Abstract
Differential scanning calorimetry (DSC) is an analytical technique that measures the molar heat capacity of samples as a function of temperature. In the case of protein samples, DSC profiles provide information about thermal stability, and to some extent serves as a structural “fingerprint” that can be used to assess structural conformation. It is performed using a differential scanning calorimeter that measures the thermal transition temperature (melting temperature; Tm) and the energy required to disrupt the interactions stabilizing the tertiary structure (enthalpy; ∆H) of proteins. Comparisons are made between formulations as well as production lots, and differences in derived values indicate differences in thermal stability and structural conformation. Data illustrating the use of DSC in an industrial setting for stability studies as well as monitoring key manufacturing steps are provided as proof of the effectiveness of this protocol. In comparison to other methods for assessing the thermal stability of protein conformations, DSC is cost-effective, requires few sample preparation steps, and also provides a complete thermodynamic profile of the protein unfolding process.
Keywords: Biochemistry, Issue 121, Differential Scanning Calorimetry, Thermal Stability, Tertiary Structure, Protein Unfolding, Thermal Transition Temperature, Enthalpy, Protein Stability
Introduction
Differential scanning calorimetry (DSC) is an experimental method that directly measures the difference in heat energy uptake taking place in a sample relative to a reference during a regulated temperature change 1 2 3 4 5 6 7 8 9 10 11 12. Carried out in a differential scanning calorimeter, the method involves introducing heat energy into a sample cell and a reference cell simultaneously while identically increasing the temperature of both cells over time 2 13 14. Due to difference in the composition of the sample and the reference, different amount of energy will be required to raise the temperature of the cells 2 12 13. Thus, the excess amount of energy required to compensate for the temperature difference between the cells is measured and directly correlated to specific thermodynamic properties of the sample 1 3.
In the 1960s, M.J. O'Neil and E. Watson of Perkin Elmer developed the first differential scanning calorimeter to measure the heat flow of solid materials 2 3 4. In parallel, P.L. Privalov and D.R. Monaseldze E.L. of the Institute of Physics, Republic of Georgia (former USSR) created a unique differential adiabatic calorimeter that can be used for biochemical research 5 6. Subsequently, Andronikashvili's team at the Institute of Physics, Republic of Georgia, reported the heat capacity of biomolecules such as fibrous and globular proteins, DNA, and RNA using DSC 7 8 9. Several teams led by Sturtevant 10 11 12, Brandts 13, and Privalov 14 15 16focused on the development of the theory and practical applications of DSC to investigate the thermodynamic details of protein unfolding. The value of DSC in studying large supramolecular structures such as phages, chloroplast, phospholipid liquid crystals, and meat proteins have also been reported 17 18 19 20.
DSC has now become commonplace in pharmaceutical research and development for the assessment of the thermal stability of biomolecules, especially proteins 1 21 22. This is mostly due to advancements in terms of sensitivity and automation of the instrumentation used to carry out the experiment 23 24. Here, the final result of the DSC experiment, namely, molar heat capacity as a function of temperature, is used to estimate the following thermodynamic parameters (change in heat capacity (∆Cp), enthalpy (∆H), entropy (∆S), and Gibbs free energy (∆G)) using the equation below:
![]() (1)
(1)
![]() (2)
(2)
![]() (3)
(3)
![]() (4)
(4)
![]() (5)
(5)
Where Cp is measured heat capacity; q is the heat flow into the test material; T0 and T are the initial and final temperatures of the transition respectively 22 25. It is also worth noting that the equations above apply to single-domain proteins that can undergo two-state transition and reversible thermal unfolding 22. Analysis of more complex proteins (e.g., non-two-state proteins, and oligomers) have been reported by Friere et al. 26; Johnson et al. 27; and Kasimova et al. 28.
To determine whether a protein undergoes two-state transition or forms intermediates during thermal denaturation, the experimentally derived enthalpy (ΔH; also referred to as calorimetric enthalpy ΔHCal) is compared to the enthalpy derived using the van't Hoff equation given below (also referred to as van't Hoff enthalpy; ΔHVH):
![]() (6)
(6)
Where Tm is the midpoint temperature of the transition, R is the ideal gas constant (1.987 cal mol-1 K-1) and Y is the fraction of the protein population in the unfolded state 16 29. If ΔHVH is equal to ΔHCal; or ΔHVH/ΔHCal is equal to 1, then the protein undergoes an "all-or-none" transition (i.e. two-state transition) 16 25 29. However, if ΔHVH is less than ΔHCal; or ΔHVH/ΔHCal is less than 1, the protein undergoes a non-two-state transition 16 25 29. The ratio of ΔHVH/ΔHCal also corresponds to the proportion of the protein structure that melts as a thermodynamic cooperative unit or domain 26.
The thermodynamic parameters mentioned above such as ΔG and ΔH provide useful information about the thermal stability of proteins, including biologics 30. However, emphasis will be laid on Tm and ΔH in this publication, as they are the reported values for this protocol. Tm is the mid-point temperature of the transition, where the folded and the unfolded states of the protein are at equilibrium (i.e., ΔG = 0) 25 31. The higher the Tm of a protein, the higher its thermal stability 31. ΔH corresponds to the area under the peak(s) of the Heat Capacity versus Temperature graph (also known as thermogram) generated at the end of the DSC experiment 16 25. It is the energy required to denature proteins and can be used to estimate the active fraction (Fa) in a protein formulation (i.e., the proportion of proteins with active conformation in a sample) using the following equation:
![]() (7)
(7)
Where ΔH is the experimentally derived enthalpy of the protein sample and Q is the enthalpy determined for a well characterized reference or standardized protein 22. The estimation of Fa is significant for monitoring the real-time stability of products as well as conducting stability studies under stress conditions as required by ICH guidelines 32. Comparison of ΔH also provides information about the compactness of the tertiary structure conformation of a protein 31.
This protocol details a procedure for assessing the thermal stability of proteins in an industrial setting and has been extensively used for the formulation of vaccines. It was developed using an automated differential scanning calorimeter that generates reproducible results for protein concentrations as low as 300 µg/mL.
Protocol
1. Instrument Start-up
Switch on the differential scanning calorimeter and increase the pressure in the cells to suppress boiling of the samples as well as prevent the formation bubbles at elevated temperatures. This is typically achieved by supplying nitrogen into the system.
Depending on the constituting material of the cell (e.g., tantalum, gold, platinum, etc.), adjust the pressure of the nitrogen gas supply according to the manufacturer's recommended pressure to avoid damaging the cell. For example, set the pressure of the nitrogen gas supply to 45 psi for the instrument used to develop this procedure, and pressure above 80 psi may damage the cell.
Ensure that all the cleaning agent reservoirs are filled to the required volume. Cleaning agents required include detergent and water to wash and clean the cell respectively after each sample run.
Set the temperature of the sample holding compartment to a suitable value, preferably 5 °C, to maintain the integrity of the sample prior to experiment.
2. Sample Preparation
Dialyze the sample against the buffer that will be used as the reference for the experiment. Alternatively, elution buffer collected at the final step of protein purification (i.e. column elution) can be used.
Determine the concentration of the protein sample using the most suitable protein concentration determination method such as Kjedahl method 33 or Lowry method 34. For objective comparison of results, use the same method consistently within the same study. The required concentration range may vary depending on the model of the instrument. For the instrument used in this protocol, the preferable working range is 0.5 – 1 mg/mL.
Degas the sample and reference buffer in vacuum to get rid of microbubbles that can cause volume inaccuracy. This step can be skipped for newer calorimeter models.
Using a micropipette and sterile tips in a laminar flow biocontainment cabinet, load the samples and their respective buffer in pairs into 96 well plates compatible with the instrument. Fill the first two pairs of wells with buffer and the last two pairs with water for the buffer-buffer and the water scan respectively. Buffer-buffer scans verify the suitability of the instrument prior to sample measurement (i.e. assessment of instrumentation error) as well as establish a baseline; while water scans are run to clean the cells.
Cover the 96-well plate with a sealing film, and ensure that the wells are properly sealed before taking the plate out of the Biosafety Cabinet to avoid sample contamination.
Place the plate in the sample holding compartment in the proper orientation.
3. Experimental Parameter Setup
Note: Depending on the instrumentation, samples can be loaded into the cell either manually using a syringe, or automatically using an autosampler. In this case (i.e. an industrial setting), an autosampler is used to save time.
Using the acquisition software, enter the sample information in the order the plate was loaded as per section 2.4. Enter concentrations if available, otherwise, enter concentration values into analysis software prior to data analysis (section 4.2).
Select the option that ensures cleaning of cells with detergent before every sample scan, which should be followed by multiple water rinse steps to ensure no detergent residue is left in the cells.
Set the starting temperature of the experiment to 20 °C, but this can vary depending on prior knowledge of the sample. For known proteins, pre-determined starting temperature can be used, while a lower starting temperature can be applied for unknown samples.
Set the final temperature of the experiment, e.g. 100 °C. The final temperature may vary depending on prior knowledge of the sample.
Set the scan rate of the experiment, e.g. 60 °C/h, which is the typical scan rate. However, scan rate may vary depending on prior knowledge of the sample, e.g. 90 °C/h, or 120 °C/h. It is advisable to scan unknown samples at different scan rates to assess the kinetics of unfolding.
Rescan samples to investigate the reversibility of the thermal unfolding. The unfolding of a protein is considered reversible if the enthalpy obtained for the second scan is at least 80% of the enthalpy value for the first scan.
Set the post-experiment thermostat to 10 °C to preserve the integrity of the calorimeter's cells.
Verify that the experiment setup parameters are correct before executing the experiment. If everything is in place, start the experiment.
4. Data Analysis
Retrieve raw data from the experiment and select one sample at a time for analysis. Subtract reference scan, i.e. buffer, from the sample scan. NOTE: Reference subtraction is carried out automatically by newer models of DSC instruments.
Enter sample concentration value if it was omitted as per section 3.1.
Fit and subtract baseline from the acquired thermogram to account for differences in the heat capacities of the folded and unfolded states of the protein which is caused by the exposure of hydrophobic groups to water upon unfolding. Linear or cubic curve fitting can be applied depending on the shape of the DSC profile for the sample. For consistency, the same type of fitting has to be used during a study, e.g. real-time stability study. This step is required to process the curve for peak integration to obtain enthalpy of transition.
Perform peak integration using non-linear least square fit. Based on product knowledge apply two-state or non-two-state model. Two-state model can be used for single cooperative thermal transitions, and for unknown proteins, apply non-two state model until further product knowledge is available. If applicable, adjust curve fitting using the iterative curve fitting function of the equipment's software until the Chi-square value remains constant.
Obtained results will show the values for midpoint of transition temperatures (Tm), calorimetric enthalpy (ΔH), and van’t Hoff enthalpy (ΔHVH) of the sample.
Representative Results
The raw data from most DSC experiments are presented as a heat flux versus temperature graph, as the calorimeter actually measures the difference in the rate of heat flow into the sample solution and buffer 35. Therefore, if both cells (i.e. sample and reference cells) contain identical solutions during an experiment, the raw data from the scan should be a flat line with no observable peaks. Any peak observed can be attributed to instrumentation error (e.g. damaged or contaminated cells), which is why running buffer scans prior to sample analysis is an adequate system suitability test. Figure 1 illustrates the result of a typical buffer scan indicating that the calorimeter was in good working condition prior to sample analysis.
Figure 2 shows the raw data for a DSC experiment carried out on different lots of two protein samples. As implied earlier, the observed peaks are the differences in the heat flux of the samples and their respective buffers. Differences in sample concentration can cause variations in heat capacity recorded by the calorimeter; however, these variations are normalized during sample analysis as per section 4.2 of the procedure. Higher concentrations can also reveal additional thermodynamic domains not contributing to the transition at lower concentrations. In addition, each transition represents a thermodynamic domain that may include one or more structural domains of the protein 36. In this case, the Protein 1 has three structural domains that melt cooperatively.
Figure 3 shows the results generated from the analysis of the raw data for protein 1 and 2 presented in Figure 2, i.e., after baseline subtraction and iterative curve fitting. The resulting thermograms have been normalized for scanning rate (automatically carried out by a pre-set algorithm in the analysis software) and concentration; thus, presenting the results of the experiment in comparable heat capacity versus temperature graphs. The analysis software uses data from the Heat Capacity versus Temperature graphs, such as Tm and ΔCp, to derive other thermodynamic parameters using variations of the equations given above depending on the cooperativity of protein unfolding.
When testing unknown samples, setting the appropriate temperature range is crucial. Otherwise, incomplete thermograms may result, as illustrated in Figure 4. Although Tm from such profiles can be derived, ΔH cannot be accurately determined. Therefore, the sample must be retested with a larger temperature range to completely capture the thermal transition. Some proteins also readily form aggregates after complete denaturation, resulting in an increasing post-transition heat capacity: this often appears as an incomplete thermogram as illustrated in Figure 2B. However, retesting with a higher final temperature can help confirm whether there is an occurrence of a conformational transition at that region of the thermogram or it is merely the heat absorbing effect of protein aggregates.
Thermal stability is one of the most significant physical properties of proteins and protein-based products in the industry 37. In pharmaceuticals, it is used to determine the stability of biologics under different conditions, including formulation buffers and environmental factors such as humidity and temperature. It is also used to monitor key manufacturing step (e.g., purification and detoxification) to ensure conformational consistency between production lots. Figures 5 and 6 illustrate the use of DSC to examine the effects of chemical detoxification and storage conditions respectively on the stability and structural conformation of two different proteins. The significant differences in Tm and ∆H indicate conformational changes and protein degradation respectively. In addition, the loss of the third transition in Figure 6 further illustrates the degradation of a domain which was confirmed by a decrease in molecular weight when the samples were analyzed using Size-Exclusion Chromatography with multi-angle light scattering (SEC-MALS) (data not shown).
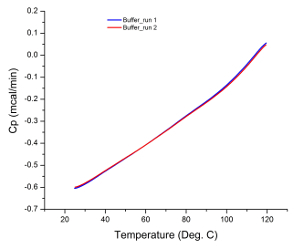 Figure 1: Buffer Scans. The similarity in gradient of each scan with no observable peaks indicates that the instrument is in good working condition and generated reproducible results. Please click here to view a larger version of this figure.
Figure 1: Buffer Scans. The similarity in gradient of each scan with no observable peaks indicates that the instrument is in good working condition and generated reproducible results. Please click here to view a larger version of this figure.
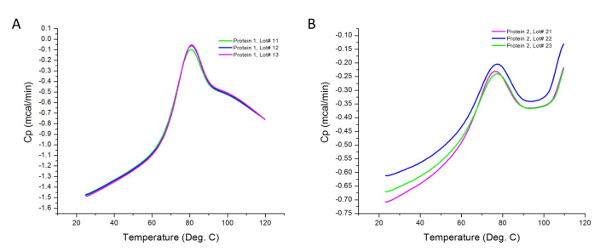 Figure 2: Raw Data Collected from DSC Experiments. These graphs are good representations of the unanalyzed (raw) data acquired after experimental runs (i.e. prior to baseline subtraction and curve fitting). Each line represents a production lot. Protein 2 tends to aggregate more readily upon heating, resulting in an increase in heat capacity above 100 °C in the post-transition region of the thermogram. Please click here to view a larger version of this figure.
Figure 2: Raw Data Collected from DSC Experiments. These graphs are good representations of the unanalyzed (raw) data acquired after experimental runs (i.e. prior to baseline subtraction and curve fitting). Each line represents a production lot. Protein 2 tends to aggregate more readily upon heating, resulting in an increase in heat capacity above 100 °C in the post-transition region of the thermogram. Please click here to view a larger version of this figure.
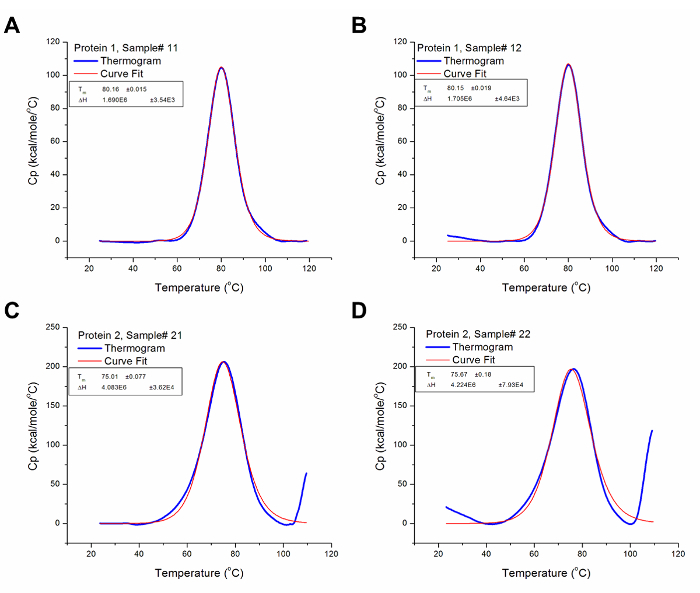 Figure 3: Analyzed DSC Data. These graphs are good representations of analyzed DSC data (i.e. after baseline subtraction and curve fitting). The blue line represents the thermogram after baseline subtraction, while the red line represents the curve with the best fit to the thermogram. (A) The Tm and ΔH for Protein 1 Sample # 12 are 80.16 °C and 1.69 x 106 cal/mol respectively. (B) The Tm and ΔH for Protein 1 Sample# 13 are 80.15 °C and 1.71 x 106 cal/mol respectively. (C) The Tm and ΔH for Protein 2 Sample # 21 are 75.01 °C and 4.08 x 106 cal/mol respectively. (D) The Tm and ΔH for Protein 2 Sample # 22 are 75.67 °C and 4.22 x 106 cal/mol respectively. Please click here to view a larger version of this figure.
Figure 3: Analyzed DSC Data. These graphs are good representations of analyzed DSC data (i.e. after baseline subtraction and curve fitting). The blue line represents the thermogram after baseline subtraction, while the red line represents the curve with the best fit to the thermogram. (A) The Tm and ΔH for Protein 1 Sample # 12 are 80.16 °C and 1.69 x 106 cal/mol respectively. (B) The Tm and ΔH for Protein 1 Sample# 13 are 80.15 °C and 1.71 x 106 cal/mol respectively. (C) The Tm and ΔH for Protein 2 Sample # 21 are 75.01 °C and 4.08 x 106 cal/mol respectively. (D) The Tm and ΔH for Protein 2 Sample # 22 are 75.67 °C and 4.22 x 106 cal/mol respectively. Please click here to view a larger version of this figure.
 Figure 4: An Incomplete Thermogram. Raw data collect for Protein 1 analyzed at an inadequate temperature range. The final temperature of experiment was set to 90 °C which did not accommodate the entire transition profile of the protein as compared to the experiment for Figure 2A which was set to 120 °C. Please click here to view a larger version of this figure.
Figure 4: An Incomplete Thermogram. Raw data collect for Protein 1 analyzed at an inadequate temperature range. The final temperature of experiment was set to 90 °C which did not accommodate the entire transition profile of the protein as compared to the experiment for Figure 2A which was set to 120 °C. Please click here to view a larger version of this figure.
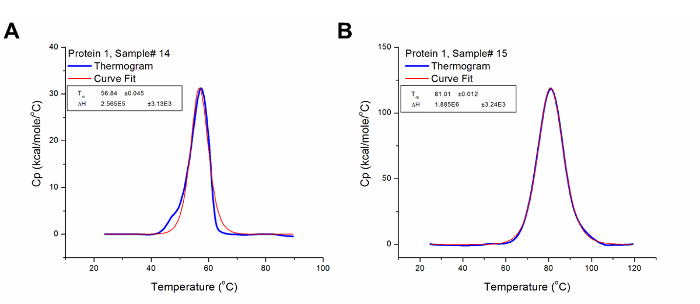 Figure 5: Analyzed Data Showing the Effect of Chemical Detoxification on the Tertiary Structure of Protein 1. (A) Protein 1 is a toxin in its native conformation and has its Tm at 56.84 °C and ΔH at 2.57 x 105 cal/mol. (B) The detoxified form of Protein 1 (i.e. toxoid) has Tm and ΔH values of 81.01 °C and 1.89 x 106 cal/mol respectively. Thus, it can be concluded that the detoxification step introduced some form of variation to the structural conformation of Protein 1 which confers greater stability (higher Tm) to its detoxified form. Please click here to view a larger version of this figure.
Figure 5: Analyzed Data Showing the Effect of Chemical Detoxification on the Tertiary Structure of Protein 1. (A) Protein 1 is a toxin in its native conformation and has its Tm at 56.84 °C and ΔH at 2.57 x 105 cal/mol. (B) The detoxified form of Protein 1 (i.e. toxoid) has Tm and ΔH values of 81.01 °C and 1.89 x 106 cal/mol respectively. Thus, it can be concluded that the detoxification step introduced some form of variation to the structural conformation of Protein 1 which confers greater stability (higher Tm) to its detoxified form. Please click here to view a larger version of this figure.
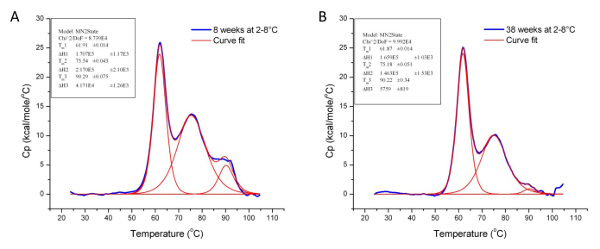 Figure 6: Analyzed Data Showing the Effect of Storage Conditions on the Conformation of Protein 3. These graphs illustrate the effect of storage temperature (2 - 8 °C) on the stability and tertiary structure of Protein 3 over 30 weeks. The Tm and ΔH values for Protein 3 at the 8th
(A) and 38th week (B) of storage are given in Table 1 below. Please click here to view a larger version of this figure.
Figure 6: Analyzed Data Showing the Effect of Storage Conditions on the Conformation of Protein 3. These graphs illustrate the effect of storage temperature (2 - 8 °C) on the stability and tertiary structure of Protein 3 over 30 weeks. The Tm and ΔH values for Protein 3 at the 8th
(A) and 38th week (B) of storage are given in Table 1 below. Please click here to view a larger version of this figure.
| Sample | Tm 1 (°C) | ∆H 1 (cal/mol) | Tm 2 (°C) | ∆H 2 (cal/mol) | Tm 3 (°C) | ∆H 3 (cal/mol) |
| Protein 3 stored at 2-8 °C for 8 weeks | 61.91 | 1.71 x 105 | 75.54 | 2.17 x 105 | 90.29 | 4.17 x 105 |
| Protein 3 stored at 2-8 °C for 38 weeks | 61.87 | 1.66 x 105 | 75.18 | 1.46 x 105 | 90.22 | 5.76 x 105 |
Table 1: Tm and ΔH Values for Protein 3 at the 8th and 38th Week of storage at 2 - 8 °C. Although the Tm values at both time points are similar, the difference in the ΔH values indicates that the tertiary structure of Protein 3 has degraded over 30 weeks under the specified storage condition.
Discussion
This procedure has been successfully incorporated in various characterization test packages, including stability and product comparability studies 21. In real-time stability studies, DSC is used to monitor the Tm, as well as estimate the Fa of biologics over time to determine their shelf-life. With regards to product comparability, it is used to assess the impact of process and facility change as well as the effect of key manufacturing steps on the structural conformation of produced lots. This typically involves the direct comparison of the ΔH of produced lot to a reference product that has been designated as the ideal product. In addition, DSC has proven to be a useful analytical tool for product formulation studies 37. The Tm of a protein in different buffers and at different concentrations can be used to determine the formulation that proffers the most stability to the protein.
To ensure the reliability of this method and objectivity of its results, it is important to keep testing parameters consistent from run to run within the same study (e.g., vaccine formulation study). However, the procedure can be modified to accommodate differences in the physical properties of various proteins. An example of a modification that can be made is altering the scanning rate of the experiment 38 39. Proteins that were prone to forming aggregates when heated were examined at a faster scanning rate (e.g., 120 °C/h) to avoid the contribution of aggregates to the thermal transition profile as well as clogging the capillaries of the calorimeter. It is worth noting that scanning rate can influence the outcome of a DSC experiment 38. Broadening of the thermal transition peak has been observed with increasing scanning rates in some proteins; however, Tm remained fairly constant 38. Furthermore, the dialysis and degassing steps for sample preparation are also very crucial for accurate results 31. Dialysis ensures that the only difference in the composition of the sample and the buffer is the protein; thus, all the excess heat absorbed by the sample can be attributed to the heat capacity of the protein. Degassing ensures precise volume analysis, as the extrapolation of the thermodynamic parameter assumes that the unfolding event is occurring under constant volume and pressure 31. The constant pressure portion of the assumption is accounted for by nitrogen pressurization of the system as per section 1.1 of the procedure.
In comparison to other methods of determining the stability of protein conformations such as Circular Dichroism (CD) and Fluorescence Spectroscopies, DSC offers a number of advantages in a commercial setting including cost and time savings. Firstly, the adiabatic design of a differential scanning calorimeter allows for the measurement of thermal stability with better temperature precision as compared to measurements with instrumentation for CD and Fluorescence Spectroscopies 6. Secondly, unlike CD, the accuracy of DSC data is not dependent on the helicity of the protein 39 40; however, CD provides additional information about the unfolding of the secondary structure, which would be complimentary to the DSC 41. Additionally, the pressurization of the DSC system allows for testing with a wide temperature range without boiling the sample; thus, a wide range of proteins can be tested by DSC.
While DSC is a relatively fast and straightforward approach to determine the thermal stability of biologics, it is not without limitations. First, the baseline subtraction step introduces some form of human inconsistency into the raw data analysis; thus, variations in results may be observed among different users. Second, differential scanning calorimeters have minimum concentration limits which might be difficult to achieve at bulk manufacturing scale. Third, the ΔH of irreversible thermal denaturation is not absolute; which implies that derived ΔG (an indicator of protein stability) in similar scenarios can be misleading. Furthermore, the method works best for purified samples. Presence of impurities may either cause a shift in the Tm if there is an interaction with the protein under investigation, or appearance of new thermal transitions if there is no interaction. In any case these extra features on the thermograms can be wrongly attributed to samples, thus impacting the interpretation of results. Despite these limitations, DSC remains a reliable method that can provide detailed thermodynamic information about the protein unfolding process if implemented properly 42.
In conclusion, DSC offers considerable advantage as a conformational readout tool for vaccine products and their intermediates. The two parameters, Tm and ΔH, collected for an array of lots of the same product can become an empirical baseline that can be used to examine the impact of process changes, formulation, and storage conditions on the tertiary structure and stability of protein and viral antigens 21 43.
Disclosures
All authors are employees at Sanofi Pasteur. The work was funded by Sanofi Pasteur, and publication fees for this video-article were paid by Sanofi Pasteur. The authors have no relevant affiliations or financial involvement with any organization or entity that has a financial conflict with the subject matter or materials discussed in the manuscript. These include employment, consultancies, stock ownership or options, or royalties.
No writing assistance was utilized in the production of this manuscript.
Acknowledgments
The authors are very thankful to Joseph Mancini (formerly with GE Healthcare), Pawel Czudec, Thomas Cage (Malvern Instruments limited) for their role in the installation and training on the differential scanning calorimeter, Sasmit Deshmukh and Webster Magcalas for their discussions.
References
- Gill P, Moghadam TT, Ranjbar B. Differential scanning calorimetry techniques: applications in biology and nanoscience. J. Biomol. Tech. 2010;21(4):167–193. [PMC free article] [PubMed] [Google Scholar]
- Watson ES, O'Neil MJ, inventors. Perkin Elmer Corp, assignee. Differential microcalorimeter. US3263484A. Patent. 1966 02 August.
- O'Neill MJ. The Analysis of a Temperature-Controlled Scanning Calorimeter. Anal. Chem. 1964;36(7):1238–1245. [Google Scholar]
- O'Neil MJ. Measurement of Specific Heat Functions by Differential Scanning Calorimetry. Anal. Chem. 1966;38(10):1331–1336. [Google Scholar]
- Privalov PL, Monaselidze DR. Mol. Biol. 1975;6:7–33. (in Russian) [Google Scholar]
- Privalov PL, Plotnikov VV. Adiabatic differential microcalorimeter. Адиабатический дифференциальный микрокалориметр. 1970. Authorship certificate No 328776 (USSR), applied 1970.
- Andronikashvili EL, et al. Calorimetric study of the nature of the intramolecular fusion of collagen, isolated from transplantable tumor. Dokl. Akad. Nauk. 1968;183(1):212–214. (In Russian) [PubMed] [Google Scholar]
- Andronikashvili EL, et al. Conformational Changes of Biopolymers in Solution. Moscow: Nauka Publishing House; 1973. pp. 171–173. (In Russian) [Google Scholar]
- Andronikashvili EL. Malignant transformation and changes in various physic-chemical properties of macromolecules and supramolecular structures. Biofizika. 1987;32(5):782–799. (In Russian) [PubMed] [Google Scholar]
- Velicelebi G, Sturtevant JM. Thermodynamics of the denaturation of lysozyme in alcohol-water mixtures. Biochemistry. 1979;18(7):1180–1186. doi: 10.1021/bi00574a010. [DOI] [PubMed] [Google Scholar]
- Sturtevant JM. Heat capacity and entropy changes in processes involving proteins. Proc. Natl. Acad. Sci. 1977;74(6):2236–2240. doi: 10.1073/pnas.74.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant JM. Biochemical applications of differential scanning calorimetry. Annu. Rev. of Phys. Chem. 1987;38:463–488. [Google Scholar]
- Jackson WM, Brandts JF. Thermodynamics of protein denaturation. A calorimetric study of the reversible denaturation of chymotrypsinogen and conclusions regarding the accuracy of the two-state approximation. Biochem. 1970;9(11):2294–2301. doi: 10.1021/bi00813a011. [DOI] [PubMed] [Google Scholar]
- Privalov PL, Khechinashvili NN, Atanasov BP. Thermodynamic analysis of thermal transitions in globular proteins. I. Calorimetric study of chymotrypsinogen, ribonuclease and myoglobin. Biopolymers. 1971;10(10):1865–1890. doi: 10.1002/bip.360101009. [DOI] [PubMed] [Google Scholar]
- Privalov PL, Khechinashvili NN. A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. J. Mol. Biol. 1974;86(3):665–684. doi: 10.1016/0022-2836(74)90188-0. [DOI] [PubMed] [Google Scholar]
- Privalov PL, Potekhin SA. Scanning microcalorimetry in studying temperature-induced changes in proteins. Methods Enzymol. 1986;131:4–51. doi: 10.1016/0076-6879(86)31033-4. [DOI] [PubMed] [Google Scholar]
- Veprintseva OD, Emelyanenko VI, Konstantinova VV, Shnyrov VL. The importance of structural changes in bacteriophage T4 tail proteins. Biofizika. 1988;33:954–961. (In Russian) [PubMed] [Google Scholar]
- Shutilova N, Semenova G, Klimov V, Shnyrov V. Temperature-induced functional and structural transformations of the photosystem II oxygen-evolving complex in spinach subchloroplast preparations. Biochem. Mol. Biol. Int. 1995;35(6):1233–1243. [PubMed] [Google Scholar]
- Sanina NM, Kostetsky EYa, Shnyrov VL. Calorimetric investigation of phosphatidyl choline from membranes of marine invertebrates. J. Evol. Biokhim. Physiol. 1987;23:451–460. [Google Scholar]
- Oreshkin EF, et al. Conformational changes in the muscle proteins of cured beef during heating. Meat Science. 1986;16(4):297–305. doi: 10.1016/0309-1740(86)90040-9. [DOI] [PubMed] [Google Scholar]
- Kirkitadze M, Hu J, Tang M, Carpick B. Qualification of a differential scanning calorimetry method for biophysical characterization of monoclonal antibodies and protein vaccine antigens. Pharm. Bioprocess. 2014;2(6):491–498. [Google Scholar]
- Jiskoot W, Daan C. Methods for structural analysis of protein pharmaceuticals. Vol. 3. Springer Science & Business Media; 2005. [Google Scholar]
- Plotnikov VV, Brandts M, Lin LN, Brandts JF. A new ultrasensitive scanning calorimeter. Anal. Biochem. 1997;250(2):237–244. doi: 10.1006/abio.1997.2236. [DOI] [PubMed] [Google Scholar]
- Plotnikov VV, et al. An autosampling differential scanning calorimeter instrument for studying molecular interactions. Assay Drug Dev. Technol. 2002;1(1):83–90. doi: 10.1089/154065802761001338. [DOI] [PubMed] [Google Scholar]
- Freire E. Differential scanning calorimetry. Protein Stability and Folding: Theory and Practice. Method in Molecular Biology. 1995;40:191–218. doi: 10.1385/0-89603-301-5:191. [DOI] [PubMed] [Google Scholar]
- Freire E, van Osdol WW, Mayorga OL, Sanchez-Ruiz JM. Calorimetrically determined dynamics of complex unfolding transitions in proteins. Annu. Rev. Biophys. Biophys. Chem. 1990;19:159–188. doi: 10.1146/annurev.bb.19.060190.001111. [DOI] [PubMed] [Google Scholar]
- Johnson CR, Morin PE, Arrowsmith CH, Freire E. Thermodynamic analysis of the structural stability of the tetrameric oligomerization domain of p53 tumor suppressor. Biochemistry. 1995;34(16):5309–5316. doi: 10.1021/bi00016a002. [DOI] [PubMed] [Google Scholar]
- Kasimova MR, Sam JM, Freire E. The conformational equilibrium of human growth hormone. J. Mol. Biol. 1998;277(2):409–418. doi: 10.1006/jmbi.1997.1613. [DOI] [PubMed] [Google Scholar]
- Saboury AA, Moosavi-Movahedi AA. Clarification of calorimetric and van't hoff enthalpies for evaluation of protein transition states. Biochem Edu. 1994;22(4):210–211. [Google Scholar]
- Privalov PL. Microcalorimetry of proteins and their complexes. Protein Structure, Stability, and Interactions. Method in Molecular Biology. 2009;490:1–39. doi: 10.1007/978-1-59745-367-7_1. [DOI] [PubMed] [Google Scholar]
- Dehghan-Nayeri N, Rezaei-Tavirani M. The Interpretation of Protein Structure Through Relationship of Melting Point (Tm) and Enthalpy of Unfolding (ΔHU) Int J Anal Pharma Biomed Sci. 2015;4(1):47–50. [Google Scholar]
- Stability testing of new drug substances and products. Guideline, ICH Harmonised Tripartite. 2003;1(2) current step. 4. [PubMed] [Google Scholar]
- Kjeldahl J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern (New method for the determination of nitrogen in organic substances) Zeitschrift für analytische Chemie. 1883;22(1):366–383. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- HÖhne G, Hemminger W, Flammersheim HJ. Differential Scanning Calorimetry. Springer Science & Business Media; 2003. [Google Scholar]
- Porter LL, George DR. A thermodynamic definition of protein domains. Proc. Natl. Acad. Sci. USA. 2012;109(24):9420–9425. doi: 10.1073/pnas.1202604109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer S, Daniel EO. Protein drug stability: a formulation challenge. Nature Reviews Drug Discovery. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- Johnson CM. Differential scanning calorimetry as a tool for protein folding and stability. Arch. Biochem. Biophys. 2013;531:100–109. doi: 10.1016/j.abb.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Chiu MH, Elmar JP. Differential scanning calorimetry: an invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J. Pharm. Bioallied Sci. 2011;3(1):39–59. doi: 10.4103/0975-7406.76463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst JD, Charles LB. Helicity, circular dichroism and molecular dynamics of proteins. J. Mol. Biol. 1994;243(2):173–178. doi: 10.1006/jmbi.1994.1644. [DOI] [PubMed] [Google Scholar]
- Kirkitadze MD, et al. Central modules of the vaccinia virus complement control protein are not in extensive contact. Biochem. J. 1999;344(1):167–175. [PMC free article] [PubMed] [Google Scholar]
- Freire E, SchÖn A, Hutchins BM, Brown RK. Chemical denaturation as a tool in the formulation optimization of biologics. Drug disc today. 2013;18(19):1007–1013. doi: 10.1016/j.drudis.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, et al. Applications of differential scanning calorimetry for thermal stability analysis of proteins: qualification of DSC. J. Pharm. Sci. 2012;101(3):955–964. doi: 10.1002/jps.22820. [DOI] [PubMed] [Google Scholar]


