Abstract
Trichinellosis is a debilitating disease in humans and is caused by the consumption of raw or undercooked meat of animals infected with the nematode larvae of the genus Trichinella. The most important sources of human infections worldwide are game meat and pork or pork products. In many countries, the prevention of human trichinellosis is based on the identification of infected animals by means of the artificial digestion of muscle samples from susceptible animal carcasses.
There are several methods based on the digestion of meat but the magnetic stirrer method is considered the gold standard. This method allows the detection of Trichinella larvae by microscopy after the enzymatic digestion of muscle samples and subsequent filtration and sedimentation steps. Although this method does not require special and expensive equipment, internal controls cannot be used. Therefore, stringent quality management should be applied throughout the test. The aim of the present work is to provide detailed handling instructions and critical control points of the method to analysts, based on the experience of the European Union Reference Laboratory for Parasites and the National Reference Laboratory of Germany for Trichinella.
Keywords: Infection, Issue 121, Trichinella larvae, food borne disease, trichinellosis, pork, game meat, magnetic stirrer method, digestion method, digestion, sedimentation, filtration
Introduction
Nematodes of the genus Trichinella can be detected in striated muscles of carnivore and omnivore mammals, birds, and reptiles worldwide. These zoonotic nematodes can reach human beings when raw or semi-raw meat and meat derived products from swine, horse, and game animals are ingested. This zoonosis can be a serious disease in humans characterized by pathognomonic signs and symptoms, e.g. diarrhea, fever, periorbital edema and myalgia and possible complications such as myocarditis, thromboembolic disease and encephalitis1.
Trichinella spiralis is the most widespread etiological agent of Trichinella infection in wild and domestic animals and causes most of the human infections worldwide. In Europe, North and West Africa, and Western Asia, Trichinella britovi is another source of human infections. In addition, ten other taxa are less commonly reported as the causative agents of the human disease and are found in different regions of the world, usually in wild animals2. In addition to wild animals such as wild boar, bears, walruses and badgers, pork represents the most important source of human infection worldwide.
The best way to prevent trichinellosis is to refrain from eating raw meat products and to cook any meat, especially game meat to safe temperatures (> 65 °C for 1 min, i.e. to change the meat color from pink to brown in the core of the meat product)3. The European Union and USDA have specified freezing and cooking times and temperatures for pork products that can be used to kill T. spiralis larvae in meat4,5. Furthermore, recommendations for the inactivation of Trichinella in meat and meat products were published by the International Commission on Trichinellosis6. In Europe, in addition to the introduction of controlled housing conditions in commercial swine herds where the risk of Trichinella infection is negligible, the prevention of human trichinellosis is based on the laboratory examination of muscle samples from susceptible animals4.
For food safety purposes, digestion assays are the only reliable procedures for the direct detection of Trichinella larvae in meat3,7. Even if there are several variations of the digestion assay, the magnetic stirrer method is the internationally accepted reference method3,4,7. This assay is based on the detection of Trichinella larvae in striated muscle tissues. After the enzymatic digestion of muscle samples and subsequent filtration and sedimentation steps, Trichinella larvae are identified by microscopy. Depending on trade obligations and national legislation, there are a multitude of small variations in the general protocol of the magnetic stirrer method. Here, technical details are based on EU requirements4, ISO specifications8, ICT guidelines6, and the experience of the European Union Reference Laboratory for Parasites (EURLP) and the German Reference Laboratory for Trichinella.
Protocol
1. Preparations
- Sample collection Note: The magnetic stirrer method can be used for single or pooled muscle samples.
- Collect samples from the appropriate predilection sites and in an appropriate amount9. Refer to the country requirements or to the recommendations of ICT, OIE or ISO.
- For the European Union, for example, take a 1 g sample from a diaphragm pillar at the transition to the sinewy part of domestic pigs. Ensure that all muscle samples are free of all fat and fascia.
- If the tongue is tested, remove the surface layer prior to testing.
- Take care if samples are frozen, as most Trichinella larvae are not freeze resistant and uncoil after death, are less resistant to digestion and tend to stick to the container walls, resulting in decreased assay sensitivity. Note: If frozen, double the sample size at least and increase the sedimentation time (see below).
- Preparation of digestion fluid
- Add 25% HCl (16 ± 0.5 mL) to 2 L of tap water preheated at 46 - 48 °C into a 3 L glass beaker. Too low of a temperature results in incomplete digestion; too high of a temperature deactivates the enzymatic properties of pepsin.
- Place a stirring rod in the beaker, and stir the solution on a preheated plate (46 - 48 °C).
- Add 10 ± 0.2 g of pepsin (1:10,000 NF, 1:12,500 BP, 2,000 FIP) to the acidic solution. Note: The quality of the pepsin is of utmost importance and enzyme activity must be certified by the producer. For laboratory safety reasons and to maintain the pepsin activity, the sequence of adding digestion fluid components must be adhered to.
- Muscle sample preparation
- Chop up to 100 g of muscle samples in a blender or grinder. To facilitate blending, add 2 mL preheated water to the samples and blend at max speed for 2 - 3 s.
- Before the second blending, open the blender and transfer any muscle sample at the top of the blending bowl edge margin to the bottom and repeat blending. Continue blending with bursts of 2 - 3 s until no visible pieces of meat remain. Note: Too little blending may result in incomplete digestion, while too much blending can damage the Trichinella larvae present in the samples6.
2. Procedure
- Muscle sample digestion
- Transfer 1 L of the digestion fluid into a cylinder. Transfer the ground meat into the beaker and disseminate in the digestion fluid with a spoon or fork. Thoroughly rinse the blender lid, blade and blender bowl with 500 mL of the preheated digestion fluid into the 3 L beaker. Note: Incomplete rinsing can result in the loss of sensitivity.
- Cover the beaker with aluminum foil and keep a constant temperature of 44 - 46 °C and monitor with a thermometer.
- Stir the digestion fluid for 30 min to create a deep vortex without splashing until the meat particles disappear. Depending on the type of meat tested (e.g. meat of wild animals), increase the digestion time until a maximum of 60 min.
- Filtration and sedimentation of the digestion fluid
- To determine the amount of undigested tissue, adjust the scale to zero, weigh the sieve before use, and note its weight. The sieve should be clean and devoid of any tissue particles, which could hinder the Trichinella larvae reaching the sedimentation funnel. Check that the separation funnel is leveled vertically.
- After digestion, carefully pour the digestion fluid through a 180 µm sieve into a separation glass funnel. The width of the separation glass funnel should not be larger than 55% of the length to allow good larva sedimentation. Take care to avoid any overflow.
- To avoid loss of sensitivity, thoroughly rinse the glass beaker and the sieve with approximately 200 mL of tap water from a squeeze wash bottle and transfer the rinsing water into the sedimentation glass funnel. Take care to carefully rinse the edges of the 3 L beaker. Lift the sieve and rinse the area under the sieve thoroughly.
- Determination of undigested tissue amount Note: Due to errors in the execution of the method, muscle tissue can remain on the sieve, as well fascia and connective tissue which are indigestible. The amount of undigested tissue is determined during the first 10 min sedimentation step (see 2.5). This allows approximately 30 min for the sieve to dry, as residual water can influence the determined weight of the undigested tissue.
- Place a paper towel on a scale, and weigh the sieve with the undigested tissues.
- Subtract the sieve weight before filtration from the sieve weight with undigested tissues. The digestion process is considered satisfactory if not more than 5% of the starting sample weight remains on the sieve (i.e. 5 g/100 g starting material).
- If the amount of undigested tissue exceeds 5%, repeat the test using fresh muscle samples. If the remaining tissue is predominantly comprised of undigested muscle tissue and fresh samples are not available, digest the undigested remains again and examine in addition to the original samples.
- Sedimentation of the digestion fluid
- Keep the filtered digestion fluid in the separatory funnel for 30 min. If left undisturbed, the Trichinella larvae will settle at the bottom of the funnel.
- If frozen samples are used, increase the sedimentation time to a maximum of 60 min.
- Once sedimentation is complete, fully open the stopcock of the separatory funnel to let at least 40 mL of digestion fluid quickly run off into a measuring cylinder (80 mL if muscle samples had been previously frozen).
- First, let half of the fluid into the cylinder and then fully open the stopcock in the opposite direction to allow 10 mL of digestion fluid to pass. Finally, open the stopcock in the opposite direction for another 10 mL. This procedure ensures that Trichinella larvae are not trapped in the stopcock. Note: Sufficient speed is needed to avoid larvae remaining in the separatory funnel, decreasing sensitivity.
- Sedimentation of the digestion fluid in the cylinder
- Leave the sediment in the cylinder for 10 min to allow larvae to sediment. Remove 30 mL supernatant by suctioning with a pipette without disturbing the 10 mL sediment.
- To reduce the amount of tissue fibers in the sample, add 30 mL tap water to the sediment and leave for another 10 min. Repeat until the liquid is clear.
- Remove supernatant and transfer the 10 mL washed sediment to a gridded Petri dish or larval counting basin. Rinse the cylinder with 10 mL tap water and then add the water to the sample. Note: As large amounts of debris in the sample can prevent the identification of the Trichinella larvae (Figure 1), further washing steps should be performed, if required.
- Microscopic examination
- Use a trichinoscope or a stereo-microscope with a sub-stage transmitted light source of adjustable intensity at a 15 to 20X magnification to examine the samples immediately after the washing steps.
- After pouring the sample into the gridded Petri dish, examine the dish/basin systematically grid by grid. If no suspect structures are found, gently move the fluid in the gridded Petri dish or larval counting basin in a circular fashion to allow any Trichinella larvae, which were potentially overlooked, to accumulate in the center of the Petri dish. Repeat the examination.
- If a suspect structure is found, increase magnification to 40 - 100X. Remove Trichinella larvae from the dish/basin with a pipette and collect in a tube with 90% ethanol.
- After the complete dish has been examined, repeat the procedure, starting from the gentle shaking of the dish/basin. Repeat the procedure at least three times or until no more larvae are found.
- Count the number of larvae. Correlate to the amount of muscle tissue tested and present as larvae per gram (lpg).
- If too many larvae are present in the digestion fluid to determine the larval number reliably, return the fluid containing the larvae to the measuring cylinder, rinse with tap water from a squeeze wash bottle, and leave larvae to settle to the bottom.
- After a 10 min sedimentation time, reduce the supernatant to a defined volume (e.g. 10 mL). To determine the exact volume, transfer the supernatant into a new cylinder with a pipette.
- Suspend larvae homogenously in the fluid by vigorous shaking. During the shaking motion, add 12 drops of 20 µL larval suspension onto a Petri dish.
- Examine each drop by microscope. Determine the number of larvae in 240 µL and delete the highest and lowest count. Extrapolate the number of larvae to the total volume (e.g. 10 mL) of supernatant. Note: The quality of the stereo-microscope is of paramount importance for the identification of Trichinella larvae.
- When larvae are collected in a tube by a pipette, check carefully that the larvae did not stick on the pipette wall but are transferred to the tube. Note: Positive Trichinella findings from pooled samples must be traced back from the pool to the carcass of origin. Here, the pool size should progressively be decreased until the positive carcass is identified. The sample size can also be increased during this process to increase sensitivity. Where examination of a collective sample produces a positive or uncertain result, a further 20 g sample is taken from each pig. The 20 g samples from five pigs are pooled and examined using the described method. In this way samples from 20 groups of five pigs will be examined. When Trichinella is detected in a pooled sample from five pigs, 20 g samples are collected from the individual pigs in the group and each is examined separately until the positive carcass of origin is detected.
Representative Results
After digestion, the shape of the infective muscle larvae can vary, making identification more difficult. Typical forms include tightly coiled larvae, lightly coiled larvae or c-shaped or completely uncoiled larvae (Figures 2B - 2C). The number of larvae found per gram can vary considerably and can range from one larva to a few hundred larvae.
The morphology of the infective muscle larva is shown in Figure 2A. Trichinella larvae are 0.7 to 1.5 mm long and approximately 0.3 mm in width. The esophagus is narrow and has a slightly rounded end. The cuticle is smooth. In the anterior half of the body cavity, the stichosome, a structure constituted of a long slender tube surrounded by a row of 45 to 55 large cells (stichocytes), can be observed. The rectum is also rounded without any projections or appendages10,11.
Other structures, besides Trichinella larvae, can be found after muscle digestion. Some examples are shown in Figures 3A - 3F. Wild animals are often infected with other parasites or the muscle samples available for testing are contaminated during the evisceration process by animate or inanimate structures/organisms. The length of the larvae, the appearance of the anterior and posterior ends and the occurrence of the stichosome help to discriminate between the different nematode genera12.
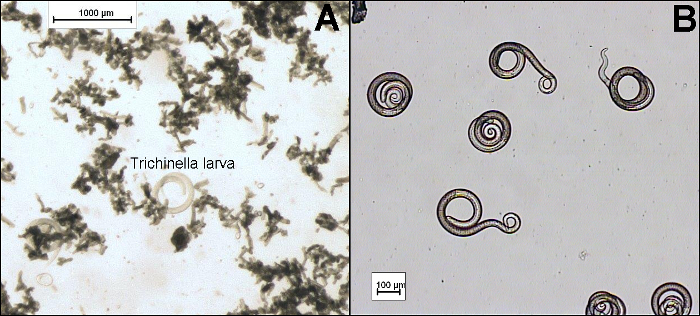 Figure 1:Microscopic Examination of Trichinella Larvae After (A) Insufficient and (B) Sufficient Rinsing Steps. Scale bars indicate 100 µm. Please click here to view a larger version of this figure.
Figure 1:Microscopic Examination of Trichinella Larvae After (A) Insufficient and (B) Sufficient Rinsing Steps. Scale bars indicate 100 µm. Please click here to view a larger version of this figure.
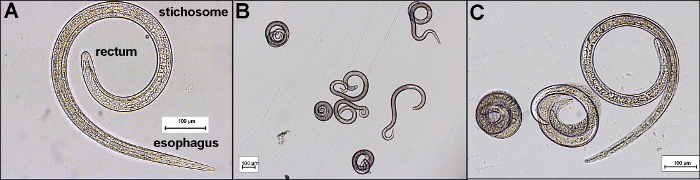 Figure 2:Morphology of Infective Trichinella Larvae Showing the Rectum, Stichosome and Esophagus of the Larva (A). Possible shapes of the muscle larvae after digestion showing (B) tightly coiled and lightly coiled moving larvae and (C) uncoiled larvae. Scale bars indicate 100 µm. Please click here to view a larger version of this figure.
Figure 2:Morphology of Infective Trichinella Larvae Showing the Rectum, Stichosome and Esophagus of the Larva (A). Possible shapes of the muscle larvae after digestion showing (B) tightly coiled and lightly coiled moving larvae and (C) uncoiled larvae. Scale bars indicate 100 µm. Please click here to view a larger version of this figure.
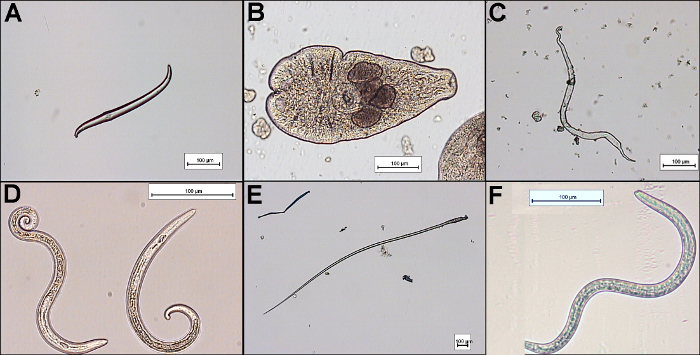 Figure 3:Examples of Incidental Findings after Digestion of Muscle Samples with the Magnetic Stirrer Method.(A) bristle-like hair of earth worm found in wild boar; (B) Alaria alata from wild boar; (C) muscle fiber from wild boar; (D)
Metastrongylus sp. from wild boar; (E) plant fiber found in wild boar (F): Toxocara sp. larva from wild boar. Scale bars indicate 100 µm. Please click here to view a larger version of this figure.
Figure 3:Examples of Incidental Findings after Digestion of Muscle Samples with the Magnetic Stirrer Method.(A) bristle-like hair of earth worm found in wild boar; (B) Alaria alata from wild boar; (C) muscle fiber from wild boar; (D)
Metastrongylus sp. from wild boar; (E) plant fiber found in wild boar (F): Toxocara sp. larva from wild boar. Scale bars indicate 100 µm. Please click here to view a larger version of this figure.
Discussion
Although the magnetic stirrer method can be easily performed, not strictly adhering to technical details leads to insufficient sensitivity, thus endangering consumer health. The critical control points have been highlighted in the text above. In addition, the use of appropriate equipment is crucial for the successful outcome of the test. All vessels should be made of glass and pipette tips coated with silicon to reduce adherence of larvae to the surface.
The sensitivity of the digestion method depends on the larval density in the muscles and the amount of muscle sample tested. For a larval density of 3 - 5 lpg of muscle tissues, a sensitivity of 100% was reported, but below 1 lpg the sensitivity dropped to 40%13. Depending on the tested host animal species, the sensitivity of the test can be improved by increasing the amount of sample used. The infection burden in wildlife is usually low, therefore larger samples sizes are used14. Predilection sites also vary among host animals. Here, the lower digestibility of some muscle tissues such as the tongue needs to be taken into account, which can also result in larger sample sizes15.
Further, it is important to understand that the magnetic stirrer method does not include internal controls. Therefore, quality assurance management from sampling to documentation is essential to obtain accurate and precise results. The quality of laboratory performance to detect Trichinella larvae in muscle samples should be monitored by regular participation of each analyst in proficiency tests.
Further limitations of the method are that the final identification stage of muscle larvae by microscopy is highly subjective and heavily dependent on the knowledge of larval morphology by the examining analyst.
An interesting alternative method to circumvent this problem is the magnetic stirrer method/on filter isolation followed by larvae detection by a latex agglutination test. However, according to EU legislation this method is only considered equivalent for the testing of meat of domestic swine but not for animals which are at higher risk of infection such as wild boar4. The identification of the larval antigen with monoclonal antibodies makes the test more objective, but denies the laboratory the possibility of determining the number of larvae per gram of meat16.
Further variations of the magnetic stirrer method include the mechanically assisted pooled sample digestion method/sedimentation technique, the mechanically assisted pooled sample digestion method/on filter isolation technique, and the automatic digestion method for pooled samples of up to 35 g technique. These techniques are all based on the artificial digestion of the meat and the visualization and counting of Trichinella larvae, but differ in the equipment used for the filtration and sedimentation steps.
The isolated larvae can be further identified at the species level by a molecular test (e.g., multiplex PCR)17. According to EU legislation, all positive samples must be forwarded to the national reference laboratory or to EURLP for the Trichinella species determination.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
We kindly thank Sabine Reckinger for excellent technical assistance and support.
References
- Gottstein B, Pozio E, Nöckler K. Epidemiology, Diagnosis, Treatment, and Control of Trichinellosis. Clin. Microbiol. Rev. 2009;22:127–145. doi: 10.1128/CMR.00026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozio E, Murrell DK. Systematics and epidemiology of Trichinella. Adv. Parasitol. 2006;63:367–439. doi: 10.1016/S0065-308X(06)63005-4. [DOI] [PubMed] [Google Scholar]
- OIE World Organisation for Animal Health. Principles and methods of validation of diagnostic assays for infectious diseases. OIE Terrestrial Manual. 2013. pp. 1–16. Available from: http://wahis2-devt.oie.int/fileadmin/Home/fr/Health_standards/tahm/1.01.05_VALIDATION.pdf.
- European Commission. Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 laying down specific rules on official controls for Trichinella in meat. Off. J. EU. 2015;212:7–34. Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32015R1375. [Google Scholar]
- United States Department of Agriculture, Food Safety and Inspection Service. 9 CFR Part 318.10 - Prescribed treatment of pork and products containing pork to destroy trichinae. U.S. Government Publishing Office. 2012. Available from: https://www.gpo.gov/fdsys/pkg/CFR-2016-title9-vol2/pdf/CFR-2016-title9-vol2-sec318-10.pdf.
- Gamble HR, et al. International Commission on Trichinellosis: recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet. Parasitol. 2000;93(3-4):393–408. doi: 10.1016/s0304-4017(00)00354-x. [DOI] [PubMed] [Google Scholar]
- Gajadhar AA, Forbes LB. An internationally recognized quality assurance system for diagnostic parasitology in animal health and food safety, with example data on trichinellosis. Vet. Parasitol. 2002;103:133–140. doi: 10.1016/s0304-4017(01)00535-0. [DOI] [PubMed] [Google Scholar]
- ISO. Microbiology of the food chain -- Detection of Trichinella larvae in meat by artificial digestion method 18743. ISO. 2015. Available from: http://www.iso.org/
- Nöckler K, Kapel CMO. FAO/WHO/OIE Guidelines for the surveillance, management, prevention and control of trichinellosis. Paris: World Organization for Animal Health (OIE); 2007. Chapter 3, Detection and surveillance for Trichinella: meat inspection and hygiene, and legislation; pp. 69–98. [Google Scholar]
- Boch J, Schnieder T, Supperer R. Veterinärmedizinische Parasitologie. Stuttgart: Parey Verlag; 2006. 6. Aufl. [Google Scholar]
- Despommier DD, Müller M. The stichosome and its secretion granules in the mature muscle larva of Trichinella spiralis. J. Parasitol. 1976;62(5):775–785. [PubMed] [Google Scholar]
- Marucci G, Interisano M, La Rosa G, Pozio E. Molecular identification of nematode larvae different from those of the Trichinella genus detected by muscle digestion. Vet Parasitol. 2013;194:117–120. doi: 10.1016/j.vetpar.2013.01.034. [DOI] [PubMed] [Google Scholar]
- Forbes LB, Gajadhar AA. A validated Trichinella digestion assay and an associated sampling and quality assurance system for use in testing pork and horse meat. J. Food Prot. 1999;62:1308–1313. doi: 10.4315/0362-028x-62.11.1308. [DOI] [PubMed] [Google Scholar]
- Malakauskas A, et al. Molecular epidemiology of Trichinella spp. in three Baltic countries: Lithuania, Latvia, and Estonia. Parasitol. Res. 2007;100(4):687–693. doi: 10.1007/s00436-006-0320-y. [DOI] [PubMed] [Google Scholar]
- Kapel CM, Webster P, Gamble HR. Muscle distribution of sylvatic and domestic Trichinella larvae in production animals and wildlife. Vet. Parasitol. 2005;132(1-2):101–105. doi: 10.1016/j.vetpar.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Interisano M, et al. Validation of a latex agglutination test for the detection of Trichinella infections in pigs. Vet. Parasitol. 2013;194:121–124. doi: 10.1016/j.vetpar.2013.01.035. [DOI] [PubMed] [Google Scholar]
- Pozio E, La Rosa , G PCR-derived methods for the identification of Trichinella parasites from animal and human samples. Methods Mol. Biol. 2003;216:299–309. doi: 10.1385/1-59259-344-5:299. [DOI] [PubMed] [Google Scholar]


