 5-HT1B receptor (Wallace et al., 2014). At doses producing this level of occupancy at these sites, the 5-HT3 receptor, which has much higher affinity for vortioxetine, would be expected to show even greater occupancy than the SERT, with relatively low occupancy at the other drug receptor targets, including the 5-HT1A receptor (Pehrson et al., 2013). However, blockade of the 5-HT3 receptor has been shown to potentiate the increase in extracellular 5-HT produced by citalopram (Mørk et al., 2012). Thus, 5-HT3 receptor antagonism by vortioxetine may amplify the elevation in extracellular 5-HT levels produced by SERT blockade, enhancing effects mediated by postsynaptic receptors occupied at low levels by vortioxetine itself at this dose. In particular, drugs that activate the 5-HT1A receptor have been reported to be anxiolytic (Gordon and Hen, 2004), including the reduction of contextually conditioned fear (Bauer, 2015), although opposing effects at pre- and postsynaptic auto- and hetero-receptors can produce inconsistent or contradictory results (Gordon and Hen, 2004). It is also possible that blockade of the 5-HT3 receptor contributed directly to the reduction of fear and anxiety by vortioxetine, as 5-HT3 antagonists have been reported to reduce fear and anxiety, but only in very limited contexts, including fear potentiated startle, a form of conditioned fear, in both humans and rodents (Olivier et al., 2000; Harmer et al., 2006a).
We have previously shown that vortioxetine reversed a deficit in cognitive flexibility in rats that have been exposed to chronic stress, which models cognitive symptoms related to prefrontal cortical dysfunction in depression (Wallace et al., 2014). The present results broaden these observations, providing further evidence of the beneficial effects of vortioxetine on other behaviors that are dysregulated by stress and that model other components of mood and anxiety disorders.
Statement of Interest
Ms. Hatherall has no financial or other interests to disclose. Dr. Morilak serves on a psychopharmacology advisory board for H. Lundbeck A/S. Dr. Sánchez was an employee of Lundbeck at the time these studies were conducted. The sponsor had no role in study design or conduct, data collection or analysis, writing the paper, or in the decision to publish.
The authors have no other statements of interest to disclose related to this work.
Acknowledgments
In addition to financial support, H. Lundbeck A/S provided the chow containing vortioxetine and the control chow used in these studies, both of which were prepared by Research Diets, Inc.
This work was supported by a research grant from H. Lundbeck A/S.
References
- Alvarez E, Perez V, Dragheim M, Loft H, Artigas F. (2012) A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol 15:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang-Anderson B, Ruhland T, Jørgensen M, Smith G, Frederiksen K, Jensen KG, Zhong H, Nielsen SM, Hogg S, Mørk A, Stensbøl TB. (2011) Discovery of 1-[2-(2,4-Dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem 54:3206–3221. [DOI] [PubMed] [Google Scholar]
- Bauer EP. (2015) Serotonin in fear conditioning processes. Beh Brain Res 277:68–77. [DOI] [PubMed] [Google Scholar]
- Bisulco S, Miller S, Moore NA, Mørk A, Patel JG, Poon P, Sanchez C, Schnelker JH, Smith DG, Hogg S. (2009) Lu AA21004, a novel multi-target drug, displays anxiolytic- and antidepressant-like effects in multiple preclinical assays. Biol Psychiatry 64 (Supp):108S. [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. (2008) Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33:320–331. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Barrera G, Lapiz MDS, Bédard T, Mahan A, Morilak DA. (2007) Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Prog Neuro-Psychopharmacol Biol Psychiatry 31:482–495. [DOI] [PubMed] [Google Scholar]
- Bremner JD. (2005) Effects of traumatic stress on brain structure and function: Relevance to early responses to trauma. J Trauma Dissociation 6:51–68. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301:386–389. [DOI] [PubMed] [Google Scholar]
- Charney DS. (2004) Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry 161:195–216. [DOI] [PubMed] [Google Scholar]
- Charney DS, Krystal JH, Delgado PL, Heninger GR. (1990) Serotonin-specific drugs for anxiety and depressive disorders. Annu Rev Med 41:437–446. [DOI] [PubMed] [Google Scholar]
- Contreras CM, Rogriguez-Landa JF, Gutierrez-Garcia AG, Bernal-Morales B. (2001) The lowest effective dose of fluoxetine in the forced swim test significantly affects the firing rate of lateral septal nucleus neurones in the rat. J Psychopharmacol 15:231–236. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. (2005) Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Revs 29:547–569. [DOI] [PubMed] [Google Scholar]
- Degroot A, Nomikos GG. (2005) Fluoxetine disrupts the integration of anxiety and aversive memories. Neuropsychopharmacology 30:391–400. [DOI] [PubMed] [Google Scholar]
- Evans L, Smagin G, Sánchez C, Morilak DA. (2015) Vortioxetine reduces exaggerated fear memory and restores active coping behavior in chronically stressed rats. Soc Neurosci Abstr 41, Online program no. 410.17. [Google Scholar]
- Fava M. (2006) Pharmacological approaches to the treatment of residual symptoms. J Psychopharmacol 20:29–34. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Hen R. (2004) The serotonergic system and anxiety. NeuroMolecular Med 5:27–40. [DOI] [PubMed] [Google Scholar]
- Green MK, Rani CSS, Joshi A, Soto-Piña AE, Martinez PA, Frazer A, Strong R, Morilak DA. (2011) Prenatal stress induces long term stress vulnerability, compromising stress response systems in the brain and impairing extinction of conditioned fear after adult stress. Neuroscience 192:438–451. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Reid CB, Ray MK, Goodwin GM, Cowen PJ. (2006. a) 5HT3 antagonism abolishes the emotion potentiated startle effect in humans. Psychopharmacology 186:18–24. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. (2006. b) Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 59:816–820. [DOI] [PubMed] [Google Scholar]
- Harris T. (2001) Recent developments in understanding the psychosocial aspects of depression. Brit Med Bull 57:17–32. [DOI] [PubMed] [Google Scholar]
- Hasselbalch BJ, Knorr U, Kessing LV. (2012) Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. J Affect Disord 134:20–31. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. (2001) The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 49:1023–1039. [DOI] [PubMed] [Google Scholar]
- Jaeger J, Berns S, Uzelac S, Davis-Conway S. (2006) Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res 145:39–48. [DOI] [PubMed] [Google Scholar]
- Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, Morilak DA. (2015) Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacology 232:3123–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ. (2009) Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res 167:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona C, Hansen T, Olsen CK. (2012) A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol 27:215–223. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. (1999) Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MDS, Morilak DA. (2010) A cognitive deficit induced in rats by chronic intermittent cold stress is reversed by chronic antidepressant treatment. Int J Neuropsychopharmacol 13:997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapmanee S, Charoenphandhu J, Charoenphandhu N. (2013) Beneficial effects of fluoxetine, reboxetine, venlafaxine, and voluntary running exercise in stressed male rats with anxiety- and depression-like behaviors. Beh Brain Res 250:316–325. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Gorman JM. (2001) A call to action: overcoming anxiety through active coping. Am J Psychiatry 158:1953–1955. [DOI] [PubMed] [Google Scholar]
- Mørk A, Montezinho LP, Miller S, Trippodi-Murphy C, Plath N, Li Y, Gulinello M, Sanchez C. (2013) Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharm Biochem Behav 105:41–50. [DOI] [PubMed] [Google Scholar]
- Mørk A, Pehrson A, Brennum LT, Nielsen SM, Zhong H, Lassen AB, Miller S, Westrich L, Boyle NJ, Sánchez C, Fischer CW, Liebenberg N, Wegener G, Bundgaard C, Hogg S, Bang-Anderson B, Stensbøl TB. (2012) Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther 340:666–675. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Gersons BPR. (2005) Effects of appraisal and coping on the neuroendocrine response to extreme stress. Neurosci Biobehav Revs 29:457–467. [DOI] [PubMed] [Google Scholar]
- Olivier B, van Wijngaarden I, Soudijn W. (2000) 5HT3 receptor antagonists and anxiety: a preclinical and clinical review. Eur Neuropsychopharmacol 10:77–95. [DOI] [PubMed] [Google Scholar]
- Pehrson A, Cremers T, Bétry C, van der Hart MGC, Jørgensen L, Madsen M, Haddjeri N, Ebert B, Sánchez C. (2013) Lu AA21004, a novel multimodal antidepressant, produces regionally selective increases of multiple neurotransmitters—A rat microdialysis and electrophysiology study. Eur Neuropsychopharmacol 23:133–145. [DOI] [PubMed] [Google Scholar]
- Price ML, Kirby LG, Valentino RJ, Lucki I. (2002) Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptation produced by repeated swimming. Psychopharmacology (Berl) 162:406–414. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. (2000) Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 47:769–776. [DOI] [PubMed] [Google Scholar]
- Roth MK, Bingham B, Shah A, Joshi A, Frazer A, Strong R, Morilak DA. (2012) Effects of chronic plus acute prolonged stress on measures of coping style, anxiety, and evoked HPA-axis reactivity. Neuropharmacology 63:1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F. (2015) Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 145:43–57. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. (2004) Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 61:168–176. [DOI] [PubMed] [Google Scholar]
- Treit D, Pesold C, Rotzinger S. (1993) Dissociating the anti-fear effects of septal and amygdaloid lesions using two pharmacologically validated models of rat anxiety. Behav Neurosci 107:770–785. [DOI] [PubMed] [Google Scholar]
- Wallace A, Pehrson AL, Sánchez C, Morilak DA. (2014) Vortioxetine restores reversal learning impaired by 5-HT depletion or chronic intermittent cold stress in rats. Int J Neuropsychopharmacol 17:1695–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrich L, Pehrson A, Zhong H, Nielsen SM, Frederiksen K, Stensbøl TB, Boyle N, Hentzer M, Sánchez C. (2012) In vitro and in vivo effects of the multimodal antidepressant vortioxetine (Lu AA21004) at human and rat targets. Int J Psychiatr Clin Pract 16 (Suppl. 1):47. [Google Scholar]