Abstract
Background. High-flow nasal oxygen (HFNO) has been shown to benefit oxygenation, ventilation and upper airway patency in a range of clinical scenarios, however its use in spontaneously breathing patients during general anaesthesia has not been described. Spontaneous respiration using i.v. anaesthesia is the primary technique used at our institution for tubeless airway surgery. We hypothesized that the addition of HFNO would increase our margin of safety, particularly during management of an obstructed airway.
Methods. A retrospective observational study was conducted using a SponTaneous Respiration using IntraVEnous anaesthesia and High-flow nasal oxygen (STRIVE Hi) technique to manage 30 adult patients undergoing elective laryngotracheal surgery.
Results. Twenty-six patients (87%) presented with significant airway and/or respiratory compromise (16 were stridulous, 10 were dyspnoeic). No episodes of apnoea or complete airway obstruction occurred during the induction of anaesthesia using STRIVE Hi. The median [IQR (range)] lowest oxygen saturation during the induction period was 100 [99–100 (97–100)] %. The median [IQR (range)] overall duration of spontaneous ventilation was 44 [40–49.5 (18–100)] min. The median [IQR (range)] end-tidal carbon dioxide (ETCO2) level at the end of the spontaneous ventilation period was 6.8 [6.4–7.1 (4.8–8.9)] kPa. The mean rate of increase in ETCO2 was 0.03 kPa min−1.
Conclusions. STRIVE Hi succeeded in preserving adequate oxygen saturation, end-tidal carbon dioxide and airway patency. We suggest that the upper and lower airway benefits attributed to HFNO, are ideally suited to a spontaneous respiration induction, increasing its margin of safety. STRIVE Hi is a modern alternative to the traditional inhalation induction.
Keywords: Anesthesia, intravenous, laryngostenosis, respiration
Editor’s key points
High-flow nasal oxygen is potentially useful in patients who are breathing spontaneously during general anaesthesia, but its efficacy has not been assessed.
A retrospective assessment of 30 patients indicated that high-flow nasal oxygen may be effective in preventing hypoxia, hypercapnoea, and complete airway obstruction in patients who are breathing spontaneously during total i.v. anaesthesia.
The use of high-flow nasal oxygen (HFNO) is gaining popularity in anaesthesia. Multiple applications in critical care have been associated with benefits to oxygenation and ventilation in both apnoeic patients and spontaneously breathing patients.1,2 In apnoeic patients, the Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) technique has been associated with a prolonged apnoeic time and a reduced rate of rise of end-tidal carbon dioxide (ETCO2).3 In spontaneously breathing patients, multiple physiological benefits of HFNO have been described, which include (i) an increased FiO2, (ii) generation of positive airway pressure, (iii) improved respiratory mechanics and (iv) reduced upper airway resistance.1,2,4,5 To date, these clinical benefits during spontaneous ventilation have been limited to awake patients (respiratory supportive care, preoperative preoxygenation, postoperative support)1,2,6, sedated patients [procedural bronchoscopy or awake fibreoptic intubation (AFOI)]5,7 or asleep patients with obstructive sleep apnoea (OSA).8 The use of HFNO in spontaneously breathing patients undergoing general anaesthesia has not been previously described.
Spontaneous respiration using i.v. anaesthesia is the primary technique used at our institution for tubeless airway surgery.9 Although we have found spontaneous ventilation to be associated with numerous benefits, general anaesthesia does have detrimental effects on respiratory mechanics, which may limit application of the technique in certain patient subgroups, particularly those with severe respiratory co-morbidity. Propofol is associated with reduced tidal and minute ventilation, increased respiratory frequency and an upper airway prone to collapse.10–12 We hypothesized that the clinical benefits of HFNO to oxygenation, ventilation and upper airway patency described in awake and sedated patients would be ideally suited to spontaneously breathing patients undergoing general anaesthesia. We report our initial experience combining HFNO with our previously described spontaneous respiration technique using i.v. anaesthesia in patients undergoing microlaryngoscopic surgery.
Methods
This study was conducted at the Princess Alexandra Hospital, which is a teaching hospital in Brisbane, Australia, with an adult tertiary ENT/Head and Neck surgical centre. Human Research Ethics Committee approval from the Metro South Hospital and Health Service (Brisbane, Queensland, Australia) was obtained before commencing the study (HREC/16/QPAH/471).
Between February 2016 and October 2016, a SponTaneous Respiration using IntraVEnous anaesthesia and High-flow nasal oxygen (STRIVE Hi) technique was performed on 30 adult patients presenting for elective microlaryngoscopic surgery. Inclusion criteria involved: (i) a clinical indication to use the technique, based on management of difficult airway pathology during the induction and/or a surgical request for tubeless anaesthesia during the procedure, (ii) use of a standardized propofol titration method (“Cp – Ce = 1”, see below) and (iii) use of HFNO with a fraction of inspired oxygen (FiO2) of 1.0 during the induction period. Patients with a pre-existing tracheostomy were excluded from the study. Every patient that received the STRIVE Hi technique and met the inclusion criteria was included into the case series after providing written consent. One patient that received STRIVE Hi was excluded after a FiO2 of 0.21 was delivered by the HFNO device during the induction. All of the STRIVE Hi cases were managed by one of the authors, or a trainee under their direct supervision.
A standardized protocol using a “Cp – Ce = 1” formula was used to titrate a Marsh-model propofol target-controlled infusion (TCI) during the STRIVE technique. This “Cp – Ce = 1” protocol has been previously described and involved a stepwise increase in the predicted plasma-site concentration target (Cpt) during the induction.9 The starting Cpt was set at 2-3 mcg.ml−1 and increased by 0.5-1 mcg.ml−1 when the predicted effect-site concentration (Ce) reached a point 1 mcg.ml−1 below the Cp (i.e. Cp – Ce = 1 mcg.ml−1). The Cp-Ce cycle was repeated until the Ce reached the endpoint for airway instrumentation.
The HFNO was delivered using an Optiflow™ THRIVE – Variable FiO2 system (Fisher and Paykel Healthcare, Auckland, New Zealand), which included a gas blender so that the oxygen concentration could be reduced to prevent an airway fire in the presence of laser, which followed the manufacturer’s recommendations.
All patients received a pre-induction protocol of i.v. glycopyrrolate 0.2 mg, metoclopramide 10 mg and lignocaine 25 mg. Pre-oxygenation with 100% oxygen occurred using the Optiflow nasal cannula at a rate of 30 l.min−1 for one min followed by 50 l.min−1 for two min in a 10–20 degrees reverse Trendelenburg position. Induction of anaesthesia commenced using the propofol TCI protocol as described above. The Optiflow rate increased to 70 l.min−1 when loss of consciousness occurred during the induction of anaesthesia and was maintained until the period of spontaneous ventilation was complete. Upper airway patency was maintained in combination with jaw thrust during the induction period. Direct laryngoscopy with a Macintosh blade was performed at a Ce of 5–6 mcg.ml−1 and Co-Phenylcaine Forte ® (lignocaine 5% + phenylephrine 0.5%, ENT Technologies Pty Ltd, Hawthorne East, Australia) was sprayed onto the epiglottis, vocal cords and subglottic trachea. Suspension laryngoscopy occurred at a Ce of 6–7 mcg.ml−1, which defined the transition between induction and maintenance phases of anaesthesia. The surgery typically proceeded tubeless, while spontaneous ventilation was maintained or in a minority with a tracheal tube and mechanical ventilation. Low dose remifentanil (e.g. 0.01–0.05 mcg.kg−1.min−1) was commenced as necessary during the maintenance phase depending on the surgical conditions and procedure being performed. The FiO2 was reduced to 0.3 using the gas blender when the laser was in use. Respiratory rate monitoring occurred using ECG impedance and/or modified qualitative capnography (e.g. Hudson mask during the induction or via a short metal cannula attached to the suspension laryngoscope, sampling from its midpoint, during the maintenance phase). After the surgery was complete, a supraglottic airway (The Ultimate Laryngeal Mask, Ultimate Medical Pty Ltd, Richmond, Australia) was inserted in the tubeless patients, followed by cessation of the HFNO and anaesthetic infusions to allow emergence.
Relevant data for all cases was retrospectively manually extracted from an electronic Automated Anaesthetic Record Keeping (AARK-Winchart) system, which was accessed online. Data relating to the conduct of the anaesthesia (e.g. propofol dosing and effect-site endpoints for airway instrumentation) were manually entered into the anaesthetic record during each case. Respiratory and haemodynamic data were automatically incorporated into the anaesthetic record. The duration of spontaneous ventilation was defined as the period extending from the induction of anaesthesia until a definitive airway was placed, or the surgery was concluded.
Oxygen saturation (SpO2) was measured with pulse oximetry. ETCO2 was recorded from a closed circuit when a definitive airway (supraglottic airway or tracheal tube) was placed at the end of the spontaneous ventilation period. Baseline ETCO2 levels were also recorded using a facemask before induction before the application of high-flow nasal oxygen in 12 patients. Where available, flow-volume loops were used to improve the accuracy of quantitative ETCO2 data collection, by ensuring sampling occurred when minimal discrepancy existed between inspiratory and expiratory volumes. Serial arterial blood gases (including baseline) were also measured and used to determine arterial oxygen and carbon dioxide partial pressure (PaO2, PaCO2 respectively) in five patients with significant cardio-respiratory co-morbidity.
Data was analysed using StatPlus:mac Pro (AnalystSoft Inc, Version v6). Simple correlation was used to assess the correlation between carbon dioxide levels at the end of the spontaneous ventilation period and the duration of spontaneous ventilation.
Results
The STRIVE Hi technique was performed on 30 patients undergoing elective microlaryngoscopy between February 2016 and October 2016. There were 17 females and 13 males with a mean age (SD [range]) of 55 (46-65 [31-78]) yrs. The median [IQR (range)] ASA grade, weight and BMI was 3 [3–3 (2–4)], 80 [65–98 (59–130)] kg and 29 [28–34 (19–46)] kg.m−2, respectively.
Twenty-seven patients required surgery for benign airway pathology, including 21 cases of laryngotracheal stenosis and six cases of laryngotracheal papilloma. Two patients had malignant laryngeal lesions causing ball-valve obstruction and one head and neck patient had a suspected recurrence after previous reconstructive surgery and radiation.
Twenty-six patients (87%) presented with significant airway and/or respiratory compromise: sixteen patients were stridulous (ten at rest, six on exertion), ten patients were dyspnoeic (two at rest, eight on exertion) and four patients were dysphonic or asymptomatic. Four patients had an airway diameter less than 5 mm, the most severe of which involved a 3 mm diameter concentric subglottic stenosis (Fig. 1).
Fig 1.
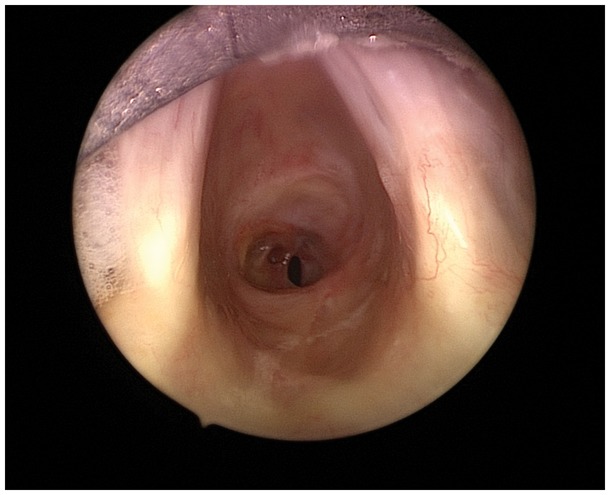
Endoscopic appearance of 3 mm diameter subglottic stenosis in a patient anaesthetized with STRIVE Hi.
Several patients were particularly challenging as a result of their combination of airway pathology and co-morbidity. These airway/co-morbidity combinations included: (i) tracheal stenosis, BMI 42 and emphysema with mixed respiratory failure (SpO2 85%, PaO2 6.5kPa, PaCO2 7.1kPa), (ii) ball-valve laryngeal tumour, BMI 32 and symptomatic emphysema (SpO2 92%, PaO2 8.1kPa, dyspnoeic at rest), (iii) tracheal stenosis, BMI 37 and OSA (SpO2 90%) and (iv) tracheal stenosis (5mm diameter), BMI 46 and 23 week pregnancy with rest stridor and severe exertional limitation. The other patient with ball-valve obstruction was referred with severe stridor at rest and had experienced a previous failed inhalation induction. One patient with previous head and neck reconstruction and radiotherapy was a known difficult intubation.
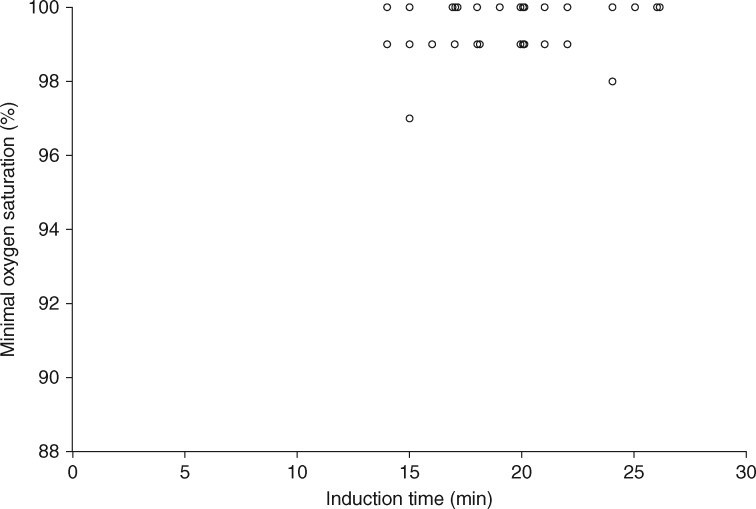
All patients tolerated the HFNO at 50 l.min−1 while conscious. No episodes of apnoea or complete airway obstruction occurred during the induction phase using STRIVE Hi. The median [(IQR (range)] lowest oxygen saturation recorded during the induction was 100 [99–100 (97–100)] % (Fig. 2). The STRIVE Hi propofol TCI dosing data and Ce endpoints for airway instrumentation are presented in Table 1.
Fig 2.
The relationship between the duration of spontaneous respiration during the induction phase and oxygen saturation levels (n = 30).
Table 1.
STRIVE Hi dosing data and Ce end-points. Data presented as median [IQR (range)] or n (%). Cpt, predicted plasma-site concentration target; Ce, predicted effect-site concentration; Cptstart, starting Cpt; Cptincrements, incremental increase in Cpt during stepwise induction;. CeLOC, Ce at loss of consciousness; CeDL, Ce at direct laryngoscopy; CeSL, Ce at suspension laryngoscopy; CeMAX, maximum Ce attained; BPM, breaths per minute
| STRIVE Hi dose variables | |
|---|---|
| Cptstart (mcg.ml−1) | 2.0 (2.0–3.0 [2.0–3.0]) |
| Cptincrements (mcg.ml−1) | 1.0 (1.0–1.0 [0.5–1.0]) |
| CeLOC (mcg.ml−1) | 1.0 (0.7–1.5 [0.5–2.4]) |
| CeDL (mcg.ml−1) | 5.0 (5.0–5.5 [4.8–6.5]) |
| CeSL (mcg.ml−1) | 6.5 (6.2–6.8 [5.9–7.5]) |
| CeMax (mcg.ml−1) | 7.0 (7.0–7.0 [6.0–8.0]) |
| BPM at CeMax | 20 (18–22 [12–27]) |
| Vasopressor use during induction | 1 (3%) |
| Vasopressor use during maintenance | 4 (11%) |
| Remifentanil use during induction | 4 (13%) |
| Remifentanil use during maintenance | 21 (89%) |
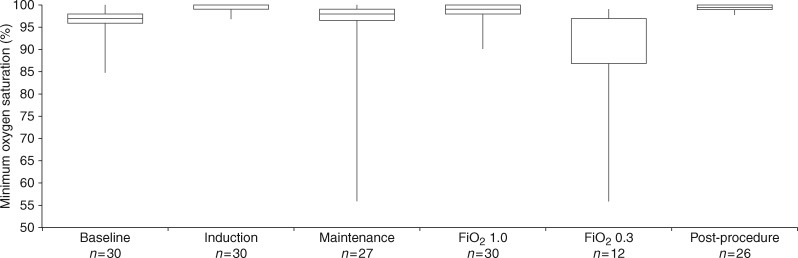
In 27 patients, the STRIVE Hi technique continued into the maintenance phase and provided tubeless anaesthesia during the surgery. In three patients, the trachea was electively intubated and mechanical ventilation facilitated the surgery. The surgery included ten laser procedures, nine balloon dilatations, two combined laser/balloon dilatations, six debridements and three diagnostic procedures. Only one case required unplanned termination of spontaneous ventilation, which occurred during the maintenance phase after remifentanil was commenced. A large overdose of misprogrammed remifentanil resulted in apnoea and uncontrolled hypoxaemia while the FiO2 was 0.3 and was successfully managed with tracheal intubation. Three cases of controlled oxygen desaturation < 90% also occurred during laser surgery when the FiO2 was 0.3. Oxygenation throughout various phases of the spontaneous ventilation period is presented in Fig. 3.
Fig 3.
A comparison of oxygen saturations throughout the different phases of spontaneous ventilation showing the effect of varying the inspired oxygen concentration (FiO2) during the maintenance phase. Box plot shows median and first and third quartiles. Vertical extensions indicate minimum and maximum observed measurements.
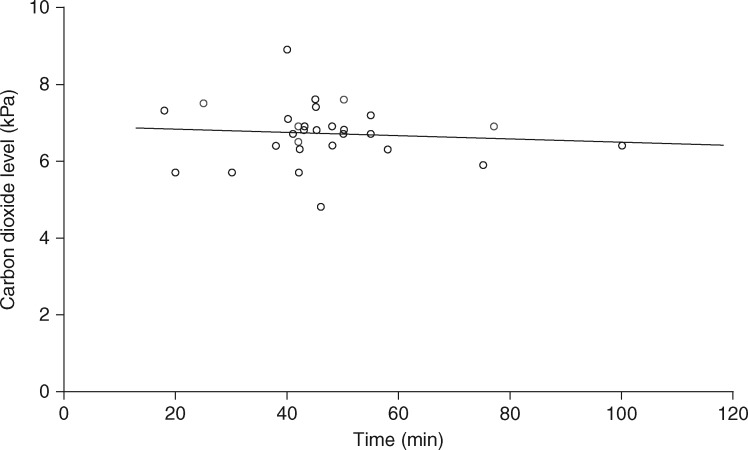
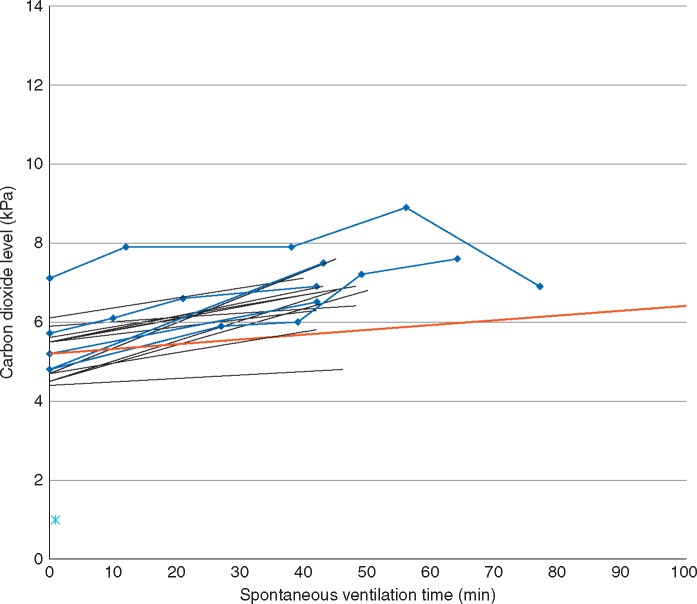
The median [IQR (range)] duration of spontaneous ventilation using STRIVE Hi was 44 [40–49.5 (18–100)] min. The median [IQR (range)] duration of the induction was 20 [17–21 (14–26)] min. The median [IQR (range)] period of FiO2 0.3 was 20 [9–25 (3-46)] min. The median [IQR (range)] ETCO2 at the end of the spontaneous ventilation period was 6.8 [6.4–7.1 (4.8-8.9)] kPa. Fig. 4 illustrates the relationship between the duration of spontaneous ventilation and ETCO2, which shows there is no correlation between these variables. Fig. 5 shows the increase in carbon dioxide measured from baseline to the end of the spontaneous ventilation period in 17 patients using two-point analysis. The mean rate of carbon dioxide increase was 0.03 kPa min−1 using end-tidal data (12 patients) and 0.035 kPa min−1 using arterial blood gas data (five patients). The complete serial arterial blood gas data sampled from five patients is presented in Table 2.
Fig 4.
The relationship between the overall duration of spontaneous respiration and end-tidal (and in five patients arterial, as indicated by the blue circles) carbon dioxide (n = 29). The line represents linear regression with r = 0.091.
Fig 5.
Carbon dioxide levels measured at baseline and at the end of the spontaneous ventilation period. Black lines indicate end-tidal measurements (n = 12). Blue lines indicate serial arterial blood gas measurements (n = 5). The red line indicates the mean overall (end-tidal + arterial) rate of increase of carbon dioxide (0.03 kPa.min−1).
Table 2.
Serial arterial blood gas data sampled from five patients
| Case | Sample time | FiO2 | pH | pCO2 (kPa) | pO2 (kPa) |
|---|---|---|---|---|---|
| 1 | 0907 | 0.21 | 7.37 | 7.1 | 6.5 |
| 0919 | 1 | 7.32 | 7.9 | 54.5 | |
| 0945 | 0.3 | 7.34 | 7.9 | 10.3 | |
| 1003 | 1 | 7.28 | 8.9 | 13.2 | |
| 1024 | 0.6 | 7.38 | 6.9 | 21.6 | |
| 2 | 1459 | 0.21 | 7.45 | 4.8 | 8.1 |
| 1542 | 0.7 | 7.3 | 6.8 | 49.1 | |
| 3 | 1000 | 0.21 | 7.45 | 4.8 | 13.3 |
| 1027 | 1 | 7.38 | 5.9 | 74.4 | |
| 1039 | 1 | 7.38 | 6.0 | 69.7 | |
| 1049 | 1 | 7.33 | 7.2 | 17.1 | |
| 1104 | 1 | 7.3 | 7.6 | 13.5 | |
| 4 | 1039 | 1 | 7.43 | 5.7 | 11.5 |
| 1059 | 1 | 7.4 | 6.1 | 70.8 | |
| 1108 | 1 | 7.38 | 6.7 | 70.5 | |
| 1131 | 1 | 7.36 | 6.9 | 13.2 | |
| 5 | 0913 | 1 | 7.43 | 5.2 | 64.4 |
| 1004 | 1 | 7.32 | 6.5 | 30.3 |
Discussion
We found that use of STRIVE Hi had beneficial effects on oxygenation, ventilation and airway patency in our patients with significant airway and respiratory compromise undergoing microlaryngoscopic surgery. During the induction of anaesthesia, there were no oxygen saturations below 97% and no episodes of apnoea or complete airway obstruction. Ventilation was preserved at deep levels of anaesthesia in a time-independent manner (up to 100 min), based on our ETCO2 data.
This is the first study to describe the use of HFNO in spontaneously breathing patients undergoing general anaesthesia. Our results are consistent with previous studies, which have described the benefits of HFNO for spontaneously breathing patients in a diverse range of clinical scenarios.1,2,4
SpO2 increased from baseline and was maintained at 97-100% during the entire induction period, which averaged 20 min. Similar improvements in oxygenation, attributed to improved oxygen delivery, have been reported using HFNO during sedation for AFOI.7 A slight but expected decrease in median SpO2 occurred during the maintenance phase, as a result of our manipulation of the FiO2 and respiratory effort (using remifentanil) depending on the procedure being performed. We routinely reduce the FiO2 to 0.3 during laser surgery (even if tubeless) to prevent an airway fire, in keeping with published recommendations.13,14 Three patients experienced controlled oxygen desaturation during the reduced FiO2, which was tolerated to facilitate safe laser surgery. The combination of HFNO and spontaneous respiration resulted in rapid re-oxygenation when the FiO2 was increased. No patients experienced oxygen desaturation <90% while the FiO2 was 1.0.
HFNO has been demonstrated to improve oxygenation and respiratory mechanics by an increase in end-expiratory lung volumes (i.e. functional residual capacity) in postoperative patients and patients with both hypoxaemic and hypercapnic respiratory failure.1,6,15,16 It is likely that these lower airway effects benefited our spontaneously breathing patients during general anaesthesia, particularly those with laryngotracheal stenosis. A spontaneous ventilation induction has traditionally been used to manage the obstructed airway,17 however recently positive pressure ventilation with neuromuscular block has been advocated, based on more favourable respiratory mechanics.18 Although negative intrathoracic pressure and reduced functional residual capacity are said to exacerbate extrathoracic airway stenosis19, we did not experience this under STRIVE Hi conditions. We believe that the positive airway pressure generated by HFNO counteracts a tendency to collapse, improves airway patency and reduces airway resistance.1,5 Furthermore, we successfully anaesthetized several particularly high-risk patients with co-existing airway and respiratory compromise, without significant detriment to their respiratory function.
The aim of a STRIVE induction, whereby spontaneous ventilation is maintained throughout the entire induction period using an upwardly titrated infusion rate of propofol, is to maintain oxygenation by preventing apnoea and complete airway obstruction, while simultaneously achieving an adequate depth of anaesthesia to tolerate airway instrumentation. We found that the addition of HFNO offered several advantages that could increase the margin of safety of a spontaneous ventilation technique during periods of inadvertent apnoea and/or total airway obstruction.
HFNO has been reported to reduce the risk of hypoxaemia from inadvertent apnoea during sedation for AFOI in obstructed airways.7 We noticed a similar benefit during two episodes of remifentanil-induced apnoea in the maintenance phase. Stable oxygenation allowed time for spontaneous respiration to recommence after the remifentanil was ceased. Although rapid hypoxaemia occurred after a misprogrammed remifentanil overdose caused apnoea in one case, this was unusual and related to suspension laryngoscope positioning. Deep insertion of the laryngoscope isolated the trachea from the oropharynx and prevented any HFNO delivery to the lower airway, such that the patient was effectively breathing room air, and had a limited oxygen reservoir when the apnoea occurred.
We also noted that the SpO2 remained completely unchanged during 60 s periods of complete airway occlusion in every case of balloon dilatation for tracheal stenosis, except the pregnant patient (who experienced a 5% decrease). This finding contrasts our previous STRIVE series without the use of HFNO9, and it is likely that the preserved oxygenation using HFNO also resulted from an increased functional residual capacity.
The tendency for the upper airway to obstruct during anaesthesia, most commonly at the velopharynx, is well described.12 Propofol causes a dose dependent increase in upper airway collapsibility, however particular vulnerability exists during the early stages of a spontaneous ventilation induction.20,21 We found that HFNO splinted the upper airway during our inductions. Although minor jaw thrust was applied to ensure airway patency, no patient required major upper airway intervention or adjuvant use. This was also in contrast to our initial STRIVE series without HFNO, where upper airway interventions were common and nasopharyngeal airways were required in most of the inductions.9 Similar upper airway benefits using HFNO, attributed to the nasopharyngeal pressure generated by high flow rates, have been reported in sedated patients undergoing bronchoscopy and patients with OSA.5,7,8
Although the THRIVE technique has been shown to extend the apnoeic window in patients with difficult airways, the ultimate benefit of this was to improve the overall process of difficult intubation.3 We found that STRIVE Hi was associated with similar human factor benefits. The process of securing a definitive airway, including suspension laryngoscope insertion, was smooth, unhurried and uncomplicated. We also noticed several areas where STRIVE Hi contrasted with THRIVE, although it is difficult to directly compare the results of each study because of their observational nature and potential for confounding and measurement bias. Firstly, the rate of ETCO2 increase was much lower in STRIVE Hi compared with THRIVE (0.03 vs 0.15 kPa.min-1), despite a longer measurement period (average 47 vs 17 min). STRIVE Hi was also extended up to an average of 56 min in seven patients with a BMI >35, without any difference in outcomes, which contrasts with THRIVE, where the safe upper limit of apnoea may be only five min in morbid obesity. Our findings are consistent with Corley and colleagues6, who reported the greatest benefits to oxygenation and respiratory mechanics from HFNO during spontaneous ventilation occurred in patients with high BMIs.
The use of i.v. anaesthesia to maintain spontaneous ventilation has several advantages compared with the more traditional inhalation induction22,23, which was associated with multiple failures in the 4th National Audit Project (NAP4).18 It has good airway reflex suppression; separates anaesthesia delivery from ventilation and its use can extend beyond the induction to facilitate tubeless airway surgery.9,22 In addition, as HFNO is an open oxygen delivery system, it cannot be used during an inhalation induction, as a result of the dilutional effect on the inspired volatile agents.24
This study is limited by its retrospective and observational nature. Although the sample size was small and performed at a single site with experience using STRIVE techniques, these patients reflect the challenges faced at a tertiary head and neck centre. As with all unprotected airways, we consider this technique contraindicated in patients at high risk of aspiration. One disadvantage using HFNO was the difficulty with carbon dioxide sampling and its effect on respiratory rate monitoring during the induction, compared with conventional capnography using a closed circuit system. Most of our carbon dioxide data involved end-tidal measurements, which may not accurately reflect arterial levels.
We have shown that STRIVE Hi has fulfilled the primary objective of airway management, which is to maintain oxygenation during the process of securing a definitive airway.25 Although there is a current vogue in difficult airway management to ablate spontaneous ventilation with neuromuscular block, there are scenarios where maintenance of spontaneous ventilation may be advantageous (e.g. anterior mediastinal mass, asleep fibreoptic intubation). We propose that STRIVE Hi is a modern alternative to the traditional inhalation induction and research is warranted to explore further applications to manage the difficult airway.
In conclusion, we have extended the application of HFNO to spontaneously breathing patients during general anaesthesia. The physiological benefits on both the upper and lower airway, which have been attributed to HFNO, are likely to increase the margin of safety of a spontaneous respiration induction. STRIVE Hi successfully managed patients with difficult and/or obstructed airways including those with respiratory compromise.
Authors’ contributions
Study design/planning: A.W.G.B., K.V., P.K.L.
Study conduct: A.W.G.B., K.V., P.K.L., C.-M.T.
Data analysis: A.W.G.B., K.V., P.K.L.
Writing paper: A.W.G.B., K.V., P.K.L.
Revising paper: all authors
Declaration of interest
A.W.G.B. has received a travel and accommodation grant from Fisher and Paykel Healthcare.
Funding
References
- 1. Roca O, Hernandez G, Diaz-Lobato S, Carratala JM, Gutierrez RM, Masclans JR. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care 2016; 20: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care 2016; 61: 529–41 [DOI] [PubMed] [Google Scholar]
- 3. Patel A, Nouraei SAR. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015; 70: 323–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high-flow nasal oxygen in adults mechanisms of action and clinical implications. Chest 2015; 148: 253–61 [DOI] [PubMed] [Google Scholar]
- 5. Diab S, Fraser JF. Maintaining oxygenation successfully with high flow nasal cannula during diagnostic bronchoscopy on a postoperative lung transplant patient in the intensive care. Case Rep Crit Care 2014; 2014: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth 2011; 107: 998–1004 [DOI] [PubMed] [Google Scholar]
- 7. Badiger S, John M, Fearnley RA, Ahmad I. Optimizing oxygenation and intubation conditions during awake fibre-optic intubation using a high-flow nasal oxygen-delivery system. Br J Anaesth 2015; 115: 629–32 [DOI] [PubMed] [Google Scholar]
- 8. McGinley BM, Patil SP, Kirkness JP, Smith PL, Schwartz AR, Schneider HA. Nasal cannula can be used to treat obstructive sleep apnea. Am J Respir Crit Care Med 2007; 176: 194–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Booth AWG, Vidhani K. Spontaneous ventilation using target-controlled propofol infusion for microlaryngoscopy in adults: a retrospective audit. Anaesth Intensive Care 2016; 44: 286–94 [DOI] [PubMed] [Google Scholar]
- 10. Dahan A. General anaesthesia and control of respiration. Semin Anesth 1996; 15: 328–34 [Google Scholar]
- 11. Allsop P, Taylor MB, Grounds RM, Morgan M. Ventilatory effect of a propofol infusion using a method to rapidly achieve steady-state equilibrium. Eur J Anaesth 1988; 5: 193–303 [PubMed] [Google Scholar]
- 12. Hillman DR, Platt PR, Eastwood PR. The upper airway during anaesthesia. Br J Anaesth 2003; 91: 31–9 [DOI] [PubMed] [Google Scholar]
- 13. Bruley ME. Surgical fires: perioperative communication is essential to prevent this rare but devastating complication. Qual Saf Health care 2004; 13: 467–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yardley IE, Donaldson LJ. Surgical fires, a clear and present danger. Surgeon 2010; 8: 87–92 [DOI] [PubMed] [Google Scholar]
- 15. Millar J, Lutton S, O’connor P. The use of high-flow nasal oxygen therapy in the management of hypercarbic respiratory failure. Ther Adv Respir Dis 2014; 8: 63–4 [DOI] [PubMed] [Google Scholar]
- 16. Nilius G, Franke KJ, Domanski U, Ruhle KH, Kirkness JP, Schneider H. Effects of nasal insufflation on arterial gas exchange and breathing pattern in patients with chronic obstructive pulmonary disease and hypercapnic respiratory failure. Advances in Experimental Medicine and Biology 2013; 755: 27–34 [DOI] [PubMed] [Google Scholar]
- 17. Mason RA, Fielder CP. The obstructed airway in head and neck surgery. Anaesthesia 1999; 54: 625–8 [DOI] [PubMed] [Google Scholar]
- 18. Patel A, Pearce A, Pracy P, Head and Neck Pathology. Chapter 18 in: Royal College of Anaesthetists. 4th National Audit Project: Major Complications of Airway Management in the UK. London: RCoA, 2011 [Google Scholar]
- 19. Nouraei SAR, Giussani DA, Howard DJ, et al. Physiological comparison of spontaneous and positive-pressure ventilation in laryngotracheal stenosis. Br J Anaesth 2008; 101: 419–23 [DOI] [PubMed] [Google Scholar]
- 20. Eastwood PR, Platt PR, Shepherd K, et al. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology 2005; 103: 470–7 [DOI] [PubMed] [Google Scholar]
- 21. Hillman DR, Walsh JH, Maddison KJ, et al. Evolution of changes in upper airway collapsibility during slow induction of anesthesia with propofol. Anesthesiology 2009; 111: 63–71 [DOI] [PubMed] [Google Scholar]
- 22. Ludbrook GL, Hitchcock M, Upton RN. The difficult airway: propofol infusion as an alternative to gaseous induction. Anaesth Intensive Care 1997; 25: 71–3 [DOI] [PubMed] [Google Scholar]
- 23. Cook TM, Morgan PJ, Hersch PE. Equal and opposite expert opinion. Airway obstruction caused by a retrosternal thyroid mass: management and prospective international expert opinion. Anaesthesia 2011; 66: 828–36 [DOI] [PubMed] [Google Scholar]
- 24. Kennedy AJ, Coakley M. Dilutional effect of nasal oxygenation. Br J Anaesth 2016; 117: 536. [DOI] [PubMed] [Google Scholar]
-
25.
Greenland K.
Art of airway management: the concept of ‘Ma’ (Japanese:
 , when ‘less is more’). Br J Anaesth 2015; 115: 809–12 [DOI] [PubMed] [Google Scholar]
, when ‘less is more’). Br J Anaesth 2015; 115: 809–12 [DOI] [PubMed] [Google Scholar]