Abstract
Standard toxicity evaluations of insecticides against insect pests are primarily conducted on adult insects. Evaluations are based on a dose-response or concentration-response curve, where mortality increases as the dose or concentration of an insecticide is increased. Standard lethal concentration (LC50) and lethal dose (LD50) tests that result in 50% mortality of a test population can be challenging for evaluating toxicity of insecticides against non-adult insect life stages, such as eggs and early instar or nymphal stages. However, this information is essential for understanding insecticide efficacy in all bed bug life stages, which affects control and treatment efforts. This protocol uses a standard dipping bioassay modified for bed bug eggs and a contact insecticidal assay for treating nymphal first instars. These assays produce a concentration-response curve to further quantify LC50 values for insecticide evaluations.
Keywords: Environmental Sciences, Issue 121, lethal concentration, dipping assay, nymph, concentration-response, Cimex lectularius, insecticide
Introduction
Bed bugs are a significant urban pest that can cause blood loss, skin irritations, sleeplessness, depression, and anxiety in their human hosts 1,2,3,4,5,6. The costs of eliminating and controlling bed bugs are high and often times require multiple visits to a home by a pest control company7. Multiple visits are usually required because of the cryptic behavior of bed bugs and the difficulties associated with killing them. In particular, bed bug eggs are difficult to control because of their small size and the protection of the embryo by the eggshell.
Currently, a common practice for many pest control companies throughout the United States is to treat a home for bed bugs using a chemical insecticide. It is well known that the eggs are difficult to control, so many companies have implemented a two-week time frame for re-treatments8. This allows bed bug eggs enough time to hatch, so that the first instar will emerge and purportedly be easier to kill with insecticides. However, there is a dearth in studies evaluating the efficacy of liquid insecticides against bed bug eggs and first instars.
Insect eggs have been documented to be the most difficult life stage to control in urban insect pests, other than bed bugs, in addition to many agricultural pests. Most of these difficulties have been attributed to the eggshell, while a few studies have reported insecticide resistance. Resistance in the egg stage has been documented in Triatoma infestans9, Pediculus humanus capitis10, Plutella xylostella11, Rhyzopertha dominica12, Cimex lectularius13, and multiple stored product beetles (i.e. Oryzaephilus surinamensis, Tribolium castaneum, Cryptolestes ferrugineus and Rhyzopertha dominica).14The development of resistance in the egg stage and immature stages, as well as complications associated with insecticide penetration through the eggshell, necessitates the need for insecticide efficacy bioassays against these life stages.
This protocol provides a step-by-step procedure for determining the efficacy of insecticides in the egg and first instar stage of common bed bugs, Cimex lectularius. Both of these protocols are concentration-response assays to allow quantification of LC50 values. Concentration-response assays are commonly used for toxicological studies, however this protocol has been adapted for easily treating groups of bed bug eggs and first instars. These assays can be adapted for various insect species' eggs and immature life stages.
Protocol
NOTE: This protocol includes two insecticide assays for separately treating bed bug eggs and first instars. Both protocols were conducted using the same insecticides, however, the protocols had to be adapted to ensure insecticide exposure and to easily manipulate the specimens.
1. Egg Dipping Insecticide Assay
- Pull mated, well-fed adult female bed bugs from a colony and place the females (5-10) into a Petri dish with filter paper. Have at least ten replicates to ensure that there will be enough eggs for the insecticide assay. Check to see if the females have laid eggs every day.
- Once they lay eggs, take the females out of the Petri dish (6 cm × 5 cm) and put them into new Petri dishes with a new filter paper daily and record the date on the old and new Petri dishes.
- Allow the eggs to age 4 to 5 days before starting the assay. Susceptibility to insecticides changes during embryonic development, so it is important to control for egg age. Hold all life stages at 12:12 L:D photoperiod, 60% RH, and 27 °C.
Once the eggs have aged appropriately, remove the bed bug eggs gently from filter papers using soft-tip forceps. Gently scrape the bottom of the egg attached to the filter paper using the forceps and then gently grab without applying much pressure on the egg to limit throwing bed bug eggs.
Place the removed bed bug eggs into their respective groups inside of plastic Petri dishes. Limit static electricity from flinging bed bug eggs by rubbing the bottom of each Petri dish on the outside with a fabric softener sheet. Treat groups of bed bug eggs (5-10 eggs per group) all at once. Consider one group a replicate and use at least five (or more) replicates for this assay.
Prepare serial dilutions of insecticides to get a range of 5 different concentrations (0.21-21 µL/mL for imidacloprid (0.1%)/β-cyfluthrin (0.5%) and a control solution with solvent (tap water) only. Make 20 mL of each concentration to have enough volume for the dipping assay.
Cut centrifuge tubes (50 mL) at the 35 mL line medially using a rotary tool with a cut-off wheel accessory and then cut a hole into the cap of the centrifuge tube (diameter = 2.54 cm) using the same rotary tool with an aluminum oxide grinding stone accessory. Leave 5 cm on the edge of the cap when cutting, so that the cap will securely screw into the tube (Figure 1).
Cut fine mesh into squares large enough to fit into the cap (~5 cm2). Screw the mesh tightly, eliminating any bumps, into the centrifuge tubes using the centrifuge tube cap.
Place a group of bed bug eggs (one replicate) using a paint brush onto the mesh inside of the centrifuge tube. Dip the group of bed bugs inside of the cut centrifuge tube into an insecticide concentration for 5 seconds.
Remove the centrifuge tube out of the insecticide solution. Dry the mesh with the eggs inside by placing the centrifuge tube directly onto a laboratory tissue.
Remove the group of eggs out of the centrifuge tube using a small, fine-bristle paint brush and place them into a clean Petri Dish with a clean filter paper.
Record egg mortality (failure to hatch) for 14 days to ensure all eggs have had sufficient time to hatch.
Correct for mortality in the control treatment using Abbott's formula (% test mortality -% control mortality/ 100 - control mortality x 100).
Insert recorded mortality with the corresponding concentrations into the analysis software15 to calculate LC50 values.

2. First Instar Insecticide Assay
Separate a group of well-fed bed bugs (5 adult males and 5 adult females), preferably fed 1 day prior, and place them into a Petri dish (6 cm × 5 cm) with filter paper provided for mating and egg laying. Allow the adult bed bugs to mate and lay eggs for approximately 7-9 days. For feeding, use an artificial feeding system that circulates hot water in glass tubes surrounding blood16. For this study, use defibronated rabbit blood.
Remove the adults from the Petri dishes (6 cm × 5 cm) and allow the eggs to hatch into first instars within the Petri dishes. It takes approximately 7-9 days for bed bug eggs to hatch at room temperature.
Collect the unfed first instars of the same age using a paint brush. Age can be determined by removing the first instars daily and recording the day they hatched. Place them into new, clean Petri dishes until the insecticide assay. First instar nymphs are not very desiccant resistant, so the insecticide assay should be completed as soon as possible.
For the assay, prepare serial dilutions of imidacloprid/β-cyfluthrin to get a range of 5 different concentrations and a control solution with solvent only. Formulated products labeled for bed bug control in the United States were used for these studies, therefore the solvent was water (according to the label).
Pipette 150 µL aliquots of each insecticide concentration and water for the control group onto individual filter papers (#1; 4.2 cm diameter). Distribute the insecticide over the entire filter paper using the pipetter by applying droplets around the entire edge and center of the filter paper.
Place the treated filter papers individually on top of individual hardboard panels (7 cm2).
Release groups of first instars (5 to 10 per group) onto the individual filter papers while still wet with insecticide and cover the first instars with an inverted bottom of a Petri dish.
Place a weight on top of the Petri dish to prevent the first instar bed bugs from escaping the treated surface.
Record mortality of first instar bed bugs after 24 h exposure to all insecticide concentrations.
Correct for mortality in the control treatment using Abbott's formula (% test mortality -% control mortality/ 100 - control mortality x 100).
Insert recorded mortality and concentrations into the analysis software15 to calculate LC50 values.
Representative Results
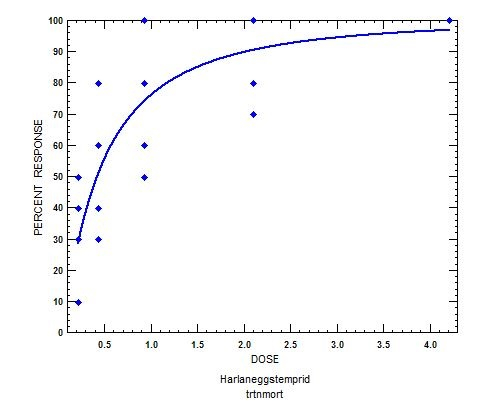
The eggs were dipped into 5 different concentrations of imidacloprid/ β-cyfluthrin (µL/mL). The first instars were placed onto treated surfaces of five different concentrations of imidacloprid/β-cyfluthrin (Figure 2).
We used three different populations of C. lectularius: Harlan, Richmond, and Epic Center (Table 1). The Harlan strain is susceptible to pyrethroid insecticides and was acquired in 2005 from Dr. Harold Harlan. The Richmond and Epic Center strains are both resistant to deltamethrin. The Richmond strain was collected from an elderly group home in Richmond, VA in 2008. The Epic Center strain was collected in 2008 in Cincinnati, OH.
Higher LC50 values indicated that a higher concentration was required to kill 50% of the test population eggs from Richmond and Epic Center strains. Similar to the eggs, the first instars from the Richmond and Epic Center populations required higher concentrations of insecticide to kill 50% of the test population. The LC50 values followed by different letters are significantly different.
Figure 1. Photograph of the modified centrifuge tube. A hole has been cut in the cap, so that a square piece of mesh can be screwed into the cap. The mesh pieces can be discarded and replaced when using different insecticides. The bed bug eggs are placed directly onto the mesh using a paint brush and then submerged into the insecticide solutions for the dipping assay. Please click here to view a larger version of this figure.
Figure 2. Representation of a dose-response graph. The percent response (mortality) of Harlan strain bed bug eggs to imidacloprid/β-cyfluthrin is plotted on the y-axis and the log concentration that the bed bugs were exposed to is represented on the x-axis. Egg mortality increased as the dose increased and mortality reached 100% at the highest concentration tested.
| Life Stage/Strain | n | LC50 (95% CI) | Slope ± SE | X2 (df) |
| Eggs | ||||
| Harlan | 250 | 0.41a (0.276-0.548) | 1.86 ± 0.24 | 33.42 (23) |
| Richmond | 320 | 1.23b (0.59-2.10) | 1.13 ± 0.14 | 82.57 (30) |
| Epic Center | 400 | 2.10b (1.049-4.587) | 0.95 ± 0.10 | 149.91 (38) |
| First Instars | ||||
| Harlan | 150 | 0.04a (0.030-0.063) | 2.16 ± 0.34 | 38.17 (28) |
| Richmond | 195 | 4.81b (1.94-10.26) | 0.66 ± 0.12 | 45.87 (37) |
| Epic Center | 190 | 19.72b (8.18-184.48) | 0.75 ± 0.17 | 45.39 (36) |
Table 1. Comparison of bed bug egg and first instar LC50 values modified from Campbell and Miller 2015 13.
Discussion
A critical step in this assay is to ensure that no eggs that are removed from a surface are damaged prior to the assay. Many insects cement their eggs to a substrate, therefore, a preliminary test may be needed to ensure that removal does not cause mortality. This test can be conducted with several replications of a treated group (bed bugs removed from filter paper) compared to a control group (bed bugs not removed). Similar low rates of mortality (or no mortality) between the treated and control group will ensure that removal did not cause egg mortality. Thus, any mortality recorded for the insecticide assay should be due to the insecticide treatment. The same preliminary experiment can be conducted using first instars to ensure that methods for collecting do not kill the experimental insects. All experiments conducted later should include control replications that do not receive insecticide but are treated with the solvent (for these experiments, tap water was the solvent).
Preliminary experiments should be conducted with the recommended label rate for formulated products and then serially diluted to determine the correct concentrations to produce a concentration response curve of mortality ranging from 20-80%. Previous insecticide studies on the same insects or compounds may also be insightful when trying to determine the appropriate concentration. Concentrations will need to be modified dependent upon the insect, life stage, and compound used. New solutions should be made each time an experiment is conducted.
The exposure of first instars to wet residues was necessary to get high mortality. Although this life stage is considered more susceptible to insecticides than older nymphs and adults, high mortality was still difficult to obtain with formulated products. Submerging the first instars into insecticides similar to the eggs was not plausible because of the resultant high mortality, thus this was a limitation to the study. The alternative first instar bioassay was manipulated to maintain first instars on surfaces to force constant contact with the treatment.
Dry residue assays and bottle assays commonly used for insecticide efficacy and resistance studies do not provide methodology for testing against multiple insect life stages17. These assays are often used to determine resistance in insects, and not to test insecticide efficacy. There are limited studies on egg dipping toxicological studies and none that have reported a standard methodology previously that can be repeated for multiple insect species.
This study provides a protocol for testing insecticide efficacy against bed bug eggs and first instars. Efficacy should be evaluated in different life stages, if not all, for a more holistic view of the efficacy of a treatment program. This consideration is important because an insect's susceptibility to insecticides changes with age and development 18. While age is an important factor in insecticide susceptibility, a factor in egg insecticide susceptibility is the eggshell. The eggshell provides a barrier to insecticides and makes insect eggs particularly difficult to control19.
These toxicological assays provide researchers a way to evaluate insecticide toxicity against various life stages of insects. Insecticidal solutions are prepared to evaluate mortality and to determine LC50 concentrations. While this protocol was used for formulated products, the steps can be manipulated for technical grade compounds and for a variety of insects and life stages to evaluate toxicity.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank Molly Stedfast for dilution and pesticide formulation assistance. We also thank Troy Anderson for probit analysis guidance and Zachary Adelman for assistance with experimental design. This research was partly supported by an Entomological Foundation award and by Virginia Pest Management scholarship funds.
References
- Sabou M, et al. Bed bugs reproductive life cycle in the clothes of a patient suffering from Alzheimer's disease results in iron deficiency anemia. Parasite. 2013;20 doi: 10.1051/parasite/2013018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt K, Kempke D, Naylor RA, Siva-Jothy MT. Sensitivity to bites by the bedbug, Cimex lectularius. Med. Vet. Entomol. 2009;23:163–166. doi: 10.1111/j.1365-2915.2008.00793.x. [DOI] [PubMed] [Google Scholar]
- Leverkus M, et al. Bullous allergic hypersensitivity to bed bug bites mediated by IgE against salivary nitrophorin. J. invest. entomol. 2006;126:91–96. doi: 10.1038/sj.jid.5700012. [DOI] [PubMed] [Google Scholar]
- Fletcher CL, Ardern-Jones MR, Hay RJ. Widespread bullous eruption due to multiple bed bug bites. Clin. Exp. Dermatol. 2002;27:74–75. doi: 10.1046/j.0307-6938.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Pritchard MJ, Hwang SW. Severe anemia from bedbugs. Can. Med. Assoc. J. 2009;181:287–288. doi: 10.1503/cmaj.090482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L) Am. J. Med. 2012;125:101–103. doi: 10.1016/j.amjmed.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Harlan HJ. Bed bugs 101: the basics of Cimex lectularius. Am. Entomol. 2006;52:99–101. [Google Scholar]
- Pinto LJ, Cooper R, Kraft SK. Bed Bug Handbook: The Complete Guide to Bed Bugs and Their Control. Mechanicsville, MD, USA: Pinto & Associates, Inc.; 2007. [Google Scholar]
- Toloza AC, et al. Differential patterns of insecticide resistance in eggs and first instars of Triatoma infestans (Hemiptera: Reduviidae) from Argentina and Bolivia. J. Med. Entomol. 2008;45:421–426. doi: 10.1603/0022-2585(2008)45[421:dpoiri]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cueto GM, Zerba EN, Picollo MI. Evidence of pyrethroid resistance in eggs of Pediculus humanus capitis (Phthiraptera: Pediculidae) from Argentina. J. Med. Entomol. 2008;45:693–697. doi: 10.1603/0022-2585(2008)45[693:eoprie]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ho SH, Goh PM. Deltamethrin as a potential ovicidal pyrethroid against Plutella xylostella L. Toxicol. let. 1984;22:161–164. doi: 10.1016/0378-4274(84)90060-2. [DOI] [PubMed] [Google Scholar]
- Bell CH, Hole BD, Evans PH. The occurrence of resistance to phosphine in adult and egg stages of strains of Rhyzopertha dominica (F.)(Coleoptera: Bostrichidae) J. Stored Prod. Res. 1977;13:91–94. [Google Scholar]
- Campbell BE, Miller DM. Insecticide Resistance in Eggs and First Instars of the Bed Bug, Cimex lectularius (Hemiptera: Cimicidae) Insects. 2015;6:122–132. doi: 10.3390/insects6010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LA, Mills KA. The toxicity of phosphine to the immature stages of resistant and susceptible strains of some common stored product beetles, and implications for their control. J. Stored Prod. Res. 1988;24:51–59. [Google Scholar]
- Robertson JL, Preisler HK, Russel RM. PoloPlus: Probit and Logit Analysis User's Guide. Petaluna, CA, USA: LeOra Software; 2003. [Google Scholar]
- Montes C, Cuadrillero C, Vilella D. Maintenance of a laboratory colony of Cimex lectularius (Hemiptera: Cimicidae) using an artificial feeding technique. J. Med. Entomol. 2002;39:675–679. doi: 10.1603/0022-2585-39.4.675. [DOI] [PubMed] [Google Scholar]
- Brogdon WG, McAllister JC. Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J. Am. Mosq. Control. Assoc. 1998;14:159–164. [PubMed] [Google Scholar]
- Katarzyna K, Saddler A, Koella JC. Effects of age and larval nutrition on phenotypic expression of insecticide-resistance in Anopheles mosquitoes. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outram I. Factors affecting the resistance of insect eggs to sulphuryl fluoride-II: the distribution of sulphuryl-35 S fluoride in insect eggs after fumigation. J. Stored. Prod. Res. 1967;3:353–358. [Google Scholar]


