Abstract
Metastasis, the spread and growth of malignant cells at secondary sites within a patient's body, accounts for > 90% of cancer-related mortality. Recently, impressive advances in novel therapies have dramatically prolonged survival and improved quality of life for many cancer patients. Sadly, incidence of brain metastatic recurrences is fast rising, and all current therapies are merely palliative. Hence, good experimental animal models are urgently needed to facilitate in-depth studies of the disease biology and to assess novel therapeutic regimens for preclinical evaluation. However, the standard in vivo metastasis assay via tail vein injection of cancer cells produces predominantly lung metastatic lesions; animals usually succumb to the lung tumor burden before any meaningful outgrowth of brain metastasis. Intracardiac injection of tumor cells produces metastatic lesions to multiple organ sites including the brain; however, the variability of tumor growth produced with this model is large, dampening its utility in evaluating therapeutic efficacy. To generate reliable and consistent animal models for brain metastasis study, here we describe a procedure for producing experimental brain metastasis in the house mouse (Mus musculus) via intracarotid injection of tumor cells. This approach allows one to produce large number of brain metastasis-bearing mice with similar growth and mortality characteristics, thus facilitating research efforts to study basic biological mechanisms and to assess novel therapeutic agents.
Keywords: Cancer Research, Issue 120, cancer metastasis, brain metastasis, preclinical, animal model, experimental model, intracarotid injection
Introduction
The metastasis of cancer to the central nervous system (CNS) is a devastating disease, and can involve either the brain parenchyma or the leptomeninges ("brain metastasis" refers to both in this article). It is the predominant intracranial malignancy, outnumbering primary gliomas by > 10:11,2. Lung cancer, breast cancer, and melanoma are the top three major neoplastic diseases that produce high incidences of brain metastasis3,4. In recent years, impressive advances in novel cancer therapies have dramatically prolonged survival and improved quality of life for many cancer patients. However, upon recurrence, the incidence of brain metastasis is rising rapidly. For example, the anti-HER2 antibody trastuzumab (Herceptin) has demonstrated significant clinical efficacy in patients with HER2+ breast cancer; yet a disturbing trend has emerged in these patients: up to 1/3 of those whose extracranial systemic disease initially benefited from trastuzumab treatment later develop brain metastasis5,6,7. Sadly, patients with brain metastasis are refractory to almost all current treatments, usually experiencing a traumatic deterioration of quality of life, and their one-year survival after diagnosis is only ~ 20%8. Current therapies for brain metastasis (including steroids, cranial radiotherapy, and surgical resection in selected patients) are merely palliative, not curative9. Therefore, brain metastasis is emerging as the next imposing challenge in this era of novel cancer therapies. To address the unmet challenge patients face every day in the clinic, we urgently need to better understand the underlying mechanism of brain metastasis and use this knowledge to develop novel therapies.
Successful colonization of the brain by metastatic cancer cells requires performance of a series of functions mediated by the intricate interaction of multiple biological players, such as the bypass of the blood brain barrier (BBB) and escape from the brain's intrinsic immune defensive mechanisms10, none of which is recapitulated in an ex vivo or in vitro system. Hence, proper and faithful in vivo models are critical for studies of brain metastasis. A conventional in vivo metastasis assay introduces cancer cells via tail vein injection, leading the majority of cells to become lodged in the lung. Brain metastatic lesions are rarely produced in these models before animal death caused by tumor burden in the lungs11. Direct intracerebral injection of cancer cells produces consistent tumor outgrowth in the CNS and is widely used in the studies of primary gliomas. However, such injection compromises the BBB and causes traumatic injury at the site of injection, both major points of concern as to the physiological relevance of this model. Another frequently used cancer cell introduction route, intracardiac injection, is easy to administer and does produce experimental metastasis to the CNS. However, concurrent metastases to organ sites other than the CNS are always produced and may cause animal mortality11; therefore, the high degree of variability of this model makes it inappropriate for quantitative evaluation of biological mechanisms or therapeutic agents with a limited number of animals.
Here we describe the procedures to produce experimental brain metastasis via the injection of cancer cells into the common carotid artery. We have used this approach to dissect individual genes' contributions to the metastatic cascade of brain metastasis and to evaluate efficacy of therapeutic interventions12,13. The major advantages of this approach are the high degree of reproducibility and low degree of variability; the major disadvantage is the sophistication and dexterity required to perform the microsurgery.
Protocol
Ethics statement: All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of The University of Texas MD Anderson Cancer Center.
1. Prepare Cancer Cells for Injection
Seed the cancer cells one or two days before injection. Use DMEM/F12 media supplemented with 10% FBS, unless a specialty medium is indicated in literature for a particular cell line.
On the day of surgical operation, harvest cells when they reach 70 - 80% confluence by first washing with serum-free media once before trypsinization (0.25%) for 1 - 2 min at 37 °C. Add into cells 10% FBS-containing DMEM/F12 media to quench the 0.25% trypsin.
Centrifuge the cells at 80 × g for 3 min. Wash cells in serum-free media twice to remove residual serum, count the cells with a hemocytometer and re-suspend in Hank's Balanced Salt Solution (HBSS) at 1 - 5 × 106 cells/ml, depending on the cell line. Keep on ice until time of injection. NOTE: MDA-MB-231 breast cancer cells and K1735 melanoma cells were used in this study, and both cell lines were used at 2 × 106 cells/ml concentration. Keep the trypsinization duration as short as possible as overtreatment may influence the outcomes of metastasis assays.
2. Prepare Mice for Tumor Cell Injection
Anesthetize mice by intraperitoneal (i.p.) injection of ketamine/xylazine cocktail (ketamine 100 mg/kg, xylazine 10 mg/kg).
Confirm complete anesthesia by pinching the animal feet and observe lack of response.
Place the mouse on a glass plate (used for casting protein gels), and secure with rubber bands.
If necessary, remove the hair of neck by shaving or applying a small amount of hair removal product and wiping the hair off with paper towel. NOTE: If using nude mice, skip this step as they do not have body hair.
Clean the neck skin by applying povidone-iodine and 70% alcohol.
Place mouse on stage of the dissecting microscope
Make an incision in the skin ~ 1 cm long with a surgical scalpel.
Bluntly dissect the muscle with toothed forceps to expose the carotid artery underneath.
Separate the carotid artery from the adjacent vagus nerve with surgical forceps.
Using surgical forceps, separate some cotton from a cotton ball, moisten, and fashion into a smaller cotton ball. Place this buffer-moisturized cotton ball underneath the carotid artery of intended site of injection.
Place a suture distal and proximal to the cotton ball and make loose knots. Tighten the proximal knot to block the blood flow into the injection site.
Proceed to cancer cell injection if the carotid artery on the cotton ball appears fully pressurized with fresh red blood.
3. Cancer Cell Injection into Carotid Artery
Vortex and draw 100 µl of cancer cells into the syringe.
Under the dissecting microscope, slowly insert the 31 G needle (with bevel up) into the lumen of the carotid artery sitting on top of the cotton ball.
Slowly inject the cells from the syringe into the carotid artery. Successful injection can be observed under the microscope by the changing color of nearby blood vessels and musculature when buffered cancer cells are pushed into the circulation.
When injection of the entire volume is complete, lift the distal carotid artery gently to prevent regurgitation.
Quickly tighten the distal knot to complete the injection process.
After completing the injection, tighten the ligatures and trim the excess silk suture with micro-scissors under the dissecting microscope. Move back the separated muscle to cover the wound site. Close the skin with two staples.
4. Surgical Recovery
Move the operated animal onto a heating pad for recovery. It may take 30 - 60 min for the animals to regain consciousness. In the meantime, administer buprenorphine (0.05 mg/kg) via subcutaneous injection as post-surgical analgesia. Note: Analgesia should be provided according to your local IACUC approved protocol.
Once the animals awaken and resume their normal behavior, return the mice to their housing location. Provide mice with gel food for several days post-surgery. Maintain a surgical record and keep it in the room.
5. Non-invasive In Vivo Imaging
For cells labelled with luciferase reporter constructs, measure cancer cell injection efficiency using in vivo imaging system14. NOTE: Imaging is not performed on the day of injection to avoid excessive stress to research animals.
Representative Results
There are two points at which the quality of injection can be evaluated. The first chance is by the operator's observation of changing blood vessel colors during injection. Leakage from poor injections can be easily observed under the dissecting microscope. The steady and secure placement of the mouse body (Figure 1A) and its carotid artery (Figure 1B) on the supporting cotton ball are critical factors for smooth and successful injection into the carotid artery. The second point to evaluate the injection quality is post-surgical non-invasive imaging. For this purpose, the cancer cells are typically labelled with a luciferase reporter ex vivo before injection. To minimize stress on the animals, in vivo imaging is usually performed 24 hr post-injection. A successful injection results in strong intracranial cancer cell load, and the mouse head is the only region with positive signals (Figure 2A).
Using in vivo imaging system to track the luciferase-labelled cancer cells, we observe an initial decline of signal intensity within days post-injection; yet, cancer cells capable of outgrowth in the CNS eventually manage to divide and produce increasing in vivo imaging system signals as brain metastases develop (Figure 2B). Intracarotid injection usually produces consistent in vivo imaging system signals with relatively low variability, which is a major advantage of using this approach to produce experimental brain metastasis. As growing tumor lesions displace brain tissues, the quality of health for animals with fast progressing brain metastasis can deteriorate rapidly, mirroring the clinical manifestation of human brain metastasis patients. Typical symptomatic behaviors indicative of the significant presence of brain metastasis include loss of weight, hunched posture, and incessant circling. At the experimental end point, the animal brains can be extracted for examination of biochemical parameters or for histological examinations. Except for melanotic metastasis lesions (Figure 3A), it is usually difficult to distinguish the tumor lesion from surrounding brain tissues prior to histological evaluation (Figure 3B). In such cases, it is advised to label tumor cells with a fluorescent reporter (e.g. GFP) for the convenience of isolating metastatic cells (Figure 3C). Alternatively, the entire brain can be dissociated into single cells and tumor cells separated by magnetic bead-conjugated antibodies.
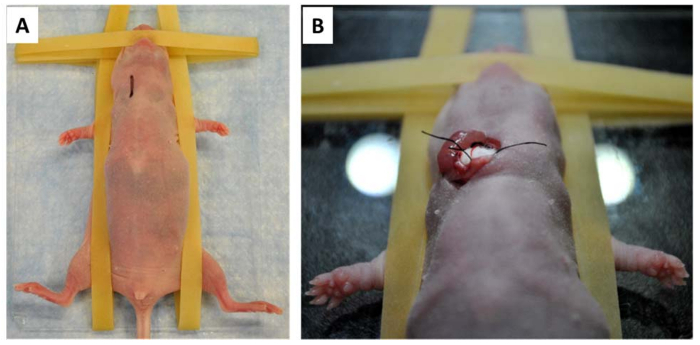 Figure 1: Preparation for carotid artery injection. A) The anesthetized mouse is placed on a glass plate and secured with rubber bands. The site and length of the incision is labelled with a black line on the neck. B) After all preparatory surgical steps are complete, the exposed carotid artery is placed for support on a firm cotton ball, ready for cancer cell injection. Please click here to view a larger version of this figure.
Figure 1: Preparation for carotid artery injection. A) The anesthetized mouse is placed on a glass plate and secured with rubber bands. The site and length of the incision is labelled with a black line on the neck. B) After all preparatory surgical steps are complete, the exposed carotid artery is placed for support on a firm cotton ball, ready for cancer cell injection. Please click here to view a larger version of this figure.
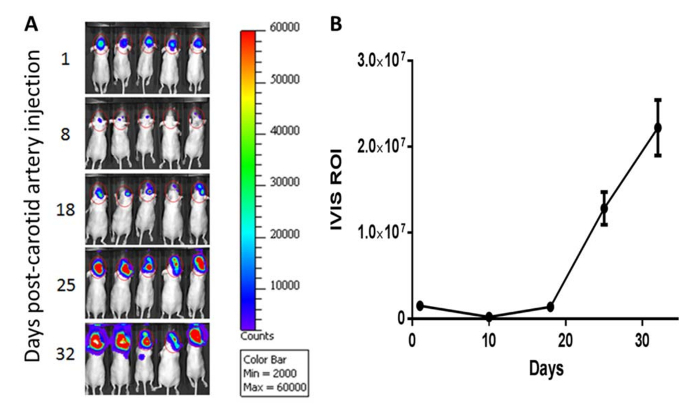 Figure 2: Non-invasive monitoring for brain metastasis outgrowth. A) Modified MDA-MB-231 breast cancer cells were labelled with luciferase reporter gene ex vivo. Starting from 24 hr post-carotid artery injection, brain metastasis development was monitored non-invasively by repeated in vivo imaging system imaging (scale bar = 50 mm). B) Quantification of in vivo imaging system imaging data indicates an initial decline of tumor burden post-carotid artery injection, followed by exponential outgrowth of brain metastasis until animal moribundity. Error bars reflect the standard error of the mean (SEM) of the total luminescence signal. Please click here to view a larger version of this figure.
Figure 2: Non-invasive monitoring for brain metastasis outgrowth. A) Modified MDA-MB-231 breast cancer cells were labelled with luciferase reporter gene ex vivo. Starting from 24 hr post-carotid artery injection, brain metastasis development was monitored non-invasively by repeated in vivo imaging system imaging (scale bar = 50 mm). B) Quantification of in vivo imaging system imaging data indicates an initial decline of tumor burden post-carotid artery injection, followed by exponential outgrowth of brain metastasis until animal moribundity. Error bars reflect the standard error of the mean (SEM) of the total luminescence signal. Please click here to view a larger version of this figure.
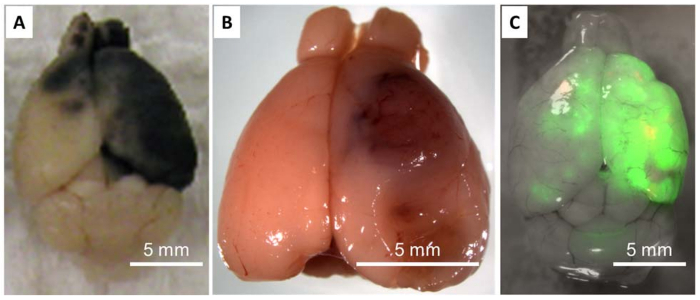 Figure 3: Harvesting Brain Metastasis Lesions. A) K1735 melanoma cells produce melanotic brain metastasis lesions in the syngeneic C3H mouse brain, which greatly facilitates the separation of brain metastatic lesions from the surrounding stroma but is limited to certain melanoma models. B) Dissociated mammary tumor cells from the transgenic MMTV-Neu/Pten-null mouse produces overt brain metastasis after implantation via carotid artery injection. The tumor/stroma margin is difficult to distinguish despite the obvious presence of angiogenic metastatic lesions. C) MDA-MB-231 breast cancer cells were pre-labelled with a green fluorescent protein (GFP) reporter ex vivo to facilitate extraction of brain metastasis lesions via surgical resection or cell sorting. Note that the majority of the metastatic lesions are concentrated on the same side of the brain where carotid arterial injection takes place. Please click here to view a larger version of this figure.
Figure 3: Harvesting Brain Metastasis Lesions. A) K1735 melanoma cells produce melanotic brain metastasis lesions in the syngeneic C3H mouse brain, which greatly facilitates the separation of brain metastatic lesions from the surrounding stroma but is limited to certain melanoma models. B) Dissociated mammary tumor cells from the transgenic MMTV-Neu/Pten-null mouse produces overt brain metastasis after implantation via carotid artery injection. The tumor/stroma margin is difficult to distinguish despite the obvious presence of angiogenic metastatic lesions. C) MDA-MB-231 breast cancer cells were pre-labelled with a green fluorescent protein (GFP) reporter ex vivo to facilitate extraction of brain metastasis lesions via surgical resection or cell sorting. Note that the majority of the metastatic lesions are concentrated on the same side of the brain where carotid arterial injection takes place. Please click here to view a larger version of this figure.
Discussion
The most critical steps for successful carotid arterial injection of cancer cells are: 1) deep anesthesia of mouse in preparation for surgical operation; 2) steady placement of carotid artery on top of the cotton support; 3) tight ligation of carotid artery after successful injection.
Deep anesthesia of ~ 30 min is usually necessary for steady surgical performance under the dissecting microscope. We use commercially ready-to-use ketamine/xylazine cocktail for mouse anesthesia. To avoid potential overdose, the ketamine/xylazine cocktail may be diluted 1:1 or 1:2 in physiological saline to provide more flexibility in adjusting the volume used for anesthesia. Alternatively, non-controlled drug tribromoethanol may also be used as anesthetic; however, we have found that the propensity of tribromoethanol to degrade makes the substance less reliable as an effective anesthetic than the ketamine/xylazine cocktail.
A steadily placed and fully pressurized carotid artery greatly facilitates the injection of cancer cells; therefore, we highly recommend spending time to carefully prepare the position of the artery under the microscope rather than proceeding with injection under suboptimal conditions.
More cancer cells will lodge within the vasculature on the side of carotid arterial injection. Sometimes a significant portion of the metastatic cells may migrate to the other side of the brain so that both hemispheres appear to bear significant amount of metastatic lesions in moribund animals.
Metastatic lesions may appear in either the parenchyma or the leptomeninges of the CNS, or in both locations. There appear to be unique tumor-microenvironment interactions that underlie these biological phenotypes15. Therefore, when a new cell line is to be tested for its capability to produce experimental brain metastasis, it is necessary to pay special attention to such phenotypes.
The internal and external carotid arteries supply blood into the brain parenchyma and leptomeninges, respectively. Accordingly, tumor cell deposition into the parenchyma or leptomeninges can be achieved by slight modification of this protocol. To allow cells only into leptomeninges, the internal carotid artery can be ligated by a suture prior to injection of tumor cells, thereby directing cells into the external carotid artery; on the other hand, ligation of the external carotid prior to injection will allow cells entry only into the internal carotid artery and the brain parenchyma.
Overall, the intracarotid injection approach produces data of higher reproducibility and lower variability compared to tail vein or intracardiac injections; it also avoids injection-induced CNS inflammation compared to the direct intracerebral injection method. Hence, this approach is currently the most valuable in quantitative studies of brain metastasis that aim to generate significant statistical conclusions using a limited number of animals. At the same time, all experimental models have limitations. Similar to other methods of hematogenous tumor cell introduction, the intracarotid injection only recapitulates later steps in the metastasis cascade after cancer cell dissemination into the circulation. Thus it is not an appropriate model to study, for example, the biological factors that determine the dissemination of primary cancer cells into the bloodstream.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors have no acknowledgements.
References
- Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29(6):533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- Barnholtz-Sloan JS, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- Bendell JC, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- Clayton AJ, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91(4):639–643. doi: 10.1038/sj.bjc.6601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romond EH, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- Mayer M. A patient perspective on brain metastases in breast cancer. Clin Cancer Res. 2007;13(6):1623–1624. doi: 10.1158/1078-0432.CCR-06-2842. [DOI] [PubMed] [Google Scholar]
- Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yu D. Microenvironment determinants of brain metastasis of brain metastasis. Cell Biosci. 2011;1(1):8. doi: 10.1186/2045-3701-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ, Nicolson GL. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J Natl Cancer Inst. 1976;57(5):1199–1202. doi: 10.1093/jnci/57.5.1199. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, et al. SRC family kinases as novel therapeutic targets to treat breast cancer brain metastases. Cancer Res. 2013;73(18):5764–5774. doi: 10.1158/0008-5472.CAN-12-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E, Modi KD, Kim J. In vivo bioluminescent imaging of mammary tumors using IVIS spectrum. J Vis Exp. 2009. [DOI] [PMC free article] [PubMed]
- Zhang C, Zhang F, Tsan R, Fidler IJ. Transforming growth factor-beta2 is a molecular determinant for site-specific melanoma metastasis in the brain. Cancer Res. 2009;69(3):828–835. doi: 10.1158/0008-5472.CAN-08-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]


