Abstract
Background: The American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC), the National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR) collaborate to provide annual updates on cancer occurrence and trends in the United States. This Annual Report highlights survival rates. Methods: Data were from the CDC- and NCI-funded population-based cancer registry programs and compiled by NAACCR. Trends in age-standardized incidence and death rates for all cancers combined and for the leading cancer types by sex were estimated by joinpoint analysis and expressed as annual percent change. We used relative survival ratios and adjusted relative risk of death after a diagnosis of cancer (hazard ratios [HRs]) using Cox regression model to examine changes or differences in survival over time and by sociodemographic factors.
Results: Overall cancer death rates from 2010 to 2014 decreased by 1.8% (95% confidence interval [CI] = –1.8 to –1.8) per year in men, by 1.4% (95% CI = –1.4 to –1.3) per year in women, and by 1.6% (95% CI = –2.0 to –1.3) per year in children. Death rates decreased for 11 of the 16 most common cancer types in men and for 13 of the 18 most common cancer types in women, including lung, colorectal, female breast, and prostate, whereas death rates increased for liver (men and women), pancreas (men), brain (men), and uterine cancers. In contrast, overall incidence rates from 2009 to 2013 decreased by 2.3% (95% CI = –3.1 to –1.4) per year in men but stabilized in women. For several but not all cancer types, survival statistically significantly improved over time for both early and late-stage diseases. Between 1975 and 1977, and 2006 and 2012, for example, five-year relative survival for distant-stage disease statistically significantly increased from 18.7% (95% CI = 16.9% to 20.6%) to 33.6% (95% CI = 32.2% to 35.0%) for female breast cancer but not for liver cancer (from 1.1%, 95% CI = 0.3% to 2.9%, to 2.3%, 95% CI = 1.6% to 3.2%). Survival varied by race/ethnicity and state. For example, the adjusted relative risk of death for all cancers combined was 33% (HR = 1.33, 95% CI = 1.32 to 1.34) higher in non-Hispanic blacks and 51% (HR = 1.51, 95% CI = 1.46 to 1.56) higher in non-Hispanic American Indian/Alaska Native compared with non-Hispanic whites.
Conclusions: Cancer death rates continue to decrease in the United States. However, progress in reducing death rates and improving survival is limited for several cancer types, underscoring the need for intensified efforts to discover new strategies for prevention, early detection, and treatment and to apply proven preventive measures broadly and equitably.
The American Cancer Society (ACS), Centers for Disease Control and Prevention (CDC), National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR) have collaborated annually since 1998 to provide updates on cancer incidence and mortality patterns in the United States (1–18). Each “Annual Report to the Nation” also features an in-depth analysis of a selected topic, and this year’s report features survival by stage, race/ethnicity, and state for common cancers.
In addition to death and incidence rates, survival is an important measure for assessing progress in efforts to improve cancer outcomes (19). As with most disease surveillance measures, the interpretation of survival trends is complicated by changes in screening and detection practices (19,20). In particular, screening may lead to the detection of cases that would not have been detected through clinical manifestation in a lifetime (overdiagnosis) or the detection of cancers that are inherently slow growing (length bias). Screening may also result in earlier diagnosis without changing the date of death, generating apparent improvements in survival without changing the actual course of disease (lead time bias) (21). In this report, we examine temporal changes in overall and stage-specific survival for all races/ethnicities combined and in overall survival by race, and contemporary overall survival by race and ethnicity and state of residence. We interpret these survival statistics in the context of changes in screening, early detection, and treatment.
Methods
Data Sources
Cancer Incidence Data
Population-based cancer incidence data by age, sex, and race/ethnicity were obtained from 39 states and two metropolitan area registries that participate in the CDC’s National Program of Cancer Registries (NPCR) and/or the NCI’s Surveillance, Epidemiology, and End Results (SEER) program. The data satisfied NAACCR’s data quality criteria and represented cases diagnosed from 1999 through 2013, (22) covering 89% of the US population. This database of 41 registries was used to derive all incidence statistics presented in this report, which is the first annual report that has drawn all incidence statistics from a single database. In the past, limitations on the number of high-quality registries extending back in time required estimation of five-year average incidence rates from one set of registries, 10-year trends from a smaller set, and long-term trends from a third even smaller set.
Anatomic site and histology were coded according to the International Classification of Diseases for Oncology (ICD-O) edition in use at the time of diagnosis and were converted to the third edition coding (23) and categorized according to SEER site groups (24). Only cases defined as malignant under ICD-O-2 and ICD-O-3 were included in this report. For solid tumors, stage was categorized as localized, regional, or distant using SEER Summary Stage 2000, which has been used for new cases (incidence) diagnosed in 1998 or later (25). All case counts were adjusted for delay in reporting or corrections (26).
Cancer Mortality Data
Cause of death by age, sex, and race/ethnicity (2000–2014) was based on death certificate information reported to state vital statistics offices and compiled into a national file for the entire United States by the CDC National Center for Health Statistics’ (NCHS’) National Vital Statistics System (27). The underlying causes of death were selected according to the International Classification of Disease (ICD) codes and rules in use at the time of death (ICD-8 through ICD-10) and categorized according to SEER causes of death recode to maximize comparability between ICD and ICD-O versions (24).
Race/Ethnicity
Information on race and ethnicity was based on medical records or death certificates. Race is categorized as white, black, Asian and Pacific Islander (API), American Indian/Alaska Native (AI/AN). Race by ethnicity according to Hispanic origin (race/ethnicity) was categorized as non-Hispanic white (NHW), non-Hispanic black (NHB), non-Hispanic Asian and Pacific Islander (NHAPI), non-Hispanic American Indian/Alaska Native (NHAI/AN), and Hispanic. Race and race/ethnicity information for AI/AN, however, is reliable only for geographic areas covered by the Indian Health Service Contract Health Service Delivery Areas (CHSDA) (10,28,29), and thus incidence, mortality, and survival data for American Indians/Alaska Natives are based on these areas. We present data by race and by Hispanic origin for incidence and mortality. Incidence was presented by race and ethnicity separately because delay adjustment factors were not available for the combined race/ethnicity categorization. Cause-specific survival and adjusted risk of cancer death are presented by race/ethnicity; however, relative survival estimates are presented only for race categories where appropriate life tables were available.
Population Data
The population estimates used as the denominators to calculate incidence and death rates were a modification of the intercensal and Vintage 2014 annual times series of July 1, county population estimates by age, sex, race, and Hispanic origin produced by the US Census Bureau’s Population Estimates Program, in collaboration with CDC’s NCHS and with support from the NCI (30). The estimates incorporate intercensal (for July 1, 2000–2009) and Vintage 2014 (for July 1, 2010–2013) bridged single-race estimates that are derived from the original multiple-race categories in the 2000 and 2010 Censuses (as specified in the 1997 Office of Management and Budget standards for the collection of data on race and ethnicity) (31,32). For most states, population estimates as of July 1 of each year were used to calculate rates that were presumed to reflect the average population of a defined geographic area for a calendar year; however, some adjustments were made to refine these estimates, as has been done in previous reports (16,17,30).
Survival Data
To examine survival over time, by race, and by geographic areas, we used survival data from three databases. We used survival data from 9-SEER cancer registries (covering about 10% of the US population) to examine temporal changes in five-year survival between patients diagnosed from 1975 to 1977 vs 2006 to 2012 and followed through 2013; 1975 was the first year when all nine registries submitted incidence data to SEER. We examined these changes for the 20 most common cancers by race (all races, whites, blacks) and by stage (all races combined only), when stage information was available. We examined differences in contemporary survival for patients diagnosed from 2006 to 2012 by race/ethnicity (NHW, NHB, NHAPI, NHAI/AN, and Hispanic) for the 20 most common cancers using survival data from 18-SEER areas, which cover about 28% of the US population. We used 33 SEER or NPCR registries compiled by NAACCR (covering 67% of the US population) to examine contemporary survival differences by state of residence for the four most common cancers (lung, colon and rectum, female breast, prostate). These 33 registries (31 states and two metropolitan areas, referred to hereafter as “states”) were considered to have sufficient vital status follow-up to conduct survival analyses because they either conducted recent National Death Index (NDI) linkages or they routinely conduct active vital status follow-up of all cases (33).
In all SEER-only-based survival analyses, only first primary cancers were used in the analysis and patients were followed for vital status through December 31, 2013. For the state-specific analysis, which included data from the SEER and NPCR registries, the first site-specific cancer was used in the analysis because of the different starting dates among the registries (34) and patients were followed for vital status through December 31, 2012, because not all registries had complete information on vital status through December 31, 2013. In SEER registries, cancers that were identified by death certificate or autopsy only were excluded, as were patients with no survival time.
Statistical Methods
Incidence and Death Rates and Trends
Cross-sectional incidence (2009–2013) and death (2010–2014) rates for all ages combined were calculated for all cancer sites combined and for the 15 most common cancer sites by sex, race, and Hispanics. These rates were calculated with their 95% confidence intervals using SEER*Stat software, version 8.3.2 (35,36). Incidence rates were delay-adjusted to account for revisions to the case counts in future submissions (http://surveillance.cancer.gov/delay/). Similarly, we calculated overall incidence and death rates for children (0–14 years). All rates were age-standardized to the 2000 US Standard Population and were expressed per 100 000 persons (35). Rates based on fewer than 16 cases were deemed to be unstable and were suppressed.
Temporal trends in age-standardized, delay-adjusted cancer incidence (1999–2013) and death (2000–2014) rates were estimated using joinpoint regression (37,38), with a maximum of two joinpoints (three line segments) allowed in each model. The resultant trends were described by the annual percentage change (APC). The five-year average annual percent changes (AAPCs) for 2009 to 2013 (incidence) and for 2010 to 2014 (mortality) were calculated using a weighted average of the slope coefficients of the underlying joinpoint regression line, with weights equal to the length of each segment over the interval. The AAPC was equal to the APC when the AAPC was entirely within the last joinpoint segment (39). Two-sided statistical significance (P < .05) for APC and AAPC was determined using a t test for the APC and for the AAPC when it lies entirely within the last joinpoint segment and a z test when the AAPC extends beyond the last joinpoint segment (40). In describing trends, the terms “increase” or “decrease” were used when the slope of the trend (APC or AAPC) was statistically significant; otherwise, terms such as “stable,” “nonsignificant increase,” and “nonsignificant decrease” were used. Trends based on fewer than 10 cases in any of the 15 data years (1999–2013 for incidence and 2000–2014 for mortality) were considered unreliable and were suppressed.
Survival Ratios and Trends
We used relative survival ratios (RSRs) to examine differences and changes in five-year survival over time or across geographic areas. RSR is a measure of excess mortality experienced by cancer patients and is calculated by dividing the observed survival from all causes of death for the patient cohort by the expected survival as estimated by life tables. Relative survival is a theoretical population-based measure representing cancer survival in the absence of other causes of death. We also calculated absolute and relative (proportional) changes in five-year RSRs between cases diagnosed in 1975 to 1977 vs 2006 to 2012 for the 20 most common cancers (all ages, by race) and for select childhood cancers (0–14 years, all races) and by SEER historic stage (localized, regional, and distant). For lymphoma, Ann Arbor staging (stage I, II, III, and IV) (41) was used; z tests were performed to examine if the changes in relative survival over time were statistically significantly different between whites and blacks.
To describe differences in survival for the 20 most common cancers across racial/ethnic groups, we calculated cause-specific survival rather than RSR because reliable life tables are not available for NHAPI and NHAI/AN populations. We also compared the risk of death in NHB, NHAPI, NHAI/AN and Hispanic populations with the NHW population using hazard ratios (HRs). These hazard ratios were calculated using Cox regression models, with a maximum of five years of follow-up and adjusting for sex, age, and summary stage (except for all cancer sites combined and leukemia). No statistically significant violations of proportional hazards assumption were found by testing the interactions between survival time and covariates. This analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC). All statistical tests were two-sided, and statistical significance level was set at a P value of less than .05.
For all survival analyses, the survival duration in months was calculated based on complete dates and 60-month survival is reported. For registries conducting active follow-up, survival duration was calculated through the date of last contact (or study cutoff, if earlier). For the remaining registries (those conducting data linkage with the National Death Index [NDI] only), survival duration was calculated through December 31, 2012, with all patients not known to be dead presumed to be alive on that date. For the analyses based only on SEER data, expected survival was estimated from race- and sex-specific life tables for the entire United States. For the analyses by state, expected survival was estimated from life tables matched to the cancer patients by age (0–99 years), sex, year, state, race, and county-level socioeconomic status (SES). Cases were censored at an achieved age of 100 years.
Results
Cancer Incidence Rates for the Most Common Cancers
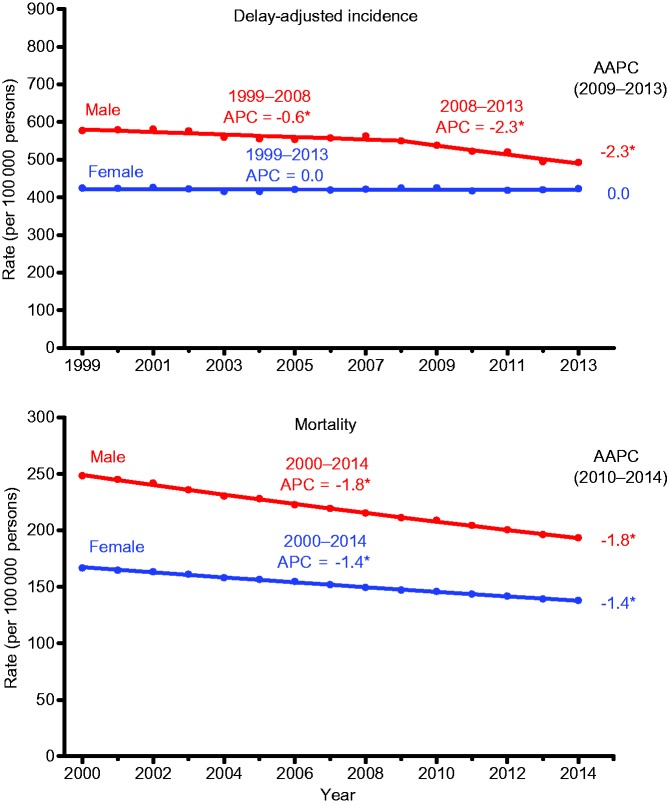
Figure 1 shows trends from 1999 to 2013 in age- and delay-adjusted incidence rates for all cancer sites combined for men and women. Incidence rates for men decreased throughout the study period, with the decrease accelerating from 0.6% (95% confidence interval [CI] = –0.9 to –0.2) per year during 1999 to 2008 to 2.3% (95% CI = –3.1 to –1.4) per year during 2008/2009 to 2013. In contrast, over the same 15-year time period, rates for women remained stable.
Figure 1.
Trends in age-standardized incidence (1999–2013) and death rates (2000–2014) for all cancers combined by sex. Rates were age-standardized to the 2000 US standard population (19 age groups Census P25–1130). Scattered points were observed rates; lines were fitted rates according to Joinpoint regression. Incidence rates were delay-adjusted and covered 89% of the US population, and mortality covered the entire United States. Registries included for incidence: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Texas, Utah, Vermont, Washington, West Virginia, Wisconsin, and Wyoming. AAPC is the average annual percent change and is a weighted average of the annual percent change (APC) over the fixed interval (2009–2013 for incidence, 2010–2014 for mortality) using the underlying joinpoint model for the period of 1999 to 2013 for incidence and the period of 2000 to 2014 for mortality. Joinpoint models with up to two joinpoints are based on rates per 100 000 persons that are age-standardized to the 2000 US standard population (19 age groups Census P25–1130). Joinpoint Regression Program, version 4.2.0.2. June 2015, Statistical Research and Applications Branch, National Cancer Institute. *The APC or AAPC is statistically significantly different from 0 (two-sided t test, P < .05). AAPC = average annual percent change; APC = annual percent change.
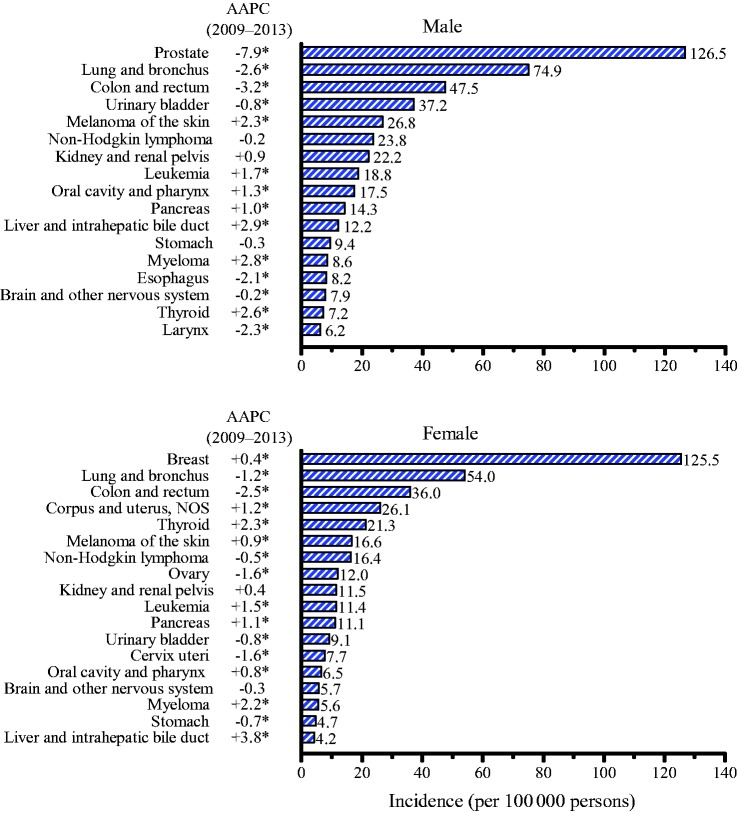
Figure 2 presents average annual incidence rates and trends during 2009 to 2013 for the 17 most common cancers in men and 18 most common cancers in women. Among men, incidence rates during 2009 to 2013 decreased statistically significantly for seven of the 17 most common cancers, including prostate (P = .003), lung and bronchus (lung; P < .001), colon and rectum (colorectal; P < .001), urinary bladder (bladder; P < .001), esophagus (P < .001), brain and other nervous system (brain; P = .005), and larynx (P < .001). The largest decline was for prostate cancer, with an average –7.9% (95% CI = –12.2% to –3.3%) decline per year. In contrast, rates increased statistically significantly for seven cancers: melanoma of the skin (melanoma), leukemia, oral cavity and pharynx (oral cavity), pancreas, liver and intrahepatic bile duct (liver), myeloma, and thyroid (P < .001, for all) and stabilized for non-Hodgkin lymphoma (NHL), kidney and renal pelvis (kidney), and stomach cancer.
Figure 2.
Age-standardized incidence rates and recent trends (five years) for the most common cancers by sex. Rates were age-standardized to the 2000 US standard population (19 age groups Census P25–1130) and were delay-adjusted and covered 89% of the US population. Registries included in analyses: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Texas, Utah, Vermont, Washington, West Virginia, Wisconsin, and Wyoming. AAPC is the average annual percent change and is a weighted average of the annual percent change (APC) over the fixed interval (2009–2013) using the underlying joinpoint model for the period of 1999 to 2013. Joinpoint models with up to two joinpoints are based on rates per 100 000 persons that are age-standardized to the 2000 US standard population (19 age groups Census P25–1130). Joinpoint Regression Program, version 4.2.0.2. June 2015, Statistical Research and Applications Branch, National Cancer Institute. *The AAPC is statistically significantly different from 0 (two-sided t test or z test, P < .05). AAPC = average annual percent change.
Among women, incidence rates during 2009 to 2013 decreased statistically significantly for seven of the 18 most common cancers: lung (P < .001), colorectal (P < .001), NHL (P = .003), ovary (P < .001), bladder (P < .001), cervix uteri (cervix; P = .001), and stomach (P < .001); however, incidence rates increased statistically significantly for nine cancers: breast (P < .03), corpus and uterus not otherwise specified (NOS; uterus; P < .001), thyroid (P < .001), melanoma (P < .01), leukemia (P < .001), myeloma (P < .001), pancreas (P < .001), oral cavity (P = .001), and liver (P < .001); incidence rates remained unchanged for kidney and brain (Figure 2). Of note is the statistically significant increase of 0.4% (95% CI = 0.1 to 0.8) per year over the past five years in breast cancer incidence, the most common cancer among women. Liver cancer among women increased by 3.8% (95% CI = 3.4 to 4.1) per year over the past five years, replacing thyroid cancer as the most rapidly increasing incident cancer among women. For most cancers, the increasing or decreasing trends during 2009 to 2013 in both men and women were continuations of past trends (Supplementary Table 1, available online).
Cancer Death Rates for the Most Common Cancers
Figure 1 also shows trends in death rates for all cancer sites combined from 2000 to 2014 by sex. Death rates decreased statistically significantly from 2000 to 2014 by 1.8% (95% CI = –1.8 to –1.8) on average per year among men and by 1.4% (95% CI = –1.4 to –1.3) per year among women. Similarly, overall cancer death rates during the most recent five years (2010 to 2014) decreased by 1.8% (95% CI = –1.8 to –1.8) per year in men by 1.4% (95% CI = –1.4 to –1.3) per year in women.
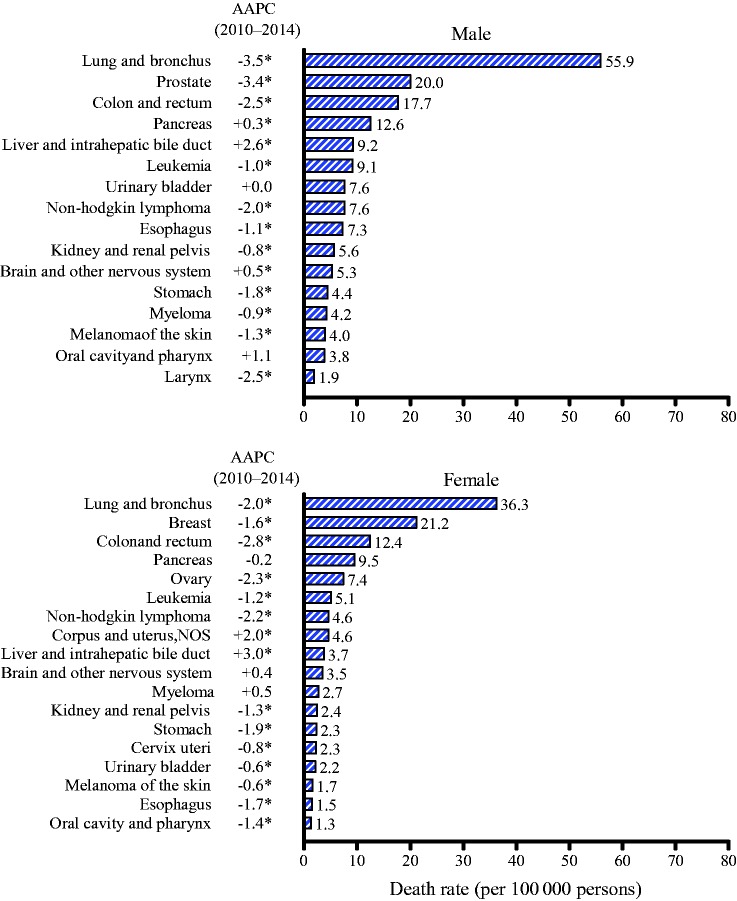
Figure 3 presents average annual death rates and trends during 2010 to 2014 for the 16 most common cancers in men and 18 most common cancers in women. Among men, death rates during this period decreased statistically significantly for 11 of the 16 cancers, including lung by 3.5% (95% CI = –3.9 to –3.2) per year, prostate by 3.4% (95% CI = –3.6 to –3.2) per year, and colorectal by 2.5% (95% CI = –2.7 to –2.4) per year. In contrast, rates increased statistically significantly for cancers of the liver by 2.6% (95% CI = 2.4 to 2.8) per year, for pancreas by 0.3% (95% CI = 0.1 to 0.4) per year, and for brain by 0.5% (95% CI = 0.0 to 1.0) per year; rates stabilized for bladder and oral cavity cancers. Among women, during the same time period death rates decreased statistically significantly for 13 of the 18 most common cancer types, including lung by 2.0% (95% CI = –2.2 to –1.8) per year, breast by 1.6% (95% CI = –1.8 to –1.4) per year, and colorectal by 2.8% (95% CI = –3.0 to –2.7) per year. In contrast, death rates increased statistically significantly for cancers of the uterus by 2.0% (95% CI = 1.4 to 2.6) per year and for liver cancer by 3.0% (95% CI = 2.6 to 3.4) per year; rates remained stable for pancreas, brain, and myeloma. As with the incidence trends, the increase or decrease in death rates for most cancers in both men and women were continuations of past trends (Supplementary Table 2, available online).
Figure 3.
Age-standardized death rates and recent trends (five years) for the most common cancers by sex. Rates were age-standardized to the 2000 US standard population (19 age groups Census P25–1130). AAPC is the average annual percent change and is a weighted average of the annual percent change (APC) over the fixed interval (2010–2014) using the underlying joinpoint model for the period of 2000 to 2014. Joinpoint models with up to two joinpoints are based on rates per 100 000 persons that are age-standardized to the 2000 US standard population (19 age groups Census P25–1130). Joinpoint Regression Program, version 4.2.0.2. June 2015, Statistical Research and Applications Branch, National Cancer Institute. *The AAPC is statistically significantly different from 0 (two-sided t test or z test, P < .05). AAPC = average annual percent change.
Current Cancer Incidence Rates and Trends by Race, Ethnicity, and Sex
Table 1 depicts average annual age-standardized and delay-adjusted incidence rates and trends for the most recent five-year period (2009–2013) by cancer site, sex, race, and ethnicity. Rates for all cancer sites combined in all racial and ethnic groups were higher in men than in women (512.9 vs 420.6 per 100 000). Black men and white women had higher overall cancer incidence rates than any of their racial/ethnic counterparts, whereas API men and women had the lowest rates. In all racial and ethnic groups, prostate cancer in men and breast cancer in women were the most frequent incident cancers, followed by lung cancer and colorectal cancer, except in Hispanics for whom colorectal preceded lung cancer. Rankings for several of the other cancers varied substantially by race and ethnicity in both men and women. Among men, for example, melanoma ranked fifth in white men and 19th in black men, whereas liver cancer ranked 11th in white men and seventh in black men.
Table 1.
Delay-adjusted incidence rates and fixed-interval trends for 2009 to 2013 for the most common cancers by sex, race, and ethnicity, for areas in the United States with high-quality incidence data*
| Sex/cancer site or type† | White‡ |
Black‡ |
API‡ |
AI/AN (CHSDA)‡ |
Hispanic‡ |
Non-Hispanic‡ |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Rate§ | AAPC‖ | P# | Rank | Rate§ | AAPC‖ | P# | Rank | Rate§ | AAPC‖ | P# | Rank | Rate§ | AAPC‖ | P# | Rank | Rate§ | AAPC‖ | P# | Rank | Rate§ | AAPC‖ | P# | |
| All sites¶ | ||||||||||||||||||||||||
| Both sexes | 461.7 | −1.1 | .007 | 474.4 | −1.4 | .001 | 302.1 | −0.4 | <.001 | 420.2 | 0.0 | .76 | 362.1 | −0.7 | <.001 | 469.8 | −1.1 | <.001 | ||||||
| Males | 511.2 | −2.2 | <.001 | 576.2 | −3.1 | <.001 | 318.2 | −1.2 | <.001 | 445.1 | −0.6 | .03 | 407.0 | −2.8 | <.001 | 525.5 | −2.2 | <.001 | ||||||
| Females | 427.5 | 0.0 | .60 | 406.1 | 0.3 | <.001 | 294.3 | 0.7 | <.001 | 405.9 | 0.6 | .002 | 334.8 | −0.1 | .05 | 430.2 | 0.0 | .53 | ||||||
| Males | ||||||||||||||||||||||||
| Prostate | 1 | 118.0 | −6.9 | .001 | 1 | 205.3 | −6.0 | .001 | 1 | 68.1 | −7.5 | .005 | 1 | 93.4 | −9.0 | .01 | 1 | 110.1 | −7.9 | <.001 | 1 | 128.4 | −7.8 | .004 |
| Lung and bronchus | 2 | 74.8 | −2.6 | <.001 | 2 | 88.2 | −3.4 | <.001 | 2 | 48.0 | −1.3 | <.001 | 2 | 73.9 | −0.5 | .25 | 3 | 43.2 | −2.5 | <.001 | 2 | 77.9 | −2.5 | <.001 |
| Colon and rectum | 3 | 46.5 | −3.3 | <.001 | 3 | 58.0 | −2.7 | <.001 | 3 | 39.0 | −2.2 | <.001 | 3 | 54.2 | −0.7 | .17 | 2 | 44.2 | −3.0 | <.001 | 3 | 48.0 | −3.2 | <.001 |
| Urinary bladder | 4 | 39.6 | −0.5 | <.001 | 5 | 20.3 | 0.7 | <.001 | 6 | 15.9 | −0.3 | .15 | 5 | 21.2 | 0.1 | .81 | 5 | 20.8 | −1.9 | <.001 | 4 | 38.7 | −0.4 | <.001 |
| Melanoma of the skin | 5 | 30.4 | 2.5 | <.001 | 19 | 1.1 | −0.6 | .32 | 18 | 1.5 | −0.4 | .47 | 12 | 8.9 | 2.0 | .03 | 14 | 5.4 | 1.0 | .15 | 5 | 29.2 | 2.5 | <.001 |
| Non-Hodgkin lymphoma | 6 | 24.5 | −0.4 | .02 | 6 | 17.7 | 0.2 | .25 | 5 | 16.3 | 0.3 | .25 | 7 | 18.4 | 0.1 | .87 | 6 | 20.8 | −0.1 | .64 | 6 | 24.1 | −0.6 | .05 |
| Kidney and renal pelvis | 7 | 22.4 | 0.6 | .005 | 4 | 24.4 | 0.8 | .13 | 9 | 11.3 | 2.7 | <.001 | 4 | 31.7 | 2.1 | .005 | 4 | 21.5 | 0.8 | .23 | 7 | 22.4 | 0.9 | .10 |
| Leukemia | 8 | 19.5 | 2.0 | <.001 | 12 | 14.6 | 1.1 | <.001 | 10 | 10.4 | 0.1 | .97 | 9 | 12.7 | −0.5 | .60 | 8 | 14.1 | 0.5 | .03 | 8 | 19.1 | 1.8 | <.001 |
| Oral cavity and pharynx | 9 | 18.0 | 1.7 | <.001 | 10 | 15.0 | −2.0 | <.001 | 8 | 11.3 | 0.3 | .36 | 8 | 16.5 | 1.1 | .21 | 11 | 11.2 | −0.7 | .02 | 9 | 18.3 | 1.5 | <.001 |
| Pancreas | 10 | 14.2 | 1.1 | <.001 | 8 | 17.0 | 0.5 | .01 | 11 | 10.4 | 0.7 | .01 | 10 | 11.6 | 0.7 | .53 | 10 | 12.4 | 0.6 | .02 | 10 | 14.5 | 1.1 | <.001 |
| Liver and intrahepatic bile duct | 11 | 11.0 | 4.2 | <.001 | 7 | 17.3 | 3.1 | <.001 | 4 | 21.1 | −0.4 | .10 | 6 | 18.9 | 3.6 | <.001 | 7 | 20.1 | 0.2 | .81 | 11 | 11.4 | 4.0 | <.001 |
| Stomach | 12 | 8.6 | 0.1 | .78 | 11 | 14.7 | −1.8 | <.001 | 7 | 14.7 | −2.9 | <.001 | 11 | 11.5 | −2.3 | .03 | 9 | 13.4 | −2.2 | <.001 | 12 | 9.0 | 0.0 | .99 |
| Myeloma | 15 | 7.9 | 2.6 | <.001 | 9 | 16.5 | 2.2 | <.001 | 13 | 5.1 | 2.6 | <.001 | 13 | 8.0 | 1.5 | .24 | 12 | 8.4 | 1.6 | <.001 | 14 | 8.6 | 2.6 | <.001 |
| Esophagus | 13 | 8.6 | −1.8 | .006 | 14 | 7.3 | −4.7 | <.001 | 15 | 3.6 | −1.4 | .05 | 14 | 7.6 | −1.7 | .21 | 17 | 5.1 | −3.8 | .003 | 13 | 8.6 | −1.9 | .001 |
| Brain and other nervous system | 14 | 8.5 | −0.1 | .07 | 15 | 4.9 | 0.4 | .11 | 14 | 4.4 | 0.3 | .36 | 15 | 5.9 | 1.2 | .30 | 13 | 6.1 | −0.5 | .006 | 15 | 8.3 | 0.0 | .68 |
| Thyroid | 16 | 7.6 | 2.6 | .008 | 16 | 3.8 | 4.9 | <.001 | 12 | 6.9 | 5.9 | <.001 | 18 | 4.3 | 3.5 | .05 | 15 | 5.3 | 4.6 | <.001 | 16 | 7.5 | 2.7 | .003 |
| Larynx | 18 | 6.1 | −2.0 | <.001 | 13 | 8.6 | −3.3 | <.001 | 16 | 2.3 | −2.9 | .002 | 17 | 5.3 | −1.9 | .07 | 16 | 5.2 | −2.9 | <.001 | 17 | 6.4 | −2.1 | <.001 |
| <.001 | ||||||||||||||||||||||||
| Females | <.001 | |||||||||||||||||||||||
| Breast | 1 | 126.9 | 0.3 | .08 | 1 | 125.3 | 1.2 | <.001 | 1 | 93.4 | 1.9 | <.001 | 1 | 105.4 | 3.6 | .05 | 1 | 95.6 | 0.4 | .06 | 1 | 129.1 | 0.5 | .01 |
| Lung and bronchus | 2 | 55.7 | −1.1 | <.001 | 2 | 50.7 | −0.7 | .006 | 2 | 28.9 | 0.4 | .01 | 2 | 58.3 | −1.7 | .02 | 3 | 26.1 | −0.7 | .001 | 2 | 56.7 | −1.1 | <.001 |
| Colon and rectum | 3 | 35.2 | −2.8 | <.001 | 3 | 42.5 | −3.1 | <.001 | 3 | 28.7 | −4.6 | <.001 | 3 | 43.7 | −1.0 | .02 | 2 | 30.6 | −2.8 | <.001 | 3 | 36.5 | −2.9 | <.001 |
| Corpus and uterus, NOS | 4 | 26.6 | 1.1 | <.001 | 4 | 25.3 | 2.3 | <.001 | 5 | 18.8 | 2.3 | <.001 | 4 | 23.9 | 1.7 | .002 | 4 | 22.4 | 2.9 | <.001 | 4 | 26.4 | 1.1 | <.001 |
| Thyroid | 5 | 22.4 | 2.1 | <.001 | 6 | 13.7 | 6.4 | <.001 | 4 | 21.5 | 3.1 | <.001 | 7 | 14.8 | 5.7 | <.001 | 5 | 20.0 | 2.7 | .02 | 5 | 21.6 | 3.1 | <.001 |
| Melanoma of the skin | 6 | 19.4 | 1.2 | .004 | 21 | 1.0 | 0.7 | .18 | 18 | 1.2 | −1.1 | .14 | 15 | 6.3 | 1.9 | .04 | 17 | 4.5 | 0.6 | .36 | 6 | 18.3 | 1.1 | .008 |
| Non-Hodgkin lymphoma | 7 | 17.0 | −0.6 | .005 | 8 | 12.4 | 0.7 | .001 | 6 | 11.0 | 0.0 | .96 | 6 | 14.8 | 0.3 | .63 | 6 | 15.8 | 0.1 | .50 | 7 | 16.5 | −0.5 | .003 |
| Ovary | 8 | 12.4 | −1.6 | <.001 | 11 | 9.7 | −0.5 | .01 | 7 | 9.4 | −0.4 | .12 | 8 | 11.6 | −0.6 | .53 | 8 | 10.7 | −1.3 | <.001 | 8 | 12.1 | −1.6 | <.001 |
| Kidney and renal pelvis | 10 | 11.6 | 0.4 | .12 | 7 | 12.8 | 0.0 | .98 | 14 | 5.1 | −1.3 | .30 | 5 | 18.9 | 2.0 | .007 | 7 | 12.4 | 2.2 | <.001 | 10 | 11.4 | −0.2 | .28 |
| Leukemia | 9 | 11.9 | 1.6 | <.001 | 12 | 9.4 | 2.5 | <.001 | 11 | 6.7 | 1.2 | .003 | 10 | 10.2 | 1.1 | .22 | 11 | 9.6 | 0.4 | .07 | 9 | 11.5 | 1.8 | .001 |
| Pancreas | 11 | 10.8 | 1.1 | <.001 | 5 | 14.4 | 0.7 | <.001 | 8 | 9.0 | 0.9 | .005 | 9 | 10.3 | 0.3 | .77 | 9 | 10.5 | 0.6 | <.001 | 11 | 11.2 | 1.1 | <.001 |
| Urinary bladder | 12 | 9.7 | −0.7 | <.001 | 14 | 6.8 | −0.3 | .26 | 15 | 3.9 | −0.5 | .35 | 13 | 6.7 | 2.3 | .02 | 15 | 5.3 | −1.3 | .002 | 12 | 9.5 | −0.7 | <.001 |
| Cervix Uteri | 13 | 7.5 | −0.9 | <.001 | 10 | 9.7 | −3.7 | .008 | 12 | 6.3 | −2.9 | <.001 | 11 | 10.1 | −5.3 | .22 | 10 | 10.0 | −3.8 | <.001 | 13 | 7.4 | −1.9 | .003 |
| Oral cavity and pharynx | 14 | 6.6 | 1.0 | <.001 | 15 | 5.3 | −0.8 | .002 | 13 | 5.2 | 3.5 | .14 | 17 | 6.2 | 0.6 | .65 | 18 | 4.3 | −1.7 | .18 | 14 | 6.7 | 0.9 | <.001 |
| Brain and other nervous system | 15 | 6.2 | −0.6 | .07 | 17 | 3.7 | 0.0 | .85 | 17 | 3.2 | 2.2 | .14 | 18 | 3.7 | −0.2 | .91 | 16 | 4.7 | −1.1 | <.001 | 15 | 5.9 | −0.5 | .11 |
| Myeloma | 16 | 4.9 | 2.4 | <.001 | 9 | 12.3 | 3.6 | <.001 | 16 | 3.2 | 0.7 | .24 | 16 | 6.3 | −1.2 | .34 | 14 | 5.6 | 0.4 | .11 | 16 | 5.6 | 2.1 | <.001 |
| Stomach | 17 | 4.1 | 0.7 | .36 | 13 | 8.1 | −1.2 | <.001 | 9 | 8.6 | −2.5 | <.001 | 14 | 6.7 | −1.4 | .15 | 12 | 8.0 | −1.6 | <.001 | 17 | 4.4 | −0.9 | <.001 |
| Liver and intrahepatic bile duct | 18 | 3.8 | 4.3 | <.001 | 16 | 5.1 | 3.7 | <.001 | 10 | 7.8 | −2.6 | .03 | 12 | 9.1 | 3.8 | .006 | 13 | 7.6 | 2.2 | <.001 | 18 | 3.9 | 3.6 | <.001 |
Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results areas reported by the North American Association of Central Cancer Registries as meeting high-quality incidence data standards for the specified time periods. AAPC = average annual percent change; AI/AN = American Indian/Alaska Native; APC = annual percent change; API = Asian/Pacific Islander; CHSDA = IHS Contract Health Services Delivery Area; IHS = Indian Health Service; NAACCR = North American Association of Central Cancer Registries; NOS = not otherwise specified; NPCR = National Program of Cancer Registries; SEER = Surveillance, Epidemiology, and End Results.
Cancers are sorted in descending order according to sex-specific rates for all races/ethnicities. More than 15 cancers may appear under males and females to include the top 15 cancers in every race/ethnicity group.
White, black, API, and AI/AN (CHSDA 2012 counties) include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive. AI/AN (CHSDA 2012) statistics exclude data from Kansas.
Rates are per 100 000 persons and were age-standardized to the 2000 US standard population (19 age groups Census P25–1130).
AAPC is the average annual percent change and is a weighted average of the annual percent change (APC) over the fixed interval 2009 to 2013 using the underlying joinpoint model for the period of 1999 to 2013. Joinpoint models with up to two joinpoints are based on rates per 100 000 persons that are age-standardized to the 2000 US standard population (19 age groups Census P25–1130). Joinpoint Regression Program, version 4.2.0.2. June 2015, Statistical Research and Applications Branch, National Cancer Institute.
Registries included in the incidence rates (2009–2013) and joinpoint models (1999–2013) for all races/ethnicities, white, black, AI/AN, API, Hispanic, and non-Hispanic (41 states): Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Texas, Utah, Vermont, Washington, West Virginia, Wisconsin, Wyoming.
For all sites, myelodysplastic syndromes are included for the rate calculations but not for the APC calculations; they are excluded from cancer-specific analysis. Ovary excludes borderline tumors.
AAPC two-sided P value based on t distribution if AAPC interval within one segment; otherwise, AAPC two-sided P value based on normal distribution.
Among men, incidence trends during 2009 to 2013 for all cancers combined and for the 20 most common cancers in each racial/ethnic group were generally similar to those of all races combined. Incidence rates decreased statistically significantly for all cancers combined and for the three most common cancers (prostate, lung, and colorectal) in each racial and ethnic group except AI/AN for lung and colorectal; rates also decreased for cancers of the stomach, esophagus, and larynx, except that rates were stable for stomach cancer in whites and for esophagus cancer and larynx cancer in AI/AN. In contrast, incidence rates increased in each racial and ethnic group for myeloma and for cancers of the pancreas and thyroid, except in AI/AN, in whom rates remained stable. Rates also increased in each racial and ethnic group for leukemia and liver cancer, except that rates remained unchanged for leukemia in API and AI/AN and for liver cancer in API and Hispanics.
Among women, overall cancer incidence rates from 2009 to 2013 increased in blacks, API, and AI/AN but remained stable in whites and Hispanics. Similarly, rates increased for breast cancer in blacks and API, whereas rates remained stable in whites, AI/AN, and Hispanic women. Rates also increased for thyroid, liver, and uterine cancers in all racial and ethnic groups, except that rates decreased for liver cancer in API women. In contrast, during 2009 to 2013, incidence rates decreased for lung cancer and colorectal cancer in all racial and ethnic groups, except that rates increased for lung cancer in API women. As with men, for most cancer sites, trends in incidence rates for women in each racial and ethnic group were similar in direction to those of all women combined.
Current Cancer Death Rates and Trends by Race, Ethnicity, and Sex
Average annual death rates and trends from 2010 to 2014 are presented by cancer site, sex, race, and ethnicity (Table 2). For all cancer sites combined, similar to incidence rates, death rates (per 100 000) were higher among men than women overall (200.4 vs 141.5) and in all racial and ethnic groups. Black men and black women had the highest cancer death rates of any racial or ethnic group. Among men, lung cancer was the leading cause of cancer death in all racial and ethnic groups, followed by prostate and colorectal in black, white, and Hispanic men, liver and colorectal in API men, and colorectal and prostate in AI/AN men. Among women, lung, breast, and colorectal cancer were the leading causes of cancer death in all racial and ethnic groups except Hispanics, in whom breast replaced lung cancer as the leading cause of cancer death.
Table 2.
US cancer death rates and fixed-interval trends from 2010 to 2014 for the most common cancers by sex, race, and ethnicity*
| Sex/cancer site or type† | White‡ |
Black‡ |
API‡ |
AI/AN (CHSDA)‡ |
Hispanic‡ |
Non-Hispanic‡ |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Rate§ | AAPC‖ | P¶ | Rank | Rate§ | AAPC‖ | P¶ | Rank | Rate§ | AAPC‖ | P¶ | Rank | Rate§ | AAPC‖ | P¶ | Rank | Rate§ | AAPC‖ | P¶ | Rank | Rate§ | AAPC‖ | P¶ | |
| All sites | ||||||||||||||||||||||||
| Both sexes | 166.2 | −1.4 | <.001 | 194.1 | −2.1 | <.001 | 102.9 | −1.3 | <.001 | 152.2 | −0.8 | .001 | 116.2 | −1.3 | <.001 | 170.3 | −1.5 | <.001 | ||||||
| Males | 199.7 | −1.7 | <.001 | 247.2 | −2.7 | <.001 | 122.7 | −1.6 | <.001 | 183.6 | −0.7 | .03 | 142.5 | −1.6 | <.001 | 205.1 | −1.7 | <.001 | ||||||
| Females | 141.8 | −1.3 | <.001 | 161.7 | −1.6 | <.001 | 88.8 | −1.0 | <.001 | 129.1 | −1.1 | <.001 | 97.7 | −1.0 | <.001 | 145.3 | −1.3 | <.001 | ||||||
| Males | ||||||||||||||||||||||||
| Lung and bronchus | 1 | 55.8 | −3.4 | <.001 | 1 | 68.0 | −3.4 | <.001 | 1 | 31.7 | −3.1 | <.001 | 1 | 46.2 | −0.9 | .06 | 1 | 27.3 | −3.0 | <.001 | 1 | 58.3 | −3.4 | <.001 |
| Prostate | 2 | 18.6 | −3.3 | <.001 | 2 | 41.9 | −4.2 | <.001 | 4 | 8.8 | −2.9 | <.001 | 3 | 19.4 | −1.9 | .007 | 2 | 16.5 | −3.0 | <.001 | 2 | 20.3 | −3.4 | <.001 |
| Colon and rectum | 3 | 17.2 | −2.5 | <.001 | 3 | 25.3 | −2.7 | <.001 | 3 | 12.4 | −2.1 | <.001 | 2 | 19.5 | −0.9 | .21 | 3 | 15.0 | −1.5 | <.001 | 3 | 17.9 | −2.5 | <.001 |
| Pancreas | 4 | 12.6 | 0.4 | <.001 | 4 | 15.0 | −0.4 | .01 | 5 | 8.2 | 0.1 | .73 | 5 | 9.8 | −1.6 | .33 | 5 | 9.5 | 0.1 | .79 | 4 | 12.8 | 0.3 | <.001 |
| Liver and intrahepatic bile duct | 6 | 8.5 | 2.7 | <.001 | 5 | 13.0 | 2.6 | <.001 | 2 | 14.3 | −0.9 | <.001 | 4 | 14.9 | 2.8 | .002 | 4 | 13.1 | 1.6 | <.001 | 6 | 8.9 | 2.6 | <.001 |
| Leukemia | 5 | 9.5 | −0.9 | <.001 | 8 | 7.5 | −1.5 | <.001 | 7 | 5.0 | −0.4 | .44 | 8 | 5.8 | −10.0 | .09 | 8 | 6.1 | −0.7 | .02 | 5 | 9.3 | −1.0 | <.001 |
| Urinary bladder | 7 | 8.1 | 0.1 | .19 | 12 | 5.4 | −0.2 | .47 | 10 | 2.9 | −0.1 | .85 | 12 | 3.6 | # | 11 | 3.9 | −0.8 | .08 | 7 | 7.9 | 0.1 | .09 | |
| Non-Hodgkin lymphoma | 8 | 7.9 | −1.9 | <.001 | 10 | 5.5 | −1.8 | <.001 | 8 | 4.9 | −1.7 | <.001 | 9 | 5.6 | −1.3 | .22 | 7 | 6.2 | −0.8 | .007 | 8 | 7.7 | −2.0 | <.001 |
| Esophagus | 9 | 7.6 | −0.6 | <.001 | 9 | 6.3 | −4.8 | <.001 | 11 | 2.9 | −0.8 | .23 | 10 | 5.6 | −1.5 | .20 | 10 | 4.0 | −0.9 | .04 | 9 | 7.6 | −1.0 | <.001 |
| Kidney and renal pelvis | 10 | 5.8 | −0.4 | .01 | 11 | 5.5 | −1.0 | .001 | 12 | 2.7 | 0.7 | .34 | 6 | 8.9 | −0.9 | .31 | 9 | 4.9 | −1.1 | .005 | 10 | 5.7 | −0.7 | <.001 |
| Brain and other nervous system | 11 | 5.7 | 0.6 | .02 | 15 | 3.2 | 0.0 | .99 | 13 | 2.4 | −0.4 | .53 | 14 | 3.0 | 1.4 | .31 | 13 | 3.4 | −0.1 | .82 | 11 | 5.5 | 0.6 | .01 |
| Stomach | 14 | 3.8 | −1.9 | .001 | 6 | 8.6 | −3.2 | <.001 | 6 | 7.1 | −4.1 | <.001 | 7 | 7.5 | −2.7 | .06 | 6 | 6.9 | −2.9 | <.001 | 14 | 4.1 | −2.2 | <.001 |
| Myeloma | 13 | 4.0 | −0.9 | <.001 | 7 | 7.5 | 0.1 | .90 | 14 | 2.1 | −5.0 | .11 | 13 | 3.3 | −2.1 | .08 | 12 | 3.4 | −1.2 | .01 | 13 | 4.3 | −0.9 | <.001 |
| Melanoma of the skin | 12 | 4.6 | −1.1 | .03 | 22 | 0.5 | −0.8 | .39 | 20 | 0.4 | # | # | 17 | 1.4 | # | # | 17 | 1.0 | 0.2 | .75 | 12 | 4.3 | −1.2 | .02 |
| Oral cavity and pharynx | 15 | 3.8 | 1.4 | .06 | 13 | 5.0 | −3.0 | <.001 | 9 | 2.9 | −1.6 | .003 | 11 | 3.7 | −0.8 | .52 | 14 | 2.4 | −0.9 | .02 | 15 | 4.0 | 1.2 | .08 |
| Larynx | 17 | 1.8 | −2.3 | <.001 | 14 | 3.4 | −3.7 | <.001 | 16 | 0.7 | −1.8 | .15 | 15 | 1.7 | # | 15 | 1.6 | −2.3 | <.001 | 16 | 1.9 | −2.5 | <.001 | |
| Females | ||||||||||||||||||||||||
| Lung and bronchus | 1 | 37.5 | −1.9 | <.001 | 1 | 34.6 | −2.0 | <.001 | 1 | 18.0 | −0.4 | .02 | 1 | 30.8 | −1.4 | .003 | 2 | 13.4 | −1.0 | <.001 | 1 | 38.3 | −1.9 | <.001 |
| Breast | 2 | 20.6 | −1.6 | <.001 | 2 | 29.2 | −1.5 | <.001 | 2 | 11.3 | −1.0 | <.001 | 2 | 14.1 | −3.7 | .002 | 1 | 14.4 | 0.5 | .46 | 2 | 21.8 | −1.6 | <.001 |
| Colon and rectum | 3 | 12.1 | −1.9 | .005 | 3 | 16.5 | −3.3 | <.001 | 3 | 8.8 | −5.1 | .07 | 3 | 14.0 | −0.4 | .62 | 3 | 9.2 | −2.1 | <.001 | 3 | 12.7 | −2.8 | <.001 |
| Pancreas | 4 | 9.4 | −0.1 | .70 | 4 | 12.1 | −0.3 | .01 | 4 | 7.3 | 0.3 | .17 | 4 | 8.1 | 0.1 | .90 | 4 | 7.7 | 0.0 | .82 | 4 | 9.7 | −0.1 | .51 |
| Ovary | 5 | 7.7 | −2.4 | <.001 | 6 | 6.4 | −1.4 | <.001 | 6 | 4.4 | −1.1 | .002 | 6 | 6.2 | −1.9 | .09 | 6 | 5.4 | −1.3 | <.001 | 5 | 7.5 | −2.3 | <.001 |
| Leukemia | 6 | 5.3 | −1.1 | <.001 | 8 | 4.6 | −1.5 | <.001 | 9 | 3.0 | −3.9 | .27 | 10 | 3.4 | −2.1 | .12 | 9 | 4.0 | −0.4 | .08 | 6 | 5.1 | −1.2 | <.001 |
| Non-Hodgkin lymphoma | 7 | 4.8 | −2.2 | <.001 | 12 | 3.4 | −0.5 | .52 | 8 | 3.2 | −2.0 | <.001 | 11 | 3.2 | −4.6 | <.001 | 7 | 4.1 | −1.9 | <.001 | 8 | 4.6 | −2.1 | <.001 |
| Corpus and uterus, NOS | 8 | 4.2 | 1.8 | <.001 | 5 | 8.1 | 2.8 | <.001 | 10 | 2.9 | 1.9 | <.001 | 8 | 3.8 | # | 10 | 3.6 | 1.4 | .001 | 7 | 4.6 | 2.3 | <.001 | |
| Liver and intrahepatic bile duct | 10 | 3.5 | 2.2 | <.001 | 9 | 4.5 | 1.7 | <.001 | 5 | 6.1 | −1.0 | .04 | 5 | 6.8 | 0.6 | .65 | 5 | 5.8 | 1.3 | <.001 | 10 | 3.6 | 2.9 | <.001 |
| Brain and other nervous system | 9 | 3.8 | −0.1 | .57 | 15 | 2.1 | 0.0 | .98 | 11 | 1.8 | 1.4 | .04 | 14 | 2.0 | # | 12 | 2.4 | 0.1 | .80 | 9 | 3.6 | 0.6 | .05 | |
| Myeloma | 12 | 2.4 | −0.2 | .52 | 7 | 5.5 | 2.1 | .09 | 13 | 1.3 | −1.8 | .04 | 13 | 2.7 | −1.6 | .36 | 13 | 2.3 | −1.4 | .002 | 11 | 2.7 | −0.1 | .95 |
| Kidney and renal pelvis | 11 | 2.5 | −1.2 | <.001 | 14 | 2.4 | −1.5 | <.001 | 14 | 1.1 | −0.6 | .39 | 7 | 4.2 | −0.2 | .85 | 14 | 2.3 | −0.3 | .51 | 12 | 2.4 | −1.4 | <.001 |
| Stomach | 15 | 2.0 | −2.5 | <.001 | 10 | 4.1 | −3.6 | <.001 | 7 | 4.3 | −3.8 | <.001 | 9 | 3.8 | −3.6 | .003 | 8 | 4.1 | −2.3 | <.001 | 15 | 2.1 | −2.9 | <.001 |
| Cervix uteri | 14 | 2.1 | −0.4 | .05 | 11 | 3.8 | −2.5 | <.001 | 12 | 1.7 | −3.2 | <.001 | 12 | 2.8 | −2.9 | .03 | 11 | 2.6 | −2.1 | <.001 | 13 | 2.3 | −0.6 | .02 |
| Urinary bladder | 13 | 2.2 | −0.4 | .003 | 13 | 2.5 | −1.4 | <.001 | 16 | 0.9 | −0.6 | .43 | 17 | 1.4 | # | # | 15 | 1.2 | −1.1 | .05 | 14 | 2.2 | −0.4 | .002 |
| Melanoma of the skin | 16 | 1.9 | −0.4 | .02 | 24 | 0.4 | −1.7 | .02 | 22 | 0.3 | 1.2 | .49 | 20 | 0.5 | # | # | 21 | 0.6 | −0.5 | .49 | 16 | 1.8 | −0.5 | .02 |
| Esophagus | 17 | 1.5 | −1.1 | <.001 | 16 | 1.9 | −4.5 | <.001 | 19 | 0.7 | −1.8 | .06 | 16 | 1.7 | # | # | 19 | 0.8 | −2.4 | <.001 | 17 | 1.6 | −1.5 | <.001 |
| Oral cavity and pharynx | 18 | 1.3 | −1.3 | <.001 | 18 | 1.3 | −2.4 | <.001 | 15 | 1.1 | −2.3 | .006 | 18 | 1.1 | # | # | 18 | 0.8 | −0.7 | .26 | 18 | 1.4 | −1.4 | <.001 |
Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results areas reported by the North American Association of Central Cancer Registries as meeting high-quality incidence data standards for the specified time periods.
Source: National Center for Health Statistics public-use data file for the total United States, 1975–2014. AAPC = average annual percent change; AI/AN = American Indian/Alaska Native; APC = annual percent change; API = Asian/Pacific Islander; CHSDA = IHS Contract Health Services Delivery Area; IHS = Indian Health Service; NAACCR = North American Association of Central Cancer Registries; NOS = not otherwise specified; NPCR = National Program of Cancer Registries; SEER = Surveillance, Epidemiology, and End Results.
Cancers are sorted in descending order according to sex-specific rates for all races/ethnicities. More than 15 cancers may appear under males and females to include the top 15 cancers in every race/ethnicity group.
White, black, API, and AI/AN (CHSDA 2012 counties) include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
Rates are per 100 000 persons and are age-standardized to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, 80–84 years, 85 years; Census publication p. 25–1130; US Bureau of the Census, Current Population Reports, p. 25–1130. Washington, DC: US Government Printing Office; 2000).
AAPC is the average annual percent change and is a weighted average of the annual percent change (APC) over the fixed interval 2010 to 2014 using the underlying joinpoint model for the period of 2000 to 2014. Joinpoint models with up to two joinpoints are based on rates per 100 000 persons that are age-standardized to the 2000 US standard population (19 age groups Census P25–1130). Joinpoint Regression Program, version 4.2.0.2. June 2015, Statistical Research and Applications Branch, National Cancer Institute.
AAPC two-sided P value based on t distribution if AAPC interval within one segment; otherwise, AAPC two-sided P value based on normal distribution.
The statistic could not be calculated. The average annual percent change is based on fewer than 10 cases for at least one year within the time interval.
From 2010 to 2014, death rates declined overall and for the most common cancers (lung, prostate, colorectal, breast) among men and women of all racial and ethnic groups, except for breast cancer among Hispanic women, lung cancer and colorectal cancer among AI/AN men, and colorectal cancer among API and AI/AN women (Table 2). Death rates for most of the other cancer sites declined or were stable among men and women in each racial and ethnic group; exceptions to these patterns were increases for liver cancer in white, black, and Hispanic men and women and AI/AN men, for pancreatic cancer in white men, and for uterine cancer in white, black, API, and Hispanic women.
Cancer Incidence and Mortality Among Children
In children age 0 to 14 years, average annual age-standardized incidence rates (per 100 000) during 2009 to 2013 ranged from 11.5 in AI/AN to 17.1 in whites (Table 3). Average annual age-standardized death rates during 2010 to 2014 ranged from 1.8 in API (95% CI = 1.6 to 2.0) and AI/AN (95% CI = 1.4 to 2.3) to 2.2 (95% CI = 2.1 to 2.3) in whites. Incidence rates during 2009 to 2013 increased statistically significantly on average by 0.4% (95% CI = 0.1 to 0.7) to 1.0% (95% CI = 0.3 to 1.6) per year in each racial and ethnic group, except in AI/AN, in whom rates remained stable. In contrast, death rates during 2010 to 2014 statistically significantly decreased on average by 1.6% (95% CI = –2.0 to –1.3) per year in all race/ethnicities combined and by 1.5% (95% CI = –1.9 to –1.2) to 2.6% (95% CI = –4.5 to –0.7) per year in each racial and ethnic group; the average annual percent change for AI/AN could not be calculated because of sparse data (Table 3).
Table 3.
Delay-adjusted childhood cancer incidence rates for areas with high-quality data* and US childhood cancer death rates by sex, race, and ethnicity, and their fixed-interval trends
| Race/ethnicity† | Children (age 0–14 years) |
|||||
|---|---|---|---|---|---|---|
| Incidence (2009–2013) |
Mortality (2010–2014) |
|||||
| Rate§ | AAPC‖ | P¶ | Rate§ | AAPC‖ | P¶ | |
| All races/ethnicities | 16.5 | 0.8 | <.001 | 2.1 | −1.6 | <.001 |
| White | 17.1 | 0.7 | <.001 | 2.2 | −1.5 | <.001 |
| Black | 12.9 | 1.5 | <.001 | 2.0 | −1.6 | <.001 |
| API | 13.6 | 1.0 | .01 | 1.8 | −2.6 | .01 |
| AI/AN (CHSDA)‡ | 11.5 | −0.7 | .33 | 1.8 | # | # |
| Hispanic | 16.1 | 0.4 | .03 | 2.1 | −2.0 | <.001 |
| Non-Hispanic | 16.6 | 1.0 | <.001 | 2.1 | −1.6 | <.001 |
Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results areas reported by the North American Association of Central Cancer Registries as meeting high-quality incidence data standards for the specified time periods. AAPC = average annual percent change; AI/AN = American Indian/Alaska Native; APC = annual percent change; API = Asian/Pacific Islander; CHSDA = IHS Contract Health Services Delivery Area; IHS = Indian Health Service; NAACCR = North American Association of Central Cancer Registries; NOS = not otherwise specified; NPCR = National Program of Cancer Registries; SEER = Surveillance, Epidemiology, and End Results.
White, black, API, and AI/AN (CHSDA 2012 counties) include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
For incidence, AI/AN (CHSDA 2012) statistics exclude data from Kansas.
Rates are per 100 000 persons and were age-standardized to the 2000 US standard population (19 age groups Census P25–1130).
AAPC is the average annual percent change and is a weighted average of the annual percent change (APC) over the fixed interval (2009–2013 for incidence; 2010–2014 for mortality) using the underlying joinpoint model for the period of 1999-2013 for incidence and the period of 2000-2014 for mortality. Joinpoint models with up to two joinpoints were based on rates per 100 000 persons that were age-standardized to the 2000 US standard population (19 age groups Census P25–1130). Joinpoint Regression Program, version 4.2.0.2. June 2015, Statistical Research and Applications Branch, National Cancer Institute. Registries included in the incidence rates (2009–2013) and joinpoint models (1999–2013) for all races/ethnicities, white, black, AI/AN, API, Hispanic, and non-Hispanic (41 states): Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Texas, Utah, Vermont, Washington, West Virginia, Wisconsin, Wyoming.
AAPC two-sided P value based on t distribution if AAPC interval within one segment; otherwise, AAPC two-sided P value based on normal distribution.
The statistic could not be calculated. The average annual percent change is based on fewer than 10 cases for at least one year within the time interval.
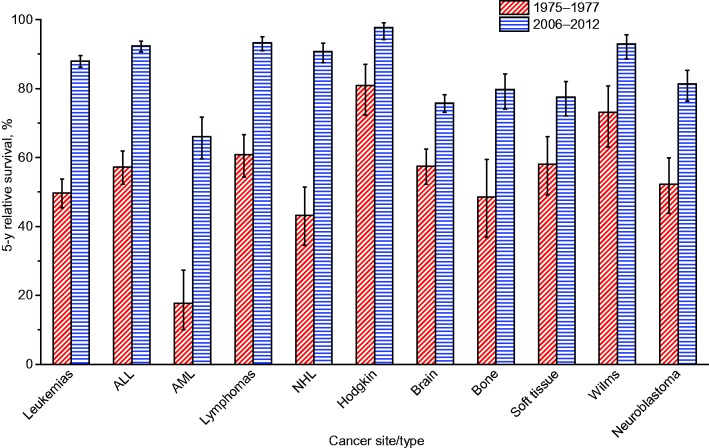
Survival Ratios and Trends
Table 4 shows changes in relative survival for all cancer sites combined (case-mix adjusted) and for 20 specific sites based on cases diagnosed in 1975 to 1977 and 2006 to 2012. When comparing cases diagnosed during these two time periods, survival increased statistically significantly in the later-diagnosed group for all but two cancer types, cervix and uterus, with the greatest absolute changes (25% or greater) observed for prostate, kidney, NHL, myeloma, and leukemia and the greatest proportional changes (100% or greater) observed for esophagus, stomach, pancreas, liver, and myeloma. Cancers with the lowest five-year relative survival rates for cases diagnosed in 2006 to 2012 were pancreas (8.5%, 95% CI = 8.0% to 9.0%), liver (18.1%, 95% CI = 17.3% to 18.9%), lung (18.7%, 95% CI = 18.4% to 19.1%), esophagus (20.5%, 95% CI = 19.4% to 21.7%), stomach (31.1%, 95% CI = 30.1% to 32.2%), and brain (35%, 95% CI = 34.0% to 36.0%); those with the highest were prostate (99.3%, 95% CI = 99.1% to 99.5%), thyroid (98.3%, 95% CI = 97.9% to 98.6%), melanoma (93.2%, 95% CI = 92.6% to 93.6%), and female breast (90.8%, 95% CI = 90.5% to 91.1%).
Table 4.
Changes in 5-year relative survival (%) for the most common cancers, all stages, all ages, SEER 9*, 1975–2012
| Cancer site | 5-y relative survival (95% CI) |
Change over time (95% CI) |
||
|---|---|---|---|---|
| 1975–1977 | 2006–2012 | Absolute, % | Proportional, % | |
| All sites (case-mix adjusted) | 50.3 (50.1 to 50.6) | 66.4 (66.2 to 66.5) | 16.0 (15.7 to 16.3) | 31.9 (31.1 to 32.6) |
| Lung and bronchus | 12.2 (11.8 to 12.6) | 18.7 (18.4 to 19.1) | 6.5 (6.0 to 7.1) | 53.6 (47.5 to 59.7) |
| Colon and rectum | 49.8 (49.1 to 50.6) | 66.2 (65.7 to 66.7) | 16.4 (15.5 to 17.3) | 32.9 (30.7 to 35.1) |
| Breast (female) | 74.8 (74.2 to 75.5) | 90.8 (90.5 to 91.1) | 16.0 (15.3 to 16.7) | 21.4 (20.3 to 22.5) |
| Prostate | 67.8 (66.7 to 68.9) | 99.3 (99.1 to 99.5) | 31.5 (30.4 to 32.6) | 46.5 (44.2 to 48.9) |
| Oral cavity and pharynx | 52.5 (51.1 to 54.0) | 67.0 (66.1 to 67.9) | 14.4 (12.7 to 16.1) | 27.4 (23.5 to 31.4) |
| Esophagus | 5.0 (4.0 to 6.2) | 20.5 (19.4 to 21.7) | 15.5 (13.9 to 17.1) | 308.1 (217.6 to 398.6) |
| Stomach | 15.2 (14.1 to 16.3) | 31.1 (30.1 to 32.2) | 15.9 (14.4 to 17.4) | 104.7 (88.2 to 121.1) |
| Pancreas | 2.5 (2.0 to 3.0) | 8.5 (8.0 to 9.0) | 6.0 (5.3 to 6.7) | 244.7 (175.9 to 313.5) |
| Liver and intrahepatic bile duct | 3.4 (2.4 to 4.7) | 18.1 (17.3 to 18.9) | 14.6 (13.3 to 16.0) | 427.6 (251.4 to 603.9) |
| Urinary bladder | 72.3 (70.9 to 73.6) | 78.5 (77.7 to 79.2) | 6.2 (4.6 to 7.7) | 8.5 (6.3 to 10.8) |
| Kidney and renal pelvis | 50.1 (48.1 to 52.0) | 74.7 (73.9 to 75.4) | 24.6 (22.6 to 26.7) | 49.2 (43.3 to 55.1) |
| Melanoma of the skin | 81.9 (80.4 to 83.3) | 93.2 (92.6 to 93.6) | 11.3 (9.8 to 12.8) | 13.8 (11.7 to 15.8) |
| Cervix uteri | 69.1 (67.4 to 70.7) | 68.8 (67.4 to 70.2) | −0.3 (−2.4 to 1.8) | −0.4 (−3.5 to 2.7) |
| Corpus and uterus, NOS | 86.9 (86.0 to 87.7) | 83.4 (82.7 to 84.0) | −3.5 (−4.6 to −2.4) | −4.0 (−5.3 to −2.8) |
| Ovary | 36.0 (34.5 to 37.6) | 46.4 (45.3 to 47.6) | 10.4 (8.5 to 12.3) | 28.9 (22.5 to 35.3) |
| Non-Hodgkin lymphoma | 46.5 (45.0 to 48.0) | 72.6 (71.9 to 73.2) | 26.1 (24.4 to 27.7) | 56.1 (50.8 to 61.3) |
| Myeloma | 24.6 (22.6 to 26.6) | 50.2 (48.9 to 51.6) | 25.7 (23.3 to 28.1) | 104.5 (87.0 to 122.0) |
| Leukemia | 34.2 (32.8 to 35.5) | 62.7 (61.8 to 63.5) | 28.5 (26.9 to 30.1) | 83.6 (75.9 to 91.2) |
| Brain and other nervous system | 22.4 (21.0 to 23.9) | 35.0 (34.0 to 36.0) | 12.5 (10.8 to 14.3) | 55.9 (44.6 to 67.1) |
| Thyroid | 92.1 (90.7 to 93.3) | 98.3 (97.9 to 98.6) | 6.1 (4.8 to 7.4) | 6.6 (5.1 to 8.2) |
The Surveillance, Epidemiology, and End Results 9 registries are Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah. CI = confidence interval; NOS = not otherwise specified; SEER = Surveillance, Epidemiology, and End Results.
When comparing cases diagnosed in 1975 to 1977 vs 2006 to 2012, survival improved substantially in the later-diagnosed group for both early (localized) and late-stage (regional, distant) diseases for most cancer types, including liver, esophagus, colorectal, female breast, and NHL (Supplementary Table 3, available online). Between 1975 to 1977 and 2006 to 2012, cancer types and stages that demonstrated a large absolute gain (20% or greater) in survival included NHL for distant stage, esophagus for localized and regional stages, oral cavity for regional and distant stages, pancreas and liver cancers for local stage, and female breast and colorectal for regional stage. Although improvements in survival for distant-stage disease over the past 30 years generally appeared to be small in absolute terms (<10% absolute gain), they were large in proportionate terms, with survival rates doubling for several cancers (Supplementary Table 3, available online). Between 1975 to 1977 and 2006 to 2012, for example, five-year relative survival for distant-stage disease increased from 5.5% (95% CI = 4.9% to 6.2%) to 13.7% (95% CI = 13.0% to 14.4%) for colorectal cancer and from 18.7% (95% CI = 16.9% to 20.6%) to 33.6% (95% CI = 32.2% to 35.0%) for female breast cancer. However, survival for many distant-stage cancers remained unchanged, for example, liver cancer (1.1%, 95% CI = 0.3% to 2.9%, in 1975–1977 and 2.3%, 95% CI = 1.6% to 3.2%, in 2006–2012).
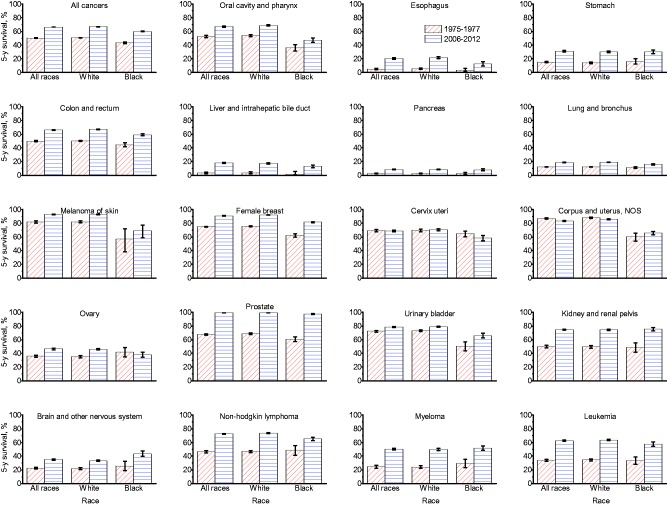
Figure 4 depicts changes in survival ratios from 1975 to 1977 and 2006 to 2012 by race for all cancer sites combined and for the 20 most common cancers. Survival improved substantially over this time period for both whites and blacks overall (all sites) and for almost all cancer types; however, survival decreased for uterine cancer in whites and cervix and ovary in blacks. The largest absolute increases were observed for prostate cancer (36.7% in blacks and 31.1% in whites), leukemia (29.0% in whites and 24.1% in blacks), NHL (26.8% in whites and 16.5% in blacks), kidney (26.6% in blacks and 24.7% in whites), and myeloma (25.7% in whites and 22.5% in blacks) (Supplementary Table 4, available online). For lung and pancreas cancers, improvements were very limited in both whites and blacks. For all sites combined and for most individual cancer types, statistically significant racial disparities (black vs white) in survival rates in 1975 to 1977 persisted in 2006 to 2012. The magnitude of the disparity between the two time periods remained similar for most cancer types, but it widened for cancers of the esophagus, lung and bronchus, ovary, cervix uteri, and NH, while it narrowed for cancers of the urinary bladder, prostate, and corpus and uterus (P < .05 for all) (Supplementary Tables 4 and 5, available online).
Figure 4.
Changes in five-year relative survival by cancer site and race, all ages, SEER 9*, 1975–2012. Error bars represent 95% confidence intervals. *The SEER 9 registries are Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah. NOS = not otherwise specified; SEER = Surveillance, Epidemiology, and End Results.
Table 5 shows five-year cause-specific survival and adjusted relative risk of cancer death by race/ethnicity in persons diagnosed in 2006 to 2012 for all cancer sites combined and for the 20 most common cancers. The five-year survival for all sites combined was highest for Hispanics (68.2%, 95% CI = 67.9% to 68.4%) and NHW (68.0%, 95% CI = 67.9% to 68.1%), followed by NHAPI (66.7%, 95% CI = 66.4% to 67.0%), NHB (62.8%, 95% CI = 62.6% to 63.0%), and NHAI/AN (60.5%, 95% CI = 59.5% to 61.5%). The adjusted relative risk of death after a diagnosis of cancer (HR) was statistically significantly higher in NHB than NHW for all cancers combined (HR = 1.33, 95% CI = 1.32 to 1.34) and for most cancer sites, with the excess risk most notable for female breast, oral cavity, and uterine cancers and non-Hodgkin lymphoma. Compared with NHW, Hispanics had statistically significantly higher risk of death for 10 of the 20 cancers, most notably for leukemia, but they have lower risk of death after diagnosis for lung and cervical cancers. Although NHAPI had higher adjusted risk of death than whites for all cancers combined, they had lower risk for 12 of the 20 cancers. However, NHAPI had higher risk of death for oral cavity cancer and for melanoma, NHL, and leukemia. NHAI/AN had a higher risk of death than NHW for all cancers combined (HR = 1.51, 95% CI = 1.46 to 1.56) and for 11 of the 20 cancers, most notably for leukemia and thyroid cancer.
Table 5.
Five-year cause-specific survival (%) and adjusted relative risk of cancer death by race/ethnicity, SEER 18*, 2006–2012
| Cancer site | 5-year cause-specific survival (95% CI) |
Adjusted relative risk† (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NHW | NHB | Hispanics | NHAPI | NHAI/AN | NHW | NHB | Hispanics | NHAPI | NHAI/AN | |
| All sites | 68.0 (67.9 to 68.1) | 62.8 (62.6 to 63.0) | 68.2 (67.9 to 68.4) | 66.7 (66.4 to 67.0) | 60.5 (59.5 to 61.5) | 1.00 | 1.33 (1.32 to 1.34) | 1.16 (1.16 to 1.17) | 1.10 (1.09 to 1.11) | 1.51 (1.46 to 1.56) |
| Lung and bronchus | 20.4 (20.2 to 20.6) | 17.2 (16.6 to 17.7) | 19.2 (18.4 to 20.0) | 22.2 (21.4 to 23.0) | 15.9 (13.6 to 18.5) | 1.00 | 1.04 (1.03 to 1.06) | 0.95 (0.93 0.97) | 0.75 (0.73 0.76) | 1.11 (1.04 to 1.18) |
| Colon and rectum | 65.9 (65.6 to 66.2) | 59.9 (59.2 to 60.7) | 66.0 (65.2 to 66.7) | 69.1 (68.3 to 69.9) | 60.0 (56.8 to 63.0) | 1.00 | 1.22 (1.19 to 1.25) | 1.02 (1.00 to 1.05) | 0.89 (0.86 0.92) | 1.28 (1.17 to 1.40) |
| Breast (female) | 89.2 (89.0 to 89.3) | 80.3 (79.8 to 80.8) | 87.8 (87.4 to 88.3) | 91.6 (91.2 to 92.0) | 85.8 (83.6 to 87.7) | 1.00 | 1.71 (1.66 to 1.76) | 1.14 (1.10 to 1.18) | 0.84 (0.80 0.88) | 1.28 (1.11 to 1.47) |
| Prostate | 94.4 (94.3 to 94.5) | 93.0 (92.7 to 93.3) | 93.3 (93.0 to 93.6) | 94.5 (94.0 to 94.9) | 89.6 (87.4 to 91.4) | 1.00 | 1.36 (1.31 to 1.42) | 1.02 (0.97 to 1.07) | 0.72 (0.67 0.78) | 1.31 (1.08 to 1.58) |
| Oral cavity and pharynx | 69.7 (69.2 to 70.2) | 51.1 (49.5 to 52.7) | 64.8 (63.1 to 66.5) | 69.3 (67.5 to 71.0) | 63.4 (57.0 to 69.0) | 1.00 | 1.81 (1.68 to 1.95) | 1.25 (1.14 to 1.37) | 1.14 (1.02 to 1.27) | 1.41 (1.02 to 1.90) |
| Esophagus | 21.8 (21.1 to 22.6) | 14.8 (13.0 to 16.7) | 19.7 (17.3 to 22.3) | 20.0 (16.8 to 23.3) | 20.6 (13.3 to 29.1) | 1.00 | 1.34 (1.27 to 1.41) | 1.10 (1.03 to 1.17) | 0.93 (0.86 to 1.01) | 1.20 (0.96 to 1.48) |
| Stomach | 30.8 (30.0 to 31.6) | 32.3 (30.7 to 33.8) | 30.8 (29.4 to 32.1) | 40.3 (38.7 to 41.8) | 22.7 (16.9 to 29.1) | 1.00 | 1.04 (1.00 to 1.09) | 1.01 (0.97 to 1.05) | 0.81 (0.77 0.84) | 1.35 (1.17 to 1.54) |
| Pancreas | 7.9 (7.6 to 8.2) | 8.4 (7.6 to 9.2) | 9.2 (8.2 to 10.2) | 9.7 (8.6 to 10.9) | 8.2 (5.2 to 11.9) | 1.00 | 1.13 (1.10 to 1.16) | 1.04 (1.01 to 1.07) | 0.93 (0.90 0.96) | 1.15 (1.02 to 1.29) |
| Liver and intrahepatic bile duct | 20.1 (19.4 to 20.8) | 16.3 (15.0 to 17.7) | 20.7 (19.6 to 21.9) | 27.2 (25.9 to 28.5) | 17.5 (13.4 to 22.2) | 1.00 | 1.17 (1.13 to 1.21) | 1.02 (0.99 to 1.05) | 0.81 (0.78 0.84) | 1.06 (0.95 to 1.18) |
| Kidney and renal pelvis | 74.9 (74.5 to 75.4) | 77.1 (76.1 to 78.2) | 76.6 (75.6 to 77.5) | 73.6 (71.9 to 75.2) | 72.1 (68.0 to 75.7) | 1.00 | 1.24 (1.17 to 1.30) | 1.02 (0.97 to 1.07) | 0.91 (0.84 0.97) | 1.06 (0.90 to 1.23) |
| Urinary bladder | 79.0 (78.7 to 79.3) | 67.0 (65.4 to 68.6) | 77.3 (76.0 to 78.6) | 78.9 (77.2 to 80.4) | 68.1 (60.7 to 74.4) | 1.00 | 1.42 (1.34 to 1.50) | 1.03 (0.97 to 1.10) | 0.85 (0.78 0.92) | 1.24 (0.97 to 1.55) |
| Melanoma of the skin | 89.6 (89.4 to 89.9) | 67.1 (61.9 to 71.8) | 84.6 (83.0 to 86.0) | 75.1 (70.6 to 79.1) | 84.8 (78.1 to 89.7) | 1.00 | 1.28 (1.07 to 1.52) | 1.21 (1.09 to 1.34) | 1.35 (1.12 to 1.61) | 1.28 (0.85 to 1.84) |
| Ovary | 45.4 (44.7 to 46.2) | 37.9 (35.7 to 40.1) | 53.4 (51.5 to 55.2) | 57.6 (55.3 to 59.9) | 44.9 (36.7 to 52.8) | 1.00 | 1.41 (1.34 to 1.49) | 1.11 (1.05 to 1.17) | 0.99 (0.92 to 1.06) | 1.06 (0.87 to 1.28) |
| Corpus and uterus NOS | 83.7 (83.4 to 84.1) | 64.3 (63.0 to 65.7) | 81.6 (80.6 to 82.6) | 83.8 (82.7 to 84.9) | 82.6 (78.0 to 86.3) | 1.00 | 1.78 (1.69 to 1.87) | 1.16 (1.09 to 1.23) | 1.05 (0.97 to 1.13) | 1.42 (1.11 to 1.79) |
| Cervix uteri | 70.0 (69.0 to 70.9) | 60.1 (58.0 to 62.0) | 74.6 (73.1 to 76.0) | 72.0 (69.6 to 74.2) | 64.7 (55.9 to 72.2) | 1.00 | 1.29 (1.20 to 1.38) | 0.87 (0.81 0.93) | 0.82 (0.74 0.90) | 1.36 (1.03 to 1.77) |
| Myeloma | 52.3 (51.4 to 53.3) | 55.8 (54.1 to 57.5) | 52.8 (50.5 to 55.0) | 53.9 (50.7 to 57.0) | 56.8 (45.0 to 67.1) | 1.00 | 1.03 (0.98 to 1.08) | 1.15 (1.08 to 1.22) | 0.98 (0.90 to 1.06) | 0.88 (0.65 to 1.16) |
| Non-Hodgkin lymphoma | 72.6 (72.2 to 73.0) | 66.7 (65.4 to 67.8) | 70.4 (69.5 to 71.4) | 69.9 (68.6 to 71.2) | 72.3 (67.5 to 76.6) | 1.00 | 1.74 (1.66 to 1.82) | 1.42 (1.36 to 1.47) | 1.27 (1.21 to 1.34) | 1.25 (1.04 to 1.50) |
| Leukemia | 63.3 (62.8 to 63.8) | 59.2 (57.6 to 60.8) | 63.5 (62.3 to 64.7) | 55.4 (53.5 to 57.3) | 62.6 (56.6 to 68.0) | 1.00 | 1.38 (1.31 to 1.45) | 1.63 (1.56 to 1.70) | 1.67 (1.58 to 1.76) | 1.52 (1.26 to 1.82) |
| Brain and other nervous system | 32.2 (31.5 to 32.8) | 42.4 (40.1 to 44.6) | 46.9 (45.2 to 48.5) | 42.3 (39.6 to 45.0) | 42.1 (33.8 to 50.1) | 1.00 | 1.00 (0.94 to 1.06) | 0.97 (0.93 to 1.02) | 0.80 (0.75 0.86) | 0.94 (0.76 to 1.15) |
| Thyroid | 97.3 (97.1 to 97.4) | 96.3 (95.6 to 96.9) | 97.4 (97.0 to 97.7) | 96.6 (96.0 to 97.0) | 96.1 (93.2 to 97.8) | 1.00 | 1.33 (1.12 to 1.58) | 0.89 (0.77 to 1.02) | 0.86 (0.74 0.99) | 1.86 (0.99 to 3.13) |
The Surveillance, Epidemiology, and End Results 18 registries are Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, Rural Georgia, the Alaska Native Tumor Registry, the Greater California, Kentucky, Louisiana, New Jersey, and Greater Georgia. CI = confidence interval; NHAI/AN = Non-Hispanic American Indian/Alaska Native; NHAPI = Non-Hispanic Asian/Pacific Islander; NHB = Non-Hispanic Black; NHW = Non-Hispanic White; NOS = not otherwise specified; SEER = Surveillance, Epidemiology, and End Results.
Adjusting for sex, age, and summary stage, except for all sites and leukemia.
Survival for patients diagnosed with the four most common cancers during 2006 to 2012 varied widely by state in both whites and blacks (Table 6). For example, female breast cancer survival in whites ranged from less than 88.0% in West Virginia and Wyoming to more than 92.0% in Colorado, North Dakota, New Hampshire, and Washington-Seattle; in blacks, it ranged from less than 76.0% in Arizona, Mississippi, and New Mexico to more than 87.0% in New Hampshire and Utah. For colorectal cancer, survival in whites ranged from less than 62.0% in Arizona, Michigan-Detroit, New Mexico, and Wyoming to more than 66.0% in Alaska, Connecticut, Hawaii, New Hampshire, and North Dakota; in blacks, it ranged from 50.1% in Iowa to more than 62.0% in Connecticut and Rhode Island. In general, survival for the four most common cancers tended to be lowest in select Southern and Midwestern states and highest in Northeastern states. Corresponding stage-specific survival data are given in Supplementary Table 7 (available online).
Table 6.
Five-year relative survival (%) for common cancers by race for select states, all ages, 2006–2012*
| State/Area | 5-y relative survival (95% CI) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colorectal cancer |
Lung cancer |
Female breast cancer |
Prostate cancer |
|||||||||
| All races | White | Black | All races | White | Black | All races | White | Black | All races | White | Black | |
| NAACCR US combined† | 63.8 (63.6 to 63.9) | 64.3 (64.1 to 64.5) | 57.6 (57.1 to 58.1) | 19.1 (18.9 to 19.2) | 19.3 (19.1 to 19.4) | 16.1 (15.8 to 16.4) | 88.8 (88.7 to 88.9) | 89.9 (89.8 to 90.0) | 79.4 (79.0 to 79.8) | 97.9 (97.7 to 98.0) | 98.0 (97.9 to 98.1) | 95.2 (94.9 to 95.5) |
| Alabama | 62.8 (61.6 to 63.9) | 64.4 (63.1 to 65.7) | 56.9 (54.6 to 59.1) | 15.9 (15.2 to 16.5) | 16.4 (15.7 to 17.1) | 13.1 (11.7 to 14.5) | 86.8 (85.9 to 87.6) | 89.2 (88.2 to 90.1) | 78.6 (76.8 to 80.4) | 96.8 (95.9 to 97.5) | 96.9 (95.8 to 97.7) | 94.4 (92.8 to 95.7) |
| Alaska | 64.8 (61.4 to 68.0) | 67.4 (63.4 to 71.1) | ‡ | 17.9 (15.8 to 20.2) | 19.4 (16.8 to 22.0) | 8.4 (1.8 to 21.7) | 87.5 (85.2 to 89.4) | 88.9 (86.3 to 91.0) | 76.4 (52.6 to 89.4) | 96.1 (94.0 to 97.4) | 96.4 (94.1 to 97.8) | 94.1 (77.8 to 98.5) |
| Arizona | 61.2 (60.0 to 62.3) | 60.9 (59.7 to 62.1) | 57.4 (50.4 to 63.7) | 18.7 (18.0 to 19.4) | 18.4 (17.7 to 19.1) | 18.4 (14.1 to 23.1) | 88.2 (87.5 to 88.9) | 88.5 (87.7 to 89.2) | 74.9 (69.6 to 79.5) | 96.0 (95.2 to 96.6) | 95.4 (94.6 to 96.1) | 91.3 (87.5 to 94.0) |
| California | 65.0 (64.5 to 65.4) | 65.0 (64.5 to 65.5) | 57.9 (56.2 to 59.5) | 18.5 (18.2 to 18.8) | 18.4 (18.0 to 18.7) | 14.7 (13.7 to 15.8) | 89.6 (89.3 to 89.9) | 90.1 (89.8 to 90.4) | 79.9 (78.7 to 81.2) | 97.1 (96.8 to 97.4) | 97.0 (96.6 to 97.3) | 95.3 (94.3 to 96.2) |
| Colorado | 65.0 (63.7 to 66.4) | 64.9 (63.5 to 66.3) | 60.6 (53.7 to 66.8) | 20.7 (19.7 to 21.6) | 20.5 (19.6 to 21.5) | 16.9 (12.6 to 21.8) | 92.0 (91.3 to 92.7) | 92.0 (91.3 to 92.7) | 83.8 (78.6 to 87.8) | 99.1 (98.6 to 99.5) | 98.2 (97.6 to 98.6) | 94.0 (89.6 to 96.6) |
| Connecticut | 66.4 (65.0 to 67.7) | 66.1 (64.6 to 67.5) | 66.3 (61.7 to 70.6) | 22.4 (21.6 to 23.3) | 22.5 (21.6 to 23.4) | 19.7 (16.5 to 23.0) | 90.4 (89.6 to 91.2) | 91.1 (90.2 to 91.9) | 79.8 (76.0 to 83.0) | 97.3 (96.5 to 97.8) | 97.3 (96.4 to 97.9) | 94.1 (91.0 to 96.1) |
| Georgia | 63.5 (62.6 to 64.4) | 65.6 (64.5 to 66.6) | 58.2 (56.5 to 59.8) | 17.7 (17.2 to 18.2) | 18.3 (17.7 to 18.9) | 15.4 (14.3 to 16.5) | 86.8 (86.2 to 87.3) | 89.1 (88.4 to 89.8) | 80.3 (79.1 to 81.4) | 98.5 (97.9 to 98.9) | 99.2 (98.6 to 99.6) | 96.7 (95.7 to 97.5) |
| Hawaii | 65.5 (63.5 to 67.5) | 70.1 (66.3 to 73.6) | ‡ | 17.4 (15.9 to 18.8) | 18.7 (16.0 to 21.5) | 10.1 (2.8 to 22.8) | 90.6 (89.3 to 91.7) | 91.8 (89.0 to 93.9) | 81.2 (60.6 to 91.7) | 94.0 (92.3 to 95.3) | 94.4 (91.3 to 96.4) | ‡ |
| Idaho | 64.7 (62.3 to 67.0) | 64.2 (61.8 to 66.5) | ‡ | 17.5 (16.1 to 19.0) | 17.5 (16.1 to 19.0) | ‡ | 89.9 (88.4 to 91.3) | 90.1 (88.6 to 91.4) | ‡ | 97.7 (96.1 to 98.6) | 97.2 (95.6 to 98.2) | ‡ |
| Illinois | 64.8 (64.1 to 65.5) | 65.6 (64.8 to 66.4) | 57.3 (55.5 to 59.1) | 20.3 (19.8 to 20.7) | 20.3 (19.8 to 20.8) | 18.2 (17.1 to 19.4) | 88.4 (87.9 to 88.9) | 89.4 (88.9 to 89.9) | 80.5 (79.0 to 81.8) | 98.5 (98.0 to 98.9) | 98.7 (98.2 to 99.1) | 94.7 (93.4 to 95.8) |
| Iowa | 64.1 (62.7 to 65.5) | 64.1 (62.7 to 65.5) | 50.1 (38.3 to 60.8) | 16.7 (15.9 to 17.5) | 16.7 (15.8 to 17.5) | 14.5 (9.1 to 20.9) | 89.8 (88.8 to 90.7) | 89.9 (88.9 to 90.8) | 80.6 (71.0 to 87.3) | 96.7 (95.6 to 97.5) | 96.2 (95.1 to 97.0) | 97.2 (87.7 to 99.4) |
| Kentucky | 63.0 (61.8 to 64.2) | 63.3 (62.0 to 64.5) | 57.8 (53.3 to 62.1) | 16.4 (15.9 to 17.0) | 16.5 (15.9 to 17.1) | 15.7 (13.3 to 18.2) | 87.7 (86.9 to 88.5) | 88.4 (87.5 to 89.2) | 77.8 (73.9 to 81.2) | 97.7 (97.0 to 98.3) | 97.8 (97.1 to 98.3) | 94.8 (91.7 to 96.8) |
| Louisiana | 63.5 (62.3 to 64.7) | 65.7 (64.2 to 67.1) | 58.6 (56.5 to 60.7) | 15.4 (14.7 to 16.1) | 16.2 (15.4 to 17.0) | 13.3 (12.1 to 14.6) | 85.9 (85.0 to 86.8) | 89.2 (88.1 to 90.1) | 78.2 (76.4 to 79.9) | 98.9 (98.1 to 99.4) | 99.8 (98.5 to 100.0) | 95.9 (94.3 to 97.1) |
| Maine | 64.5 (62.3 to 66.6) | 64.1 (61.9 to 66.2) | ‡ | 18.9 (17.7 to 20.2) | 18.9 (17.7 to 20.1) | ‡ | 91.6 (90.2 to 92.8) | 91.6 (90.2 to 92.8) | ‡ | 98.7 (96.6 to 99.5) | 98.3 (96.4 to 99.2) | 97.6 (78.9 to 99.7) |
| Michigan-Detroit | 60.4 (59.1 to 61.7) | 61.6 (60.1 to 63.2) | 55.6 (53.0 to 58.0) | 19.3 (18.5 to 20.0) | 19.9 (19.0 to 20.8) | 16.8 (15.3 to 18.3) | 86.7 (85.8 to 87.5) | 88.8 (87.8 to 89.7) | 78.9 (76.9 to 80.8) | 98.4 (97.4 to 99.0) | 98.4 (97.2 to 99.1) | 96.2 (94.4 to 97.5) |
| Mississippi | 61.3 (59.8 to 62.7) | 63.9 (62.0 to 65.7) | 56.4 (53.9 to 58.8) | 16.7 (15.9 to 17.5) | 17.9 (17.0 to 18.9) | 12.9 (11.5 to 14.3) | 84.6 (83.4 to 85.7) | 89.1 (87.7 to 90.3) | 75.4 (73.2 to 77.4) | 97.9 (96.8 to 98.7) | 100.0 (99.2 to 100.0) | 93.9 (92.0 to 95.4) |
| Montana | 64.0 (61.3 to 66.6) | 65.0 (62.3 to 67.7) | ‡ | 18.4 (16.9 to 20.1) | 18.8 (17.2 to 20.5) | ‡ | 90.2 (88.4 to 91.7) | 90.6 (88.8 to 92.1) | ‡ | 95.2 (93.2 to 96.6) | 95.5 (93.5 to 96.9) | ‡ |
| Nebraska | 65.1 (63.2 to 67.0) | 65.0 (63.0 to 66.9) | 58.5 (49.0 to 66.9) | 18.1 (17.0 to 19.3) | 18.1 (16.9 to 19.3) | 16.9 (11.5 to 23.2) | 89.6 (88.2 to 90.7) | 90.0 (88.6 to 91.2) | 79.5 (69.9 to 86.3) | 97.1 (95.6 to 98.1) | 96.9 (95.4 to 98.0) | 95.1 (83.8 to 98.6) |
| New Hampshire | 67.8 (65.4 to 70.0) | 67.7 (65.3 to 69.9) | ‡ | 22.2 (20.9 to 23.7) | 22.3 (20.9 to 23.7) | ‡ | 93.1 (91.7 to 94.2) | 93.2 (91.9 to 94.3) | 88.0 (59.5 to 96.9) | 99.0 (96.8 to 99.7) | 99.2 (96.6 to 99.8) | ‡ |
| New Jersey | 61.3 (60.4 to 62.2) | 62.3 (61.3 to 63.2) | 52.4 (49.9 to 54.9) | 21.4 (20.8 to 21.9) | 22.1 (21.5 to 22.7) | 14.8 (13.3 to 16.3) | 88.4 (87.8 to 88.9) | 89.9 (89.3 to 90.4) | 76.7 (74.8 to 78.4) | 98.7 (98.1 to 99.1) | 99.3 (98.8 to 99.6) | 95.1 (93.6 to 96.3) |
| New Mexico | 61.5 (59.5 to 63.4) | 61.4 (59.4 to 63.5) | 47.2 (31.3 to 61.5) | 15.9 (14.6 to 17.2) | 15.7 (14.4 to 17.1) | 14.1 (6.1 to 25.4) | 88.0 (86.7 to 89.2) | 88.3 (87.0 to 89.6) | 72.3 (56.0 to 83.5) | 96.2 (94.8 to 97.2) | 96.5 (95.1 to 97.5) | 83.4 (69.0 to 91.5) |
| New York | 63.5 (62.9 to 64.1) | 63.5 (62.9 to 64.2) | 58.8 (57.4 to 60.2) | 22.9 (22.5 to 23.3) | 22.9 (22.5 to 23.3) | 18.9 (17.9 to 20.0) | 89.3 (88.9 to 89.6) | 90.4 (90.0 to 90.8) | 80.6 (79.6 to 81.6) | 97.5 (97.1 to 97.8) | 98.0 (97.7 to 98.2) | 94.3 (93.5 to 95.0) |
| North Carolina | 64.6 (63.8 to 65.5) | 65.7 (64.7 to 66.7) | 60.3 (58.4 to 62.2) | 19.0 (18.5 to 19.5) | 19.4 (18.9 to 20.0) | 16.8 (15.6 to 18.0) | 88.9 (88.4 to 89.5) | 90.9 (90.2 to 91.4) | 81.1 (79.8 to 82.4) | 98.4 (97.8 to 98.8) | 99.2 (98.7 to 99.5) | 95.8 (94.5 to 96.7) |
| North Dakota | 67.9 (64.8 to 70.8) | 68.2 (65.0 to 71.2) | ‡ | 19.6 (17.5 to 21.8) | 19.7 (17.6 to 22.0) | ‡ | 92.3 (90.1 to 94.0) | 92.4 (90.1 to 94.2) | ‡ | 98.4 (96.5 to 99.3) | 98.6 (96.6 to 99.4) | ‡ |
| Pennsylvania | 63.6 (62.9 to 64.3) | 63.9 (63.1 to 64.6) | 56.7 (54.4 to 59.0) | 19.6 (19.1 to 20.0) | 19.5 (19.1 to 20.0) | 17.5 (16.2 to 18.9) | 88.6 (88.2 to 89.1) | 89.5 (89.0 to 90.0) | 78.6 (76.8 to 80.3) | 99.0 (98.6 to 99.3) | 98.7 (98.3 to 99.0) | 94.8 (93.2 to 96.0) |
| Rhode Island | 63.9 (61.3 to 66.4) | 63.9 (61.2 to 66.4) | 63.7 (50.6 to 74.2) | 22.0 (20.5 to 23.6) | 21.9 (20.4 to 23.5) | 22.2 (14.6 to 30.9) | 91.0 (89.4 to 92.3) | 91.4 (89.8 to 92.8) | 80.7 (70.3 to 87.8) | 98.1 (96.7 to 98.9) | 97.6 (96.0 to 98.5) | 96.5 (88.4 to 99.0) |
| South Carolina | 61.8 (60.5 to 63.0) | 63.2 (61.7 to 64.6) | 56.9 (54.5 to 59.2) | 17.7 (17.0 to 18.4) | 18.1 (17.4 to 18.9) | 15.6 (14.2 to 17.2) | 86.9 (86.1 to 87.7) | 88.9 (87.9 to 89.8) | 80.7 (79.0 to 82.3) | 97.9 (97.1 to 98.5) | 98.5 (97.6 to 99.1) | 95.6 (94.1 to 96.7) |
| Texas | 63.1 (62.5 to 63.7) | 63.8 (63.2 to 64.4) | 56.0 (54.3 to 57.5) | 18.2 (17.8 to 18.6) | 18.3 (17.9 to 18.7) | 15.4 (14.4 to 16.4) | 87.9 (87.5 to 88.3) | 89.1 (88.7 to 89.5) | 77.6 (76.3 to 78.8) | 96.8 (96.4 to 97.2) | 96.8 (96.4 to 97.2) | 94.0 (92.8 to 94.9) |
| Utah | 65.5 (63.5 to 67.5) | 65.9 (63.8 to 68.0) | 58.5 (37.3 to 74.7) | 17.9 (16.2 to 19.7) | 18.2 (16.4 to 20.0) | ‡ | 89.6 (88.3 to 90.8) | 89.7 (88.4 to 90.9) | 89.6 (63.2 to 97.4) | 98.0 (97.0 to 98.7) | 98.1 (97.1 to 98.7) | 97.5 (85.3 to 99.6) |
| Washington-Seattle | 65.8 (64.5 to 67.1) | 65.7 (64.2 to 67.1) | 61.4 (54.6 to 67.5) | 19.1 (18.4 to 20.0) | 19.0 (18.2 to 19.8) | 17.0 (13.0 to 21.6) | 92.1 (91.4 to 92.8) | 92.7 (91.9 to 93.4) | 83.1 (77.9 to 87.2) | 98.4 (97.7 to 98.9) | 98.7 (97.9 to 99.2) | 96.1 (92.5 to 98.0) |
| West Virginia | 62.6 (60.9 to 64.3) | 62.6 (60.8 to 64.3) | 60.9 (50.1 to 70.0) | 16.4 (15.5 to 17.3) | 16.4 (15.5 to 17.3) | 13.0 (8.8 to 18.1) | 87.7 (86.4 to 89.0) | 87.7 (86.3 to 89.0) | 86.2 (77.1 to 91.9) | 98.4 (96.5 to 99.3) | 98.6 (96.5 to 99.4) | 90.7 (80.0 to 95.8) |
| Wisconsin | 64.1 (62.9 to 65.3) | 64.5 (63.2 to 65.6) | 59.2 (53.8 to 64.2) | 18.8 (18.1 to 19.5) | 19.1 (18.4 to 19.8) | 18.1 (14.9 to 21.5) | 90.0 (89.2 to 90.6) | 90.4 (89.7 to 91.1) | 80.1 (75.8 to 83.7) | 97.3 (96.6 to 97.9) | 97.7 (96.9 to 98.3) | 96.0 (92.0 to 98.0) |
| Wyoming | 59.4 (55.6 to 63.0) | 59.7 (55.8 to 63.3) | ‡ | 17.5 (15.1 to 20.0) | 17.8 (15.3 to 20.3) | ‡ | 86.7 (83.9 to 89.0) | 86.9 (84.0 to 89.2) | ‡ | 99.4 (95.3 to 99.9) | 99.5 (94.2 to 100.0) | ‡ |
Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results (SEER) registries as compiled by the North American Association of Central Cancer Registries. First and subsequent primary cancers included, using the first applicable record per patient in each analysis. The survival duration in months was calculated based on complete dates. For registries meeting SEER follow-up standards (SEER registries plus Montana and Wyoming), the survival duration for alive patients was calculated through the date of last contact (or study cutoff, if earlier). For these registries, alive cases with no survival time were excluded from analysis. For the remaining registries, survival duration for alive patients was calculated through December 31, 2012, with all patients not known to be dead presumed to be alive on this date. CI = confidence interval; NAACCR = North American Association of Central Cancer Registries; NPCR = National Program of Cancer Registries; SEER = Surveillance, Epidemiology, and End Results.
Thirty-one states plus two metropolitan areas.
Statistics are suppressed when fewer than 10 cases were reported for the specific cancer, when the standard error was greater than or equal to 10%, or when the difference of the upper and lower confidence intervals was greater than 40%.
Figure 5 shows five-year survival for select childhood cancers diagnosed during two calendar periods, 1975 to 1977 and 2006 to 2012. Survival improved substantially between the two periods for all cancer types, ranging from an absolute increase of 16.8% (95% CI = 9.2% to 24.3%) for Hodgkin lymphoma to 48.3% (95% CI = 37.7% to 59.0%) for acute myeloid leukemia (Supplementary Table 6, available online). By race, five-year survival for all childhood cancers included in Supplementary Table 6 (available online) increased from 57.9% (95% CI = 55.4% to 60.3%) to 85.3% (95% CI = 84.1% to 86.4%) in white children and from 57.3% (95% CI = 49.3% to 64.5%) to 82.1% (95% CI = 79.0% to 84.9%) in black children. Five-year cancer survival for children of all races diagnosed from 2006 to 2012 ranged from 66.1% (95% CI = 59.7% to 71.7%) for acute myeloid leukemia to 97.7% (95% CI = 94.3% to 99.1%) for Hodgkin lymphoma.
Figure 5.
Changes in five-year relative survival for select childhood cancers (0–14 years), SEER 9*, 1975–2012. Error bars represent 95% confidence intervals. *The SEER 9 registries are Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah. ALL = acute lymphoid leukemia; AML = acute myeloid leukemia; NHL = non-Hodgkin lymphoma; SEER = Surveillance, Epidemiology, and End Results.
Discussion
Overall cancer death rates continue to decrease in both men and women for all major racial and ethnic groups. Rates decreased for 11 of the 16 most common cancers in men and for 13 of the 18 most common cancers in women, including lung, colorectal, female breast, and prostate cancers. In contrast, death rates increased for liver cancer in men and women, for pancreas cancer and brain cancer in men, and for uterine cancer in women. In contrast to overall mortality trends, overall incidence rates decreased in men but stabilized in women. Incidence rates decreased for seven of the 17 most common cancers in men and seven of the 18 most common cancers in women, including lung and colorectal cancers, whereas rates increased for seven cancers in men and nine cancers in women, including liver, myeloma, melanoma, oral cavity, and thyroid in both men and women and pancreatic cancer in men. Survival increased substantially overall and for both early and late-stage diseases for several but not all cancer sites, and survival varied statistically significantly by race/ethnicity and state.
Factors that have contributed to the continued decrease in cancer death rates overall and for the most common cancers have been described in previous reports (14–18) and include reduced tobacco use, which is a well-established cause of 16 cancer types and accounts for nearly one-third of cancer deaths (42), improved early detection (eg, colorectal, breast, and cervix), and improved treatments for many cancers. In particular, cigarette smoking prevalence among adults over the past 50 years decreased by more than 50% because of public health policies against tobacco and increased awareness about the health hazards of smoking (42). However, there are still about 40 million adult smokers (43), and smoking remains the leading cause of cancer death (42,44–46). These facts underscore the need for expansions of federal and state tobacco control programs and the development of new strategies, such as pricing strategies and plain tobacco packaging to accelerate the reduction in tobacco use (47).
Unlike mortality trends, where increases are generally unfavorable and declines are indicators of progress, increases in incidence may reflect changes in detection practice and may have both positive and negative implications with respect to cancer control (19,20). For example, the continued increase in melanoma and thyroid cancer incidence rates over the past several decades is in part thought to reflect increased diagnostic scrutiny (48–50); however, incidence rates for both cancer types increased for late-stage and large tumors (48,49,51), suggesting the role of underlying risk factors such as increases in intermittent recreational sun exposures for melanoma (49) and radiation and other unrecognized carcinogens for thyroid cancer (52). Likewise, trends in prostate cancer incidence rates have been affected by changes in the uptake of prostate-specific antigen (PSA) testing. Prostate cancer incidence rates decreased substantially (53,54) following the US Preventive Services Task Force recommendations against routine screening for PSA testing in men age 75 years or older (2008) and in men age 50 years or older (2011) (55,56). In contrast, the continued increase in liver cancer incidence rates is likely due to the high prevalence of chronic hepatitis C virus infection resulting from intravenous drug use by baby boomers during the 1960s to 1980s, as well as the obesity epidemic beginning in the 1980s (18). The obesity epidemic also may have contributed in part to the increases in endometrial, pancreas, and kidney cancer incidence rates (14) because obesity is estimated to account for 49%, 28%, and 24% of the total cases, respectively, in the United States (57).
In contrast to adult cancers, there are few known environmental risk factors for childhood cancers (58). According to the 2016 Cancer Statistics Review; however, the increase in the overall childhood cancer incidence rates during the last five data years (2009–2013) was largely confined to acute lymphocytic leukemia (ALL) and NHL (59), and ALL incidence increased only in Hispanic white children (60). Known and suspected risk factors for ALL include parental smoking during pregnancy, pesticide exposure, high birthweight, and Down syndrome, and for NHL they include Epstein Barr virus and inherited or acquired immunodeficiency (34,58,60–62). However, we could not find data to support that these risk factors may have contributed to the increasing incidence trends in ALL or NHL.
This is the second annual report to include a special section on population-based survival. The previous report compared relative five-year survival for two diagnostic periods (1975–1979 and 1995–2000) and examined risks of dying from cancer, once diagnosed, in each racial and ethnic population compared with non-Hispanic whites (7). This report extends the most recent diagnosis period by 12 years compared with the previous report, includes temporal changes in stage-specific survival, and presents contemporary survival data by race/ethnicity and state. As in the previous report, survival improved over time for almost all cancers at every stage of diagnosis. However, survival remains very low for some cancer sites and for most of the cancer sites diagnosed at distant stage. Disparities in survival by race have persisted over time, and variations in survival by state of residence are evident in recent data. Although increasing survival over time reflects progress in treating many cancer types, survival trends for some cancers must be interpreted with caution due to biases related to screening and early detection (21) and should be interpreted in the broader context of trends in incidence, stage at diagnosis, and mortality (63).
Cancer screening can lengthen the survival interval by moving back the time of diagnosis without changing the eventual date of death (lead time bias), as well as by identifying relatively slow-growing cancers that have good prognoses (length bias) (21). Screening may also lead to overdiagnosis by finding cancers that never would have been clinically detected during the course of the patients’ natural lives (21). It is especially important to keep these biases in mind when interpreting survival trends during time periods when screening for particular cancers (eg, female breast cancer and prostate cancer) has been widely implemented in the general population.
Lead time, length bias, and overdiagnosis are also a concern when interpreting survival trends for cancer types for which changes in diagnostic technology or medical care practice have increased the detection of asymptomatic diseases (21). Among the cancers for which screening has been widely implemented in the general population, the greatest absolute increase in five-year relative survival has occurred for prostate cancer, along with the greatest controversies about the benefits of screening and treatment (64). Widespread adoption of PSA screening began in 1987, resulting in a dramatic increase in incidence, and survival trends are thought to have been influenced by lead time bias, length bias, and overdiagnosis (64). In addition to screening-related biases, analysis of prostate cancer survival trends is complicated by inconsistent stage and grade classification over time (65–67). Changes in prostate cancer treatment for which there is evidence of survival benefit include increased use of radical prostatectomy beginning in the 1980s, radiation therapy in combination with androgen deprivation therapy beginning in the mid to late 1990s, and protocols for evaluation and treatment of biochemical recurrence (68). Despite uncertainties about the benefits and harms of prostate cancer screening and which treatment approaches are optimal, it is clear that prostate cancer death rates have declined substantially in the United States since the early 1990s (67). Modeling studies that used survival data from clinical trials and population-based data on incidence and treatment suggest that stage shift due to screening and changes in treatment have contributed to declining prostate cancer mortality (68).
Increases in survival have also been observed since 1975 for breast and colorectal cancer, but have been much more limited for cervical cancer. The introduction of mammography screening in the 1980s and increasing use in the 1990s led to increased detection of localized and smaller breast cancers (69,70); within-stage shifts in tumor size and other prognostic features likely contributed to increased survival for localized and regional disease. Survival for distant-stage breast cancer has also been steadily improving since the early 1990s (71). Concurrent with the introduction of mammography screening, multiple improvements in breast cancer therapy occurred, including the use of hormonal therapies for hormone receptor–positive cancers and multi-agent chemotherapy (72). Modeling studies suggest that both screening and adjuvant therapy contributed to declines in breast cancer mortality (73).
Several colorectal screening modalities have been used since the 1980s, but with much slower population uptake than mammography screening (12,74). Detection of colorectal cancer at an early stage through screening may have contributed in part to the improvement in the overall survival (12). However, much of the improvement likely resulted from treatment advances, including improved surgical care, adjuvant chemotherapy for patients with regional (node-positive) disease (75), resection (surgical removal) of distant disease, and neoadjuvant therapy for rectal cancer (76). Unlike breast and colorectal cancer, there has been little progress in overall cancer survival for cervical cancer during the period studied, in part because Pap test screening had already been widely disseminated by 1987 (77).
For most cancer types for which screening has not been implemented in the general population, changes in long-term survival are easier to interpret, although increases in earlier detection, stage shifts, and changes in staging rules may have influenced some survival trends. For a number of solid tumors where surgery is the primary treatment and surgical mortality is relatively high (eg, esophageal and lung cancer), declines in surgical mortality have likely contributed to improved survival (78). Lower surgical mortality may have been achieved through improvements in anesthesia and supportive care, institution of quality improvement programs, and regionalization of high-risk surgeries (78).
The survival improvements over time highlighted in this report also reflect major advances in systemic therapies for some cancers, including imatinib mesylate for chronic myelogenous leukemia in the early 1990s (79), rituximab for B-cell non-Hodgkin lymphoma in the 1990s (80), and combination of chemotherapies for childhood cancers beginning in the 1960s (81,82). In particular, the continued statistically significant improvements in five-year survival rates for most cancers occurring in children—with over 80% of children surviving five years during recent diagnosis years—have been attributed to the systematic conduct of clinical trials assessing the efficacy of multimodal approaches involving combination chemotherapy, radiotherapy, and/or surgery with increased expertise in supportive care in specialized cancer centers (83). Member institutions of the Children’s Oncology Group, a National Cancer Institute supported trials group, care for 90% of children diagnosed with cancer in the United States (61,84) .
In this era of increasingly personalized cancer therapy, it is hoped that dramatic progress in treatment and survival will be observed for other cancer types as well. It may not be possible in this analysis to detect the impact of very recent therapeutic improvements on population survival due to the time lag for case reporting and follow-up in cancer registry data; examples include protein kinase inhibitors for non–small cell lung cancer, colorectal cancer, and chronic myeloid leukemia; anti-angiogenics (which inhibit blood vessel growth) for colorectal and ovarian cancers; and immunotherapy for melanoma and non–small cell lung cancer (85). Such improvements may also be difficult to discern in population-based registry data for therapies that apply only to subsets of patients for a cancer site.
Although five-year survival for most cancers types improved among both blacks and whites over the past 30 or more years, the racial disparities observed for most cancer sites and for all cancer sites combined in the earlier period (1995–1997) for many common cancers have persisted, and they may have increased for prostate cancer and female breast cancer. Much work remains in order to understand the likely multiple causes of these observed differences; however, they may in part reflect differences among racial/ethnic groups in receipt or timeliness of recommended treatments (8,86–90). For example, black women with breast cancer are less likely to receive and adhere to adjuvant chemotherapy and more likely to experience delayed initiation of such therapy (86,91,92). The risk of death in blacks compared with whites was higher overall (all sites) and for the most common cancers in stage-adjusted analyses.
Similarly, compared with whites, AI/AN had higher risk of death for almost all cancer types and Hispanics had higher risk of death for many cancers, which may in part reflect treatment differences (8,93). In contrast, API had lower risk of death than whites for most cancers, including lung, colorectal, prostate, and female breast. While there are limited data showing that receipt of standard cancer treatments is higher in API than in whites, API have higher median income than whites and a higher proportion of persons with college-level education (94). However, higher survival among API and Hispanics need to be interpreted with caution because of known issues related to the follow-up of these patients (95). Further investigation of the factors that contribute to racial/ethnic survival differences is needed.
We also found evidence to suggest geographic differences in survival. For most of the common cancer sites, several northeastern states (eg, Connecticut, New Hampshire, Rhode Island) often had higher survival than elsewhere, whereas several southern states (eg, Alabama, Mississippi) often had lower survival. However, variations in survival by state have to be interpreted with caution as they may reflect differences in population demographics (race, age, ethnicity, and socioeconomic status), cancer screening rates, residents’ access to and quality of cancer care, and cancer registration practices that impact case ascertainment, date of diagnosis, and follow-up, and/or other factors (33,96).
Strength and Limitations
This is the first “Annual Report to the Nation” that has used a single database (41 quality certified cancer registries, covering 89% of the US population) to provide all delay-adjusted incidence statistics. In future years, as the number of quality certified registries increases, we hope to cover a larger proportion of the US population and/or present trends using a longer time series. Monitoring cancer incidence trends is one of the most important uses of population-based registry data, and the ability to apply delay adjustment at the national level improves the accuracy and consistency of these results. Similarly, we used a single database (31 state registries and two metropolitan area registries) covering 67% of the US population to analyze survival variations by race/ethnicity and area of residence. High-quality survival data are vital for identifying disparities in cancer treatment and outcomes.
A limitation of this report is that we used the SEER historic stage variable for survival analysis to ensure consistency over time for all cancer sites. The collection of historic stage data by SEER since 1975 has been valuable for understanding long-term trends in incidence, survival, and mortality, but in the past decade it has been increasingly difficult to maintain historically comparable stage data over time. Stage definitions change as new diagnostic procedures are incorporated and American Joint Committee on Cancer staging rules are modified to reflect advances in clinical knowledge. As the collection of increasingly more information will be necessary to create clinically meaningful disease classifications for prognosis and treatment, examining and reporting of long-term survival trends by stage may become difficult or impossible.
On the other hand, the availability of detailed information on stage and other prognostic factors for almost all incident cancers in the United States creates unprecedented opportunities to study the increasing use of new treatments and their impacts on survival in population-based samples. The NCI has created a valuable national resource for such studies by linking SEER registry data to Medicare records to augment data on treatments and comorbidities (97). Ongoing related efforts by the NCI include the development of a new tool, the “SEER Cancer Survival Calculator” (SEER*CSC), to provide information on prognosis of individual patients to help patients and their families and doctors in making difficult decisions about treatment (98,99). In addition, the CDC has invested resources in utilizing NPCR registries for conducting population-based comparative effectiveness research (100) and in making these data available to researchers through NCHS’s Research Data Center (http://www.cdc.gov/rdc/index.htm).
Another limitation of this report is misclassification of race/ethnicity information in medical records (incidence), death certificates, and Census. Since 2000, the Census has given respondents the option to self-select multiple race/ethnicity categories, creating incompatibility with race/ethnicity information on medical records and death certificates, which often have single race/ethnicity categories. To address this problem, the US Census Bureau, in collaboration with CDC’s NCHS and NCI, has developed methods to generate single-race population estimates, but with some uncertainties about the population estimates and resultant rates (101). Furthermore, race/ethnicity information on death certificates is underascertained for AI/AN, API, and Hispanics (102), leading to underestimation of cancer rates. In addition, cancer rates for broad racial and ethnic groups (eg, Hispanic and API) may mask important variations in cancer burden by country of origin (103,104).
Finally, as with survival trends, incidence trends need to be interpreted with caution as changes in incidence rates may result from changes in risk factor prevalence, increased or decreased use of screening or diagnostic techniques, or a combination of these. Further, the AAPC was used as a summary measure to average trends in magnitude and direction over the most recent five-year data period using joinpoint regression (39), but one cannot necessarily conclude that rates continue to increase or decrease throughout the five-year period.
Future Directions
Cancer survival, particularly for advanced-stage diseases, is expected to increase markedly in light of recent advances in precision medicine and immunotherapy for late-stage cancers (eg, melanoma, lung cancer) (81,82). Further, the White House’s Cancer Moonshot initiative and other similar initiatives to accelerate progress against cancer aim to build on these recent advances and find cures (105,106). However, despite many reasons for optimism about the potential for research to accelerate the development of highly effective treatments, important challenges remain in the access and delivery of cancer care to enable all populations to benefit from treatment advances. Some of the new cancer drugs cost $10 000 per month and are not affordable even by most insured patients because of the high out-of-pocket expenses, which are about 20% of the drug’s cost for Medicare-insured patients (107–109). The high cost of cancer treatment dubbed “financial toxicity”(110) has been associated with reduced spending on groceries and clothing, skipped medications and physician appointments to save money, bankruptcy, and mortality (111,112). It has been suggested that if measures are not taken to contain the escalating trend in treatment costs, cancer care in the United States could become less affordable and could impede the very goal of the Affordable Care Act, which is to make high-quality health care accessible to all (113). Cognizant of this problem, the American Society of Clinical Oncology recently developed a conceptual framework for medical oncologists to assess the value of cancer treatment options, with an emphasis on the clinical benefit (efficacy), toxicity (safety), and cost (efficiency) of drugs (113).
To accelerate progress in reducing cancer mortality, we must not only intensify efforts to develop effective targeted therapies and find cures, but also heighten our efforts to broadly and equitably apply proven preventive measures. A large percentage of the reduction in cancer death rates since 1990 has come from preventive measures rather than treatment advances (114–117). For example, Thun and Jemal estimated that reduced tobacco use over the past four decades alone accounted for about 40% of the decrease in overall male cancer death rates from 1991 to 2003 (116). Tobacco use still accounts for nearly 30% of cancer deaths in the United States (44–46), and about 17% of US adults (40 million adults) are current cigarette smokers, with prevalence varying extensively across states (43,118). Recent reports documented that only North Dakota funded a state tobacco control program at the CDC-recommended level during 2016 and nearly half of the states did not have statewide comprehensive smoke-free laws that ban smoking in bars, restaurants, and workplaces (119,120). Devoting increased resources and enacting laws and regulations to strengthen tobacco control policies at both state and federal levels—such as tobacco product pricing strategies, plain packaging, statewide comprehensive smoke-free laws, and reducing nicotine content in tobacco products to nonaddictive levels (47)—could greatly reduce morbidity and mortality from smoking-related cancers and other smoking-related diseases.
Additional interventions for patient and provider education and outreach programs and interventions aimed at removing barriers to accessing preventive services are needed to increase the low or suboptimal uptake of screening for colorectal cancer (121) and lung cancer (122), human papilloma virus vaccination (123), and testing for hepatitis C virus infection (124). Additional resources are also required to create neighborhoods that encourage physical activity and healthy eating habits and to identify new approaches to prevent and reverse the obesity epidemic (125), which accounts for 15% to 20% of total cancer deaths in the United States (126). Furthermore, more attention and resources are needed for identifying major risk factors for common cancers such as colorectal, breast, and prostate. Also needed are concerted efforts to understand the increasing incidence trends in uterine, female breast, and pancreas cancer, as well as to plan and implement proven preventive measures (57,127).
Conclusions
Overall cancer death rates continue to decrease in the United States, reflecting improvements in prevention, early detection, and treatment. However, progress in reducing mortality and improving survival is limited for several cancers. This requires renewed commitment to redouble our efforts to discover new strategies for prevention, early detection, and treatment and to apply proven interventions broadly and equitably.
Funding
This work was supported by the American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC), the National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR).
Notes
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. Further, the findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the author’s agencies (ACS, CDC, NCI, NAACCR). The authors have no disclosures to make.
We gratefully acknowledge the contributions of the state and regional cancer registry staff for their work in collecting the data used in this report. In addition, we thank Rick Firth, Daniel Miller, Joe Zou, and Steve Scoppa of Information Management Services, Inc., for assistance in compiling the data used in this report.
References
Supplementary Material
References
- 1. Wingo PA, Ries LA, Rosenberg HM, et al. Cancer incidence and mortality, 1973–1995: A report card for the U.S. Cancer. 1998;82(6):1197–1207. [DOI] [PubMed] [Google Scholar]
- 2. Wingo PA, Ries LA, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91(8):675–690. [DOI] [PubMed] [Google Scholar]
- 3. Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000;88(10):2398–2424. [DOI] [PubMed] [Google Scholar]
- 4. Howe HL, Wingo PA, Thun MJ, et al. Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst. 2001;93(11):824–842. [DOI] [PubMed] [Google Scholar]
- 5. Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94(10):2766–2792. [DOI] [PubMed] [Google Scholar]
- 6. Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95(17):1276–1299. [DOI] [PubMed] [Google Scholar]
- 7. Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101(1):3–27. [DOI] [PubMed] [Google Scholar]
- 8. Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97(19):1407–1427. [DOI] [PubMed] [Google Scholar]
- 9. Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006;107(8):1711–1742. [DOI] [PubMed] [Google Scholar]
- 10. Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110(10):2119–2152. [DOI] [PubMed] [Google Scholar]
- 11. Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100(23):1672–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103(9):714–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual report to the nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(9):2338–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jemal A, Simard EP, Dorell C, et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edwards BK, Noone AM, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120(9):1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107(6):XXX(X):XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryerson AB, Eheman CR, Altekruse SF, et al. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Measurement of progress against cancer. Extramural Committee to Assess Measures of Progress Against Cancer. J Natl Cancer Inst. 1990;82(10):825–835. [PubMed] [Google Scholar]
- 20. Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med. 2006;260(2):103–117. [DOI] [PubMed] [Google Scholar]
- 21. Croswell JM, Ransohoff DF, Kramer BS. Principles of cancer screening: Lessons from history and study design issues. Semin Oncol. 2010;37(3):202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. North American Association of Central Cancer Registries. NAACCR data quality criteria. Available at http://www.naaccr.org/Certification/Criteria.aspx. Accessed July 8, 2015.
- 23. World Health Organization. International Classification of Diseases for Oncology. 3rd ed.Geneva, Switzerland: World Health Organization Press; 2000. [Google Scholar]
- 24. Howlader N, Noone AM, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975–2012. Bethesda, MD: National Cancer Institute; 2015. http://seer.cancer.gov/csr/1975_2012/. Accessed June 10, 2016. [Google Scholar]
- 25. Young JL, Jr, Roffers SD, Ries LAG, et al. SEER Summary Staging Manual 2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute; 2001. [Google Scholar]
- 26. Clegg LX, Feuer EJ, Midthune DN, et al. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537–1545. [DOI] [PubMed] [Google Scholar]
- 27. Kochanek KD, Murphy SL, Xu J, et al. Deaths: Final data for 2014. Natl Vital Stat Rep. 2016;65(4):1–122. [PubMed] [Google Scholar]
- 28. Espey DK, Jim MA, Richards TB, et al. Methods for improving the quality and completeness of mortality data for American Indians and Alaska Natives. Am J Public Health. 2014;104(suppl 3):S286–S294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Espey DK, Wiggins CL, Jim MA, et al. Methods for improving cancer surveillance data in American Indian and Alaska Native populations. Cancer. 2008;113(5 suppl):1120–1130. [DOI] [PubMed] [Google Scholar]
- 30. Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute. Population estimates used in NCI's SEER*Stat software. http://seer.cancer.gov/popdata/methods.html. Accessed July 13, 2015.
- 31. Centers for Disease Control and Prevention. National vital statistics system. https://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm. Accessed December 22, 2016.
- 32. Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories: Data evaluation and research. Vital Health Stat. 2003:2(135). [PubMed] [Google Scholar]
- 33. Weir HK, Johnson CJ, Mariotto AB, et al. Evaluation of North American Association of Central Cancer Registries' (NAACCR) data for use in population-based cancer survival studies. J Natl Cancer Inst Monogr. 2014;2014(49):198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosso S, De Angelis R, Ciccolallo L, et al. Multiple tumours in survival estimates. Eur J Cancer. 2009;45(6):1080–1094. [DOI] [PubMed] [Google Scholar]
- 35. Surveillance Research Program, National Cancer Institute. SEER*Stat software version 8.3.2. www.seer.cancer.gov/seerstat. Accessed June 10, 2016.
- 36. Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569. [DOI] [PubMed] [Google Scholar]
- 37. National Cancer Institute. Joinpoint regression program, version 4.2.0.2. 2015. http://surveillance.cancer.gov/joinpoint/. Accessed July 14, 2015.
- 38. Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 39. Clegg LX, Hankey BF, Tiwari R, et al. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. National Cancer Institute. AAPC confidence intervals--Joinpoint Help System 4.3.1.0. https://surveillance.cancer.gov/help/joinpoint/setting-parameters/advanced-tab/aapc-confidence-intervals. Accessed August 22, 2016.
- 41. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag; 2010. [Google Scholar]
- 42. US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014. [Google Scholar]
- 43. Jamal A, Homa DM, O'Connor E, et al. Current cigarette smoking among adults United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. [DOI] [PubMed] [Google Scholar]
- 44. Jacobs EJ, Newton CC, Carter BD, et al. What proportion of cancer deaths in the contemporary United States is attributable to cigarette smoking? Ann Epidemiol. 2015;25(3):179–182, e1. [DOI] [PubMed] [Google Scholar]
- 45. Lortet-Tieulent J, Goding Sauer A, Siegel RL, et al. State-level cancer mortality attributable to cigarette smoking in the United States. JAMA Intern Med. 2016;176(12):1792–1798. [DOI] [PubMed] [Google Scholar]
- 46. Siegel RL, Jacobs EJ, Newton CC, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med. 2015;175(9):1574–1576. [DOI] [PubMed] [Google Scholar]
- 47. Centers for Disease Control and Prevention. Best Practices for Comprehensive Tobacco Control Programs—2014. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 48. Ward EM, Jemal A, Chen A. Increasing incidence of thyroid cancer: Is diagnostic scrutiny the sole explanation? Future Oncol. 2010;6(2):185–188. [DOI] [PubMed] [Google Scholar]
- 49. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S17–S25, e1–e3. [DOI] [PubMed] [Google Scholar]
- 50. Vaccarella S, Franceschi S, Bray F, et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–617. [DOI] [PubMed] [Google Scholar]
- 51. Aschebrook-Kilfoy B, Grogan RH, Ward MH, et al. Follicular thyroid cancer incidence patterns in the United States, 1980–2009. Thyroid. 2013;23(8):1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pellegriti G, Frasca F, Regalbuto C, et al. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054–2061. [DOI] [PubMed] [Google Scholar]
- 54. Jemal A, Ma J, Siegel R, et al. Prostate cancer incidence rates 2 years after the US Preventive Services Task Force recommendations against screening. JAMA Oncol. 2016;2(12):1657–1660. [DOI] [PubMed] [Google Scholar]
- 55. Prasad SM, Drazer MW, Huo D, et al. 2008 US Preventive Services Task Force recommendations and prostate cancer screening rates. JAMA. 2012;307(16):1692–1694. [DOI] [PubMed] [Google Scholar]
- 56. US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(3):185–191. [DOI] [PubMed] [Google Scholar]
- 57. World Cancer Research Fund American Institute for Cancer Research. Policy and Action for Cancer Prevention. Food, Nutrition, and Physical Activity: A Global Perspective. Washington, DC: AICR; 2009. [Google Scholar]
- 58. Ross JA, Spector LG. Cancers in children. In: Schottenfeld D, Fraumeni JF. Jr, eds. Cancer Epidemiology and Prevention. 3rd ed.New York, NY: Oxford University Press; 2006:1251–1268. [Google Scholar]
- 59. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013. Bethesda, MD: National Cancer Institute; 2016. http://seer.cancer.gov/csr/1975_2013/. [Google Scholar]
- 60. Barrington-Trimis JL, Cockburn M, Metayer C, et al. Trends in childhood leukemia incidence over two decades from 1992 to 2013. Int J Cancer. 2017;140(5):1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. [DOI] [PubMed] [Google Scholar]
- 62. Linet MS, Brown LM, Mbulaiteye SM, et al. International long-term trends and recent patterns in the incidence of leukemias and lymphomas among children and adolescents ages 0–19 years. Int J Cancer. 2016;138(8):1862–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cho H, Mariotto AB, Schwartz LM, et al. When do changes in cancer survival mean progress? The insight from population incidence and mortality. J Natl Cancer Inst Monogr. 2014;2014(49):187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65(6):1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jani AB, Master VA, Rossi PJ, et al. Grade migration in prostate cancer: An analysis using the Surveillance, Epidemiology, and End Results registry. Prostate Cancer Prostatic Dis. 2007;10(4):347–351. [DOI] [PubMed] [Google Scholar]
- 66. Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: Interpreting trends in prostate cancer—part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91(12):1017–1024. [DOI] [PubMed] [Google Scholar]
- 67. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012(45):152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Etzioni R, Gulati R, Tsodikov A, et al. The prostate cancer conundrum revisited: Treatment changes and prostate cancer mortality declines. Cancer. 2012;118(23):5955–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Miller BA, Feuer EJ, Hankey BF. The increasing incidence of breast cancer since 1982: Relevance of early detection. Cancer Causes Control. 1991;2(2):67–74. [DOI] [PubMed] [Google Scholar]
- 70. Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among US women. Breast Cancer Res. 2007;9(3):R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen L, Linden HM, Anderson BO, et al. Trends in 5-year survival rates among breast cancer patients by hormone receptor status and stage. Breast Cancer Res Treat. 2014;147(3):609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sledge GW, Mamounas EP, Hortobagyi GN, et al. Past, present, and future challenges in breast cancer treatment. J Clin Oncol. 2014;32(19):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. [DOI] [PubMed] [Google Scholar]
- 74. Swan J, Breen N, Graubard BI, et al. Data and trends in cancer screening in the United States: Results from the 2005 National Health Interview Survey. Cancer. 2010;116(20):4872–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66:83–95. [DOI] [PubMed] [Google Scholar]
- 76. Salem ME, Hartley M, Unger K, et al. Neoadjuvant combined-modality therapy for locally advanced rectal cancer and its future direction. Oncology (Williston Park). 2016;30(6):546–562. [PubMed] [Google Scholar]
- 77. Hiatt RA, Klabunde C, Breen N, et al. Cancer screening practices from National Health Interview Surveys: Past, present, and future. J Natl Cancer Inst. 2002;94(24):1837–1846. [DOI] [PubMed] [Google Scholar]
- 78. Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hunter T. Treatment for chronic myelogenous leukemia: The long road to imatinib. J Clin Invest. 2007;117(8):2036–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dotan E, Aggarwal C, Smith MR. Impact of rituximab (rituxan) on the treatment of B-cell non-Hodgkin's lymphoma. P T. 2010;35(3):148–157. [PMC free article] [PubMed] [Google Scholar]
- 81. DeVita VT, Jr, Rosenberg SA. Two hundred years of cancer research. N Engl J Med. 2012;366(23):2207–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. DeVita VT, Schein PS. The use of drugs in combination for the treatment of cancer: Rationale and results. N Engl J Med. 1973;288(19):998–1006. [DOI] [PubMed] [Google Scholar]
- 83. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Children's Oncology Group. https://www.childrensoncologygroup.org/. Accessed January 4, 2017.
- 85. Pennock GK, Chow LQ. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist. 2015;20(7):812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fedewa SA, Ward EM, Stewart AK, et al. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: A national cohort study 2004–2006. J Clin Oncol. 2010;28(27):4135–4141. [DOI] [PubMed] [Google Scholar]
- 87. Griggs JJ, Hawley ST, Graff JJ, et al. Factors associated with receipt of breast cancer adjuvant chemotherapy in a diverse population-based sample. J Clin Oncol. 2012;30(25):3058–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schmid M, Meyer CP, Reznor G, et al. Racial differences in the surgical care of Medicare beneficiaries with localized prostate cancer. JAMA Oncol. 2016;2(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang EH, Yu JB, Abouassally R, et al. Disparities in treatment of patients with high-risk prostate cancer: Results from a population-based cohort. Urology. 2016;95:88–94. [DOI] [PubMed] [Google Scholar]
- 90. Warner ET, Tamimi RM, Hughes ME, et al. Racial and ethnic differences in breast cancer survival: Mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33(20):2254–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hershman DL, Tsui J, Wright JD, et al. Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. J Clin Oncol. 2015;33(9):1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Freedman RA, Virgo KS, He Y, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117(1):180–189. [DOI] [PubMed] [Google Scholar]
- 93. Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–357. [DOI] [PubMed] [Google Scholar]
- 94. US Bureau of Labor Statistics. Labor force and characteristics by race and ethnicity, 2014. Report 1057. BLS Reports. 2015. www.bls.gov. [Google Scholar]
- 95. Pinheiro PS, Morris CR, Liu L, et al. The impact of follow-up type and missed deaths on population-based cancer survival studies for Hispanics and Asians. J Natl Cancer Inst Monogr. 2014;2014(49):210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Johnson CJ, Weir HK, Yin D, et al. The impact of patient follow-up on population-based survival rates. J Registry Manag. 2010;37(3):86–103. [PubMed] [Google Scholar]
- 97. Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 suppl):IV–3–18. [DOI] [PubMed] [Google Scholar]
- 98. Feuer EJ, Lee M, Mariotto AB, et al. The Cancer Survival Query System: Making survival estimates from the Surveillance, Epidemiology, and End Results program more timely and relevant for recently diagnosed patients. Cancer. 2012;118(22):5652–5662. [DOI] [PubMed] [Google Scholar]
- 99. Feuer EJ, Rabin BA, Zou Z, et al. The Surveillance, Epidemiology, and End Results Cancer Survival Calculator SEER*CSC: Validation in a managed care setting. J Natl Cancer Inst Monogr. 2014;2014(49):265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chen VW, Eheman CR, Johnson CJ, et al. Enhancing cancer registry data for comparative effectiveness research (CER) project: overview and methodology. J Registry Manag. 2014;41(3):103–112. [PMC free article] [PubMed] [Google Scholar]
- 101. Liebler CA, Halpern-Manners A. A practical approach to using multiple-race response data: A bridging method for public-use microdata. Demography. 2008;45(1):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Arias E, Heron M, Hakes JK. The validity of race and Hispanic-origin reporting on death certificates in the United States: An update. National Center for Health Statistics. Vital Health Stat. 2016;2(172). [PubMed] [Google Scholar]
- 103. Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation US Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2162–2169. [DOI] [PubMed] [Google Scholar]
- 104. Pinheiro PS, Sherman RL. Why an alternative algorithm for identification of Hispanic subgroups is useful. J Registry Manag. 2009;36(1):3–4. [PubMed] [Google Scholar]
- 105. Lowy D, Singer D, DePinho R, et al. Cancer moonshot countdown. Nat Biotechnol. 2016;34(6):596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.The Vice President's Cancer Moonshot. https://www.whitehouse.gov/cancermoonshot. Accessed August 19, 2016.
- 107. Fojo T, Grady C. How much is life worth: Cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst. 2009;101(15):1044–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kantarjian HM, Fojo T, Mathisen M, et al. Cancer drugs in the United States: Justum Pretium—the just price. J Clin Oncol. 2013;31(28):3600–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med. 2011;364(21):2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zafar SY, Abernethy AP. Financial toxicity, Part I: A new name for a growing problem. Oncology (Williston Park). 2013;27(2):80–81, 149. [PMC free article] [PubMed] [Google Scholar]
- 111. Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood). 2013;32(6):1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34(9):980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology statement: A conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33(23):2563–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Aelion CM, Airhihenbuwa CO, Alemagno S, et al. The US Cancer Moonshot initiative. Lancet Oncol. 2016;17(5):e178–e180. [DOI] [PubMed] [Google Scholar]
- 115. Neugut AI, Gross CP. Targeting the Cancer Moonshot. JAMA Oncol. 2016;2(4):421–422. [DOI] [PubMed] [Google Scholar]
- 116. Thun MJ, Jemal A. How much of the decrease in cancer death rates in the United States is attributable to reductions in tobacco smoking? Tob Control. 2006;15(5):345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer. PLoS One. 2010;5(3):e9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jemal A, Thun M, Yu XQ, et al. Changes in smoking prevalence among US adults by state and region: Estimates from the Tobacco Use Supplement to the Current Population Survey, 1992–2007. BMC Public Health. 2011;11:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Holmes CB, King BA, Babb SD. Stuck in neutral: Stalled progress in statewide comprehensive smoke-free laws and cigarette excise taxes, United States, 2000–2014. Prev Chronic Dis. 2016;13:E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. American Cancer Society Cancer Action Network. How do you measure up? A progress report on state legislative activity to reduce cancer incidence and mortality. 14th ed.American Cancer Society Cancer Action Network; 2016. http://www.acscan.org/content/report-cards/2016/. Accessed August 22, 2016. [Google Scholar]
- 121. Fedewa SA, Sauer AG, Siegel RL, et al. Prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2015;24(4):637–652. [DOI] [PubMed] [Google Scholar]
- 122. Doria-Rose VP, White MC, Klabunde CN, et al. Use of lung cancer screening tests in the United States: Results from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jemal A, Fedewa SA. Prevalence of hepatitis C virus testing in cohorts born between 1945 and 1965 in the US Am J Prev Med. 2015;48(5):e7–e9. [DOI] [PubMed] [Google Scholar]
- 125. Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348(17):1625–1638. [DOI] [PubMed] [Google Scholar]
- 127. Cogliano VJ, Baan R, Straif K, et al. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103(24):1827–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.